Abstract
An interaction exists between stress and alcohol in the etiology and chronicity of alcohol use disorders; yet a knowledge gap exists regarding the neurobiological underpinnings of this interaction. In this regard, we employed an 11-day unpredictable, chronic, mild stress (UCMS) procedure to examine for stress-alcohol cross-sensitization of motor activity, as well as alcohol consumption/preference and intoxication. We also employed immunoblotting to relate the expression of glutamate receptor-related proteins within subregions of the nucleus accumbens (NAC) to the manifestation of behavioral cross-sensitization. UCMS mice exhibited a greater locomotor response to an acute injection of 2 g/kg alcohol than unstressed controls and this cross-sensitization extended to alcohol intake (0–20%), as well as to the intoxicating and sedative properties of 3 and 5 g/kg alcohol, respectively. Regardless of prior alcohol injection (2 g/kg), UCMS mice exhibited elevated NAC shell levels of mGlu1α, GluN2b and Homer2, as well as lower PLCβ within this subregion. GluN2b levels were also lower within the NAC core of UCMS mice. The expression of stress-alcohol locomotor cross-sensitization was associated with lower mGlu1α within the NAC core and lower ERK activity within both NAC subregions. As Homer2 regulates alcohol sensitization, we assayed also for locomotor cross-sensitization in Homer2 wild-type (WT) and knock-out (KO) mice. WT mice exhibited a very robust cross-sensitization, that was absent in KO animals. These results indicate that a history of mild stress renders an animal more sensitive to the psychomotor and rewarding properties of alcohol, which may depend upon neuroplasticity within NAC glutamate transmission.
Keywords: alcoholism, glutamate, Homer proteins, intoxication, nucleus accumbens, unpredictable chronic mild stress
INTRODUCTION
Globally, alcohol use is the leading risk factor for premature death & disability among people between the ages of 15–49 (Lim et al., 2012). Alcohol use disorders (AUDs) exhibit a very high degree of co-morbidity with all affective disorders (e.g., bipolar disorder, major depression, generalized anxiety disorder, post-traumatic stress disorder); with the life-time co-prevalence rates ranging from 20–47% (e.g., Hasin and Grant 2004; Hasin et al. 2005, 2007; Kessler et al. 2005; Presley et al. 1994; SAMHSA 2010). It is unclear from epidemiological data which disorder preceded the other in comorbid individuals. However, the median age of onset for comorbid AUD-affective disorder is estimated to be 10 years younger than that for a substance abuse disorder alone; alcoholics are 2–3 times more likely than non-alcoholics to be diagnosed with an affective disorder (e.g., Grant et al. 2005; Hasin and Grant 2004; Kessler et al. 2005). Moreover, individuals suffering from affective disorders are more likely to develop AUDs than the general population (e.g., Blanco et al. 2013; Grant et al. 2005; Hasin and Grant 2004; Jacobsen et al. 2001; Kessler et al. 2005) and report affective symptom relief as a motivating factor for their problematic drinking (e.g., Annis et al. 1998; Brown et al. 1995; Dawson et al. 2005; Heilig et al. 2010; Nishith et al., 2001; Noone et al. 1999; Sinha 2009; Sinha et al. 2009; Uhart and Wand 2009). Although such reports implicate affective disorders as antecedents of problem drinking in humans, a large number of confounding variables (incl. other drug abuse or therapeutic drug treatment, trauma &/or frequent aversive life events, frequency/amount of alcohol consumption, duration of drug/alcohol abstinence) render it difficult to disentangle cause-effect relations between life histories of adverse events or excessive alcohol intake and behavioral anomalies in human subjects.
In this regard, the use of partial animal models have proven indispensable tools with which to parse apart the role for subject factors (e.g., a life history of adversity/stress) as underpinning excessive alcohol consumption and to determine the impact of these subject factors upon the brain. In this regard, stress increases alcohol intake and potentiates the effects of alcohol upon the brain’s reward system in animal models of AUDs (e.g., Breese et al., 2005; Delis et al., 2013; Heilig & Koob, 2007; Koob, 2013; Richardson et al., 2008). Stress is also a well-known trigger for alcohol craving and relapse to alcohol drinking in both humans with AUDs and in animal models of this disorder (c.f., Becker et al. 2011; Logrip et al. 2012). Moreover, the locomotor hyper-activating phenotype produced by the repeated exposure to stressors can cross-sensitize with that of alcohol in both adolescent and adult mice (Camarini 2012; Roberts et al., 1995; Rocha et al., 2012), suggesting that stress and alcohol activate common neural circuits that impact behavioral sensitivity to alcohol. These later findings have relevance for the neurobiology of AUDs, affective disorders and their comorbidity as studies in both humans (Newlin and Thomson, 1990; Schuckit, 1985; Schuckit and Gold, 1988; Schuckit et al., 2001; Wilhelmsen et al., 2003) and laboratory animals (e.g., Becker et al. 2011; Brabant et al. 2014; Crabbe et al., 2005), indicate a relation between sensitivity to alcohol’s psychomotor effects and the propensity to consume alcohol. Thus, the present study employed a technically facile unpredictable chronic mild stress (UCMS) model to examine the cause-effect relation between a history of adversity and behavioral sensitivity to the psychomotor-activating and intoxicating properties of alcohol, as well as the preference for, and consumption of, alcohol versus a palatable sweet solution.
It is very difficult to determine cause-effect relations between a history of adverse life events and cellular/molecular perturbations in the brain of humans as it is impossible to study these relations in any systematic, experimentally-controlled, fashion. Group1 metabotropic glutamate receptors (mGluRs), NMDA receptors, and their major scaffolding proteins Homers, are highly implicated in regulating behavioral sensitivity to alcohol, including alcohol-induced psychomotor sensitization (c.f., Bird and Lawrence 2009; Chandrasekar 2013; Cui et al. 2013; Gass and Olive 2008; Holmes et al. 2013; Szumlinski et al. 2008a; Woodward and Szumlinski 2014). The protein expression of these molecules, as well as the major intracellular effector phospholipase Cβ (PLCβ), is changed markedly within mesocorticolimbic structures, most notably within the nucleus accumbens (NAC), as a consequence of either early life stress (Ary et al. 2007) or repeated alcohol experience (c.f., Cui et al. 2013; Woodward and Szumlinski 2014; see also Lum et al. 2014). Moreover, these glutamate-related proteins are important for the locomotor cross-sensitization between stress and other drugs of abuse (e.g., Tolliver et al., 1996; Wedzony and Czyrak, 1994). Thus, herein, we determined the impact of our UCMS procedures upon the protein expression of Group1 mGluRs, NMDA receptor subunits, Homer proteins, and PLCβ within NAC subregions of adult mice. We also included an analysis of the relative expression of phosphorylated to total extracellular signal-regulated kinase (ERK) as an index of neuronal activity within NAC subregions (e.g., Koya et al. 2009). Finally, as the results of our immunoblotting studies indicated an up-regulation in the expression of Homer2 proteins by our UCMS procedures, we determined the functional relevance of Homer2 for the stress-alcohol cross-sensitization by studying the behavioral responsiveness to alcohol of Homer2 knock-out (KO) mice and their wild-type (WT) counter-parts following a history of unpredictable stressor exposure. Based on prior work indicating that Homer2 is necessary for the development of alcohol-induced behavioral sensitization (Szumlinski et al. 2005) and other work demonstrating an active and necessary role for Homer2 within the NAC shell in regulating alcohol intake (c.f., Cui et al. 2013), it was hypothesized that Homer2 is necessary for stress-alcohol cross-sensitization.
MATERIALS AND METHODS
Subjects
The majority of the subjects in this report were adult (8 weeks old), male Swiss Webster (SW) mice (Charles River Laboratories, Hollister, CA). For studies examining the role for Homer2 in mediating alcohol-stress cross-sensitization, we employed wild-type (WT) and Homer2 gene knock-out (KO) mice on a mixed C57BL/6J X 129Xi/SvJ background (see Shin et al. 2003 for details). These latter mice were bred in-house at the University of California Santa Barbara from heterozygous breeders originally provided by the laboratory of Dr. Paul F. Worley (Johns Hopkins University). All studies employing mutant mice examined mice from a minimum of 3 different litters for each replicate of study to avoid litter confounds and mice were tested between 10 and 15 weeks of age. SW mice were housed in groups of 4, while WT/KO mice were housed in groups of 2–5, on ventilated racks, under a regular 12-h light:dark cycle (lights off at 19:00 hrs), with food and water available ad libitum, unless mandated by the UCMS procedures (see below). One exception was the SW mice involved in the study of UCMS effects upon alcohol/saccharin intake. These UCMS mice were routinely housed in groups of 4 under a reverse light cycle (lights off at 11:00 h), unless mandated by the UCMS procedures. During UCMS procedures, the control mice in the drinking study were group-housed and then housed singly to assess individual fluid intake. All experimental protocols were consistent with the guidelines put forth by the NIH and were approved by the Institutional Animal Care and Use Committee of the University of California Santa Barbara.
Unpredictable Chronic Mild Stress (UCMS) Procedures
Following a minimum of 5 days acclimation to the vivarium conditions in the Psychology building, mice were subjected to our UCMS procedures (see Table 1), which were adapted from those employed in previous studies of SW mice (e.g., Camarini 2012; Rocha et al., 2012), as well as murine models of depression (e.g., Nollet et al. 2012), Post-Traumatic Stress Disorder (e.g., Harvey et al. 2003) and prenatal stress (e.g., Campbell et al. 2009). UCMS procedures occurred over an 11-day period, with morning (AM) stressors occurring between 09:00–11:00 h, afternoon (PM) procedures occurring between 14:00–17:00 h. Over-night (O/N) procedures commenced at 17:00 h and were terminated between 08:30–09:30 h the next day. With the exception of O/N procedures or changes to the animal’s light cycle, all UCMS procedures were conducted in the laboratory. For damp bedding exposure, approximately 300 ml of water was added to the absorbent home cage bedding. For restraint stress, mice were placed in a 50 ml plastic tube with breathing holes for 1 h (e.g., Campbell et al., 2009). For isoflurane inhalation, mice were placed in an induction chamber for 15 min, in which 2% isoflurane was delivered with oxygen as the carrier gas (e.g. Harvey et al., 2003). For exposure to soiled rat bedding, mice were placed with cage-mates into a Plexiglas cage (40 cm W X 32 cm L X 19 cm H), formerly inhabited by a rat for 1 h. For forced swim, mice were placed into a pool (30 cm in diameter; 45 cm high) filled with room-temperature water up to 35 cm (e.g., Ary et al. 2013) and allowed to swim, under experimenter supervision, for 15 min. For exposure to a brightly light arena, mice were placed in groups of 8–12 in a large open field with white Plexiglas walls and a clear Plexiglas floor (100 cm L X 100 cm W X 50 cm H) where they were allowed to freely locomote for 1 h in a procedural room illuminated by standard overhead fluorescent light bulbs. For cage tilt, the home cages were removed from the ventilated rack, placed on a table in the colony room and a spacer (10 cm W X 10 cm L X 4 cm H) was placed under one corner of the cage O/N (e.g., Nollet et al. 2012). For housing with bedding from strange males, the mice were transferred to a novel cage that was formerly inhabited by non-cage-mate males who were involved in the study. For housing under a shifted light cycle, mice were transferred, in their home cages, to a different colony room under an opposite day-light cycle and placed on the ventilated rack therein. For exposure to bobcat urine, mice were placed with their cage-mates into a standard rat cage, the walls of which were wiped with 100% bobcat urine (The PeeMart, Sandy Point, ME) and remained in the cage for 1 h. For isolation housing, mice were single-housed in a new cage with fresh bedding O/N. For all O/N procedures, the mice were returned either to their original home cage (following single-housing) or a fresh home-cage (following damp bedding or bedding from strange males) the next morning between 08:30 and 09:30 h. As a control for the UCMS procedures, mice received once daily intraperitoneal (IP) injections of saline (0.01 ml/kg) for 11 days. This control procedure was employed to habituate the mice to the handling and injection procedures administered on the test for locomotor cross-sensitization (see below).
Table 1.
Summary of the UCMS procedures employed in this study.
| Day | Time | Procedure |
|---|---|---|
| 1 | O/N | Exposure to damp bedding |
| 2 | AM | 1-h immobilization in restraint tube |
| 3 | PM O/N |
5-min exposure to 2% isoflurane anesthesia Cage tilt |
| 4 | AM PM |
1-h exposure to soiled rat bedding 5-min forced swim |
| 5 | All day O/N |
Exposure to damp bedding Food and water deprivation |
| 6 | AM PM |
5-min forced swim 12-h housing under shifted light cycle |
| 7 | PM O/N |
1-h immobilization in restraint tube Individual/isolation housing |
| 8 | AM PM O/N |
5-min exposure to 2% isoflurane anesthesia 1-h exposure to brightly lit inescapable arena Housing with bedding from strange males |
| 9 | AM PM O/N |
1-h immobilization in restraint tube 1-h exposure to soiled rat bedding Food and water deprivation |
| 10 | PM O/N |
15-min forced swim Cage tilt |
| 11 | AM PM O/N |
1-h exposure to bobcat urine 3-h housing under shifted light cycle Housing with bedding from strange males |
| 12 (Test) |
AM | 15-min locomotor cross-sensitization test + 2-h 4-bottle-choice drinking session or Rotarod |
Monitoring of Health Outcomes
The body weight of all mice subjected to the UCMS and control procedures was recorded prior to any experimental manipulation and every day during the experimental procedures. No mouse exhibited any significant reduction in body weight (i.e., >10%) over the course of the study. In addition to recording body weight daily, we examined also the coat condition of the animals at the start of each day. This involved visual inspection of the face, top of head, abdomen, shoulders, back, as well as left and right flanks for signs of dishevelment/lack of self-care. A score was assigned to each body part as follows: 0 = well-groomed, 0.5 = moderate degradation, 1 = unkempt. A high score indicated that the coat is in poor condition, implying a low level of grooming/self-care, and this index of anxiety/stress has been pharmacologically validated (Santarelli et al., 2003; Surget et al., 2008; Tanti et al. 2012).
