Abstract
Objective
Sexual dimorphism is evident in attention-deficit/hyperactivity disorder (ADHD), including subtype prevalence, adverse outcomes, and neural phenotype. Neurobiological studies of ADHD suggest boys show more abnormalities in motor and premotor structure and function, whereas girls differ from typically developing (TD) peers in prefrontal circuitry. We applied diffusion tensor imaging (DTI) to identify ADHD-related sex-specific differences in motor/premotor and prefrontal white matter (WM) microstructure in children.
Method
DTI estimated differences in WM microstructure among 120 children aged 8–12 years, 60 with ADHD (30 boys) and 60 controls (30 boys), matched on age, IQ, and handedness. Effects of diagnosis and sex on fractional anisotropy (FA) were assessed in motor/premotor and prefrontal regions. Group differences in FA and associations with response control (e.g., reaction time variability [CVRT] and commission error rate) were examined separately within sex.
Results
Sex-by-diagnosis interactions were observed for FA in primary motor (M1) and medial orbitofrontal (MOFC) cortex. Post hoc tests revealed that boys with ADHD showed bilateral reductions in FA within M1, compared with TD peers; in contrast, girls with ADHD showed higher FA bilaterally within MOFC. Decreased M1 FA was associated with higher CVRT in boys and higher commission error rates in girls. For MOFC, lower FA was associated with greater CVRT and commission error rates across all participants with ADHD.
Conclusion
ADHD affects the white matter of boys and girls differently; boys appear more affected in regions responsible for control of basic actions, whereas girls show more abnormalities in regions responsible for higher-level, top-down control.
Keywords: intra-subject variability, attention, diffusion tensor imaging, sex differences, response control
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is characterized by developmentally inappropriate levels of inattention, hyperactivity, and impulsivity, resulting in significant functional impairment.1 Specific cognitive deficits are thought to underlie the behavioral symptoms of ADHD, including difficulties with response control (e.g., response inhibition and variability), attention regulation, and working memory.2 Among individuals with ADHD, important differences have emerged between sexes in terms of prevalence rates, symptom presentation, and comorbidities.3–7 Functional outcomes differ between sexes as well.3,8,9
Although evidence for sex differences in brain development and connectivity in youth with ADHD is emerging,10,11 much of the existing literature to date has relied upon either male samples only12 or samples with too few girls with ADHD to enable across- and within-sex comparisons.13,14 Given the differences in ADHD prevalence rates and symptom presentation between boys and girls, and the differences in pace of cortical development and myelination of frontal and associated regions essential for response control and attentional regulation,15–17 studies carefully examining sex–specific neuroanatomical differences in ADHD and their functional correlates are needed. Increasing evidence suggests sex-based differences in neuropsychological functioning in children with ADHD,18–21 with girls generally showing more deficits in planning and strategy mediated by prefrontal circuits, and boys showing greater impairments in more basic aspects of response control mediated by motor/premotor circuits. Recent work21 examining sex differences in intrasubject variability in response time (ISV, which is robustly increased among children with ADHD22) revealed a sexually dimorphic effect of task complexity such that increased ISV among girls with ADHD was specific to a Go/No-go task with substantial cognitive load and working memory demands, whereas boys with ADHD exhibited increased ISV regardless of working memory demands. This dimorphic profile was also seen in error rates. Taken together with the differences in outcomes and behavior, these sex-based differences in cognitive control suggest the likelihood of underlying differences between boys and girls in neuroanatomy and functional connectivity associated with ADHD.
Among neuroimaging studies including enough girls in the sample to investigate sex-specific differences, findings have revealed differences between children with ADHD and typically-developing (TD) peers in regional gray and white matter (WM) volumes11 as well as basal ganglia volume and shape.23 These include sex-specific differences of reduced gray matter in the left lateral premotor cortex in girls with ADHD, reduced left medial prefrontal cortex (PFC) WM volumes in boys with ADHD, as well as smaller basal ganglia volumes in boys but not girls with ADHD, as compared to same-sex controls. In a careful examination of frontal lobe morphology in children with ADHD,24 reduced cortical surface area was found in orbitofrontal, medial prefrontal, and anterior cingulate cortices relative to TD children; however, differences were also seen between boys and girls with ADHD. Boys with ADHD were found to have reduced premotor cortical volume, while girls with ADHD showed prefrontal reductions relative to same-sex controls.24
Examination of WM microstructure using diffusion tensor imaging (DTI) has begun to provide additional evidence of WM pathology and disruptions in anatomical connectivity in ADHD, although findings have been inconsistent.25,26 For example, Silk et al12 found increased fractional anisotropy (FA) values in left prefrontal-temporal regions and right occipito-parietal cortex in boys with ADHD, while others have found decreased FA in premotor and left parieto-occipital regions.27,28 A recent diffusion spectrum imaging (DSI) study14 found reduced FA in four bilateral frontostriatal tracts in youth (predominantly males) with ADHD. Overall, DTI and DSI studies suggest abnormalities in large WM tracts such as superior longitudinal fasciculus, arcuate fasciculus, and anterior corona radiata in youth with ADHD,25,26,29 areas connecting PFC with basal ganglia and thus thought to play an important role in response control. However, findings have been based on predominantly male samples, with too few girls included to examine sex-specific differences. Further work investigating frontal subregions is needed to better elucidate the associations between WM abnormalities and response variability in ADHD, and to clarify neural substrates underlying the sex-specific differences in ADHD features.
Evidence regarding the functional implications of WM abnormalities in ADHD is also inconsistent. For example, left WM volumes and reduced left orbitofrontal FA were associated with ADHD-related symptom severity (in boys11); however, these differences were not associated with response control on motor inhibition tasks (i.e., Go/No-go reaction time [RT], commission rate, ISV). In contrast, Lin et al.14 reported an association among ISV and FA in the cingulum bundles and frontostriatal tracts. Similarly, Hong et al.13 found specific tract-based anomalies in frontal-subcortical and fronto-cerebellar regions were associated with performance (commission/omission errors) on a continuous performance task (CPT), with FA values within a primarily right hemisphere network including superior frontal gyrus, anterior cingulate, and supplementary motor cortex (SMC) negatively correlated with CPT omissions and RT variability. However, these samples contained too few girls for between-sex comparisons. Although these studies support prior research implicating prefrontal circuitry in ADHD,11,30,31 and lay a foundation for further investigation, the issues raised by Weyandt et al.32 regarding consistency and clinical relevance of abnormal imaging findings remain to be addressed. Furthermore, given the well-documented difference in symptom presentation and functional outcomes between girls and boys with ADHD, studies that are well-powered to examine both sex-specific differences in WM regions suspected to be critical for response control and functional implications of any findings are much needed.
