Abstract
Objective
Given that depression in men is associated with risk for seriously adverse consequences, evaluating how putative neural mechanisms of depression—such as reward-related frontostriatal connectivity—may be altered in late adolescent boys with a history of depression is an important research aim. Adolescents and adults with depression have been demonstrated to show blunted striatal response and heightened medial prefrontal cortex (mPFC) activation to winning reward. Function in reward circuits appears to be best understood as coordination of regions within frontostriatal circuitry, and alterations to this circuitry could occur in those with a history of depression.
Method
The current study evaluated functional connectivity between the nucleus accumbens and mPFC in a sample of 166 ethnically-diverse boys with and without a history of depression. Participants completed an fMRI monetary reward paradigm at age 20. Lifetime history of depression and other psychiatric illnesses was measured prospectively and longitudinally, using structured clinical interviews at 7 time points from ages 8 to 20.
Results
Boys with a history of depression showed heightened positive connectivity between the nucleus accumbens and the mPFC relative to boys with no psychiatric history when winning rewards relative to losing rewards. This altered frontostriatal connectivity pattern was also associated with greater number of depressive episodes in the boys’ lifetime.
Conclusions
History of depression in late adolescent boys may be associated with altered coordination between the nucleus accumbens and mPFC when winning reward. This coordination could reflect over-signaling of the mPFC to dampen typical VS response or enhance weak VS response.
Keywords: Reward, Depression, Regulation, Positive Affect, Frontostriatal Connectivity
Increasing evidence demonstrates that diminished positive affect and blunted reward responding in the ventral striatum distinguish clinical depression from other affective disorders in adults (Epstein et al., 2006; Surguladze, Keedwell, & Phillips, 2003) and adolescents (Forbes et al., 2006; Forbes et al., 2009), are associated with biological risk for depression (Gotlib et al., 2010; Monk et al, 2008; Olino et al., 2013), and are evident even between episodes (Dichter, Kozink, McClernon, & Smoski, 2012). These findings provide strong evidence that altered neural processing of reward is a significant component of the developmental pathophysiology of clinical depression (Eshel & Roiser, 2010, Price & Drevets, 2010; Steele, Kumar, & Ebmeier, 2007). In addition, altered reward processing may also represent an endophenotype for depression, or a behavioral manifestation of genetic vulnerability present regardless of episode state (Hasler, Drevets, Manji, & Charney, 2004). Although function in reward circuitry—rather than simply response in candidate reward regions—captures reward processing, few studies have examined functional connectivity in young people with a history of depression.
Reward function is best understood within a coordinated circuit of neural regions, including the ventral striatum (VS) and medial prefrontal cortex (mPFC) (Haber & Knutson, 2010). Within the VS, activation in the nucleus accumbens appears to be the most specific to motivational aspects (“wanting”) and subjective experience (“liking”) of reward processing in the brain (Berridge, 2009, Haber & Knutson, 2010, Knutson & Greer, 2008), although the link to “wanting” appears to be stronger suggesting this region’s strong influence in anticipatory positive affect (Haber & Knutson, 2010). Within the mPFC, the pregenual anterior cingulate cortex (pgACC) is thought to play a role in top-down regulation of affect (Etkin, Egner, & Kalisch, 2011). Evidence also demonstrates that the dorsal medial region of the mPFC (medial BA 8, 9, and 10 and dorsal BA 32) including the dorsal ACC is involved in other-related processing (Denny, Kober, Wager, & Ochsner, 2012), such as analyzing the emotions and perceptions of others. Furthermore, the mPFC is functionally connected with the ventral striatum and thus may serve regulatory functions for reward responding and positive affect (Haber & Knutson, 2008). The dorsal ACC of the mPFC is also thought to be part of a larger salience network, a cluster of brain regions also comprised of the anterior insula, thalamus, amygdala, and substania nigra and implicated in orienting to and valuating of personally-relevant stimuli (Seeley et al., 2007). Thus, the dorsal mPFC’s strong role in reward functioning may be due in part to its link to salience processing. Altogether, the VS and mPFC have consistently been implicated as regions involved in reward processing; however the way in which these two regions may act in tandem in depression is still relatively unknown.
Ventral striatal response is low during reward receipt in adolescent and adult depression, and for adolescents at biological risk for depression (Epstein et al., 2006; Forbes et al., 2009; Gotlib et al., 2010; Monk et al., 2008; Surguladze et al., 2003). Similar to altered reward processing in the VS, greater activation in the mPFC has been demonstrated in clinically depressed adolescents and adults (Knutson & Greer, 2008), and this altered activation pattern in the mPFC has also been associated with greater increases in depressive symptoms during adolescence in boys (Morgan, Olino, McMakin, Ryan, & Forbes, 2013). The mPFC may be particularly sensitive to social experiences, as suggested by literature on the influence of stress on reward response (Bogdan & Pizzagalli, 2006). These altered reward findings, taken together, may suggest that over-regulation of positive affect elicited by the ventral striatum via the mPFC (pgACC, dACC, and dmPFC) may hinder enjoyment of rewards, may signify over-concern with or heightened salience of reward-related self-performance in relation to others, and may increase risk for depression.