Locomotor Test for Cross-sensitization
Following the 11-day UCMS or control procedures, groups of SW, WT and Homer2 KO mice were assayed for alcohol-stress cross-sensitization in a test for locomotor hyperactivity. For this, mice from the UCMS and control conditions were randomly assigned to receive an IP injection of either 2 g/kg alcohol or an equivalent volume of saline vehicle (vol = 0.02 ml/g). This dose of alcohol was selected as it typically elicits locomotor activity when administered acutely to SW mice (e.g., Faria et al. 2008; Miquel et al. 1999; Quoilin et al. 2010) and is sufficient to reveal alcohol-stress cross-sensitization in both adult and adolescent SW mice subjected to similar UCMS procedures as those employed in the current study (Camarini 2012; Rocha et al., 2012). Immediately following injection, mice were placed into Plexiglas activity chambers (20 W cm X 37 cm L X 25 cm H) for 15 min. The total distance traveled was recorded using digital video-tracking and ANYMaze software (Stoelting Company, Wood Dale, IL).
Alcohol Consumption
To determine whether or not the potentiation of alcohol-induced locomotion observed in UCMS animals extended to alcohol preference and/or intake, a group of UCMS and control SW mice were first tested in the morning for alcohol-induced locomotor activity (2 g/kg) to verify the efficacy of our UCMS procedures to augment alcohol-induced locomotion. The afternoon following this test (14:00 h), mice were presented simultaneously with 4 identical sipper tubes, containing 0, 5, 10 and 20% alcohol (v/v) and given continuous access to these alcohol concentrations for 5 consecutive days (e.g., Lominac et al. 2006; Szumlinski et al. 2005). As unpublished data indicate that the cross-sensitizing effects of UCMS procedures persist for at least 2 weeks in SW mice (Camarini and Szumlinski laboratories, unpublished data), we also determined whether or not the potentiation of alcohol intake exhibited by UCMS mice generalized to a non-caloric, sweet solution by continuing to test these same mice for saccharin intake/preference under 3 bottle-choice procedures. For this, mice had continuous access to 0, 0.0125 and 0.025% saccharin (w/v) for a total of 3 days (e.g., Cozzoli et al. 2014). During both phases of the study, bottles were weighed once daily and the amount of fluid consumed over the 24-h period was determined by subtracting the recorded weight from the weight recorded the previous day. Spillage from each concentration was determined by conducting the 4-bottle or 3-bottle procedures on 2 empty cages in the colony room and the average spillage from each concentration was subtracted from the volume consumed for each animal daily. The amount of alcohol/saccharin intake was then expressed in g/kg body weight and the preference for the different concentrations determined daily from the total volume consumed as an index of sensitivity to the rewarding properties of alcohol/saccharin (e.g., Cozzoli et al. 2014; Lominac et al. 2006; Szumlinski et al. 2005).
Rotarod Performance
To relate UCMS effects upon the psychomotor-activating and rewarding properties of alcohol to influences upon alcohol-induced behavioral intoxication, a group of UCMS and control mice were trained to walk on a fixed speed (10 rpm) rotarod (IIT Life Science, Woodland Hills, CA), using procedures akin to those described in Cronise et al. (2005). The rotarod apparatus consisted of 5 rotating rods (10 cm long; 3.3 cm in diameter; covered in sand paper to prevent slippage) that were individually encased to prevent socialization and escape of the animals. The floor beneath each rod was equipped with sensors that detected the fall of an animal from each individual rod and recorded the time of the fall. Initially, mice were habituated to walking on the rotating rod during a 2-min session. If a mouse fell from the rod during this 2-min session, the fall was noted and the mouse was immediately placed back onto rod until the completion of the 2-min session. After all the mice were exposed to the apparatus, the animals then underwent proper training on the apparatus. For this, mice underwent a series of 3-min training trials, with 30-sec rests in between. An animal was considered “trained” once it successfully remained on the rotating rod for a total of 3 training trials. During each of these 3-min training trials, if an animal fell from the rod, the time of the fall was recorded and the animal was left on the floor of the apparatus until the start of the next 3-min training trial. The total number of trials required to train the animal was used to index for group differences in basal motor coordination. The next day, all mice were injected with 3 g/kg alcohol and locomotor activity assessed for 15 min, as described above. Fifteen minutes following the cross-sensitization test (i.e., 30 min post-injection), mice were subjected to three 3-min rotarod test trials. As during the 3-min test trials, if an animal fell from the rod, the fall was noted, the time of the fall recorded and the animal remained on the floor of the apparatus until the start of the next test trial. Again, a 30-sec rest period was allowed between trials. The average time spent on the rod under the influence of alcohol was then compared to that obtained during the 3-min baseline training trials to index alcohol intoxication.
Regain of Righting Reflex
As an additional index of alcohol sensitivity, mice from the rotarod study were tested also for their ability to regain their righting reflex following IP administration of a heavily sedating dose of alcohol (5 g/kg). Righting reflex testing occurred the day following rotarod testing (i.e., on the 3rd day following the end of the UCMS procedures). The procedures employed were similar to those used previously to examine for genotypic differences in the sedative properties of alcohol (Szumlinski et al. 2005). Mice were injected with alcohol and upon loss of muscle tone, were placed in a supine position in an empty cage. The time taken to right themselves (i.e., place all 4 paws on the floor of the cage) was recorded on a stopwatch by an experiment blind to the prior treatment of the animals. For practical reasons, a maximum of 180 min was allowed for an animal to regain its righting reflex at which point the experiment was terminated.
Blood Alcohol Concentrations
At the end of the regain of righting reflex study (i.e., 180 min post-injection with 5 g/kg alcohol, IP), blood was sampled from all mice and samples were analyzed with an Analox Analyzer (model GL5, Analox Instruments USA, Lunenburg, MA, USA) to relate the differential sensitivity of the groups to the sedative properties of alcohol to alcohol levels.
Immunoblotting
Immunoblotting studies were conducted on whole tissue homogenates from the NAC core and shell subregions to examine for biochemical correlates of alcohol-stress cross-sensitization, with a focus on Group1 mGluRs, NMDA glutamate receptor subunits, Homer scaffolding proteins, PLCβ and the relative expression of total ERK to phosphorylated ERK (tERK:pERK), as these molecules have been highly implicated in regulating behavioral sensitivity to alcohol, including alcohol-induced locomotor hyperactivity, sedation and intake/preference (c.f., Bird and Lawrence 2009; Chandrasekar 2013; Gass and Olive 2008; Woodward and Szumlinski, 2014). Tissue was dissected from SW mice in 1 mm-thick coronal sections, using an 18-gauge tissue punch, over ice, at 3 h following the test for alcohol-stress cross-sensitization (see above). The procedures employed for this study were very similar to those recently described by our group (e.g., Ary et al. 2013; Cozzoli et al. 2014; Goulding et al. 2011; Lum et al. 2014). The samples were homogenized in a medium consisting of 0.32 M sucrose, 2 mM EDTA, 1% w/v sodium dodecyl sulfate, 50 µM phenyl methyl sulfonyl fluoride and 1 µg/ml leupeptin (pH=7.2) and 50 mM sodium fluoride, 50 mM sodium pyrophosphate, 20 mM 2-glycerol phosphate, 1 mM p-nitrophenyl phosphate, and 2 µM microcystin LR were included to inhibit phosphatases. All samples were stored at −80°C until assay. Samples were subjected to a SDS-polyacrylamide gel electrophoresis using 3–8% Tris-Acetate gels or 4–12% Bis-Tris gels (Invitrogen). Proteins were transferred to PVDF membranes and pre-blocked with phosphate-buffered saline containing 0.1% (v/v) Tween-20 and 5% (w/v) nonfat dried milk powder for a minimum of one hour prior to overnight incubation with a primary antibody. Anti-Homer2a/b (Cosmo Bio USA Inc, Carlsbad, CA; 1:1000 dilution), anti-Homer1b/c (GeneTex Inc, Irvine, CA; 1:1000 dilution), anti-mGlu5 (Millipore, Billerica, MA; 1:1000 dilution), anti-NR2a and anti-NR2b (Calbiochem, San Diego, CA; 1:1000 dilution), as well as anti-PLCβ3 and anti-ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:2000 dilution) rabbit polyclonal antibodies were used. Mouse polyclonal anti-mGlu1α antibody (BD Transduction Laboratories, Sparks, MD; 1:1000 dilution) and anti-p(Tyr204)ERK1/2 (Santa Cruz Biotechnology; 1:1000 dilution) were also used. Membranes were then washed and incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Upstate, Charlottesville, VA; 1:40,000–1:80,000 dilution) or anti-mouse secondary antibody (Millipore; 1:40,000–1:80,000) for 90 min. Membranes were washed again and immunoreactive bands were detected by enhanced chemiluminescence using either ECL Plus (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) or Pierce SuperSignal West Femto (Thermo Fisher Scientific, Rockford, IL). A rabbit anti-calnexin polyclonal primary antibody (Stressgen, Victoria, BC) was used to standardize protein loading across samples. Membranes were stripped between assays for proteins of similar molecular weight (ReStore Plus, Thermo Fisher Scientific). Immunoreactivity of each protein was quantified using Image J (NIH, Betheseda, MD). Protein/calnexin ratios were utilized to normalize immunoreactivity of each protein with its respective calnexin value and for group comparisons, these data for experimental animals were expressed as a percent of the 4 saline-injected control animals on each gel to normalize across membranes.
Statistical Analyses
The health outcome data were analyzed using a Stress (UCMS vs. Control) X Day or Stress X Genotype (Homer2 WT vs. KO) X Day analysis of variance (ANOVA), with repeated measures on the Day factor (11 days). The locomotor data from the cross-sensitization studies were analyzed using Stress X Injection (Alcohol vs. Saline) or a Stress X Genotype X Injection ANOVA, while the drinking data were analyzed using a Stress X Concentration ANOVA, with repeated measures on the Concentration factor (2–4 levels, depending upon the dependent variable). Pearson correlational analyses were also performed to determine whether or not alcohol-induced locomotor activity could predict total alcohol consumption. The rotarod data were analyzed using a Stress X Test ANOVA, with repeated measures on the Test factor (Baseline vs. Alcohol) and the righting reflex, as well as the BAC, data were analyzed using t-tests for independent samples. Significant interactions were deconstructed for an examination of simple effects, followed by LSD post-hoc tests or t-tests, as appropriate. α=0.05 for all analyses.
RESULTS
Health outcomes
Table 2 provides a summary of the average change in body weight of the SW mice within each of the 3 experiments, as well as that of Homer2 WT and KO mice in the cross-sensitization study. As indicated in Table 2, our UCMS procedures either did not affect (cross-sensitization and intoxication studies) or reduced (drinking study) body weight in SW mice over the course of study. UCMS procedures also significantly reduced body weight in both Homer2 WT and KO mice (Table 2). Despite negatively impacting body weight gain in some experiments, none of the SW mice studied exhibited any signs of poor self-care, with all animals in both the UCMS and the control groups receiving a total coat condition score of 0 (i.e., the animals never exhibited dishevelment on any body part during the course of study) and thus, statistics were not performed on these data. In Homer2 WT and KO mice, there was also very little evidence for a negative effect of the UCMS procedures upon self-care, with groups exhibiting no or very few, minor, signs of dishevelment (Table 2) and no genotypic differences were noted in this regard.
Table 2.