The present study examined WM microstructure within specific frontal regions of interest in a relatively large sample (N=120) of children with ADHD, oversampled for girls, and matched TD controls. Based upon recent previous work,24 we hypothesized a differential effect of ADHD on FA across the sexes, with boys showing more abnormalities within bilateral motor/premotor areas, and girls showing more abnormalities within prefrontal regions. Additionally, we hypothesized that WM microstructure (measured by FA) would be associated with ISV and commission errors in regions showing an effect of diagnosis.
METHOD
Participants
The sample included 120 children, 60 with ADHD (30 boys, 30 girls) and 60 controls (30 boys, 30 girls) matched on age, intellectual ability, and handedness (Table 1). Participants were predominantly Caucasian (74.2%), with 15.8% African American, 1.7% Asian, and 8.3% Biracial. Families were largely of middle class socioeconomic status (Hollingshead estimate M = 50.23, SD = 9.97, range = 22–66). Following Institutional Review Board approval, participants aged 8 to 12 years were recruited through local schools, community-wide advertisement, volunteer organizations, and medical institutions. Following telephone screening, participants were screened for psychiatric diagnoses via structured parent interview, then scheduled for a study visit and mailed parent and teacher behavioral ratings used to confirm diagnostic status, including Conners’ Parent and Teacher Rating Scales-Revised, Long Version (CPRS-R:L and CTRS-R:L).33 Consent and assent were obtained from parents and children, respectively.
Table 1.
Sample Demographics and Performance on Behavioral Measures
| Control | ADHD | p a | p b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All Controls | Boys | Girls | All ADHD | Boys | Girls | All | Boys | Girls | All | |
| n | 60 | 30 | 30 | 60 | 30 | 30 | ||||
| Age in years | 10.2 (1.1) | 10.2 (1.3) | 10.2 (0.9) | 9.9 (1.3) | 9.9 (1.3) | 9.9 (1.3) | 0.25 | 0.36 | 0.48 | |
| FSIQ | 111.9 (10.0) | 111.7 (9.8) | 112.2 (10.3) | 108.6 (12.3) | 109.6 (10.7) | 107.6 (13.8) | 0.11 | 0.44 | 0.15 | 0.72 |
| VCI | 114.7 (11.7) | 116.2 (11.6) | 113.3 (11.9) | 113.0 (15.1) | 116.5 (13.5) | 109.6 (16.0) | 0.49 | 0.92 | 0.31 | 0.05 |
| PRI | 107.1 (11.3) | 106.7 (11.4) | 107.4 (11.4) | 109.2 (13) | 110.9 (13.7) | 107.5 (12.3) | 0.34 | 0.20 | 0.97 | .57 |
| Conners Inatt. Raw | 2.30 (2.93) | 2.90 (3.03) | 1.69 (2.02) | 18.36 (5.07) | 18.59 (4.60) | 18.14 (5.57) | <10−8 | <10−8 | <10−8 | 0.68 |
| Conners Hyp. Raw | 1.83 (1.83) | 2.07 (2.02) | 1.57 (1.61) | 13.28 (6.35) | 13.10 (5.31) | 13.45 (7.34) | <10−8 | <10−8 | <10−8 | 0.99 |
| ADHD subtype H:I:C | N/A | N/A | N/A | 2:17:41 | 1:9:20 | 1:8:21 | N/A | N/A | N/A | N/A |
| Handedness R:L:M | 49:5:6 | 24:3:3 | 25:2:3 | 51:6:3 | 26:4:0 | 25:2:3 | 0.41 | 0.17 | 1.00 | 0.48 |
| Go/No-go CVRT | 0.33 (0.13) | 0.36 (0.16) | 0.29 (0.10) | 0.39 (0.18) | 0.45 (0.22) | 0.32 (0.10) | 0.03 | 0.06 | 0.21 | 0.001 |
| Commission Error Rate | 0.38 (0.20) | 0.44 (0.19) | 0.32 (0.20) | 0.46 (0.20) | 0.55 (0.18) | 0.37 (0.19) | 0.04 | 0.03 | 0.33 | <0.001 |
Note: Values represent mean (SD). Attention-deficit/hyperactivity disorder (ADHD) subtype: H = Hyperactive-Impulsive, I = Inattentive, C = Combined; Handedness: R = Right, L = Left, M = Mixed. CVRT = coefficient of variation in reaction time; FSIQ = Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV), Full Scale IQ score; N/A = not applicable; PRI = Perceptual Reasoning Index; VCI = WISC-IV Verbal Comprehension Index.
ADHD vs. Control comparison.
Boys vs. Girls comparison (across groups).
ADHD diagnosis and group assignment were confirmed using the following criteria: 1) positive DSM-IV ADHD diagnosis using a structured parent interview (Diagnostic Interview for Children and Adolescents, Fourth Edition—DICA-IV);34 and 2) T-scores >60 on the DSM-IV Hyperactive/Impulsive or Inattentive scales of the Conners’ Parent Rating Scales-Revised, Long Version (CPRS-R:L),33 and 3) 6 of 9 DSM-IV symptoms met (item rating of 2 or 3) on the Hyperactive/Impulsive or Inattention scales of the ADHD Rating Scale-IV, Home version (ADHD-RS).35 This information was reviewed by a child neurologist (S.H.M.) for final confirmation of ADHD diagnosis. Control participants with T-scores >60 on either the DSM-IV Inattentive or Hyperactive/Impulsive scales of the CPRS-R or CTRS-R, or ratings of 2 or greater for four or more symptoms of inattention or hyperactivity/impulsivity (ADHD Rating Scale-IV) were excluded. Determination of ADHD subtypes was based on rating scale responses, DICA-IV, and clinical judgment.