One possibility is that late adolescents with a history of depression show heightened co-activation of the striatum and cortical reward regions relative to individuals without a history of depression, perhaps indicating heightened engagement of the mPFC to dampen activation from the striatum when its activation is elicited during the pursuit of valued and salient rewards (i.e., greater positive connectivity between VS and regulatory regions). Indeed, recent work has indicated that stronger positive frontostriatal connectivity in response to positive social feedback is associated with higher levels of social anhedonia during the transition from adolescence to adulthood (Healey, Morgan, Musselman, Olino, & Forbes, 2014), providing further evidence that heightened mPFC-accumbens connectivity in response to reward may be associated with a dampening of positive affect. Numerous studies have investigated responding in these specific reward regions (e.g., Epstein et al., 2006; Surguladze et al., 2003; Forbes et al., 2006, 2009; Smoski et al., 2009) among others, but little research has investigated reward-circuitry function, particularly for adolescents with psychopathology. Functional connectivity, a method of psychophysiological interaction (PPI) that measures co-activation between neural regions, is an emerging method for measuring neural regulation of affect processing. A growing body of research has begun using PPI to investigate the link between functional connectivity and psychopathology, using both categorical group designs (Almeida et al., 2009, 2011) and dimensional characteristics of symptom level (Healey et al, 2014; Keller et al., 2013). Also, whereas a wealth of research has evaluated functional connectivity within the default mode network (DMN) in depression (e.g., see Sheline et al., 2009), studies evaluating reward-circuitry function in depressed individuals are needed to determine whether depression is associated with alterations in frontostriatal connectivity, particularly in response to rewarding stimuli. As suggested by Forbes and Dahl (2012), the demonstrated alterations in the VS and the mPFC could reflect altered coordination between these two regions (rather than just independent disruptions in these regions) and this disrupted coordination may be present even out of episode in adolescents with a history of depression. No study, to our knowledge, has specifically evaluated functional connectivity of the VS to other reward-related circuitry in adolescents with a history of depression.
Understanding aberrations in reward functioning during the transition from adolescence to adulthood, a developmental period when maturation in the mPFC is occurring rapidly and aiding abstract goal-directed planning (Blakemore, 2008) is particularly important. Adolescents and young adults with trait-like dysregulated reward function may have difficulties during this period with normative processes (i.e., engaging in new, risky behaviors with peers; forming meaningful relationships) and this may increase their vulnerability to developing depression (Davey, Yucel, & Allen, 2008). Given that depression typically onsets and worsens during adolescence and early adulthood, evaluating processes associated with depression vulnerability from childhood through adolescence into the transition to adulthood is important and few studies have the longitudinal data to examine this important question.
Also, importantly, although depression is more common in women, depressed men are at even greater risk for devastating outcomes, such as death by suicide, compared with depressed women (Dumais et al., 2005), suggesting that characterizing reward alterations in adolescent males with a history of depression is important for informing basic research on neural attributes of depression and to inform prevention and intervention efforts.
We evaluated how connectivity between the nucleus accumbens (Van den Bos, Cohen, Kahnt, & Crone, 2012) and the mPFC (Price & Drevets, 2010; Amodio & Frith, 2006; Phillips et al., 2008) differs for male adolescents with and without a lifetime history of clinical depression. We used a seed-based approach to evaluate task-based functional connectivity (O’Reilly et al., 2012) between these two reward regions. We focused on mPFC, including dorsal ACC, as our region of interest based on prior and consistent evidence of its role in reward functioning in depression. However, we considered other regions of the salience network—anterior insula, thalamus, substania nigra, amygdala—in order to test the specificity of mPFC’s association with the nucleus accumbens. Based on our assessments in the longitudinal, prospective study of mental health and associated problems in boys, we included two comparison groups: boys with no prior history of psychiatric illness (healthy comparison group) and boys without a history of depression but with a prior history of other psychiatric illnesses (psychiatric comparison group) in order to evaluate the specificity of reward-related alterations in history of depression relative to history of other psychiatric illnesses. We also explored other salience network regions (e.g., amygdala, anterior insula) in order to test the specificity of boys’ response to reward stimuli relative to salient stimuli in general. Given previous empirical findings that social anhedonia is associated with stronger positive VS-mPFC connectivity in response to rewarding stimuli (Healey et al., 2014), we hypothesized that boys with history of depression would exhibit greater mPFC-accumbens connectivity during a rewarding event relative to boys without this history. We predicted that our findings would be specific to boys with a history of depression (relative to both boys with other psychiatric illness and healthy boys) and to the mPFC, a region consistently implicated in reward processing, rather than in other regions implicated in salience processing.