Summary of the health outcomes of the mice following UCMS procedures. The average change in body weight of UCMS and control Swiss-Webster (SW), Homer2 wild-type (WT) and knock-out (KO) mice from Day 1 to 11 of UCMS procedures is presented, in addition to a summary of the total coat condition score of Homer2 WT and KO mice. Swiss-Webster mice did not receive any score for coat condition and thus, their data are not presented herein. Data were analyzed by either t-tests or ANOVAs and the results of these analyses are presented below. Sample sizes are indicated in parentheses.
| Study | Control | UCMS | Statistic |
|---|---|---|---|
| Change in Body Weight (g) | |||
| SW Cross-sensitization | 1.50 ± 0.22 (24) | 1.18 ± 0.21 (24) | t(46)=1.05, p=0.30 |
| SW Drinking | 0.31 ± 1.03 (12) | −2.17 ± 0.57 (12) | t(22)=2.11, p=0.05 |
| SW Intoxication | 1.24 ± 0.32 (10) | 0.85 ± 0.47 (10) | t(18)=0.69, p=0.50 |
| Homer2 WT | 0.88 ± 0.35 (13) | −0.35 ± 0.31 (14) | UCMS effect: F(1,54)=8.80, p=0.005 Genotype effect: p=0.51 UCMS X Genotype: p=0.57 |
| Homer2 KO | 0.45 ± 0.29 (12) | −0.38 ± 0.39 (16) | |
| Total Coat Score | |||
| Homer2 WT | 0.12 ± 0.06 (13) | 0.00 ± 0.00 (14) | UCMS effect: F(1,54)=0.99, p=0.33 Genotype effect: p=0.23 UCMS X Genotype: p=0.20 |
| Homer2 KO | 0.08 ± 0.05 (12) | 0.94 ± 0.63 (16) | |
Locomotor activity in Swiss-Webster mice
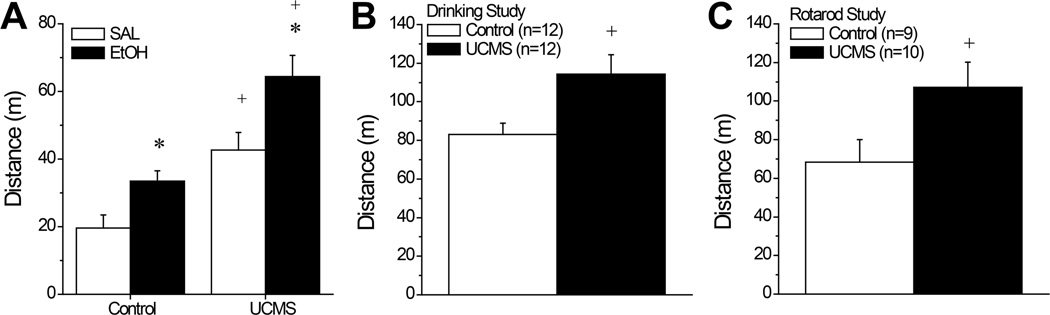
Fig. 1A summarizes the total distance traveled by the first cohort of UCMS and control SW mice during a 15-min cross-sensitization test (n=11–12/group). As illustrated, an acute injection with 2 g/kg alcohol stimulated locomotor activity in both UCMS and control SW mice above that exhibited by their saline-injected counterparts [Injection effect: F(1,42)=34.59, p<0.0001]. Moreover, UCMS procedures promoted locomotor hyperactivity overall [Stress effect: F(1,42)=14.92, p<0.0001]. While the results of the statistical analysis did not indicate a significant Stress X Injection interaction [p=0.39], it is obvious from an inspection of Fig. 1A that the UCMS-alcohol group exhibited the greatest locomotor activity of all the groups tested, indicative of cross-sensitization and t-tests conducted between the UCMS-alcohol and UCMS-SAL [t(21)=3.63, p=0.002], as well as between the UCMS-alcohol and the Control-alcohol groups [t(19)=3.59, p=0.002] confirmed the presence of a potentiation of alcohol-induced locomotor activity in UCMS animals.
Figure 1.
Summary of the effects of our unpredictable chronic mild stress (UCMS) procedures upon alcohol-induced locomotion in 3 different cohorts of Swiss-Webster mice. (A) In the cross-sensitization study (n=12/group), an acute injection with 2 g/kg alcohol (EtOH; solid bars) elevated locomotor activity during a 15-min test, relative to an equivalent volume injection of saline (SAL; open bars). Mice subjected to our UCMS procedures (UCMS) exhibited higher locomotor activity versus controls, regardless of alcohol administration. However, the greatest locomotor activity was observed in UCMS-EtOH mice. In both the studies of stress interactions with alcohol drinking (n=12/group) (B) and alcohol intoxication (n=9 for Control; n=10 for UCMS) (C), mice subjected to our UCMS procedures also exhibited a greater locomotor response to 2 g/kg alcohol during a 15-min test than did animals subjected to our control procedures. *p<0.05 vs. SAL; +p<0.05 vs. Control (t-tests).
Alcohol and saccharin intake by Swiss-Webster mice
The alcohol-induced locomotor activity exhibited by the cohort of SW mice slated for the alcohol/saccharin drinking study was overall greater than that exhibited by mice in the locomotor activity study, presumably reflecting the fact that mice were assayed for locomotor activity during the dark phase of the circadian cycle (Fig. 1B vs. 1A). Regardless of this, the SW mice slated for the alcohol/saccharin drinking study also exhibited a greater locomotor response to 2 g/kg alcohol following our UCMS procedures than did their corresponding controls (Fig.1B) [t(22)=2.66, p=0.01]. Thus, the UCMS procedures were sufficient to augment psychomotor sensitivity to alcohol prior to the commencement of drinking procedures.
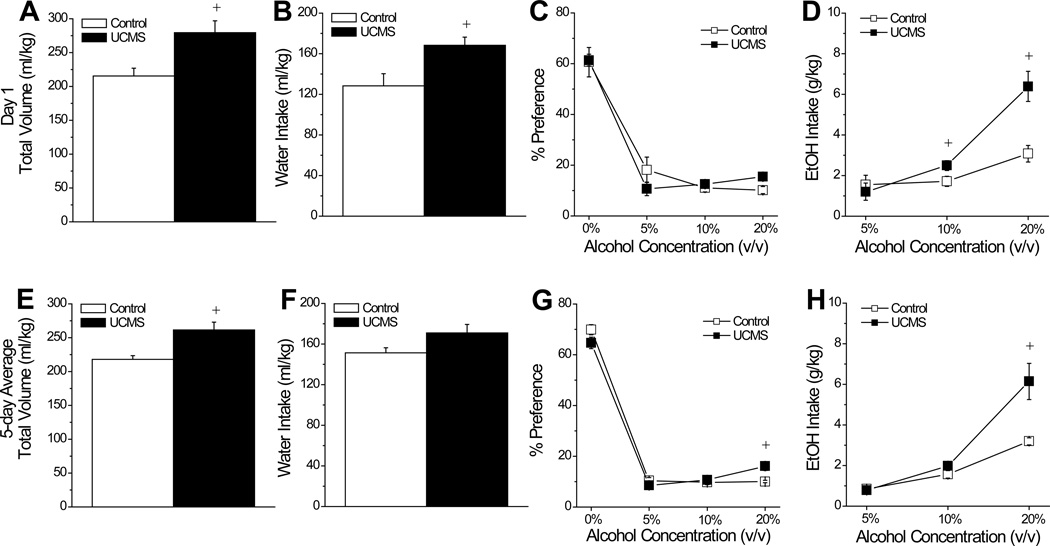
We first examined for UCMS effects upon initial alcohol preference and intake through analyses of the data from the first 24-h drinking session. As illustrated in Fig.2, group differences were noted with respect to the total volume consumed by the mice on this first session (Fig. 2A) [t(22)=3.03, p=0.006) and UCMS animals exhibited higher water intake than controls (Fig.2B) [t(22)=2.83=0.01]. Although the UCMS procedures did not influence the dose-response function for alcohol preference on the first day of drinking (Fig.2C) [Concentration effect: F(3,66)=87.06, p<0.0001; Stress effect: p=1.0; interaction, p=0.36], there were significant group differences with respect to the dose-response function for alcohol intake (Fig.2D) [Concentration effect: F(2,44)=37.22, p<0.0001; Stress effect: F(1,22)=8.26, p=0.009; Stress X Concentration: F(2,44)=10.51, p<0.0001], with UCMS animals exhibiting significantly higher intakes of 10% [t(22)=2.69, p=0.03] and 20% alcohol [t(22)=3.90, p=0.001], compared to controls.
Figure 2.
Summary of the effects of unpredictable chronic mild stress (UCMS) upon alcohol intake and preference. During the first 24 h of alcohol availability, mice subjected to our UCMS procedures consumed a marginally higher total volume of fluid (A) and a significantly larger volume of water (B), compared to mice subjected to our control procedures (Control). (C) During this time, group differences were not observed in the dose-response function for alcohol preference, however (D) UCMS mice consumed a greater amount of both 10% and 20% alcohol, relative to controls. When averaged across the 5 days of alcohol availability, there was no group difference in the total volume consumed (E) or in water intake (F). In contrast, UCMS mice exhibited a greater average preference for (G), and intake of (H), 20% alcohol. n=12/group. +p<0.05 vs. Control (t-tests).
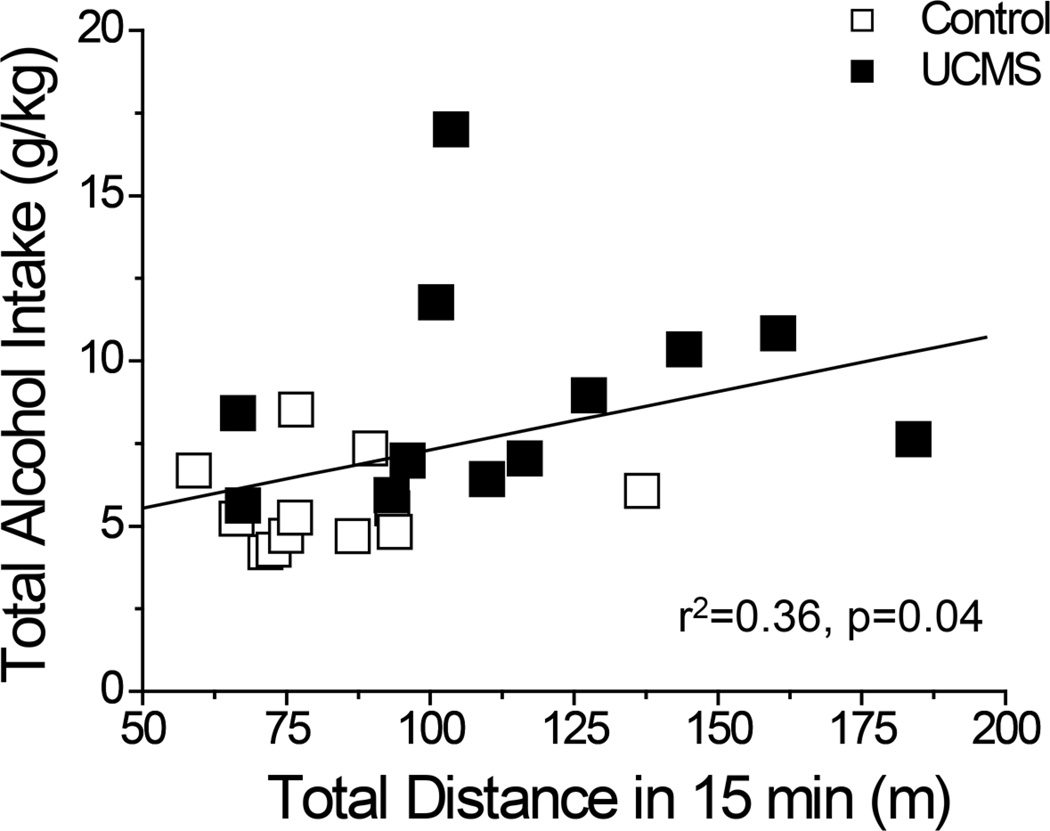
A similar, but not identical, pattern of group differences were noted when the data for intake and preference were averaged across the 5 days of drinking (Fig.2E–H). Again, we observed a statistically significant group difference in total fluid consumption (Fig.2E) [t(22)=3.46 p=0.002]. However, the group difference in average total fluid intake did not reflect an effect of UCMS on the average water intake (Fig.2F; t-test, p=0.06). However, group differences emerged for the dose-response functions for both average alcohol preference (Fig.2G) [Stress X Concentration: F(3,66)=3.98, p=0.01] and average alcohol intake (Fig.2H) [Stress X Concentration: F(2,44)=9.15, p<0.0001]. The significant interactions for both variables reflected higher preference for, and intake of, the 20% concentration in UCMS versus control mice [20% preference: t(22)=3.46, p=0.002; 20% intake: t(22)=3.24, p=0.004; p’s for comparisons at other concentrations > 0.10]. In support of a predictive relation between greater sensitivity to alcohol’s psychomotor effects and alcohol intake, a significant correlation was observed between the total distance traveled in response to 2 g/kg alcohol (from Fig.1B) and total alcohol intake of the mice over the 5-day drinking period (Fig.3) [r2=0.36, p=0.04].
Figure 3.