Exclusion criteria for both groups included history of seizures, other neurological conditions, or identified genetic disorder. Children were also excluded if they met criteria for conduct disorder, mood disorder, generalized anxiety disorder, separation anxiety disorder, or obsessive-compulsive disorder on the DICA-IV. Children with ADHD were not excluded for oppositional defiant disorder (ODD), considering the high rates of comorbidity. In the control group, additional exclusionary criteria included diagnosis of any psychiatric disorder based upon DICA-IV and history of speech/language or other developmental disorder. Intellectual ability was assessed using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV).36 Children were excluded if they earned scores below 79 on either the Perceptual Reasoning Index (PRI) or the Verbal Comprehension Index (VCI), without restriction on full scale IQ (FSIQ), given the impact of attentional symptoms and/or slower processing speed on FSIQ. Of 486 participants enrolled on several related protocols, 47 were excluded based upon psychiatric diagnoses, color-blindness, low cognitive ability, or abnormal imaging findings. Additionally, children who had developed beyond Tanner stage II as identified by parent interview were deemed ineligible. Of those eligible, DTI imaging was attempted on 349 children, and 281 (102 ADHD) had useable data (i.e., free of visually appreciable motion artifact or other imaging artifacts) from both DTI and T2 scans and passed pre-processing; at the time of data analysis, 193 (78 ADHD) scans were processed. Of those, 30 were girls with ADHD to whom the boys with ADHD were matched; these were matched to 60 control participants as noted above.
Within the group with ADHD, 70% (n=42) were prescribed stimulant medication. To preclude effects on task performance, medications were discontinued the day prior to and the day of testing. Children with ADHD taking any other psychoactive medications were excluded. No controls were taking psychoactive medication.
Response Control Measure
Go/No-go Task
Participants completed an 8 minute, computer-based, Go/No-go paradigm (outside the scanner) in which a well-ingrained stimulus-response association (green=go, red=no-go) was used to minimize working memory and other cognitive demands. Participants used the dominant hand index finger to push a button immediately in response to green spaceships. Stimuli were presented for 300ms with an interstimulus interval of 2000ms for 217 trials. Presentation cues were weighted towards green spaceships at a ratio of 4:1, intensifying the need to inhibit a habituated motor response. Only “go” RTs were analyzed; RTs faster than 200ms and on failed inhibition trials were omitted from the analysis. ISV was computed as the SD of RT/mean RT for correct go responses (coefficient of variation of RT: CVRT), providing a measure of response variability controlling for response speed.
Magnetic Resonance Imaging (MRI) Acquisition and Measurement
Before scanning, participants underwent a mock scanning session in an inactive, realistic scanner to ensure participant comfort, limited motion, and optimal scan quality. Images were acquired with single-shot echo planar imaging (EPI; SENSE factor 2.5) on a 3T Phillips scanner. Two runs were collected per participant, with 32 gradient directions (b=800 s/mm3) and one b0 in each run. Sixty 2.2mm axial slices were acquired for each volume, with an imaging matrix of 96 × 96 zero-filled to 256 × 256 for a 0.9 mm resolution reconstructed resolution. Preprocessing was performed using CATNAP,37 with the RADAR motion correction procedure. The gradient table was adjusted according to the motion correction transformation, and the tensors estimated using RESTORE38 in order to reduce impact of motion artifacts. Participants with any visible artifacts on their FA images, or evident large in-scanner movements, were excluded from analysis. There were no significant between-group (ADHD: M= .39, SD= .27; TD: M= .32, SD= .18; t[97] = −1.86, p =.07) or between sex (within ADHD) (Boys: M= .41, SD= .18; Girls: M= .37, SD= .33; t[58] = −.581, p = .564) differences on motion, as measured by average framewise displacement. Based on these data, we chose not to match further on motion, nor covary for motion in the brain-behavior correlations.
EPI distortions were corrected by transformation of each participant’s mean b0 image to a non-EPI axially acquired T2-weighted image that was taken as part of the same imaging protocol. Transformations were estimated using Large Deformation Diffeomorphic Metric Mapping (LDDMM).39 Tensor images were then resampled into 1mm isotropic resolution to match the resolution of the atlas template image. Each participant’s DTI images were parceled according to the frontal lobe atlas developed by Ranta et al40,41 using a semi-automated atlas-based protocol.42 First, each participant’s DTI images were warped to a standard template using multi-channel LDDMM. Next, the reverse transformation was applied to the atlas-space regions of interest (ROIs), bringing the standard labels into native space. Finally, in order to interrogate only the WM within each native-space ROI, an FA cutoff was applied, in which only voxels where FA was greater than 0.25 were considered.
