Abstract
Purpose
The effect of BCAA (branched chain amino acid) administration on muscle atrophy during growth phases is not well known. We investigated whether BCAA administration can prevent the muscle atrophy induced by hindlimb suspension in growing male rats.
Methods
Male Wistar rats were assigned to 1 of 2 groups (n = 7/group): hindlimb suspension and hindlimb suspension with oral BCAA administration (600 mg·kg−1·day−1, valine 1: leucine 2: isoleucine 1). After 14 days of hindlimb suspension, the weight and mRNA levels of the soleus muscle were measured.
Results
BCAA administration prevented a decrease in soleus muscle weight. BCAA administration attenuated atrogin-1 and MuRF1 mRNA expression, which has been reported to play a pivotal role in muscle atrophy.
Conclusion
BCAA could serve as an effective supplement for the prevention or treatment of muscle atrophy, especially atrophy caused by weightlessness.
Keywords: branched chain amino acids, hindlimb suspension, muscle atrophy, atrogin-1, MuRF1, rat
INTRODUCTION
Muscle mass is regulated by the balance between protein synthesis and protein degradation [1,2]. Muscle atrophy is characterized by a decrease in protein synthesis, an increase in protein degradation, or simultaneous changes in both processes and occurs in a variety of catabolic conditions such as prolonged disuse, denervation, aging, and cancer [3,4]. Among several methods such as hindlimb suspension, limb casting or surgical tenotomy, the hindlimb suspension model is the most effective and well-designed animal model for investigating muscle atrophy due to weightlessness [5,6]. During hindlimb suspension, there is muscle atrophy accompanied by decreased muscle weight and fiber size in addition to a general shift from slow twitch to fast twitch muscles [7]. This muscle atrophy can lead to decreased muscle strength and fatigue resistance, and can affect health and physical activity levels [8,9]. Therefore, it is important to find effective nutritional intervention to decrease muscle atrophy during weightlessness in clinical rehabilitation during the growth phase.
The decrease of myofibrillar protein is controlled by the transcription level of key proteins that regulate the autophagy/lysosomal protein breakdown pathway and the ubiquitin proteasome system (UPS) [10–12]. Of these, UPS is commonly known as the primary pathway associated with disuse muscle atrophy. The process of protein degradation via the UPS involves two separate and consecutive steps: 1) tagging of the target protein by covalent attachment of ubiquitin molecules and 2) degradation of the tagged target protein by the 26S proteasome complex [13].
Muscle specific E3 ligase atrogin-1 (muscle atrophy F-box, also called MAFbx) and MuRF1 (muscle ring finger 1) are significantly up-regulated in various muscle atrophy situations such as fasting, diabetes, cancer, hindlimb unloading, immobilization and denervation. The functional importance of these genes was demonstrated using MAFbx and MuRF1 null mice. Both atrogin-1 and MuRF1 null mice show normal growth curves and their skeletal muscles and hearts had normal weights and morphology following denervation. Therefore, these data indicate that atrogin-1 and MuRF1 expression by the Ubiquitin Proteasome System in skeletal muscle atrophy can be considered a reliable marker [14–18]. Among several experimental models inducing disuse muscle atrophy, the hindlimb suspension model is a well-designed animal model [5,6,19]. Atrogin-1 and MuRF1 are up-regulated up to 3 fold in hindlimb suspension, immobilization and denervation [4,17].
BCAAs inhibit atrogin-1 and MuRF1 expression in the C2C12 mouse muscle cell line as well as the Qt6 quail muscle cell line [4,20]. In addition, it is well known that BCAAs stimulate protein synthesis. The main signaling pathway regulating protein synthesis is the IGF-1/Akt/mTOR pathway and the ability of BCAA to increase protein synthesis seems to rely on mTOR activation [21]. Furthermore, mTOR has an inhibitory effect due to BCAA on atrogin-1mRNA expression in C2C12 myocytes [4]. Sandri et al. [22] reported that IGF-1 treatment or Akt overexpression inhibit FoxO and atrogin-1 expression in cultured myotubes undergoing atrophy. These results suggest that BCAAs may have a protective effect against muscle atrophy by inhibiting protein degradation and stimulating the protein synthesis pathway. However, the effect of BCAA administration on the alteration of both protein synthesis and degradation pathways in prolonged weightless muscle atrophy is not well known.