Best-fit regression line of the relationship between locomotor activity expressed by saline- and alcohol-injected SW mice during a 15-min test (from Fig. 1A) of the effects of UCMS (closed symbols) or control procedures (open symbols) and the average total alcohol intake exhibited by the mice during subsequent assessment of drinking (from Fig.2H). n=12/group
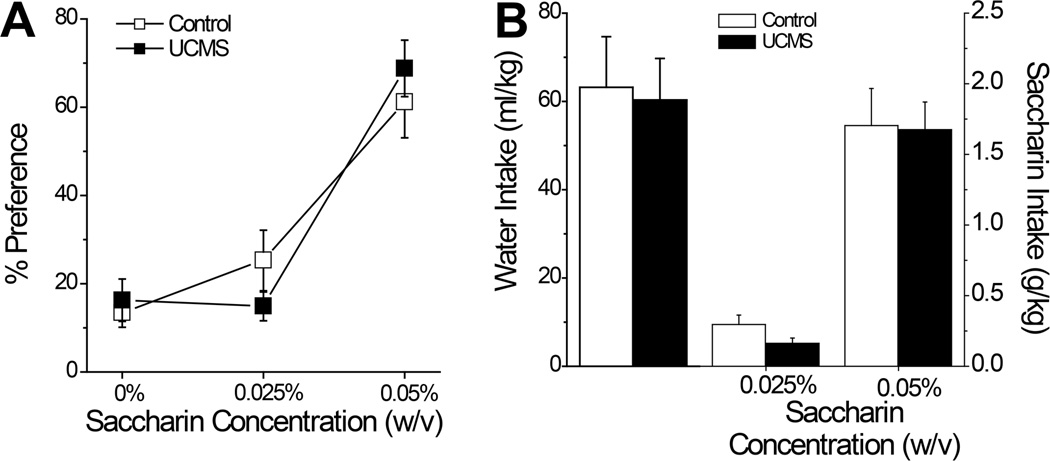
In contrast to alcohol intake (Fig.2), we observed no group differences in fluid consumption whatsoever when animals were assayed for saccharin preference and intake (Fig.4). Regardless of whether we examined initial intake (data not shown) or the average intake across the saccharin drinking days, UCMS did not impact: the total volume consumed (Control: 15.30 ± 1.15 ml vs. UCMS: 15.30 ± 1.12 ml; t-tests, p>0.25), preference for saccharin (Fig.4A) [Concentration effect: F(2,44)=30.55, p<0.001; no main Stress effect or interaction, p’s>0.40], water intake (Fig.4B; t-tests, p’s>0.60) or saccharin intake at either concentration (Fig.4B) [Concentration effect: F(1,22)=65.02, p<0.0001; no main Stress effect or interaction, p’s>0.75]. Together, the above data indicate that our UCMS procedures augmented high-dose alcohol intake, , without impacting consumption of a palatable sweet reinforcer.
Figure 4.
Summary of the effects of our unpredictable chronic mild stress (UCMS) procedures upon saccharin preference and intake. (A) UCMS mice did not differ from mice subjected to our control procedures (Control) with respect to the dose-response for average saccharin preference across 3 consecutive days of drinking. (B) Group differences were also not noted for the average intake of water or either of the 2 saccharin concentrations. n=12/group.
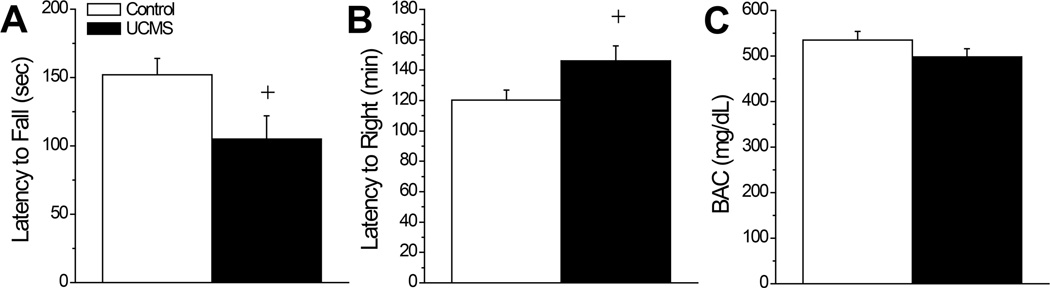
Alcohol Intoxication
As observed in the drinking study (Fig.1B), the UCMS mice slated for testing on the rotarod exhibited greater locomotor activity than controls when challenged with a 2 g/kg alcohol injection (Fig.1B) [t(17)=2.19, p=0.04] prior to assays for alcohol intoxication. In the rotarod assay, UCMS mice exhibited a shorter latency to fall when injected with 3 g/kg, compared to controls (Fig.5A) [t(17)=2.21 ,p=0.04], indicative of greater alcohol intoxication. Consistent with this, these same UCMS mice exhibited a longer latency to right than controls when injected with 5 g/kg (Fig.5B) [t(17)=2.13, p=0.04]. In fact, 4/10 UCMS failed to regain their righting reflex at the end of the 3-h testing period, while all 9 of the control mice righted themselves before the end of the testing period. Although BACs determined from collection of trunk blood were marginally lower in UCMS mice versus controls at the end of the 3-h testing period (Fig.5C), the difference did not approach statistical significance (t-test, p=0.20). Thus, our UCMS procedures not only increase sensitivity to the psychomotor-activating properties of moderate alcohol doses, they increase sensitivity to the sedative-hypnotic effects of higher alcohol doses and this effect is not obviously related to effects of repeated stress upon an index of alcohol metabolism.
Figure 5.
Summary of the effects of our unpredictable chronic mild stress (UCMS) procedures upon alcohol intoxication and sedation. (A) When assessed on a fixed speed rotarod, UCMS mice (n=10) exhibited a shorter latency to fall when injected with 3 g/kg alcohol, compared to mice subjected to our control procedures (Control; n=9). (B) When injected with 5 g/kg, UCMS mice exhibited a longer latency to right in a regain of righting reflex study than did Controls. (C) The group differences in the sedative properties of 5 g/kg alcohol were not obviously related to an effect of UCMS upon BACs determined at 180 min post-injection (n=9/group). +p<0.05 vs. Control (t-tests).
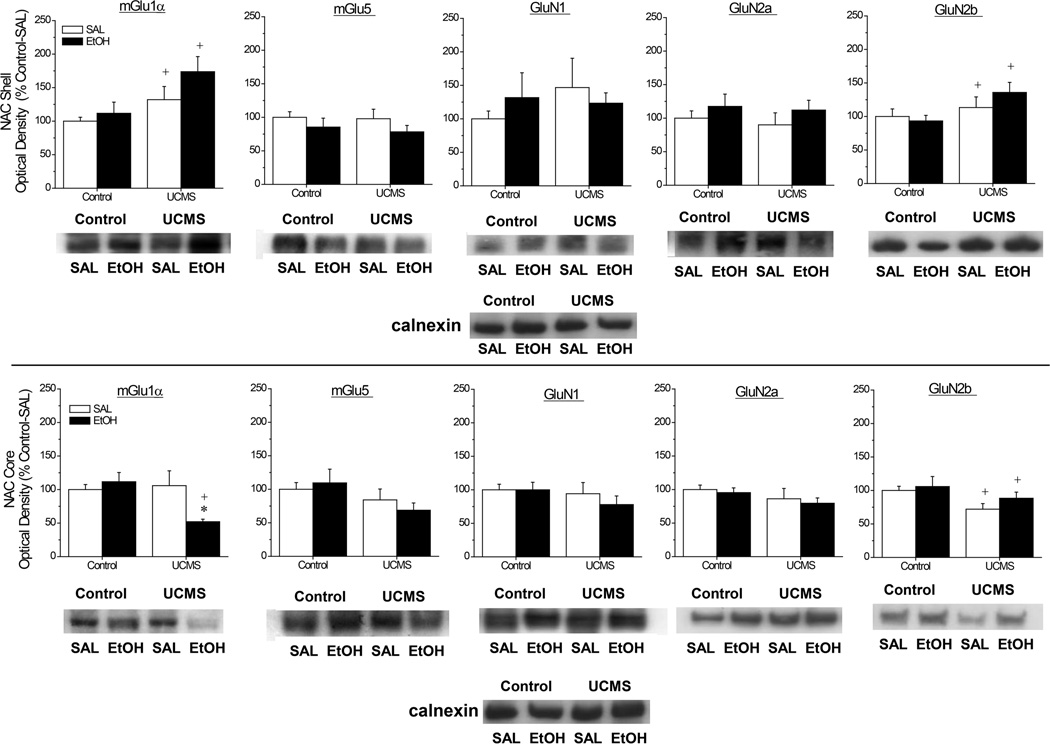
Immunoblotting for Glutamate Receptor Proteins
As illustrated in Fig.6, our UCMS procedures and their interactions with an acute injection of alcohol affected selectively the expression of mGlu1α and GluN2b subunits within NAC subregions and did so in an opposing manner. In the NAC shell, mGlu1α and GluN2b levels were higher in UCMS mice, relative to controls, but these effects were not influenced by alcohol injection (Fig.6A) [for mGlu1α, Stress effect: F(1,48)=7.43, p=0.009; no Injection effect or interaction, p’s>0.05; for GluN2b: [Stress effect: F(1,47)=4.67, p=0.04; other p’s>0.05]. In contrast, a stress-alcohol interaction was observed for mGlu1α within the NAC core (Fig.6B) [Stress X Injection: F(1,47)=5.71, p=0.02], with UCMS-alcohol mice exhibiting significantly lower mGlu1α levels than both their unstressed [t(22)=4.20, p<0.0001] and their saline-injected [t(22)=2.40, p=0.03] counterparts. Moreover, UCMS procedures reduced GluN2b levels overall within the NAC core, but in a manner independent of alcohol injection (Fig.6B) [Stress effect: F(1,46)=4.93, p=0.03; other p’s>0.05]. Although UCMS procedures appeared to lower mGlu5 levels within the NAC core (Fig.6B), this effect failed to reach statistical significance (Stress effect: p=0.06) and no group differences were apparent for mGlu5 levels within the NAC shell (Fig.6A; all p’s>0.10). Lastly, no significant group differences were noted for the NAC shell expression of GluN1 (all p’s>0.35) or GluN2a (all p’s>0.20) or for the expression of these NMDA receptor subunits within the NAC core (for GluN1, all p’s>0.25; for NR2A, all p’s>0.10).
Figure 6.
Summary of the interactions between our unpredictable chronic mild stress (UCMS) procedures and an acute injection of alcohol (EtOH) or saline (SAL) upon the total protein expression of Group1 mGluRs and NMDA receptor subunits within the nucleus accumbens (NAC) shell (A) and the NAC core (B). Representative immunoblots for each protein of interest are also included. The data are expressed as a percent change in optical density of non-stressed controls mice (Control) injected with SAL. The data represent the means ± SEMs of 12 mice/group. *p<0.05 vs. respective SAL group; +p<0.05 vs. respective Control (unstressed) group (LSD post-hoc tests).
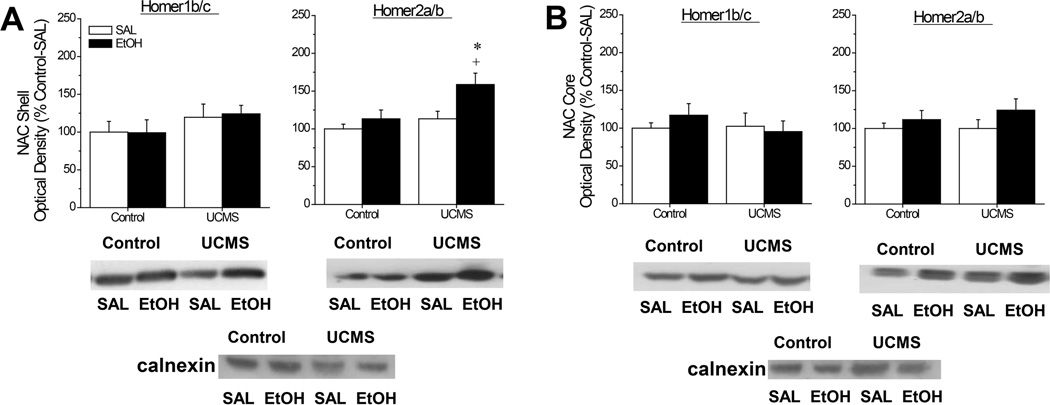
Immunoblotting for CC-Homer Proteins
Analysis of the data for Homer2a/b within the NAC shell (Fig.7A) indicated an elevation in protein expression by both our UCMS procedures [Stress effect: F(1,45)=6.86, p=0.01] and an acute alcohol injection [Injection effect: F(1,45)=6.75, p=0.01], but no interaction between these two factors (p=0.16). However, visual inspection of the data strongly suggests that both main effects are driven by the high level of protein expression in UCMS-EtOH mice, relative to the other groups tested. Indeed, orthogonal comparisons indicated an effect of acute alcohol in UCMS animals [t(21)=2.52, p=0.02], but not in controls (t-test p=0.35) and a UCMS effect in alcohol-injected animals [t(21)=2.44, p=0.02], but not in saline-injected mice (t-test, p=0.27). In contrast, no group differences were noted for Homer1b/c within the NAC shell (Fig.7A; all p’s>0.1) and neither Homer isoform was influenced by our procedures within the NAC core (Fig.7B; for Homer1/c, all p’s>0.35; for Homer2a/b, all p’s>0.10).
Figure 7.
Summary of the interactions between our unpredictable chronic mild stress (UCMS) procedures and an acute injection of alcohol (EtOH) or saline (SAL) upon the total protein expression of constitutively expressed Homer proteins within the nucleus accumbens (NAC) shell (A) and the NAC core (B). Representative immunoblots for each protein of interest are also included. The data are expressed as a percent change in optical density of non-stressed controls mice (Control) injected with SAL. The data represent the means ± SEMs of 12 mice/group. *p<0.05 vs. respective SAL group; +p<0.05 vs. respective Control (unstressed) group (LSD post-hoc tests).