Data Analysis
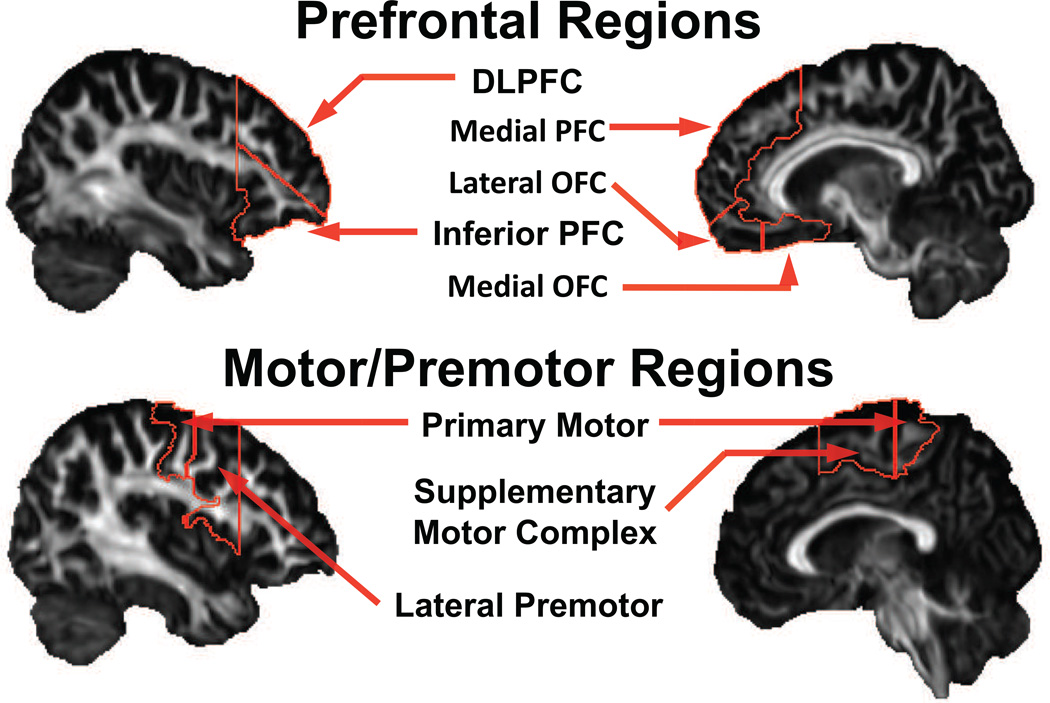
Diagnostic group differences in Go/No-go performance (i.e., CVRT and commission error rate) were examined via independent samples t-tests for the entire group and separately for boys and girls. Given the hypothesized differential effect of ADHD diagnosis on WM microstructure in boys and girls, two-way analyses of variance (ANOVAs: Sex × Diagnosis) were performed in motor/premotor regions (primary motor cortex, SMC, lateral premotor area) and in prefrontal regions (dorsolateral, inferior, and medial PFC, lateral and medial orbitofrontal cortex) (Figure 1), with Bonferroni correction applied in each analysis. Following significant findings, a follow-up t-test for FA was performed within-sex to clarify the nature of the statistical interaction. In order to correct for multiple comparisons, motor/premotor region and prefrontal region analyses were considered separate hypotheses. For motor/premotor (three regions of interest), a Bonferroni-corrected alpha-level was set at α= 0.05/3 =0.015. Likewise for premotor regions, an alpha-level of α= 0.05/5 =0.01 was used. Additionally, in regions showing a statistical interaction in FA, Pearson correlations between FA and response control, as measured by CVRT and commission error rates, were examined among participants with ADHD.
Figure 1.
Regions of interest in the frontal lobe atlas, grouped by premotor or prefrontal regions. Note: DLPFC = dorsolateral prefrontal cortex; OFC = orbitofrontal cortex; PFC = prefrontal cortex.
RESULTS
Demographics
Mean intellectual ability (WISC-IV PRI and VCI) was within the average range and not significantly different between diagnostic groups (Table 1). Age, handedness, racial background, and family socioeconomic status also did not differ between groups. Within the group with ADHD, 41 participants (20 male) exhibited combined, 17 participants (9 male) exhibited primarily inattentive, and 2 participants (1 male) exhibited hyperactive/impulsive presentation/subtype. Subtype distribution did not differ between sexes (p=.92). Across the sample, 18.3% met criteria for ODD and 13.3% met criteria for specific phobia (e.g., the dark, thunderstorms).
Go/No-go performance
As expected, CVRT was higher in children with ADHD (p=0.03; Table 1) versus controls, with effects primarily in boys (p=.06), not girls (p=.21). Likewise, children with ADHD made more commission errors (p=.037), evident primarily in boys (p=.029) rather than girls (p=.33).
Sex × Diagnosis interactions
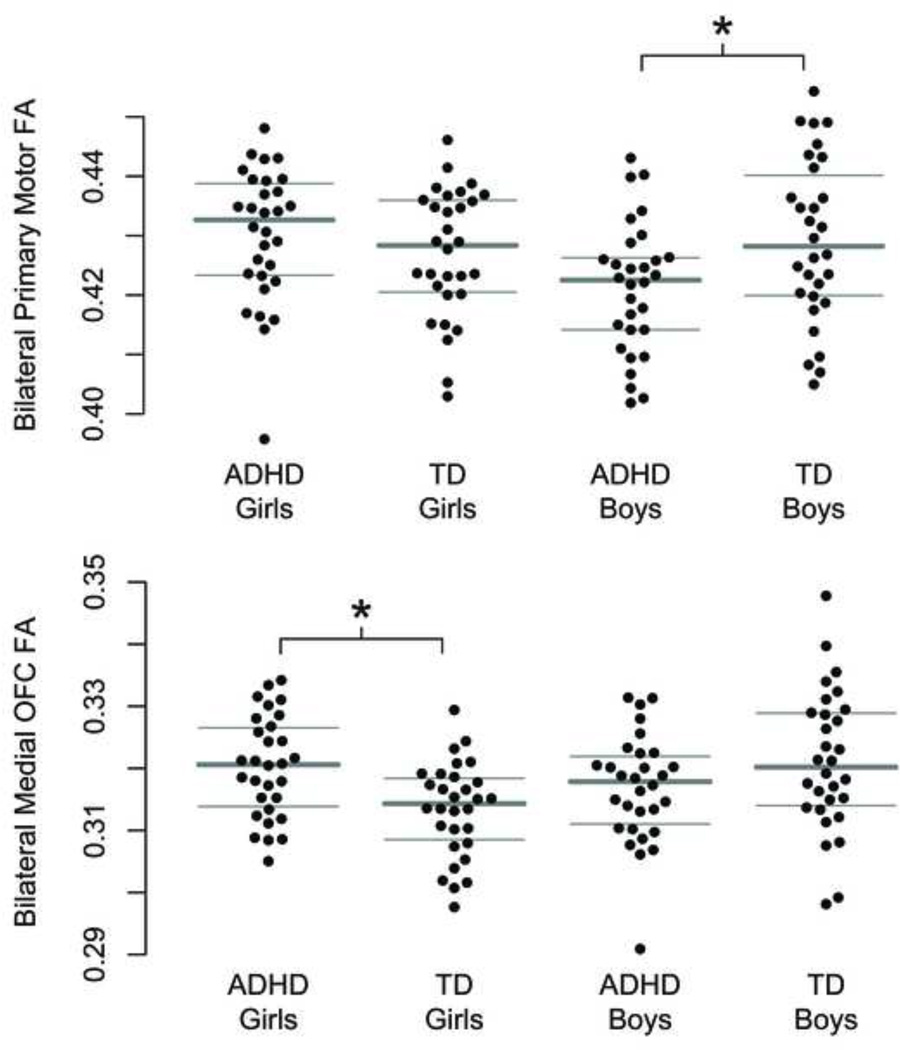
Within motor/premotor regions, two-way ANOVAs (Sex × Diagnosis) (Table 2) revealed a significant interaction between sex and diagnosis on FA within the primary motor cortex (M1) (p=.011), but not among any other motor/premotor regions. Statistically significant main effects of diagnosis and sex were not observed; likewise, there was no effect of diagnosis, sex, or sex-by-diagnosis interaction on premotor FA as a whole. The pattern of results held when co-varying for age. Post hoc investigation of group differences in M1 FA separately in boys and girls (Figure 2) revealed significantly lower FA among boys with ADHD (p=.015) relative to TD boys, with no difference among girls.