Therefore, we hypothesized that BCAAs may have a protective effect on muscle atrophy induced by prolonged weightless through the protein synthesis and degradation pathway. To examine this hypothesis, we used growing rats and selected a hindlimb suspension model as the disuse muscle atrophy model.
METHODS
Animals
Male Wistar rats were purchased from Orient Bio (Seongnam, Korea) at 6 weeks of age (n = 14; mean weight = 273 g). Animals were housed in a controlled environment with a 12 h light/dark cycle and were fed rat chow and water ad libitum during the entire experimental period. All experimental procedures were carried out with approval from the Institutional Animal Care and Use Committee of Konkuk University. The protocol was approved by the Committee on the Ethics of Animal Experiments at Konkuk University (Permit number: KU11055-3).
Experimental design
After 1 week of acclimatization, rats were randomly assigned to a hindlimb suspension group (n = 7) or a hindlimb suspension with BCAA administration group (n = 7). The BCAA group received a diet supplemented with 600mg BCAA/kg (valine: leucine: isoleucine = 1:2:1, Ajinomoto, Tokyo, Japan) dissolved in water. The body weight was measured every morning at 09:00. The experiment was conducted for 14 days.
Hindlimb suspension
The hindlimb suspension procedure was performed according to the method described by Morey-Holton and Globus [5]. Briefly, the tail of each rat was cleaned, dried, then sprayed with a generous amount of adhesive spray (Mueller Sports Medicine Inc., Germany) and dried for 5 min. The rats were allowed 360° rotation and to walk freely on their forelimbs for access to food and water. The rats were suspended by the tail at an angle of ~30° from the head down to avoid contact between the hindlimbs and the ground.
Sample collection
After 14 days of hindlimb suspension, the rats were killed under Avertin-induced anesthesia. For analysis, soleus and extensor digitorum longus (EDL) muscle samples were excised. All muscle samples were weighed and then stored at −80°C until used for analysis.
Total RNA extraction and reverse transcription
Total RNA was extracted from the soleus muscles using Trizol reagent (life technologies, Inc.) according to the manufacturer’s instructions. Samples were treated with DNase, chloroform extracted and re-suspended in 20μl RNase-free water. Reverse transcription was carried out using cDNA Synthesis Master Mix (GenDEPOT, CA, USA). Subsequently, the cDNA was stored in aliquots at −20°C. RT-PCR was performed using amfico Taq DNA polymerase (GenDEPOT, CA, USA). The primer sequences were as follows: atrogin-1 (FORWRAD 5′ GAC TGG ACT TCT CGA CTG CC 3′, REVERSE 5′ GAC TTG CCG ACT CTC TGG AC 3′); MuRF1 (FORWARD 5′ ACA TCT TCC AGG CTG CCA AT 3′, REVERSE 5′ GTT CTC CAC CAG CAG GTT CC 3′); Akt1 (FORWARD 5′ TGC TGG AGG ACA ACG ACT AT 3′, REVERSE 5′ TGT CAT CTT GAT CAG GCG GT 3′); mTOR (FORWARD 5′ TTG AGG TTG CTA TGA CCA GAG AGA A 3′, REVERSE 5′ TTA CCA GAA AGG ACA CCA GCC AAT G 3′); and GAPDH (FORWARD 5′ TGC TGG TGC TGA GTA TGT CG 3′, REVERSE 5′ TGA TGG CAT GGA CTG TGG TC 3′).
Data analysis
All data are presented as the mean ± SE. All statistical analyses were performed with SPSS version 19.0 software (SPSS, Inc., Chicago, IL, USA). The statistical significance of differences in the mean values of the two groups of rats was evaluated using the independent t-test. A p-value of < 0.05 denoted statistical significance.
RESULTS
Food consumption and body weight
Hindlimb suspension for 14 days, BCAA administration did not affect food intake, final body weight in both groups of rats (Fig. 1).
Fig. 1.