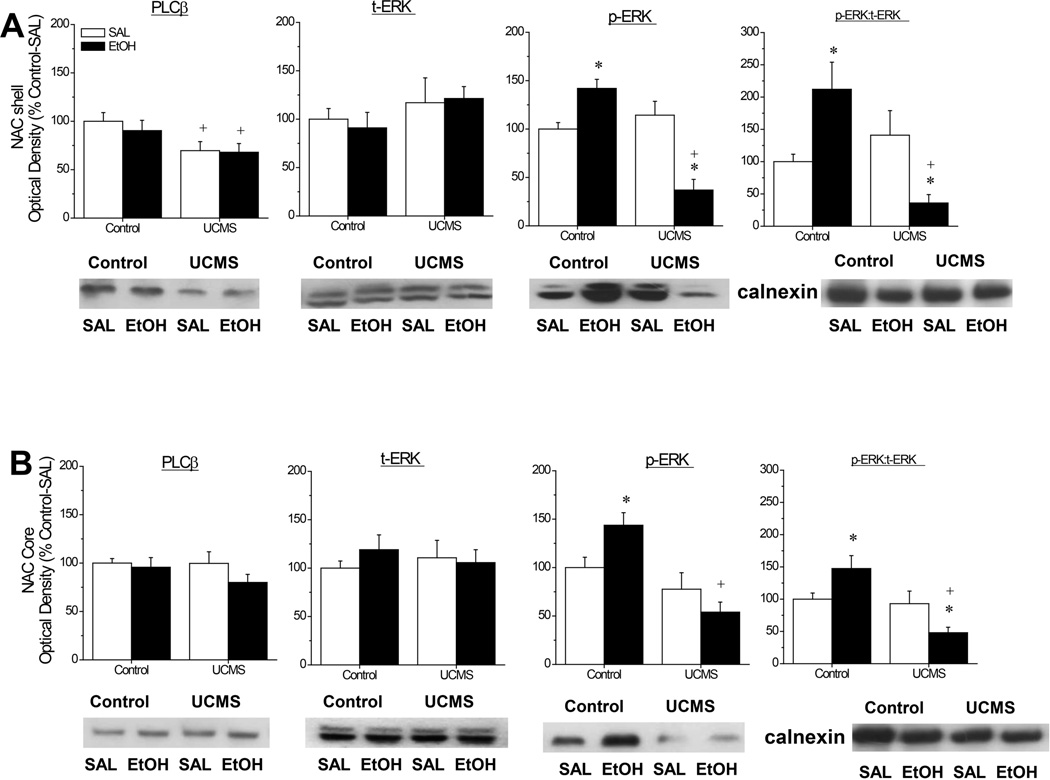
Immunoblotting for PLCβ and ERK
As illustrated in Fig.8, UCMS procedures reduced PLCβ levels overall within the NAC shell [Stress effect: F(1,48)=7.79, p=0.008; other p’s>0.55], without influencing enzyme levels within the core (all p’s>0.15). Neither our UCMS procedures nor the alcohol injection affected t-ERK levels in the NAC shell or core (Stress X Injection ANOVAs, all p’s>0.4); however, in both subregions, we observed a stress-alcohol interaction for p-ERK expression as assessed by an analysis of either p-ERK levels [for NAC shell, F(1,45)=30.25, p<0.0001; for NAC core: F(1,43)=6.60, p=0.01] or the relative expression of the phosphorylated form of the kinase [for NAC shell, F(1,43)=12.96, p=0.001; for NAC core: F(1,43)=8.35, p=0.006]. In the NAC shell (Fig.8A), the interaction reflected opposing responsiveness of p-ERK to the acute alcohol injection in control versus UCMS mice; alcohol elevated both the total and relative levels of p-ERK within the NAC shell in controls [p-ERK: t(21)=3.56, p=0.002; ratio: t(21)=2.46, p=0.02], while lowering both of these measures in UCMS mice [p-ERK: t(21)=4.25, p<0.0001; ratio: t(21)=2.65, p=0.02]. The interaction also reflected differential effects of our UCMS procedures upon p-ERK expression, as no control-UCMS differences were observed in saline-injected animals for either the total or relative expression of p-ERK (t-tests, p’s>0.30), while p-ERK levels were significantly lower in alcohol-injected UCMS mice versus alcohol-injected controls [p-ERK: t(21)=7.30, p<0.0001; ratio: t(21)=4.00, p=0.001]. A very similar pattern of group differences in p-ERK expression were noted for the NAC core (Fig.8B), with the acute alcohol injection significantly elevating total p-ERK expression in control mice [t(20)=2.56, p=0.02], but not in UCMS animals (t-test: p=0.25). When the relative expression of p-ERK was examined post-hoc, the alcohol-induced increase in relative p-ERK expression exhibited by control animals was more modest [t(20)=2.01, p=0.06], but a significant difference emerged between saline- and alcohol-injected UCMS animals [t(20)=2.09, p=0.05]. As observed for the NAC shell, the UCMS procedures did not significantly alter total or relative p-ERK levels in saline-injected mice (t-tests: p’s>0.25), but lowered p-ERK expression in alcohol-injected animals [p-ERK: t(21)=5.35, p<0.0001; ratio: t(21)=4.41, p<0.00001].
Figure 8.
Summary of the interactions between our unpredictable chronic mild stress (UCMS) procedures and an acute injection of alcohol (EtOH) or saline (SAL) upon the total protein expression of PLCβ, total ERK1/2 (t-ERK) and phospho(Tyr204)-ERK1/2 (p-ERK), as well as the relative expression of the phosphorylated and unphosphorylated forms of ERK (pERK:tERK) within the nucleus accumbens (NAC) shell (A) and the NAC core (B). Representative immunoblots for each protein of interest are also included. The data are expressed as a percent change in optical density of non-stressed controls mice (Control) injected with SAL. The data represent the means ± SEMs of 12 mice/group. *p<0.05 vs. respective SAL group; +p<0.05 vs. respective Control (unstressed) group (LSD post-hoc tests).
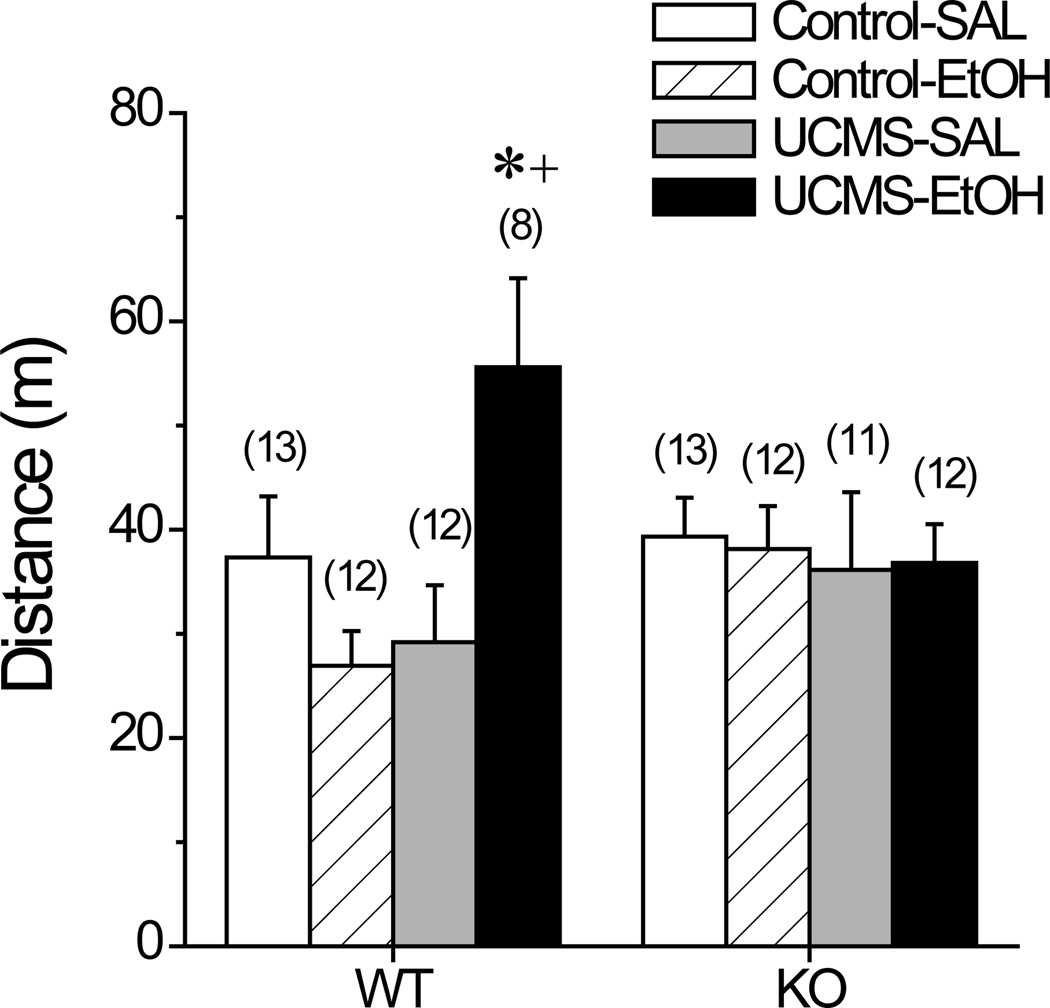
Locomotor Cross-sensitization in Homer2 WT and KO Mice
Fig.9 summarizes the effects of our UCMS procedures upon the locomotor activity induced by a challenge injection of 2 g/kg alcohol in Homer2 WT and KO mice. As illustrated, the capacity of alcohol and UCMS to cross-sensitize depended upon intact Homer2 gene products [Injection X Stress X Genotype: F(1,92)=5.44, p=0.02]. Deconstruction of the significant 3-way interaction along the Genotype factor confirmed that WT mice exhibited UCMS-alcohol cross-sensitization [Injection X Stress: F(1,44)=9.99, p=0.003], with UCMS WT mice exhibiting greater alcohol-stimulated locomotor activity than either control WT mice injected with alcohol [t(18)=3.58, p=0.002] or UCMS WT mice injected with saline [t(18)=2.72, p=0.01]. In contrast, no group differences were observed in KO mice [2-way ANOVA, all p’s>0.05].
Figure 9.
Summary of the effects of our unpredictable chronic mild stress (UCMS) procedures upon saline (SAL)- and alcohol (EtOH; 2 g/kg)-induced locomotion in Homer2 wild-type (WT) and knock-out (KO) mice during a 15-min test. Sample sizes are indicated in parentheses. *p<0.05 vs. SAL; +p<0.05 vs. Control (t-tests).
DISCUSSION
Clinical evidence supports a life history of adverse events as an antecedent for problematic drinking (e.g., Annis et al. 1998; Blanco et al. 2013; Brown et al. 2012; Dawson et al. 2005; Heilig et al. 2010; Nishith et al., 2001; Noone et al. 1999; Sinha et al. 2009; Uhart and Wand 2009) and stress-alcohol cross-sensitization has been theorized, in part, to contribute to this relation (c.f., Burke and Miczek 2014; Hopf et al 2011). It has long been recognized that the ability to predict a stressor is a major factor determining an individual’s biobehavioral response to the stressor (c.f., Koolhaus et al. 2005) and that chronic exposure to unpredictable stressors produces a robust, depressive-like, phenotype in laboratory rodents that is associated with perturbations within the biochemistry of neural circuits subserving motivation (c.f., Willner, 2005). However, relatively few preclinical reports exist that focus on understanding the impact of unpredictable chronic mild stress (UCMS) upon behavioral sensitivity to alcohol, particularly alcohol intake (D’Aquila et al. 1994; Camarini et al. 2012; Rocha et al. 2012; Smith et al., 1996) of relevance to understanding the etiology of alcoholism and the high prevalence of comorbidity between alcohol use and affective disorders (e.g., Grant et al. 2005; Hasin and Grant 2004; Jacobsen et al. 2001; Kessler et al. 2005).