Table 2.
Sex by Diagnosis Interactions in Fractional Anisotropy (FA) Among Motor/Premotor Regions and Prefrontal Regions
| TD Boys (n=30) | ADHD Boys (n=30) | TD Girls (n=30) | ADHD Girls (n=30) | Sex × Diag | ADHD vs TD p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Region (all bilateral) | mean | sd | mean | sd | mean | sd | mean | sd | p-value | among boys | among girls |
| Motor/Premotor regions: | |||||||||||
| Primary Motor | 0.4293 | 0.0138 | 0.4211 | 0.0110 | 0.4270 | 0.0108 | 0.4301 | 0.0113 | 0.01* | 0.015* | 0.28 |
| Supplementary Motor | 0.4251 | 0.0113 | 0.4197 | 0.0110 | 0.4262 | 0.0132 | 0.4242 | 0.0108 | 0.41 | ||
| Lateral Premotor | 0.4094 | 0.0109 | 0.4046 | 0.0104 | 0.4052 | 0.0096 | 0.4046 | 0.0095 | 0.26 | ||
| Prefrontal regions: | |||||||||||
| Dorsolateral Prefrontal | 0.3838 | 0.0101 | 0.3811 | 0.0091 | 0.3804 | 0.0075 | 0.3820 | 0.0094 | 0.21 | ||
| Inferior Prefrontal | 0.3787 | 0.0096 | 0.3744 | 0.0106 | 0.3762 | 0.0079 | 0.3753 | 0.0101 | 0.34 | ||
| Medial Prefrontal | 0.3874 | 0.0077 | 0.3840 | 0.0093 | 0.3856 | 0.0077 | 0.3864 | 0.0084 | 0.48 | ||
| Medial Orbitofrontal | 0.3211 | 0.0113 | 0.3169 | 0.0087 | 0.3134 | 0.0076 | 0.3202 | 0.0081 | 0.001* | 0.11 | 0.0014* |
| Lateral Orbitofrontal | 0.3891 | 0.0093 | 0.3894 | 0.0090 | 0.3875 | 0.0101 | 0.3905 | 0.0116 | 0.17 | ||
Note: ADHD = attention-deficit/hyperactivity disorder; TD = typically developing.
p<.05
Figure 2.
Stripcharts showing fractional anisotropy (FA) within white matter of the primary motor (M1) region bilaterally and medial orbitofrontal cortex (MOFC) bilaterally, across sex and diagnosis. Note: There was a significant sex-by-diagnosis interaction on FA in both MOFC (p=.001) and M1 (p=.011): boys with attention-deficit/hyperactivity disorder (ADHD) showed significantly lower M1 FA relative to typically developing (TD) boys (p=.015), whereas girls with ADHD showed significantly higher MOFC FA (p=.001) relative to TD girls.
Within prefrontal regions, two-way ANOVAs revealed a sex-by-diagnosis interaction in medial orbitofrontal cortex (MOFC) FA (p=.0011), and not among other prefrontal regions. No significant main effects of diagnosis and sex were observed, and the pattern held when co-varying for age. Again, there was no effect of diagnosis, sex, or sex-by-diagnosis interaction on prefrontal FA as a whole. Post hoc investigation of diagnostic differences in FA separately in boys and girls revealed higher FA among girls with ADHD compared with TD peers (p=.0014), and no difference among boys.
In both the M1 and the MOFC, no FA values fell outside of three SD away from the mean in the full cohort.