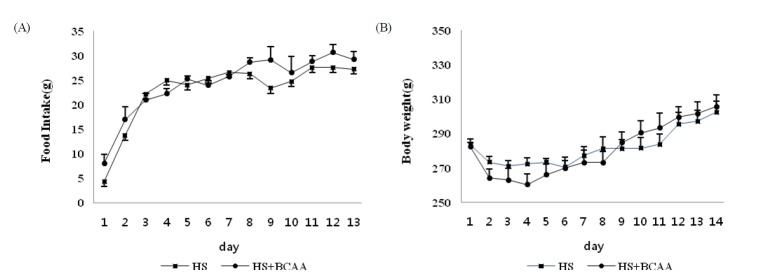
Effects of BCAA on food intake and body weight. A, Food intake was measured every day during hindlimb suspension. B, Body weight was measured every day during hindlimb suspension. These results are expressed as the mean ± SE. HS, hindlimb suspension; HS + BCAA, hindlimb suspension + BCAA.
Soleus and EDL muscle weight
The BCAA administration group exhibited significantly attenuated soleus muscle to body weight ratio compared to the hindlimb suspension group (Fig. 2A). However, the EDL muscle weight to body weight ratio did not differ between the groups (Fig. 2B).
Fig. 2.
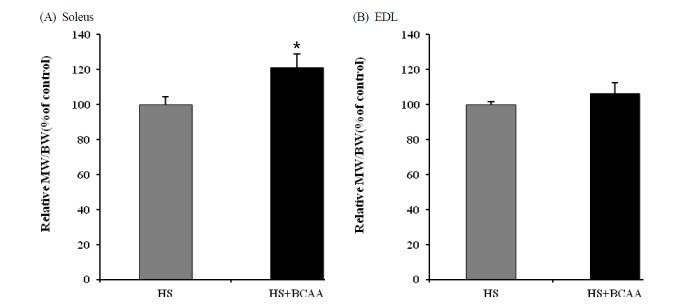
Effects of BCAA on soleus and EDL muscle weight. A, Soleus muscle weight to body weight after hindlimb suspension. B, EDL muscle weight to body weight after hindlimb suspension. These results are expressed as the mean ± SE. *indicates a significant difference to the HS group (p < 0.05). HS, hindlimb suspension; HS + BCAA, hindlimb suspension + BCAA.
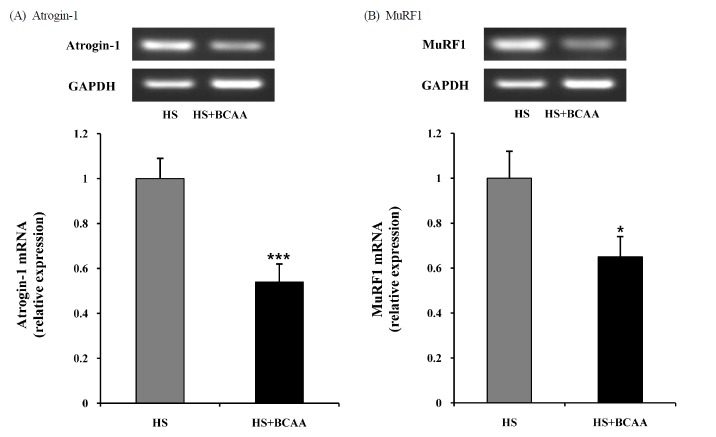
Atrogin-1 and MuRF1 mRNA levels in Soleus muscles
To determine the mechanism underlying the inhibitory effect of BCAA on hindlimb suspension induced muscle atrophy, we measured atrogin-1 and MuRF1 mRNA levels in the soleus muscle. Atrogin-1 and MuRF1 mRNA expression was significantly attenuated by BCAA (Fig. 3).
Fig. 3.
Effects of BCAA on atrogin-1 and MuRF1 mRNA expression in soleus muscles. A, Atrogin-1 mRNA expression in soleus muscle after hindlimb suspension. B, MuRF1 mRNA expression in soleus muscle after hindlimb suspension. These results are expressed as the mean ± SE. *indicates a significant difference to the HS group (p < 0.05); *** indicates a significant difference to the HS group (p < 0.001). HS, hindlimb suspension; HS + BCAA, hindlimb suspension + BCAA.