As reported for chronic, intermittent, stressor exposure (Roberts et al., 1995), an 11-day history of UCMS increased behavioral sensitivity to the psychomotor properties of alcohol as evidenced not only by increased alcohol-induced locomotor hyperactivity (2 g/kg), but also increased motor incoordination and sedation elicited by higher alcohol concentrations (3 and 5 g/kg, respectively). As the alcohol dose-motor response function is inverted U-shaped, the observation that UCMS mice exhibited a shorter latency to fall from the rotarod and longer latencies to regain their righting reflex (Fig. 5) at the higher alcohol doses tested argues a shift to the left in the dose-response function for alcohol’s psychomotor effects by UCMS. Notably, the potentiation of alcohol-induced locomotor activity by UCMS was reliable across distinct cohorts of SW mice and under different experimenters (e.g., Fig.1). Moreover, the potentiation of alcohol-induced locomotion was observed in SW mice subjected to our UCMS procedures during either the light or the dark phase of the circadian cycle (Fig.1A,C vs. Fig.1B). These observations demonstrate the reproducibility of the UCMS effect upon psychomotor responsiveness to alcohol of importance to an animal model of stress-alcohol interactions. Marked strain differences exist regarding behavioral and physiological responsiveness to stressors, with SW mice exhibiting higher stressor responsiveness than other mouse strains (e.g., van Bogaert et al., 2006a,b). Consistent with this, our UCMS procedures augmented the locomotion exhibited by saline-injected SW mice when placed in a novel environment (Fig.1A), while these same procedures did not influence novelty-induced locomotion in mice on a mixed C57BL/6J X 129SvJ/Xi background (Fig.9). The development of alcohol-induced behavioral sensitization can be strain-dependent (c.f., Phillips et al. 1997; but see Szumlinski et al. 2008b). Despite this and despite the apparent strain differences in susceptibility to UCMS effects upon spontaneous locomotor activity, a history of repeated stressor exposure potentiated alcohol-induced locomotion in both SW and hybrid mice (Fig. 1 vs. Fig.9). Such findings suggest that our procedurally facile, 11-day, UCMS procedure may be applied across different strains of mice in order to further our understanding of the underlying mechanisms through which a life history of adversity potentiates sensitivity to alcohol’s psychomotor, and other behavioral, effects.
The motivational valence of many drugs of abuse, including alcohol, is typically inversely related to sensitivity to the drug’s psychomotor properties; in the case of alcohol, intake and reinforcement tends to be negatively related to individual sensitivity to the drug’s sedative-hypnotic properties (e.g., Fergusson et al. 2003; Petrakis et al. 2004; Schuckit et al. 1997; Shabani et al. 2011; Szumlinski et al. 2005). However, despite exhibiting greater sensitivity to alcohol’s motor-impairing and sedative effects (Fig.5), UCMS animals exhibited greater, not lower, alcohol intake under a continuous-access voluntary alcohol drinking paradigm (Fig.2). Interestingly, the UCMS-induced increase in alcohol drinking was apparent only at the higher alcohol concentrations tested; relative to unstressed controls, UCMS mice consumed a greater amount of both 10% and 20% alcohol (v/v) during the very first 24-h drinking session (Fig.2D), and more alcohol from the 20% solution on average across the 5 days of drinking (Fig.2H). Thus, our 11-day UCMS procedures not only heightened initial alcohol intake, but also exerted more protracted effects upon the voluntary consumption of high-dose alcohol. Although UCMS SW mice did not differ from unstressed controls with respect to their initial preference for each alcohol solution (Fig.2C), their average preference for 20% alcohol was greater than that of control mice (Fig.2G). Such findings suggest that with drinking experience, the motivational valence of high-dose alcohol may sensitize at a faster rate in individuals with a prior life history of unpredictable stress, compared to those without. Studies employing operant self-administration and place-conditioning procedures should be conducted in order to vet this possibility more thoroughly. Nevertheless, the heightened intake of, and preference for, high-dose alcohol observed in UCMS animals are consistent with the results of prior work employing repeated stressor paradigms of various types and durations (for reviews, Becker et al. 2011; Breese et al., 2005; Burke and Miczek 2014; Delis et al., 2013; Heilig & Koob, 2007; Koob, 2013; Logrip et al. 2012; Richardson et al., 2008) and further support a cause-effect relation between a life history of unpredictable stress and excessive alcohol consumption. Moreover, as a stress-induced potentiation of alcohol’s psychomotor effects was first confirmed in mice prior to commencing drinking procedures(Fig.1B) and alcohol-induced psychomotor activity predicted subsequent alcohol intake under continuous access procedures (Fig.3), our data provide evidence for greater sensitivity to alcohol-induced psychomotor activation as an encouragement, rather than a deterrent, for high-dose alcohol drinking. This observation is consistent with earlier theories stemming from the alcohol sensitization literature arguing that sensitization might increase the probability of uncontrolled alcohol intake (Hunt and Lands, 1992) and/or heighten the reward value of the drug (Newlin and Thomson, 1990).
UCMS mice exhibited significantly greater water intake on day 1 of alcohol presentation, compared to control animals (Fig. 2B). As mice had ad libitum access to water for 64 h prior to commencing the alcohol drinking procedures (i.e., water restriction ended on the morning of day 10 of UCMS procedures; see Table 1), it is not likely that the elevated water intake exhibited by UCMS mice reflected greater thirst in these animals. Notably, UCMS mice in the drinking study did exhibit a significant drop in body weight over the 11-day course of procedures that was not apparent in control animals (Table 2). Thus, the elevated water intake exhibited on day 1 of alcohol presentation might be commensurate with an increase in food intake during recovery from the UCMS procedures; unfortunately, food intake was not monitored during the course of study to determine whether or not a correlative relation exists between food and water intake in early recovery. Nevertheless, the heightened water intake exhibited initially by UCMS mice was short-lived; no group differences were apparent for the average water intake of the mice during either the alcohol drinking study (Fig. 2F) or the saccharin drinking study (Fig. 4B). Such observations argue against some protracted effect of our UCMS procedures upon water homeostasis or, importantly, upon general reward processes.
Furthering this latter point, UCMS mice did not differ from controls with respect to their preference for, or intake of, palatable saccharin solutions (Fig. 4). In contrast to the alcohol phase of the study (Fig. 2), SW mice consumed the majority (~60%) of their daily fluid intake from the 0.025% (w/v) saccharin solution and did so in a manner indistinguishable from control animals (Fig.4A). The negative results for saccharin intake/preference are consistent with the very moderate to negligible effects of our UCMS procedures upon our health outcome measures (Table 2), but contrast starkly with the marked reduction in sweet solution reward, as well as body weight and self-care, reported to occur under more prolonged (3–10 week) UCMS paradigms more frequently used to model major depression in rodents (see Willner, 2005). Indeed, the majority of studies that have employed prolonged UCMS procedures have reported also reduced indices of drug reward, including blunted alcohol preference and intake (e.g., D’Acquila et al. 1994; Papp et al. 1991; Smith et al. 1996) and the cooccurrence of low preference for both non-drug and drug rewards has been interpreted to reflect UCMS-induced anhedonia (c.f., Willner 2005). However, the rate of comorbidity between alcohol use disorders and affective disorders, including major depression, is remarkably high (e.g., Hasin and Grant 2004; Hasin et al. 2005, 2007; Kessler et al. 2005; Presley et al. 1994; SAMHSA 2010). As such, higher, not lower, alcohol intake is predicted of an animal model of affective disorder and/or alcoholism-affective disorder comorbidity. Indeed, our 11-day UCMS procedures promoted alcohol intake, without consistently affecting water intake and without impacting saccharin reward; this collection of findings argues the predictive validity of our model for the neurobiological study of stress-alcohol interactions of relevance to the etiology of alcohol use disorder and its comorbity with affective disorders. This all being said, we would like to acknowledge that we assayed for saccharin intake 8 days following cessation of our UCMS procedures. Thus, while our negative results for saccharin drinking most certainly argue against any protracted effect of our UCMS procedures upon general reward processes, it is possible that any UCMS effects upon general reward processes may have waned prior to the presentation of the saccharin solutions. Humans who suffered psychological trauma exhibit problematic drinking for years following the event(s) (e.g., Blanco et al. 2013; Grant et al. 2004; Hasin et al. 2007; Jacobson et al. 2001; Nishith et al. 2001). Now that we have established initial predictive validity for our 11-day UCMS procedures for alcohol preference/intake in the short-term, it is important to extend these observations and more thoroughly vet the longevity of stress-alcohol interactions of relevance to health outcomes during protracted recovery from psychophysiological trauma. Studies are currently on-going in our laboratories to address this important issue.
The fact that UCMS mice exhibited higher baseline psychomotor activity, as well as greater sensitivity to both the acute psychomotor effects and the rewarding properties of alcohol suggests that our UCMS procedures were sufficient to elicit plasticity within neural circuits governing both the psychomotor and incentive motivational properties of this drug. While there exists a considerable amount of work related to stress-alcohol interactions vis-à-vis both hypothalamic and extra-hypothalamic stress pathways, as well as mesocorticolimbic dopamine transmission (c.f., Becker et al. 2011; Brabant et al. 2014; Breese et al., 2005; Koob, 2013; Lee et al. 2013; Lu and Richardson 2014), we have reported lasting effects of developmental exposure to repeated stressors upon indices of basal and drug-stimulated changes in glutamate transmission within the NAC and/or interconnected structures (Ary et al. 2007; Kippin et al. 2008). Indeed, glutamate transmission, particularly within the NAC, has been long-implicated in the development of drug-induced motor sensitization, including that to alcohol (e.g., Carrara-Nascimento et al. 2011; Cornish and Kalivas, 2001; Di Franza and Wellman 2007; Schmidt and Pierce 2010; Szumlinski et al. 2005, 2008a) and NAC glutamate is well-established to contribute critically to alcohol’s rewarding/reinforcing properties (for recent reviews, Chandrasekar, 2013; Griffin 2014; Holmes et al. 2013; Woodward and Szumlinski, 2014). Compared to controls, UCMS mice exhibited elevated expression of mGlu1α, and GluN2b within the NAC shell irrespective of whether or not the animals were challenged with a 2 g/kg alcohol injection and UCMS animals exhibited a rise in Homer2 expression in response to an acute alcohol injection (Figs.6 and 7). The protein profile of the NAC shell of UCMS mice is particularly interesting as it is very similar to that observed in rodents with chronic alcohol experience (Cozzoli et al. 2009, 2012; Goulding et al. 2011; Lum et al. 2014; Obara et al. 2009; Szumlinski et al. 2008b). Moreover, an increase mGlu1α, GluN2b and/or Homer2a/b is also observed within the NAC shell of inbred and selectively bred mouse strains that exhibit excessive alcohol drinking phenotypes (Cozzoli et al. 2009, 2012; Goulding et al. 2011) and considerable neuropharmacological and neurogenetics evidence points to a cause-effect relation between elevated NAC (shell) expression of Group1 mGluRs, GluN2b and Homer2a/b in promoting or maintaining a “pro-alcoholic” phenotype (c.f., Bird and Lawrence, 2009; Cui et al., 2013; Gass and Olive, 2008; Holmes et al., 2013, Woodward and Szumlinski, 2014). Furthermore, NAC Homer2 actively regulates the development of motor sensitization elicited by repeated alcohol injections (Szumlinski et al., 2004; 2008b). Although the mechanism(s) through which a history of UCMS elevates NAC shell levels of mGlu1α, GluN2b and Homer2a/b remain to be elucidated, we theorize that this neuroadaptation might underpin the psychomotor/emotional hyper-reactivity of UCMS mice, which is posited to increase the negative reinforcing properties of alcohol and drive high-dose alcohol consumption (Fig.10A–C). Consistent with this notion, Homer2 KO mice exhibit an alcohol-avoiding and intolerant phenotype (Szumlinski et al. 2005) and fail to exhibit UCMS-alcohol cross-sensitization (Fig. 9).
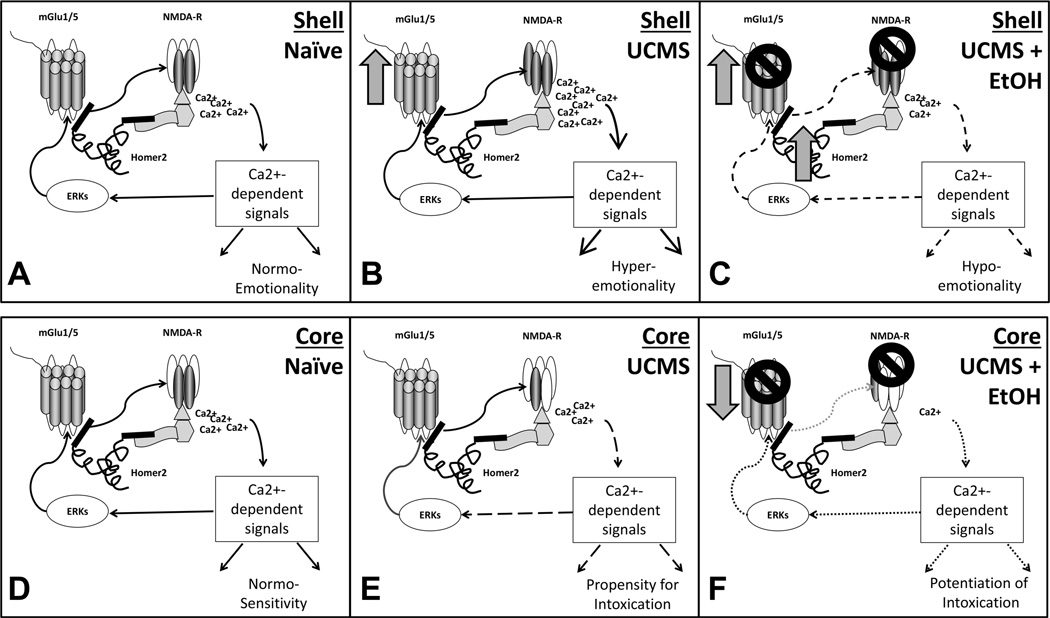
Figure 10.