Method
Participants were 166 boys from the Pitt Mother and Child Project, a longitudinal project on vulnerability and resilience in boys from low-income families, a population at heightened risk for various emotional and behavioral problems (Shaw, Hyde, & Brennan, 2012). Families were recruited to the study when boys were between the ages of 7 and 17 months of age from the Women, Infants, and Children Nutritional Supplement (WIC) centers in the greater Pittsburgh area. At age 18 months, average family income was $1,135.36 per month with a Hollingshead Index of 23.01, indicating working class status. All participants were boys because of the project’s original focus on the developmental precursors of externalizing problems. The sample was 55% European-American, 41% African-American, and 4% were of other races/ethnicities (e.g., biracial, Hispanic). All procedures received Institutional Review Board approval at the University of Pittsburgh. Although previous studies from this larger project have evaluated how parenting characteristics and stressful life events predict activation in reward regions in this sample (e.g., Casement, Shaw, Sitnick, Musselman, & Morgan, 2014; Morgan, Shaw, & Forbes, 2014), this study was unique in evaluating how reward circuitry connectivity differs depending on boys’ own clinical history of depression and other psychiatric disorders across a long longitudinal time frame (age 8–20).
Originally, 310 boys and their families were recruited to participate in the longitudinal project. Of the 310 boys, 186 boys participated in fMRI scan at age 20 with 184 of those having lifetime psychiatric history data. Of those 184 participants, 166 had usable fMRI data (n = 10 removed due to low behavioral response to task or misunderstanding the task; n = 5 with < 80% coverage in the VS; n = 2 due to warped images; n = 1 due to being psychotic during the scan, none due to excessive head movement). Boys were required to be free of stimulants and other psychiatric medications to participate in the scan. Of these 166 boys, 43 boys had current or past clinical depression (7 met criteria for current MDD and were retained in the depression group for analyses; the remaining met criteria for past MDD or Dysthymia), 55 had a prior history of other psychiatric illnesses (Attention Deficit Hyperactivity Disorder, Oppositional Defiant Disorder, Conduct Disorder, Anxiety Disorders, Substance Dependence, Psychotic Disorder, Antisocial Personality Disorder) but not MDD or Dysthymia, and 68 boys had no prior history of clinical depression or other psychiatric illnesses. Boys in the history of depression group had other psychiatric illnesses. Table 1 lists demographic information and the distribution of other psychiatric illnesses for the history of depression and history of psychiatric illnesses groups. There were no significant differences in boys’ race and yearly income by diagnostic group. At the time of the scan, boys were medically and neurologically healthy.
Table 1.
Demographic and Diagnostic Data per Group
| History of MDD (N = 43) | History of Other Psych Illnesses (N = 68) | Healthy (N = 55) | Statistic | |
|---|---|---|---|---|
| Race | 61% Caucasian, 33% African American, 7% Other |
54% Caucasian, 35% African American, 10% Other |
48% Caucasian, 41% African American, 11% Other |
χ2 = 10.33; p = .59 |
| Yearly Income at age 20 |
$15,712.20 | $16,270.79 | $16,710.75 | F = .01; p = .98 |
| Diagnostic History |
51% (9%) Anxiety Disorder, 47% ADHD, 47% ODD, 30% Conduct Disorder, 21% Substance Dependence, 9% ASPD |
50% ADHD, 44% ODD, 38% (4%) Anxiety Disorder, 25% Conduct Disorder, 19% with Substance Dependence, 16% ASPD, 3% (3%) Bipolar Disorder |
-- | -- |
Note. ADHD = Attention Deficit Hyperactivity Disorder. ODD = Oppositional Defiant Disorder. ASPD = Antisocial Personality Disorder. Race and Diagnostic percentages may not total to 100%. Current rates of Anxiety Disorders are in parentheses. ADHD, ODD, and Conduct Disorder were not assessed at age 20. ASPD and Substance Dependence were only assessed at age 20.
Measures
History of psychiatric illness
Boys’ history of psychiatric illness was measured using semi-structured interviews with clinically trained bachelor’s or master’s level research associates trained to reliability by a licensed psychologist at ages 8, 10, and 11 via the Kiddie Schedule for Affective Disorders (KSADS; Kaufman et al., 1997) with parent report, at age 12 via the KSADS with parent report for externalizing disorders and boys’ report for internalizing disorders, at ages 15 and 17 via KSADS with boys’ report, and at age 20 via the Structured Clinical Interview for Depression (SCID; Spitzer et al., 1992). At age 20, boys also completed the SCID-II module on Antisocial Personality Disorder. Diagnoses were determined during case conference with the two licensed principal investigators of the study.