Brain-behavior analyses
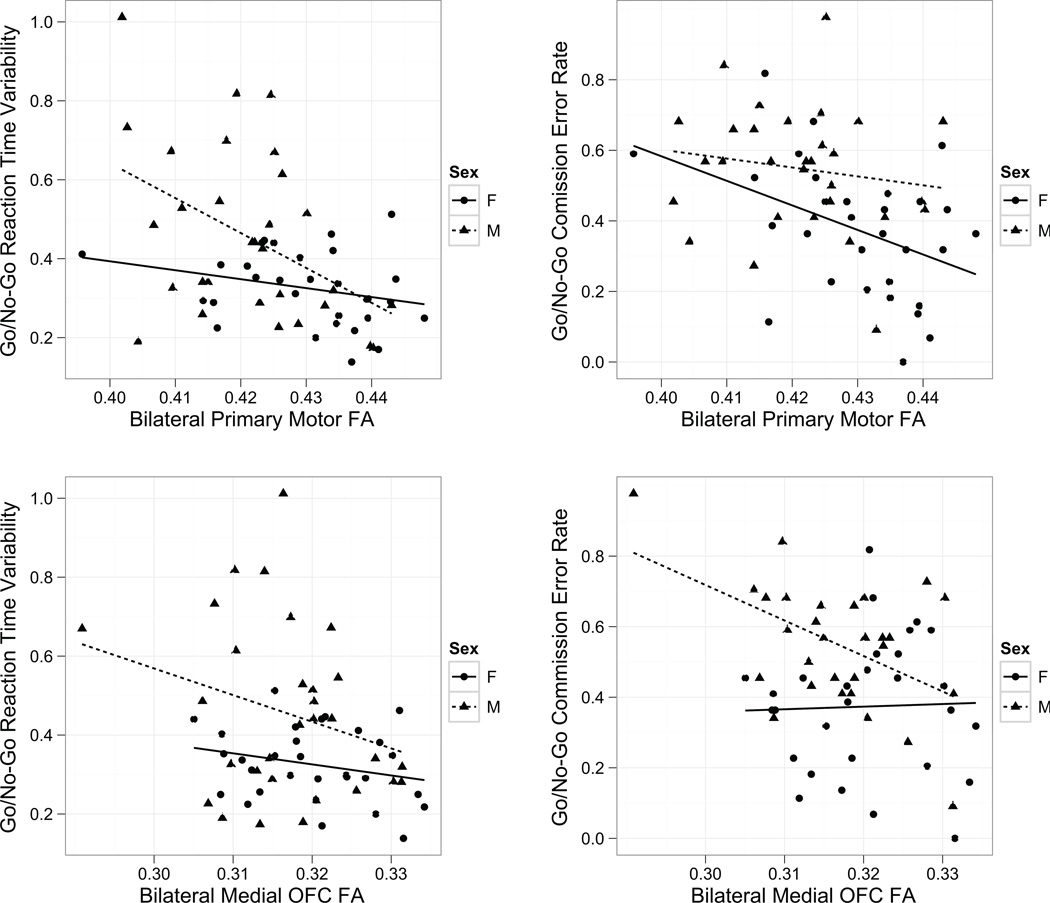
Given the sex-by-diagnosis interaction in FA, we examined the association between WM microstructure within identified regions and response control among participants with ADHD (Figure 3). Considering boys and girls with ADHD together, M1 FA was negatively associated with both CVRT (r=−0.45, p<.001) and commission error rate (r=−0.41, p=.0012). Examining associations within sex, a significant correlation was observed between M1 FA and CVRT among boys (r=−0.45, p=.014), but not among girls (r=−0.27, p=.14). Conversely, commission error rate was negatively correlated with M1 FA in girls (r=−0.41, p=.023), but not boys (r=−0.16, p=.41).
Figure 3.
Association between response control measures (Coefficient of Variation in Reaction Time, Commission error rate) and regional fractional anisotropy (FA) values in the primary motor region (M1) and bilateral medial orbitofrontal cortex (MOFC) in attention-deficit/hyperactivity disorder (ADHD). Note: Increased variability was significantly associated with lower FA in both regions (M1: p<.001, MOFC: p=.022); for M1 this effect was primarily observed in boys. Higher commission error rate was associated with lower FA in both regions (M1: p=.001, MOFC: p=.026). This association was observed primarily in girls for M1 and in boys for MOFC.
Examining associations with response control and medial OFC FA among all participants with ADHD, medial OFC FA was negatively correlated with CVRT (r=−0.29, p=.022) as well as commission error rate (r=−0.29, p=.026). When examining associations within sex, a significant correlation was observed for commission error rate among boys (r=−0.49, p=.0054), but not girls (r=0.032, p=.87). In contrast, CVRT was not significantly associated with medial OFC among boys (r=−0.27, p=.15) or girls (r=−0.24, p=.19) with ADHD, suggesting an equivalent contribution of both sexes to the overall group finding of negative correlation of CVRT with medial OFC FA. These differences in effect size between sexes are practically significant to moderate; the difference in r2 between groups for the M1-CVRT correlation is 0.13 (practically significant), between M1-Commission errors r2 is 0.14 (practically significant), and between MOFC-Commission errors r2 is 0.24 (practically significant to moderate).
DISCUSSION
The present findings provide novel evidence for sex-specific differences in neural substrates potentially underlying ADHD symptomatology. Among boys, ADHD-related abnormalities were observed in WM microstructure in regions commonly associated with basic motor response control, specifically M1. In contrast, ADHD-related abnormalities among girls were observed in the microstructure of prefrontal regions subserving motivation and top-down emotional regulation, specifically medial OFC. Furthermore, diffusion anisotropy within identified regions (M1 and medial OFC) was correlated with ADHD-associated deficits in both CVRT and commission error rate. Within sex, M1 FA was associated with CVRT in boys and with commission error rates in girls, while medial OFC FA was associated with commission errors in boys.
This study represents the first attempt to deliberately examine sex differences in investigation of WM microstructure in regions underlying response control in children with ADHD. Of the existing neuroimaging studies in this area, both of which were anatomic, not DTI studies, only Mahone et al.11 and Dirlikov et al.24 included enough girls with ADHD in the sample to examine sex-related differences. Mahone et al.11 found that premotor grey matter volumes were associated with response variability in boys, but not girls; in contrast, Dirlikov et al.24 found different patterns of reduced cortical surface area in boys and girls, suggesting premotor reductions in boys with ADHD, but prefrontal reductions in girls with ADHD. Our findings support and extend this work, suggesting that this sex-based dissociation is also present in the underlying white matter.
Consistent with the anatomic findings of Dirlikov et al.,24 our investigation revealed prefrontal rather than motor/premotor WM abnormalities in girls with ADHD. This is consistent with prior behavioral studies showing more deficits in planning and strategy in girls with ADHD,19 as the medial OFC has been shown to have a role performing such planning tasks.43,44 Our finding of higher FA among girls with ADHD appears counterintuitive, as reduced FA is most commonly associated with pathology. However, previous work has shown higher FA values associated with ADHD status, including within prefrontal regions.12,17,29,45 This finding may be due to reduced neuronal branching near the WM/GM interface among girls with ADHD, as reduced “fanning” of fibers on a sub-voxel scale may result in elevated FA.