Hypothetical models of how interactions between Group1 mGluRs, Homer2, NMDA receptors (NMDA-Rs) and ERK that may account, in part, for (A–C) the hyper-emotional state and excessive alcohol-drinking phenotype, as well as (D–F) the hypersensitivity of UCMS animals to the intoxicating properties of alcohol. (A&D) In naïve individuals, Homers scaffold mGlu1/5 receptors to NMDA-Rs via interactions with a chain of other scaffolding molecules, including Shank and PSD-95. Amongst the many functional consequences of Homer interactions with glutamate receptors, Homer scaffolding to mGlu1/5 negatively regulates the ability of ERKs and other proline-directed kinases to phosphorylate these receptors and this attenuates their ability to facilitate slow inward currents through NMDA-Rs upon their activation (Park et al. 2013). (B) In the NAC shell, UCMS upregulates mGlu1 (arrow) and GluN2b (dark subunits in NMDA-R) and this adaptation should increase calcium (Ca2+)-dependent signaling by either or both receptor(s). It is this heightened glutamate signaling within the NAC shell that may account for the emotional hyper-reactivity of UCMS animals. (C) Alcohol inhibits mGluRs and NMDA-Rs (e.g., Lovinger et al. 1989; Minami et al. 1998) and higher GluN2b content [as observed within the NAC shell of UCMS animals (Fig.6A)], increases the alcohol sensitivity of NMDA-Rs (c.f., Woodward and Szumlinski 2014). Alcohol inhibition of glutamate receptor function is theorized to contribute, in part, to the anxiolytic properties of the drug (e.g., Koob 2003). Thus, the excessive alcohol intake of UCMS animals might reflect an attempt to overcome the glutamate hyper-activity induced within the NAC shell by their history of repeated stress. Acute alcohol administration also elevates NAC shell levels of Homer2 (Fig.7A) of UCMS mice and this molecular response is predicted to restrict mGluR scaffolding to the perisynaptic region, away from NMDA-Rs (c.f., Szumlinski et al. 2008a), as well as physically occlude the ERK-dependent phosphorylation of mGluRs required for the potentiation of NMDA-R current (Park et al. 2013). Thus, an alternate/additional mechanism through which alcohol may exert its negative reinforcing properties might relate to a disruption of ERK-mGluR-NMDA-R signaling via increased Homer2 expression. (E) In the NAC core, a history of UCMS down-regulates (arrow) GluN2b expression (Fig.6B) and this effect is predicted to reduce the signaling efficiency of NMDA-Rs, as well as their ability to scaffold to PSD-95/Shank/Homer2 complexes (c.f., Szumlinski et al 2008a). This compromised glutamate signaling through NMDA-Rs within the NAC core of UCMS animals is posited to increase sensitivity to alcohol’s motor effects (c.f., Nona et al. 2014). (F) Consequently, when administered alcohol acutely, UCMS animals exhibit greater sensitivity to the drug’s motor effects, as manifested by heightened psychomotor-activation in response to moderate alcohol doses and greater intoxication/sedation in response to higher alcohol doses. This increased sensitivity to the motor effects of alcohol is predicted to be potentiated by the alcohol-induced reduction in mGlu1 observed in UCMS animals (Fig.7B), which is predicted to blunt the capacity of mGlu1 to facilitate calcium current through NMDA-Rs.
Despite the above, the UCMS protein profile of the NAC shell is not identical to that produced by repeated alcohol as binge drinking history augments PLCβ levels within the NAC shell (Lum et al. 2014), whereas enzyme levels were lowered within this subregion following UCMS (Fig.8A). Whether or not the reduction in PLCβ observed in UCMS mice reflects some compensatory response to increased mGlu1α function cannot be discerned from the present study. Although the present immunoblotting data are correlative in nature, the opposing effects of UCMS upon the NAC shell expression of mGlu1α (up-regulation) and PLCβ (down-regulation) suggests that a history of stressor exposure may uncouple Group1 mGluRs from their canonical signaling pathway. By extension then, increased mGlu1α or mGlu5 signaling through PLCβ does not likely underpin the “alcohol pre-sensitized” phenotype of UCMS mice-an argument supported by our prior work demonstrating that pharmacological inhibition of mGlu1, mGlu5 or PLCβ within the NAC shell reduces, not potentiates, alcohol intake (Cozzoli et al. 2009, 2012; Lum et al., 2014).
As reported previously in alcohol-experienced animals (e.g., Cozzoli et al. 2009, 2012; Goulding et al. 2011; Obara et al. 2009; Szumlinski et al., 2008b), distinctions exist with respect to the protein profile elicited by UCMS between NAC shell and core subregions (Figs. 6–8). However, in contrast to repeated alcohol, which either does not change (Cozzoli et al. 2009, 2012) or increases (Goulding et al. 2011; Obara et al., 2009) GluN2b levels within the NAC core, UCMS mice exhibited lowered GluN2b expression within this subregion and a reduction in NAC core mGlu1α levels was observed only in alcohol-injected UCMS mice, corresponding to their cross-sensitized state (Fig. 6). While we have yet to detect a reduction in Group1 mGluR or GluN2 expression within the NAC core of alcohol-experienced animals, similar receptor changes are observed in cocaine-sensitized rodents (Ary and Szumlinski, 2007). Indeed, the psychomotor-activating effects of stress have been long-known to cross-sensitize with those of stimulant drugs, including cocaine (c.f., Burke and Miczek, 2014; Kalivas and Stewart, 1991; Robinson and Becker, 1986). GluN2b-containing NMDA receptors exhibit higher affinity for glutamate and slower desensitization kinetics than other NMDA receptor subtypes (c.f., Woodward and Szumlinski, 2014). Thus, reduced basal expression of GluN2b subunits within the NAC core is predicted to result in less efficient NMDA receptor signaling in alcohol-naïve UCMS animals. NMDA receptor blockade by alcohol contributes, in part, to the sedative-hypnotic effects of this drug [REF]. By extension then, UCMS-induced reduction in GluN2b expression within the NAC core might underpin the hyper-sensitivity of UCMS animals to alcohol’s intoxicating effects (Fig.10D–F). Indeed, compromised NMDA and mGlu1/5 receptor function has been theorized to account for the hyper-sensitivity of Homer2 KO mice to the sedative-hypnotic properties of alcohol (Szumlinski et al. 2005) and systemic pretreatment with the mGu1 antagonist CPCCOEt augments alcohol-induced behavioral intoxication in C57BL/6J mice (Lominac et al. 2006). Thus, reduced glutamatergic signaling through GluN2b-containing NMDA receptors and/or mGlu1α within the NAC core might also contribute to UCMS-alcohol cross-sensitization of the drug’s psychomotor effects.
Interestingly, although the effects of UCMS upon glutamate receptor-related protein expression were more or less opposite between the NAC shell and core subregions (Fig. 6), we observed a nearly identical UCMS-alcohol interaction for p-ERK levels within both subregions (Fig. 8). As reported by others (Ibba et al., 2009; Thorsell et al., 2013), an acute alcohol injection potentiated p-ERK levels in unstressed controls and this effect was observed at 3 hrs post-injection (Fig.8). While a history of UCMS did not produce any detectable change in basal NAC p-ERK expression, p-ERK levels dropped below baseline in response to the alcohol challenge in UCMS animals (Fig. 8) – an effect reported to occur in response to the acute administration of alcohol doses greater than 2.5 g/kg (Anderson et al., 2010; Torres et al., 2013; Zhu et al., 2013) or in alcohol-dependent animals (Sanna et al., 2002). Thus, a history of UCMS sensitizes mice to alcohol-induced changes in NAC ERK activation. Supporting a functional link between UCMS-alcohol interactions in NAC p-ERK levels and stress-alcohol behavioral cross-sensitization, systemic administration of an ERK inhibitor augments operant responding for alcohol (Faccidomo et al., 2009). To the best of our knowledge, no study has yet to assess the effects of local ERK inhibition with NAC subregions upon behavioral sensitivity to alcohol, although ERK inhibition within other extended amygdala structures is reported to augment alcohol’s discriminative stimulus and rewarding/reinforcing properties (Campbell et al., 2014; Besheer et al., 2012; Pandey et al., 2006). Such findings argue that a deficiency in the alcohol responsiveness of ERK within NAC subregions might play a critical role in regulating stressor-induced potentiation of alcohol drinking behavior. Indeed, recent evidence indicates that the capacity of mGlu5, and perhaps also mGlu1, to potentiate slow inward calcium currents through NMDA receptors is regulated by ERK phosphorylation of the receptor near the Homer interacting domain. Furthermore, the expression of long-form Homers, incl. Homer2 isoforms, occludes this subtle cross-talk between mGluRs and NMDA receptors and mutation of the ERK phosphorylation sites eliminates the potentiation of NMDA current by mGlu5 upon receptor stimulation, while leaving the canonical αq signaling pathway through mGlu5 receptors intact (Park et al. 2013). While our understanding of the behavioral relevance of this recently characterized interaction between ERK, Group1 mGluRs, Homers and NMDA receptors is relatively poor, mice with a mutation of the ERK phosphorylation sites on mGlu5 exhibit hypersensitivity to the acute psychomotor-activating properties of cocaine (Park et al. 2013). Such data suggest that ERK-mGluR interactions are important negative regulators of sensitivity to the acute psychomotor properties of drugs of abuse and perhaps normally curb alcohol intake (Campbell et al., 2014; Faccidomo et al., 2009; Pandey et al., 2006) and a hypothetical model to account for how changes in ERK activation within the NAC shell and core might be sufficient to augment behavioral sensitivity to alcohol via interactions with glutamate receptors is provided in Fig. 10.
In conclusion, an 11-day history of UCMS renders an animal more sensitive to both the psychomotor and rewarding properties of alcohol, providing predictive validity of our procedures for the study of the psychobiological underpinnings of stress-alcohol interactions of relevance to the etiology of alcoholism and its comorbidity with affective disorders. To this end, the potentiation of behavioral responsiveness to alcohol by UCMS history was associated with increased indices of post-synaptic glutamate transmission within the NAC shell, lowered indices of post-synaptic glutamate transmission within the NAC core and impaired alcohol responsiveness of ERK within both NAC subregions. All of these molecular adaptations have been implicated previously in greater behavioral sensitivity to alcohol and/or other drugs of abuse, rendering them attractive as targets to understand how a life history of adversity predisposes individuals to alcoholism and the neurobiological underpinnings of alcoholism-affective disorder comorbidity.
Acknowledgements
Funding provided by: NIAAA grant AA016650 to KKS, FAPESP grant 2012/10260-7 and CNPq grant to RC.
Footnotes
Authors’ Contributions
Sema G. Quadir conducted a large portion of the behavioral studies, contributed to manuscript composition and editing.
Jaqueline Rocha established experimental procedures, contributed to experimental design, conducted a portion of both the behavioral and the immunoblotting studies, conducted data analyses, and edited the manuscript.
Rianne R. Campell conducted a portion of the behavioral studies and edited the manuscript.
Melissa G. Wroten conducted a large portion of the immunoblotting studies and edited the manuscript.
Nimrita Singh conducted a portion of the behavioral studies and edited the manuscript.
John J. Holloway conducted a portion of the behavioral and immunoblotting studies and edited the manuscript.
Sukhmani Bal conducted a portion of the behavioral studies and edited the manuscript.
Rosana Camarini assisted in experimental design and edited the manuscript.
Karen K. Szumlinski supervised all aspects of behavioral and immunoblotting studies, contributed to experimental design, conducted data analyses and composed the manuscript.