Neural response to reward
Boys completed a widely-used monetary reward fMRI paradigm at age 20 to assess response to reward (Forbes et al., 2010). The fMRI paradigm was a slow event-related card-guessing game that evaluates neural response to the anticipation and receipt of monetary reward feedback and reliably engages the VS and mPFC in adolescents and adults with affective disorders (Forbes et al., 2009, 2010; Nusslock et al., 2012). Participants received win, loss, or no-change (neutral) feedback for each trial. Participants were told that their performance would determine a monetary reward after the scan, with $1 for each win and 50 cents deducted for each loss. Trials were presented in pseudorandom order with predetermined outcomes. Earnings totaled $6. Trials were presented in a single run, with 24 trials total and a balanced number of trial types within runs (i.e., 12 possible-win vs. no-change trials and 12 possible-loss vs. no-change trials). During each trial, participants guessed via button press whether the value of a visually presented card, with a possible value of 1–9, was higher or lower than 5 (4s), learned the trial type (possible-win, possible-loss) to anticipate feedback (6s) and received feedback (won money, loss money, or no change; 1s plus 9s inter-trial interval). Participants were unaware of fixed outcome probabilities.
fMRI Acquisition and Preprocessing
Each participant was scanned using a Siemens 3T Trio scanner. BOLD functional images were acquired with a gradient echo planar imaging (EPI) sequence and covered 39 axial slices (3.1mm thick) beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE=2000/25ms, FOV=20cm, matrix=64×64). All scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data. Before the collection of fMRI data for each participant, we acquired a reference EPI scan that we visually inspected for artifacts (e.g., ghosting) and for good signal across the entire volume of acquisition. The fMRI data from all included participants were cleared of such problems.
Preprocessing and whole-brain image analyses were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). For each scan, structural images for each participant were segmented, and functional images were realigned to correct for head motion, co-registered to the segmented structural data, spatially normalized into standard stereotaxic space (Montreal Neurological Institute template) using a 12-parameter affine model, and smoothed with a 6mm full-width at half-maximum Gaussian filter. Participants’ data were inspected for adequate coverage of the VS (> 80%) and adequate task responding. All remaining participants had movement < 2mm in each plane on average across all frames.
Data Analytic Strategy
For each participant, first-level general linear model (GLM) predetermined condition effects were calculated to produce an image for our contrast of interest: win outcome > loss outcome. We chose to contrast win trials with loss trials as PPI analyses require that coordination during physiological regions of interest depend on correlation with psychological states as inferred from active task conditions (O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012). By subtracting response to loss, this contrast isolates response to winning from response to feedback in general.
Second level analyses then evaluated group differences in frontostriatal connectivity for this contrast of interest using psychophysiological interaction (PPI) in SPM8 (for description of PPI, see O’Reilly et al., 2012). The bilateral nucleus accumbens was our seed region of interest because of the strong and consistent evidence of its role in reward processing (Haber & Knutson, 2010). Rather than using a seed-based region larger in size (e.g., the entire VS), we focused on the nucleus accumbens as our seed region as a conservative test of reward processing. Furthermore, we focused on one region of the striatum based on studies of connectivity and neural activation demonstrating that sub-regions of the striatum (i.e., nucleus accumbens, caudate, putamen) appear to differentially co-activate other reward regions (DiMartino et al., 2008; Postuma & Dagher, 2006) and have differing reward functions (Haber & Knutson, 2010). We used a task-based approach (versus resting-state connectivity) to examine neural co-activation in response to an event or stimulus because we were interested in depression-related neurobiological differences in reward circuitry during rewarding events (Davey et al., 2008; Forbes & Dahl, 2012, Hulvershorn, Cullen, & Anand, 2011).
The nucleus accumbens was defined anatomically using the WFU PickAtlas (v. 3.03). Based on our hypotheses and the literature on depression effects on nucleus accumbens and mPFC response to reward, we evaluated the strength of association between our seed region of interest with other neural regions in the context of winning reward (vs. losing reward) and in the context of anticipating the potential receipt of reward (vs. anticipating the potential loss of reward). We then evaluated main effect of group (history of depression, history of other psychiatric illness, and healthy controls) on frontostriatal connectivity using mPFC (includes dorsal ACC), insula, amygdala, substantia nigra, and thalamus region of interest (ROI) masks. The mPFC ROI mask was defined anatomically using PickAtlas as a 25-mm radius sphere encompassing medial BA 10 and BA 32 (see Forbes et al., 2010). Our insula, amygdala, substantia nigra, and thalamus masks were selected anatomically using the PickAtlas tool. Simulations in the AlphaSim program in AFNI (Forman et al., 1995; Ward, 2000) were used to estimate the minimum number of contiguous voxels required to avoid Type I error (cluster level threshold p < .05). The minimum number of contiguous voxels for each mask was 178 voxels for mPFC, 62 voxels for amygdala, 11 voxels for substantia nigra, 102 voxels for thalamus, and 119 voxels for insula.
Next, to conduct post-hoc tests of group differences, significant findings from main effect of group were saved as a mask to be used for follow up t-tests evaluating differences within groups (e.g., history of depression vs. healthy controls). Once again, simulations in the AlphaSim program in AFNI were used to estimate the minimum number of contiguous voxels required to avoid Type I error (cluster level threshold p < .05) for our saved significant main effects of group masks (Ward, 2000). The minimum number of contiguous voxels was 36 voxelsfor the mPFC main effects mask and 44 voxels for our insula main effects mask. Whole brain analyses were then used to confirm that our ROI remained significant in unconstrained results, using a threshold of p< .01 and extent threshold of 30 voxels.