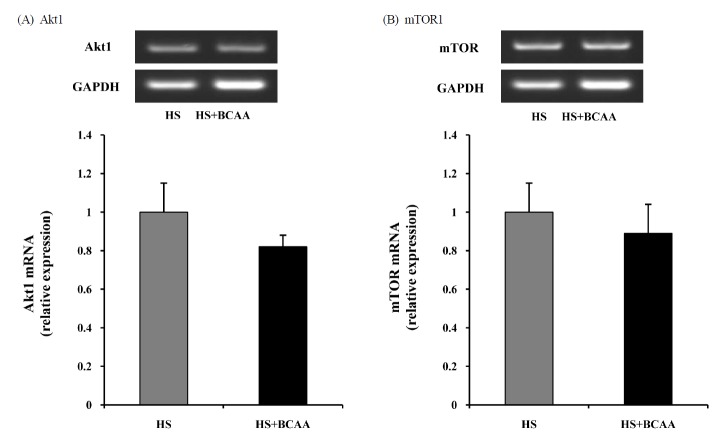
Akt1 and mTOR mRNA levels in Soleus muscles
To determine whether Akt1 and mTOR are affected by BCAA, we measured the levels of Akt1 and mTOR mRNA in the soleus muscle. After hindlimb suspension for 14 days, there was no significant deference in the hindlimb suspension groups with or without BCAA (Fig. 4).
Fig. 4.
Effects of BCAA on Akt1 and mTOR mRNA expression in soleus muscles. A, Akt1 mRNA expression in soleus muscle after hindlimb suspension. B, mTOR mRNA expression in soleus muscle after hindlimb suspension. These results are expressed as the mean ± SE.. HS, hindlimb suspension; HS + BCAA, hindlimb suspension + BCAA.
DISCUSSION
In the present study, body weight in both groups was slightly increased after 14 days of hindlimb suspension, and food intake stabilized after the first 3 days. Our findings align with previous research showing that food consumption may decrease then increase for a few days and that body weight would stabilize or increase slightly throughout the 14 days of hindlimb suspension in growing rats [5].
The present study describes the effect of BCAA administration on hindlimb suspension-induced muscle atrophy. Recent study has shown that BCAA administration prevents dexamethasone-induced soleus muscle atrophy in rats [23]. In the present study, BCAA administration suppresses loss of weight in the soleus muscle, but not the EDL. It has been well established that the soleus muscle exhibits pronounced atrophy induced by hindlimb unloading compared to the EDL muscle; this is because hindlimb unloading predominantly decreases activity in slow-twitch and anti-gravitational muscles like the soleus [6,20,23–26]. Although these results are consistent with our findings, the administration of BCAA still reduced weight decrease in the soleus muscle during hindlimb suspension compared to the hindlimb suspension group without BCAA. BCAA administration appears to have the potential to prevent disuse atrophy in rats.
Protein degradation is controlled by several mechanisms. One of the most predominant mechanisms, ATP-dependent ubiquitin proteasome system (UPS), is mostly known as a primary pathway associated with disuse muscle atrophy [10–12]. The process of muscle protein degradation via the UPS is controlled by two muscle-specific ubiquitin ligases. Muscle atrophy F-box (MAFbx/atrogin-1) and muscle ring finger protein1 (MuRF1), both muscle-specific ubiquitin ligases, are involved in protein degradation in muscle and in increasing muscle atrophy [11,14,17]. In this study, we found that administration of BCAA significantly suppresses the expression of atrogin-1 and MuRF1 mRNA in soleus muscle compared to the hindlimb suspension group. This result agrees with the recent study by Yamamoto et al. [27] showing that BCAA significantly reduced the atrogin-1 mRNA level but not the MuRF1 mRNA level in dexamethasone induced muscle atrophy in rats. Herningtyas et al. [4] reported that BCAAs and arginine decreased atrogin-1 and MuRF1 mRNA levels induced by starvation in C2C12 myocytes. Also, Maki et al. [2] reported that oral BCAA administration suppressed atrogin-1 and MuRF1 protein expression during hindlimb suspension. Although the underlying mechanism through which BCAA decreases atrogin-1 and MuRF1 mRNA expressions is not fully clarified, BCAA prevents hindlimb suspension-induced soleus muscle atrophy, at least partly through the inhibition of mRNA expression of E3 ligases such as atrogin-1 and MuRF1.