References
- Agosti V. Predictors of alcohol dependence relapse during recurrence of major depression. J Addict Dis. 2013;32:79–84. doi: 10.1080/10550887.2012.759861. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34:2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis HM, Sklar SM, Moser AE. Gender in relation to relapse crisis situations, coping, and outcome among treated alcoholics. Addict Behav. 1998;23:127–131. doi: 10.1016/s0306-4603(97)00024-5. [DOI] [PubMed] [Google Scholar]
- Ary AW, Aguilar VR, Szumlinski KK, Kippin TE. Prenatal stress alters limbo-corticostriatal Homer protein expression. Synapse. 2007;61:938–941. doi: 10.1002/syn.20439. [DOI] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK. Imbalances in prefrontal cortex CC-Homer1 versus -Homer2 expression promote cocaine-seeking behavior. J Neurosci. 2013;33:8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ary AW, Szumlinski KK. Regional differences in the effects of withdrawal from repeated cocaine upon Homer and glutamate receptor expression: A two-species comparison. Brain Res. 2007;1184:295–305. doi: 10.1016/j.brainres.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology. 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Fisher KR, Cannady R, Grondin JJ, Hodge CW. Intra-amygdala inhibition of ERK(1/2) potentiates the discriminative stimulus effects of alcohol. Behav Brain Res. 2012;228:398–405. doi: 10.1016/j.bbr.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird MK, Lawrence AJ. Group I metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Pérez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: Results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2013;132:630. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant C, Guarnieri DJ, Quertemont E. Stimulant and motivational effects of alcohol: Lessons from rodent and primate models. Pharmacol Biochem Behav. 2014;122C:37–52. doi: 10.1016/j.pbb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ, Navarro M. Prior multiple ethanol withdrawals enhance stress induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a–receptor agonist. Neuropsychopharmacology. 2005;30:1662–1669. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, Williams J, Bray RM, Hourani L. Postdeployment alcohol use, aggression, and post-traumatic stress disorder. Mil Med. 2012;177:1184–1190. doi: 10.7205/milmed-d-11-00119. [DOI] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R. Cross sensitization between stress and ethanol in adolescent and adult mice: Role of neuronal nitric oxide synthase. Alcohol Clin Exp Res. 2012;36(S2):83A. [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE. Contribution of early environmental stress to alcoholism vulnerability. Alcohol. 2009;43:547–554. doi: 10.1016/j.alcohol.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RR, Waltermire RS, Courson JA, Greentree DI, McGregor H, Szumlinski KK. ERK phosphorylation of mGluR5 within the BNST inhibits binge alcohol intake in mice. Alcohol Clin Exp Res. 2014;39(S1):13A. [Google Scholar]
- Carrara-Nascimento PF, Griffin WC, 3rd, Pastrello DM, Olive MF, Camarini R. Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol. 2011;45:451–460. doi: 10.1016/j.alcohol.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar R. Alcohol and NMDA receptor: current research and future direction. Front Mol Neurosci. 2013;6:14. doi: 10.3389/fnmol.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Cozzoli DK, Courson J, Wroten MG, Greentree DI, Lum EN, Campbell RR, Thompson AB, Worley PF, Jonquieres G, Klugmann M, Finn DA, Szumlinski KK. Binge alcohol drinking by mice requires intact Group1 metabotropic glutamate receptor signaling within the central nucleus of the amygdala. Neuropsychopharmacology. 2014;39:435–444. doi: 10.1038/npp.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cronise K, Finn DA, Metten P, Crabbe JC. Scheduled access to ethanol results in motor impairment and tolerance in female C57BL/6J mice. Pharmacol Biochem Behav. 2005;81:943–953. doi: 10.1016/j.pbb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Cui C, Noronha A, Morikawa H, Alvarez VA, Stuber G, Szumlinski KK, Kash TL, Roberto M. New insights on neurobiological mechanisms underlying alcohol addiction. Neuropharmacology. 2013;67C:223–232. doi: 10.1016/j.neuropharm.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aquila P, Brain PF, Willner P. Effects of chronic mild stress in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Ruan WJ. The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol. 2005;40:453–460. doi: 10.1093/alcalc/agh176. [DOI] [PubMed] [Google Scholar]
- Delis F, Thanos PK, Rombola C, Rosko L, Grandy D, Wang GJ, Volkow ND. Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav Neurosci. 2013;127:95–105. doi: 10.1037/a0030750. [DOI] [PubMed] [Google Scholar]
- Di Franza JR, Wellman RJ. Sensitization to nicotine: how the animal literature might inform future human research. Nicotine Tob Res. 2007;9:9–20. doi: 10.1080/14622200601078277. [DOI] [PubMed] [Google Scholar]
- Faccidomo S, Besheer J, Stanford PC, Hodge CW. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology. 2009;204:135–147. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, Giorgetti Britto LR, Camarini R. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PA. Early reactions to cannabis predict later dependence. Arch Gen Psychiat. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Hervé D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: A factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011;10:111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Patricia Chou S, June Ruan W, Huang B. Co-occurrence of 12-month mood and anxiety disorders and personality disorders in the US: results from the national epidemiologic survey on alcohol and related conditions. J Psychiatr Res. 2005;39:1–9. doi: 10.1016/j.jpsychires.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Griffin WC., 3rd Alcohol dependence and free-choice drinking in mice. Alcohol. 2014;48:287–293. doi: 10.1016/j.alcohol.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983:97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: Association with past alcohol dependence. Arch Gen Psychiatry. 2004;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology. 2013;229:539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Sparta DR, Bonci A. Translational models of interactions between stress and alcohol consumption: strengths and limitations. ILAR J. 2011;52:239–2350. doi: 10.1093/ilar.52.3.239. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Lands WEM. A role for behavioral sensitization in uncontrolled ethanol intake. Alcohol. 1992;9:327–328. doi: 10.1016/0741-8329(92)90075-l. [DOI] [PubMed] [Google Scholar]
- Ibba F, Vinci S, Spiga S, Peana AT, Assaretti AR, Spina L, et al. Ethanol-induced extracellular signal regulated kinase: role of dopamine D1 receptors. Alcohol Clin Exp Res. 2009;33:858–867. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG. Volume I: Secondary school students (NIH Publication No. Rockville, MD: National Institute on Drug Abuse; 1999. National Survey Results on Drug Use from the Monitoring the Future Study, 1975–1997; pp. 98–4345. [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippin TE, Szumlinski KK, Kapasova Z, Rezner B, See RE. Prenatal stress enhances responsiveness to cocaine. Neuropsychopharmacology. 2008;33:769–782. doi: 10.1038/sj.npp.1301447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Nazarenko SE. Comparison of the excitatory and anaesthetic effects of ethanol in C57BL/6 and BALB/c mice; relation to blood ethanol concentration. Med Biol. 1978;56:281–285. [PubMed] [Google Scholar]
- Lee MR, Bollinger JW, Leggio L. Increased Ethanol Consumption Following Chronic Psychosocial Stress: Do Oxytocin and Baclofen Hold any Therapeutic Promise? Front Psychiatry. 2013;4:148. doi: 10.3389/fpsyt.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. Erratum in (2013) Lancet 381:1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacol. 2012;62:552–564. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Dep. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lu YL, Richardson HN. Alcohol, stress hormones, and the prefrontal cortex: A proposed pathway to the dark side of addiction. Neuroscience. 2014;277C:139–151. doi: 10.1016/j.neuroscience.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum EN, Campbell RR, Rostock C, Szumlinski KK. mGluR1 within the nucleus accumbens regulates alcohol intake in mice under limited-access conditions. Neuropharmacology. 2014;79:679–687. doi: 10.1016/j.neuropharm.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Strange J. The global burden of drug use and mental disorders. The Lancet. 2013;382:1540–1542. doi: 10.1016/S0140-6736(13)61781-X. [DOI] [PubMed] [Google Scholar]
- Minami K, Gereau RW4th, Minami M, Heinemann SF, Harris RA. Effects of ethanol and anesthetics on type 1 and 5 metabotropic glutamate receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;53:148–156. doi: 10.1124/mol.53.1.148. [DOI] [PubMed] [Google Scholar]
- Miquel M, Correa M, Sanchis-Segura C, Aragon CM. The ethanol-induced open-field activity in rodents treated with isethionic acid, a central metabolite of taurine. Life Sci. 1999;64:1613–1621. doi: 10.1016/s0024-3205(99)00098-3. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Nishith P, Resick PA, Mueser KT. Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. J Trauma Stress. 2001;14:469–479. doi: 10.1023/A:1011152405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 2012;37:2210–2221. doi: 10.1038/npp.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone M, Dua J, Markham R. Stress, cognitive factors, and coping resources as predictors of relapse in alcoholics. Addict Behav. 1999;24:687–693. doi: 10.1016/s0306-4603(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes CM, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Subregional selectivity in the effects of alcohol withdrawal upon Homer/glutamate receptor expression in the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M, Lappas S, Muscat R, Willner P. Attenuation of place preference conditioning but not place aversion conditioning by chronic mild stress. J Psychopharmacol. 1992;6:352–356. doi: 10.1177/026988119200600302. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: Attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology. 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Park JM, Hu JH, Milshteyn A, Zhang PW, Moore CG, Park S, Datko MC, Domingo RD, Reyes CM, Wang XJ, Etzkorn FA, Xiao B, Szumlinski KK, Kern D, Linden DJ, Worley PF. A prolyl-isomerase mediates dopamine-dependent plasticity and cocaine motor sensitization. Cell. 2013;154:637–650. doi: 10.1016/j.cell.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–1782. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Roberts AJ, Lessov CN. Behavioral sensitization to ethanol: genetics and the effects of stress. Pharmacol Biochem Behav. 1997 Jul;57(3):487–493. doi: 10.1016/s0091-3057(96)00448-0. 1997. [DOI] [PubMed] [Google Scholar]
- Presley CA, Meilman PW, Lyerla R. Development of the Core Alcohol and Drug Survey: Initial findings and future directions. J Amer Coll Health. 1994;42:248–255. doi: 10.1080/07448481.1994.9936356. [DOI] [PubMed] [Google Scholar]
- Quoilin C, Didone V, Tirelli E, Quertemont E. Ontogeny of the stimulant and sedative effects of ethanol in male and female Swiss mice: gradual changes from weaning to adulthood. Psychopharmacology. 2010;212:501–512. doi: 10.1007/s00213-010-1971-z. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275:790–797. [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rocha JBS, Zuniga JP, Camarini R. Effects of 7-nitroindazole in behavioral sensitization between stress and ethanol in mice. Alcohol Clin Exp Res. 2012;36(S):111A. [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 2010;1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Studies of men at high risk for future alcoholism. In: von Wartburg JP, Magnenat P, Muller R, Wyss S, editors. Currents in alcohol research and the prevention of alcohol problems. Berne Stuttgart, Toronto’ Hans Huber Publishers; 1985. pp. 45–51. [Google Scholar]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res. 2001;25:323–329. [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch Gen Psychiatry. 1998;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Smith TL, Wiesbeck GA, Kalmijn J. The relationship between Self-Rating of the Effects of alcohol and alcohol challenge results in ninety-eight young men. J Stud Alcohol. 1997;58:397–404. doi: 10.15288/jsa.1997.58.397. [DOI] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed CR, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCbeta GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Maurel Remy S, Schreiber R, DeVry J. Chronic mild stress causes a decrease in the preference for low alcohol concentrations in male Wistar rats. Eur Neuropsychopharmacol. 1996;6:S4–S131. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings (Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586 Findings) 2010
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: Implications for addiction. Biochem Pharmacol. 2008a;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 over-expression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008b;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Rohrer J, Griffin W, III, Klugmann M, Toda S, Champtiaux NP, Berry T, Shealy S, During M, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate vulnerability to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During MT, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for ethanol-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Kiianmaa K. Does tolerance develop to the activating, as well as the depressant, effects of ethanol? Pharmacol Biochem Behav. 1982;17:1073–1076. doi: 10.1016/0091-3057(82)90496-8. [DOI] [PubMed] [Google Scholar]
- Tanti A, Rainer Q, Minier F, Surget A, Belzung C. Differential environmental regulation of neurogenesis along the septo-temporal axis of the hippocampus. Neuropharmacology. 2012;63:374–384. doi: 10.1016/j.neuropharm.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Tapocik JD, Liu K, Zook M, Bell L, Flanigan M, et al. A novel brain penetrant NPS receptor antagonist, NCGC00185684, blocks alcohol-induced ERK-phosphorylation in the central amygdala and decreases operant alcohol self-administration in rats. J. Neurosci. 2013;33:10132–10142. doi: 10.1523/JNEUROSCI.4742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolliver BK, Ho LB, Reid MS, Berger SP. Evidence for dissociable mechanisms of amphetamine- and stress-induced behavioral sensitization: effects of MK-801 and haloperidol pretreatment. Psychopharmacology. 1996;126:191–198. doi: 10.1007/BF02246448. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, O’dell LE. Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alcohol Clin Exp Res. 2013;38:108–115. doi: 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Wand GS. Stress, alcohol and drug interaction: an update of human research. Addict Biol. 2009;14:43–64. doi: 10.1111/j.1369-1600.2008.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bogaert MJ, Groenink L, Oosting RS, Westphal KG, van der Gugten J, Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006a;5:139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- van Bogaert M, Oosting R, Toth M, Groenink L, van Oorschot R, Olivier B. Effects of genetic background and null mutation of 5-HT1A receptors on basal and stress-induced body temperature: modulation by serotonergic and GABAA-ergic drugs. Eur J Pharmacol. 2006;550:84–90. doi: 10.1016/j.ejphar.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Czyrak A. The role of corticosteroids in the acquisition of sensitization to locomotor stimulant effects of MK-801. Brain Res. 1994;657:351–356. doi: 10.1016/0006-8993(94)90991-1. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen KC, Schuckit M, Smith TL, Lee JV, Segall SK, Feiler HS, Kalmijn J. The search for genes related to a low-level response to alcohol determined by alcohol challenges. Alcohol Clin Exp Res. 2003;27:1041–1047. doi: 10.1097/01.ALC.0000075551.02714.63. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Szumlinski KK. Glutamate signaling in alcohol abuse and dependence. In: Norohna ABC, Cui C, Harris A, Crabbe JC, editors. Neurobiology of Alcohol Dependence. Academic Press; 2014. pp. 173–206. [Google Scholar]
- Zhu Y, Wang Y, Zhao B, Wei S, Xu M, Liu E, et al. Differential phosphorylation of GluN1-MAPKs in rat brain reward circuits following long-term alcohol exposure. PLoS One. 2013;8:e54930. doi: 10.1371/journal.pone.0054930. [DOI] [PMC free article] [PubMed] [Google Scholar]