Analyses in SPSS using extracted data from significant connectivity clusters evaluated whether age of onset and number of depressive episodes were associated with connectivity between the nucleus accumbens and our ROIs.
Results
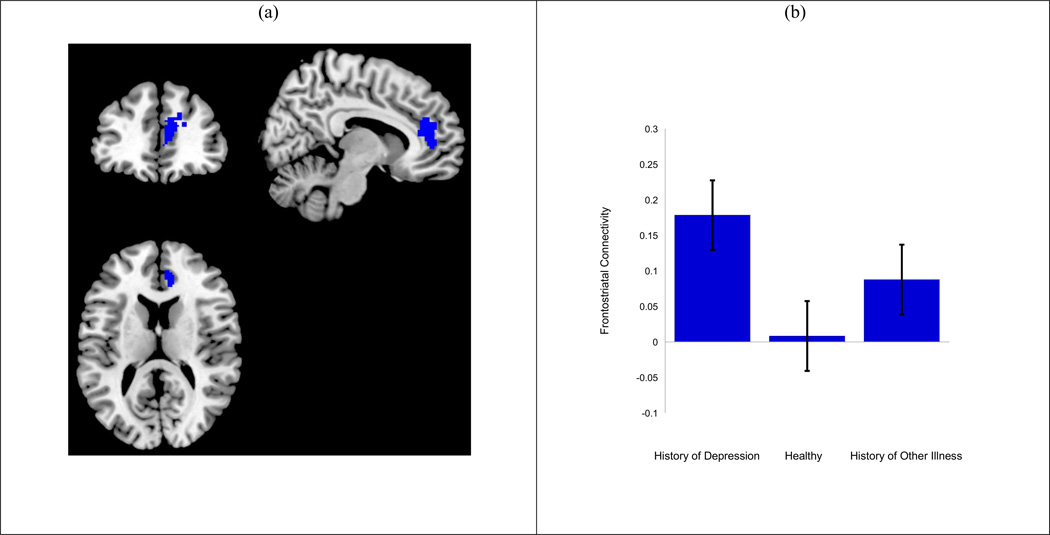
There was a significant main effect of group for frontostriatal connectivity during reward outcome vs. loss outcome (pgACC/adACC and dmPFC, includes BA 32 and medial BA 9/10, F = 9.46, 320 voxels, [10, 42, 15]; Figure 1) (Table 2). Follow-up pairwise analyses in SPM8 indicated that boys with a history of depression showed significantly greater positive connectivity relative to boys with no psychiatric history after correcting for multiple comparisons.1
Figure 1.
Heightened positive connectivity between the nucleus accumbens and dmPFC/pgACC when winning rewards distinguishes boys with lifetime history of depression from boys without any psychiatric history.
Table 2.
History of Depression and Frontostriatal Functional Connectivity in Response to Monetary Reward
| Condition/Contrast | Region | BA | x y z | F or t | Cluster |
|---|---|---|---|---|---|
| Reward Win vs. Loss Lose | |||||
| Main Effect of Group | pgACC/adACC, dmPFC | BA 32; Medial BA 9/10 | 10, 42, 15 | 9.46 | 320* |
| Pairwise Group Differences | |||||
| MDD > Healthy | pgACC/adACC, dmPFC | BA 32; Medial BA 10 | 20, 32, 19 | 2.97 | 184* |
| MDD > Psychiatric | pgACC/adACC | Medial BA 9 | 12, 38, 22 | 2.42 | 28 |
| Psychiatric > Healthy | pgACC | Medial BA 10 | 8, 45, 11 | 2.71 | 22 |
| Reward Anticipation vs. Loss Anticipation | |||||
| -- | -- | -- | -- | -- | -- |
Note. Group main effect is an F statistic, and pairwise effects are t statistics (degrees of freedom = 163). Pairwise group differences are based on post-hoc tests restricted to clusters revealed by the test for main effect of group. MDD = History of Major Depressive Disorder. pgACC= Pregenual anterior cingulate cortex. adACC = Anterior dorsal anterior cingulate cortex. dmPFC = Dorsal medial prefrontal cortex. BA = Brodmann Area. Coordinates are in Talairach space and refer to peak voxel of the cluster.
As predicted by our specificity hypothesis, there were no significant differences between boys with a psychiatric illness compared to healthy boys. Unexpectedly, there was also no difference between boys with a psychiatric illness and boys with a lifetime history of depression, after correcting for multiple comparisons. Findings for our mPFC-accumbens connectivity for our reward anticipation vs. loss anticipation contrast also did not pass AlphaSim corrections.
To further test for specificity to regions implicated in reward processing, we next ran analyses evaluating connectivity with other salience network regions of interest (amygdala, thalamus, anterior insula, and substantia nigra). There were no significant findings for our reward anticipation vs. loss anticipation or for our reward outcome vs. loss outcome contrasts, after correcting for multiple comparisons, for any of our remaining salience network regions of interest. Whole brain analyses indicated that our mPFC cluster still emerged as a region of interest for the main effect of group (44 voxels, p< .01) and for the MDD > healthy contrast (109 voxels, p < .01), even among unconstrained analyses.