Examining associations between WM microstructure and measures of response control among children with ADHD, we find that across sexes, FA in the identified regions (M1 and MOFC) correlates with commission error rate as well as with ISV in reaction time (as measured by CVRT). This provides further evidence that disrupted WM microstructure within these regions contributes to the core deficits in response control that characterize ADHD. However, some discrepancy emerged between the microstructure differences and their behavioral correlates within sexes, as commission error rate was associated with FA in medial OFC among boys (not girls), but girls differed most from their TD peers in this region. Likewise, commission error rate was associated with M1 FA only in girls, while diagnosis-related differences in M1 FA were seen only in boys. It is conceivable that an aberrant developmental process in the medial OFC in girls with ADHD and in the M1 in boys with ADHD results in insufficient support of response control, and thus, reduced correlations with commission errors. Regarding CVRT, lack of significant correlations in girls may reflect reduced ISV in CVRT (e.g., restricted range). Notably, the CVRT-medial OFC correlation was significant across the group with ADHD as a whole, while showing a similar but non-significant correlation strength within girls and boys with ADHD. Considering that M1 FA and medial OFC FA show similar strength of relationship with CVRT, both across and within sex, findings of non-significance at the within-sex level may be attributable to lack of statistical power for subgroup analyses.
Consistent with prior work, our findings of WM abnormalities in motor regions in boys and in prefrontal regions in girls may reflect maturational differences in girls and boys of this age. Specifically, posterior regions (e.g., motor cortex) mature before more anterior regions, particularly medial OFC, which shows a protracted developmental course.46 Thus, findings of prefrontal differences in girls but not boys with ADHD fit within the framework of the relatively more advanced developmental trajectory of cortical development in girls relative to boys.16 Furthermore, OFC has been associated with affective regulation,47 a capacity that matures later in life, consistent with the extended developmental span of the OFC. Given findings of greater vulnerability to mood disorders in girls with ADHD,8 differences in WM microstructure development in OFC may underlie some of these risks. Relative to controls, present findings of reduced M1 FA in boys with ADHD but greater medial OFC FA in girls with ADHD may also reflect the developmental course of these brain regions; it is possible that lower FA in earlier-developing M1 reflects decreased integrity of more streamlined WM projections characteristic of this region, with this reduction in FA associated with poorer response control. In contrast, in later-developing regions such as medial OFC, higher FA in girls with ADHD may reflect relative lack of complexity in the emergence of more complex projections.
Strengths of this study include the relatively large sample and deliberate oversampling for girls with ADHD, permitting examination of sex-related differences in WM response variability associations. Furthermore, the sample was carefully selected and screened for comorbidities, and groups were matched on age, sex, handedness, and IQ, suggesting that any differences likely reflect ADHD-related findings rather than sampling artifacts. Potential limitations of this study include this careful screening, which eliminated participants with typical comorbidities seen in ADHD. Screening was designed to provide a relatively “pure” sample of ADHD, enabling examinations of brain-behavior associations not limited by other potentially confounding factors. However, such screening may make findings somewhat less generalizable to community youth with ADHD, in whom comorbidity is quite common. Additionally, screening likely contributed to the relatively high intellectual ability of the sample, likely due in part to exclusion of commonly occurring comorbidities. The lack of sex difference in symptom severity and rate of comorbid ODD in this sample also suggests that girls in the sample exhibited a more severe ADHD phenotype than is typical for community samples. Furthermore, DTI has a number of recognized limitations, including susceptibility to partial volume effects, inability to resolve intra-voxel crossing fibers, and reduced spatial resolution compared to T1 anatomical imaging. This investigation used methods designed to reduce these limitations as much as possible.
In sum, our findings suggest a sexually dimorphic pattern of frontal WM abnormalities in children with ADHD, such that boys show greater involvement of motor regions crucial to more basic aspects of motor response control, whereas girls show greater involvement of prefrontal regions important to top-down regulation of higher-order emotional and behavioral responses. This study is among the first to examine sex differences in WM microstructure and associations with response control measures in children with ADHD. As such, results advance our understanding of the differential contribution of frontal WM anomalies in ADHD to the characteristic difficulties with response control associated with the disorder.
Acknowledgments
This work was supported by NIH/NINDS: RO1 MH078160; RO1 MH085328; K23 MH101322; R01NS084957; P41EB015909, and the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award program UL1 TR 000424-06, and was carried out in part at the F.M. Kirby Center for Functional Brain Imaging, using resources provided under P41 EB015909.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Jacobson, Rosch, Mori, Mostofsky, Mr. Peterson, and Ms. Crocetti report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Lisa A. Jacobson, Kennedy Krieger Institute, Baltimore, MD.; Johns Hopkins University School of Medicine, Baltimore.
Daniel J. Peterson, Kennedy Krieger Institute, Baltimore, MD..
Keri S. Rosch, Kennedy Krieger Institute, Baltimore, MD.; Johns Hopkins University School of Medicine, Baltimore.
Deana Crocetti, Kennedy Krieger Institute, Baltimore, MD..
Susumu Mori, Kennedy Krieger Institute, Baltimore, MD.; Johns Hopkins University School of Medicine, Baltimore.