Furthermore, it is well known that BCAA plays a pivotal role in protein synthesis through the direct activation of mTOR without the activation of upstream signaling molecules like IGF-1 and Akt1 [28]. However, recent study shows that leucine activates Akt as well as mTOR [29]. Akt, also known as protein kinase B (PKB), has emerged as a critical mediator of mTOR activity. The Akt/mTOR signaling pathway activates the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) and ribosomal protein S6 kinase (S6K), a key molecule in protein synthesis, and eventually increases protein synthesis [30–36].
Activated Akt inhibits FoxOs, which is a transcription factor for muscle specific E3 ligases such as atrogin-1 and MuRF1, and hence increases protein synthesis through the suppression of protein degradation. Inversely, a crucial regulatory factor for protein synthesis, mTOR, is inhibited by the activation of FoxOs activation, leading to protein degradation [37–38]. These results suggest that cross-talk between protein synthesis and degradation is not limited to Akt, but also involves FoxOs.
Thus, we examined the Akt and mTOR mRNA to assess the cross-talk between protein synthesis and degradation following oral BCAA administration during hindlimb suspension. In this study, we found that BCAA did not increase the Akt1 and mTOR mRNA levels compared to the hindlimb suspension group. This result agrees with some human studies showing that infusion of BCAA suppressed muscle protein breakdown without increasing protein synthesis, although species differences may be present [39–40]. In contrast with our results, leucine increased the phosphorylation of Akt and mTOR against denervation induced muscle atrophy in middle aged rats [29]. These results suggest that although BCAA administration did not increase the levels of Akt1 and mTOR mRNA, it has the potential to affect the activation of Akt1 and mTOR at the protein level. However, Maki et al. [2] reported that BCAA did not affect the phosphorylation of Akt1. Although we did not confirm cross-talk between protein synthesis and degradation during atrophy, our results clearly revealed that oral BCAA administration during hindlimb suspension could attenuate muscle atrophy through the suppression of the ubiquitin proteasome pathway without the expression of Akt1 and mTOR mRNA.
In conclusion, our data shows that BCAA administration attenuates hindlimb suspension-induced soleus muscle atrophy in rats. We also suggest that oral BCAA administration may prevent hindlimb suspension-induced soleus muscle atrophy via the inhibition of the ubiquitin-proteasome system, but not by activating the protein synthesis pathway. We confirmed that BCAA could serve as a very effective supplement in the prevention or treatment of muscle atrophy.
REFERENCES
- 1.Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up regulated in aged rat tibialis anterior muscle. Mechanism of Ageing and Development. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Maki T, Yamamoto D, Nakanishi S, Iida K, Iguchi G, Takahashi Y, Kaji H, Chihara K, Okimura Y. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutrition Research. 2012;32:676–683. doi: 10.1016/j.nutres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Attaix D, Mosoni L, Dardevet D, Combaret L, Mirand PP, Grizard J. Altered responses in skeletal muscle protein turnover during aging in anabolic and catabolic periods. The International Journal of Biochemistry & Cell Biology. 2005;37:1962–1973. doi: 10.1016/j.biocel.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Herningtyas EH, Okimura Y, Handayaningsih AE, Yamamoto D, Maki T, Iida K, Takahashi Y, Kaji H, Chihara K. Branched-chain amino acids and arginine suppress MaFbx/atrogin-1 mRNA expression via mTOR pathway in C2C12 cell line. Biochemistry et Biophysian Acta. 2008;1780:1115–1120. doi: 10.1016/j.bbagen.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 6.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Servais S, Letexier D, Favier R, Duchamp C, Desplanches D. Prevention of unloading-induced atrophy by vitamin E supplementation: Links between oxidative stress and soleus muscle proteolysis? Free Radical Biology & Medicine. 2007;42:627–635. doi: 10.1016/j.freeradbiomed.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg L. During muscle atrophy, thick but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. Journal of Cell Biology. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. American Journal of Physiology. 2004;284:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 10.Jagoe RT, Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183–190. doi: 10.1097/00075197-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 12.Price SR. Increased transcription of ubiquitin-proteasome system components: molecular responses associated with muscle atrophy. IJBCB. 2003;35:617–628. doi: 10.1016/s1357-2725(02)00385-0. [DOI] [PubMed] [Google Scholar]