Correlations using extracted values from our significant connectivity findings showed that number of depressive episodes was positively correlated with mPFC-accumbens connectivity (r = .16, p< .05). Age of onset was not significantly related to mPFC-accumbens connectivity.
Discussion
Our findings indicate that lifetime history of depression for low-SES, late-adolescent boys is associated with altered functional connectivity between the nucleus accumbens and the mPFC in response to winning monetary reward. Specifically, late-adolescent boys with a history of depression showed greater positive functional connectivity between the bilateral nucleus accumbens and the mPFC when winning rewards relative to peers without any psychiatric history from a similar sociodemographic background. Importantly, this altered connectivity pattern was found to be specific to boys with a lifetime history of depression and specific to the mPFC, a region consistently implicated in reward processing, rather than in other brain regions implicated in processing salient stimuli in general. Together, the nucleus accumbens and mPFC may act ineffectively in tandem and may over-engage one another for adolescents with a history of depression. One possible explanation for this, given our results and anatomical projections from the mPFC to the nucleus accumbens (Haber & Knutson, 2010) and given the ACC’s putative role in affect regulation (Etkin, Egner, & Kalisch, 2011) including implicit emotion regulation (Gyurak, Gross, & Etkin, 2011), is that the mPFC may be over-regulating accumbens response in the face of a pleasant event as a means of dampening boys’ subjective experience of positive affect when winning reward. The mPFC is also implicated in self- and other-related processing, which may suggest that boys could be over-evaluating their performance in relation to others’ performance when also experiencing pleasure from winning reward. Another plausible explanation, especially given PPI’s inability to determine timing or direction of effects, is that the mPFC is functionally over-connected with an underactive nucleus accumbens and thus responds strongly to initiate activation in the nucleus accumbens (Forbes & Dahl, 2012).
Boys with a history of depression may find it difficult to enjoy positive and rewarding experiences perhaps due to prior experience with disappointment and loss (Davey et al., 2008; Olino et al., 2011) and may either consciously or unconsciously dampen their affective response to prevent feelings of disappointment and sadness that may have accompanied positive events in the past. Indeed, pursuing reward is risky in that it can lead to intense feelings of joy or deep feelings of disappointment, depending on the outcome (Davey et al., 2008). Even after a positive outcome of reward pursuit, depressed adolescents appear to dampen their affective response potentially as means of not getting their hopes up (Olino et al., 2011). This process is particularly relevant during mid to late adolescence, when rewards take on new value and meaning such as self-attainment and status (Davey et al., 2008). Given that the dorsal ACC of the mPFC is thought to be involved in both affect regulation and salience detection (Etkin et al.,2011; Seeley et al., 2007), our findings may indicate that heightened communication between the VS and mPFC may suggest dampening of positive affect during rewarding contexts
We also found that greater positive frontostriatal connectivity was associated with greater number of episodes of depression but not to age of onset of depression. These findings may indicate that these reward alterations are associated with more depressive symptomatology rather than to duration of time since becoming depressed. Altered reward processing may be associated with the developmental progression of the disease rather than developmental age. Multiple depressive episodes could influence how frontostriatal circuitry responds to rewarding events (i.e., a scar effect). Experiencing feelings of loss, low mood, and diminished interest in fun events repeatedly may have negatively influenced these boys’ ability to respond to rewarding events adaptively. However, it should be noted that due to the nature of our design, we cannot rule out that altered frontostriatal connectivity in response to reward may be a biological characteristic of recurrent depression (thus emerging prior to onset of depression). Future research evaluating connectivity and depression prospectively is needed to test whether this altered connectivity leads to recurrent depression or is a scar effect of multiple depressive episodes.
Coordination between the VS and mPFC is important, as these reward regions are connected through the ventral pallidum and thus should function in tandem (Haber & Knutson, 2010). Altered coordination between these regions may reflect dysfunction in reward circuitry and may be associated with affective and behavioral consequences. These findings suggest that vulnerability to depression—or, possibly, scarring as a result of past episodes—may be associated with neural disruptions when winning rewards and that this disrupted reward functioning may persist following remission of the depressive episode. Social reward processing becomes more important during adolescence and early adulthood, developmental periods in which individuals typically create and sustain deeper, more meaningful social relationships and pursue new personal accomplishments and developmental periods. Adolescence and early adulthood are also periods in which depression typically onsets and becomes chronic. Thus, trait-like dysregulated reward function may contribute to vulnerability to recurrent depressive episodes during adolescence and the transition to adulthood.
Interestingly, our group difference findings were specific to dmPFC (including dorsal ACC) rather than other regions of the salience network, suggesting that the regulatory roles of the dmPFC may be most relevant to reward functioning in depression. Indeed, in their review of neural systems implicated in depression, Hamilton, Chen, & Gotlib (2013) suggested that the salience network may be specifically elicited in response to negative stimuli rather than positive stimuli in individuals with depression. Thus, although disruptions in the salience network have been found in individuals with depression (Hamilton et al., 2013), our hypothesis was that lifetime history of depression (and the task we used) would be linked to reward-related (dys)function and frontostriatal regions are most closely tied to this function (Haber & Knutson, 2010).