Stewart H. Mostofsky, Kennedy Krieger Institute, Baltimore, MD.; Johns Hopkins University School of Medicine, Baltimore.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 2.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Gaub M, Carlson CL. Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997;36(8):1036–1045. doi: 10.1097/00004583-199708000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Hinshaw SP, Owens EB, Sami N, Fargeon S. Prospective follow-up of girls with attention deficit/hyperactivity disorder into adolescence: Evidence for continuing cross-domain impairment. J Consult Clin Psychol. 2006;74(3):489–499. doi: 10.1037/0022-006X.74.3.489. [DOI] [PubMed] [Google Scholar]
- 5.Keltner NL, Taylor EW. Messy purse girls: adult females and ADHD. Perspectives in psychiatric care. 2002;38(2):69–72. [PubMed] [Google Scholar]
- 6.Mikami AY, Hinshaw SP, Lee SS, Mullin BC. Relationships between Social Information Processing and Aggression among Adolescent Girls with and without ADHD. J Youth Adolesc. 2008 Aug;37(7):761–771. doi: 10.1007/s10964-007-9237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. The Psychiatric clinics of North America. 2010 Jun;33(2):357–373. doi: 10.1016/j.psc.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Biederman J, Monuteaux MC, Mick E, et al. Young adult outcome of attention deficit hyperactivity disorder: a controlled 10-year follow-up study. Psychol Med. 2006 Feb;36(2):167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- 9.Derks EM, Hudziak JJ, Boomsma DI. Why more boys than girls with ADHD receive treatment: a study of Dutch twins. Twin Res Hum Genet. 2007 Oct;10(5):765–770. doi: 10.1375/twin.10.5.765. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Wang PN, Chuang KH, Jong YJ, Chao TC, Wu MT. Absence of gender effect on children with attention-deficit/hyperactivity disorder as assessed by optimized voxel-based morphometry. Psychiatry Res. 2008 Dec 30;164(3):245–253. doi: 10.1016/j.pscychresns.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Mahone EM, Ranta ME, Crocetti D, et al. Comprehensive examination of frontal regions in boys and girls with attention-deficit/hyperactivity disorder. Journal of the International Neuropsychological Society : JINS. 2011 Nov;17(6):1047–1057. doi: 10.1017/S1355617711001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009 Sep;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong SB, Zalesky A, Fornito A, et al. Connectomic Disturbances in Attention-Deficit/Hyperactivity Disorder: A Whole-Brain Tractography Analysis. Biol Psychiatry. 2014;76:656–663. doi: 10.1016/j.biopsych.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Lin HY, Gau SS, Huang-Gu SL, Shang CY, Wu YH, Tseng WY. Neural substrates of behavioral variability in attention deficit hyperactivity disorder: based on ex-Gaussian reaction time distribution and diffusion spectrum imaging tractography. Psychol Med. 2014 Aug 9;44:1751–1764. doi: 10.1017/S0033291713001955. [DOI] [PubMed] [Google Scholar]
- 15.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999 Oct;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 16.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007 Jul 15;36(4):1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008 Jun;29(6):696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J Atten Disord. 2012 Apr;16(3):190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2010 Nov;25(7):656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wodka EL, Loftis C, Mostofsky SH, et al. Prediction of ADHD in boys and girls using the D-KEFS. Arch Clin Neuropsychol. 2008 May;23(3):283–293. doi: 10.1016/j.acn.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seymour KE, Mostofsky SH, Rosch KS. Cognitive Load Differentially Impacts Response Control in Girls and Boys with ADHD. J Abnorm Child Psychol. 2015 Jan 28; doi: 10.1007/s10802-015-9976-z. [Epub ahead of print]. PMID: 25624066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kofler MJ, Rapport MD, Sarver DE, et al. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clinical psychology review. 2013 Aug;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Qiu A, Crocetti D, Adler M, et al. Basal ganglia volume and shape in children with ADHD. Am J Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirlikov B, Shiels Rosch K, Crocetti D, Denckla MB, Mahone EM, Mostofsky SH. Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage. Clinical. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liston C, Malter Cohen M, Teslovich T, Levenson D, Casey BJ. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biol Psychiatry. 2011 Jun 15;69(12):1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 26.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2012 Apr;36(4):1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Ashtari M, Kumra S, Bhaskar SL, et al. Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biol Psychiatry. 2005 Mar 1;57(5):448–455. doi: 10.1016/j.biopsych.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Yang L, Wang Y. Applying imaging genetics to ADHD: the promises and the challenges. Molecular neurobiology. 2014 Oct;50(2):449–462. doi: 10.1007/s12035-014-8683-z. [DOI] [PubMed] [Google Scholar]
- 29.Peterson DJ, Ryan M, Rimrodt SL, et al. Increased regional fractional anisotropy in highly screened attention-deficit hyperactivity disorder (ADHD) J Child Neurol. 2011 Oct;26(10):1296–1302. doi: 10.1177/0883073811405662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE. Smaller prefrontal and premotor volumes in boys with ADHD. Biol Psychiatry. 2002;52(8):785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 31.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006 May;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 32.Weyandt L, Swentosky A, Gudmundsdottir BG. Neuroimaging and ADHD: fMRI, PET, DTI findings, and methodological limitations. Dev Neuropsychol. 2013;38(4):211–225. doi: 10.1080/87565641.2013.783833. [DOI] [PubMed] [Google Scholar]
- 33.Conners CK. Conners' Rating Scales - Revised. North Tonawanda, New York: Multi-Health Systems Inc.; 1997. [Google Scholar]
- 34.Reich W, Welner Z, Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. North Tonawanda: Multi-Health Systems; 1997. [Google Scholar]
- 35.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV. New York: Guilford Press; 1998. [Google Scholar]
- 36.Wechsler DL, Kaplan E, Fein D, Kramer JH, Morris R, Delis DC. Wechsler Intelligence Scale for Children - Fourth Edition - Integrated, technical and interpretive manual. San Antonio, Tx: Harcourt Assessment, Inc.; 2004. [Google Scholar]
- 37.Landman BA, Farrell JA, Jones CK, Smith SA, Prince JL, Mori S. Effects of diffusion weighting schemes on the reproducibility of DTI-derived fractional anisotropy, mean diffusivity, and principal eigenvector measurements at 1.5T. Neuroimage. 2007 Jul 15;36(4):1123–1138. doi: 10.1016/j.neuroimage.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 38.Chang L-C, Jones D, Pierpaoli C. RESTORE: Robust estimation of tensors by outlier rejection. Magn Reson Med. 2005;53(5):1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- 39.Beg MF, Miller MI, Trouve A, Younes L. Computing large deformation metric mapping via geodesic flows of diffeomorphisms. International Journal of Computer Vision. 2005;61:139–157. [Google Scholar]
- 40.Ranta ME, Chen M, Crocetti D, et al. Automated MRI parcellation of the frontal lobe. Hum Brain Mapp. 2014 May;35(5):2009–2026. doi: 10.1002/hbm.22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranta ME, Crocetti D, Clauss JA, Kraut MA, Mostofsky SH, Kaufmann WE. Manual MRI parcellation of the frontal lobe. Psychiatry Res. 2009 May 15;172(2):147–154. doi: 10.1016/j.pscychresns.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008 Apr 1;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000 Mar;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 44.Wunderlich K, Dayan P, Dolan RJ. Mapping value based planning and extensively trained choice in the human brain. Nat Neurosci. 2012 May;15(5):786–791. doi: 10.1038/nn.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen L, Huang X, Lei D, et al. Microstructural abnormalities of the brain white matter in attention-deficit/hyperactivity disorder. J Psychiatry Neurosci. 2015 Jul;40(4):280–287. doi: 10.1503/jpn.140199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011 Aug;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011 Feb;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]