Somewhat surprisingly, findings were specific to winning reward and not anticipating reward, indicating that prior history of depression may be linked to alterations in the experience of liking or enjoying rewarding experiences. Although other research suggests that depression may arise from difficulty with anticipatory positive affect (Davidson, 1998), it may be possible that alterations in reward anticipation may characterize earlier phases of the development of depression (i.e., prior to onset of depression, during first episode). Boys with an established history of depression may show differences in how they process rewards once they obtain them (Berridge, 2009; Olino et al., 2011), because they may have more prior experiences of deeply-felt disappointment and loss. Indeed, our finding that greater number of episodes of depression is associated with stronger positive frontostriatal connectivity suggests that this reward outcome finding may be related to recurrent depression rather than to emerging, sub-threshold depression or first episode of depression (which could be more closely linked to altered reward anticipation). However, further testing and replication of our findings is needed to support this possibility.
Our findings were significant when comparing boys with a history of depression to clinically healthy boys, but not to boys with other psychiatric illnesses. However, the pattern of our findings illustrates that boys with a history of depression appear to show greater positive frontostriatal connectivity compared to boys with a history of other psychiatric illness and those boys appear to show greater connectivity relative to healthy boys. It should be noted that our psychiatric comparison group had diagnoses also associated with altered reward responding in the striatum (e.g., substance dependence, ADHD, anxiety disorders). However given that only our history of depression group significantly differed than our healthy comparison group, disruptions in connectivity between the nucleus accumbens and the dmPFC and pgACC of the mPFC may be associated with clinical depression but not other reward-related disorders.
We note that our study evaluated reward-related brain function in a unique sample of late adolescent boys with a history of depression, a population often neglected in depression research but nonetheless important to understanding this devastating disorder. To fully understand the nature of frontostriatal connectivity in depression, it will be valuable for future studies to include varying SES, male and female participants, and to use prospective designs. Our depressed group showed the predicted pattern of altered frontostriatal connectivity during reward processing that has been proposed to characterize depression (Forbes & Dahl, 2012) and has been observed in prior research on social anhedonia (Healey et al, 2014). Boys from similar SES backgrounds with a history of depression, regardless of their current depressive symptoms, may show these altered patterns due to inheriting vulnerable reward systems that are impacted by their environment.
Although we propose that frontostriatal connectivity differences in our history of depression group reflects further evidence of a depression endophenotype, our findings could also be a result of scar effects of experiencing multiple episodes of depression. Future work that compares remitted individuals to individuals with a familial risk for depression can clarify this question. A larger group of boys with a history of depression may have allowed us to evaluate altered connectivity in boys with current depression vs. past depression. Although we did not have the power to evaluate this comparison, it should be noted that analyses that excluded the seven boys with current MDD produced substantively similar findings (i.e., greater positive frontostriatal connectivity in boys with a history of MDD relative to healthy boys). Also, as all of the participants were late adolescent, low SES boys from an urban community, our findings may not generalize to samples of girls or boys of higher SES or from other types of communities (i.e., rural, suburban) or in other developmental periods. Furthermore, importantly, PPI does not allow the determination of direction/timing of connectivity, which limits our ability to detect which region is responding to the other.
Our study had many strengths including prospective and longitudinal examination of history of depression from childhood through late adolescence in a sample of young men who were at risk for affective and behavioral problems due to low sociodemographic status. The use of this rich sample of high risk young men allowed us to evaluate alterations in frontostriatal connectivity and its association with history of depression. Another strength of the study was the use of a widely utilized reward paradigm that has been validated with populations with various clinical disorders and with a wide range of ages (Forbes et al., 2009, 2010; Nusslock et al., 2012).
The current study is the first, to our knowledge, to be able to demonstrate that boys with a history of depression show altered coordination of reward circuits relative to boys without this history. These findings are important for intervention development. Modules that teach high-risk adolescent boys how to effectively regulate their positive affect and pursue rewards adaptively may be useful for promoting effective neural coordination of reward circuits and preventing feelings of distress and sadness when experiencing rewarding events.
Acknowledgements
This research was supported by Grant R01 MH050907 from the National Institutes of Health to Daniel S. Shaw and R01 DA02622 to Daniel S. Shaw and Erika E. Forbes. We thank the staff and study families of the Pitt Mother and Child Project for their time and commitment to this research.
Footnotes
Declaration of Interest. All authors report no biomedical financial interests or potential conflicts of interest.
Analyses in which we removed the seven participants with current MDD were substantively similar and passed AlphaSim corrections.
Contributor Information
Judith K. Morgan, Email: morganjk@upmc.edu.
Daniel S. Shaw, Email: casey@pitt.edu.
Thomas M. Olino, Email: olinotm@upmc.edu.
Samuel C. Musselman, Email: musselmansc@upmc.edu.
Nikhil T. Kurapati, Email: ntk@pitt.edu.
Erika E. Forbes, Email: forbese@upmc.edu.
References
- Almeida JRC, Versace A, Mechelli S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Kronhaus DM, Sibille E, Langenecker SA, Versace A, LaBarbara EJ, Phillips ML. Abnormal left-sided orbitomedial prefrontal cortical-amygdala connectivity during happy and fear face processing: A potential neural mechanism of female MDD. Frontiers in Psychiatry. 2011;2:1–14. doi: 10.3389/fpsyt.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Wanting and liking: Observations from the neuroscience and psychology laboratory. Inquiry. 2009;52:378–398. doi: 10.1080/00201740903087359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Review Neuroscience. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: Implications for depression. Biological Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casement MD, Shaw DS, Sitnick SL, Musselman S, Forbes EE. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, &Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience Biobehavioral Review. 2008;32:1–19. doi: 10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24:1742–1752. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Kozink RV, McClernon JF, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. Journal of Affective Disorders. 2012;136:1126–1134. doi: 10.1016/j.jad.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMartino A, Shcheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: A resting state fMRI study. Cerebral Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dumais A, Lesage AD, Alda M, Rouleau G, Dumont M, Chawky N, Roy M, Mann JJ, Benkelfat C, Turecki G. Risk factors for suicide completion in major depression: A case-control study of impulsive and aggressive behaviors in men. American Journal of Psychiatry. 2005;162:2116–2124. doi: 10.1176/appi.ajp.162.11.2116. [DOI] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;38:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulated and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, May CJ, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: An fMRI study. Journal of Child Psychology and Psychiatry. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research Review: Altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry. 2012;53:3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Tarr JA, Sciarrillo SR, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of American Academy of Child and Adolescent Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Hamilton JP, Cooney RE, Singh MK, Henry ML, Joorman J. Neural processing of reward and loss in girls at risk for major depression. Archives of General Psychiatry. 2010;67:380–386. doi: 10.1001/archgenpsychiatry.2010.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: A dual-process framework. Cognition and Emotion. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JP, Chen MC, Gotlib IH. Neural systems approaches to understanding major depressive disorder: An intrinsic functional organization perspective. Neurobiology of Disease. 2013;52:4–11. doi: 10.1016/j.nbd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charnery DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Healey KL, Morgan JK, Musselman SC, Olino TM, Forbes EE. Social anhedonia and medial prefrontal response to mutual liking in late adolescents. Brain and Cognition. 2014;89:39–50. doi: 10.1016/j.bandc.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn LA, Cullen K, Anand A. Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging & Behavior. 2011;5:307–328. doi: 10.1007/s11682-011-9134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J, Young CB, Kelley E, Prater K, Levitin DJ, Menon V. Trait anhedonia is associated with reduced reactivity and connectivity of mesolimbic and paralimbic reward pathways. Journal of Psychiatric Research. 2013;47:1319–1328. doi: 10.1016/j.jpsychires.2013.05.015. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for school-age children present and lifetime version. (K-SADSPL): Initial reliability and validity data. Journal of American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: Neural correlates and consequences for choice. Philosophical Transactions of the Royal Society. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expression in children and adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan N, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK, Shaw DS, Forbes EE. Maternal depression and warmth during childhood predict age 20 neural response to reward. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:108–117. doi: 10.1016/j.jaac.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, Frank E, LaBarbara EJ, Klein CR, Phillips ML. Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14:249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Dahl RE, Ryan ND, Silk JS, Birmaher B, Axelson DA, Forbes EE. I won, but I’m not getting my hopes up”: Depression moderates the relationship of outcomes and reward anticipation. Psychiatry Research. 2011;194:393–395. doi: 10.1016/j.pscychresns.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Williamson DE, Dahl RE, Ryan ND, Forbes EE. Reduced reward anticipation in youth at high-risk for unipolar depression: A preliminary study. Developmental Cognitive Neuroscience. 2013 doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TEJ, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience. 2012;7:604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Grecius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, Brennan LM. Early predictors of boys’ antisocial trajectories. Development and Psychopathology. 2012;24:871–888. doi: 10.1017/S0954579412000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID) I: History, rationale, and description. JAMA Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Steele JD, Kumar P, Ebmeier KP. Blunted response to feedback information in depressive illness. Brain. 2007;130:2367–2374. doi: 10.1093/brain/awm150. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Keedwell P, &Phillips M. Neural systems underlying affective disorders. Advances in Psychiatric Treatment. 2003;9:446–455. [Google Scholar]
- Van den Bos W, Cohen MX, Kahnt T, Crone E. Striatum-medial prefrontal cortex connectivity predicts developmental changes in reinforcement learning. Cerebral Cortex. 2012;22:1247–1255. doi: 10.1093/cercor/bhr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. AFNI 3dDeconvolve Documentation, Medical College of Wisconsin. 2000 [Google Scholar]