- 13.Glickman MH, Ciechanover A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol rev. 2001;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 14.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlen R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nature Cell Biology. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 15.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 ligases MuRF1 Degrades Myosin Heavy Chain Protein in Dexamethasone-Treated Skeletal Muscle. Cell metabolism. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. Journal of Cell Biology. 2009;185:1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes MD, Lecker SH, Jagoe RT, Goldberg AL. Atrigin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proceedings of the National Academic Sciences. 2001;98:1440–1445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1(IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin(PI3K/Akt/mTOR)pathway. The Journal of Biological Chemistry. 2005;280:2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- 19.Oishi Y, Ogata T, Yamamoto K, Terada M, Ohira T, Ohira Y, Taniguchi K, Roy R. Cellular adaptation in soleus muscle during recovery after hindlimb unloading. Acta Pshysiol. 2008;192:381–395. doi: 10.1111/j.1748-1716.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 20.Tesseraud S, Métayar-Coustard S, Boussaid S, Crochet S, Audouin E, Derouet M, Seiliez I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cell. Biochemical and Biophysical Research Communications. 2007;357:181–186. doi: 10.1016/j.bbrc.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 21.Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. American Journal of Physiology-Endocrinology And Metabolism. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandri M, Sandri C, Gilbert E, Picard A, Walsh K, Schiaffino S, Lecker S, Goldberg A. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Criswell DS, Booth FW, DeMayo F, Schwartz RJ, Gordon SE, Fiorotto ML. Overexpression of IGF-1 in skeletal muscle of transgenic mice does not prevent unloading-induced atrophy. Am J Physiol Endocrinol Metab. 1998;275:E373–E379. doi: 10.1152/ajpendo.1998.275.3.e373. [DOI] [PubMed] [Google Scholar]
- 24.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspension in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 25.Fitts RH, Riley DA, Widrick JJ. Physiology of a micro-gravity environment invited review: microgravity and skeletal muscle. J Appl Physiol. 2000;89:823–839. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 26.Hurst JE, Fitts RH. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J Appl Physiol. 2003;95:1405–1417. doi: 10.1152/japplphysiol.00516.2002. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto D, Maki T, Herningtyas EH, Ikeshita N, Shibahara H, Sugiyama Y, Nakanishi S, Lida K, Iguchi G, Takahashi Y, Kaji H, Chihara K, Okimura Y. Branched-chain amino acids protect against dexamethasone-induced soleus muscle atrophy in rats. Muscle & Nerve. 2010;41:819–827. doi: 10.1002/mus.21621. [DOI] [PubMed] [Google Scholar]
- 28.Lynch CJ. Role of leucine in the regulation of mTOR by amino acids: revelations from structure-activity studies. J Nutr. 2001;131:861S–865S. doi: 10.1093/jn/131.3.861S. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro CB, Christofoletti DC, Pezolato VA, Durigan Rita de CM, Prestes J, Tibana RA, Pereira ECL, de Sousa Neto IV, Durigan JLQ, da Silva CA. Leucine minimizes denervation-induced skeletal muscle atrophy of rats through akt/mtor signaling pathways. Frontiers in physiology. 2015:6. doi: 10.3389/fphys.2015.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR(mammalian target of rapamycin) signaling by intracellular amino acid availability. Biochem J. 2003;372:555–566. doi: 10.1042/BJ20021266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busquetes S, Alvarez B, Liovera M, Agell N, Lopez-Soriano FJ, Argiles JM. Branched-chain amino acids inhibit proteolysis in rat skeletal muscle; mechanisms involved. J Cell Physiol. 2000;184:380–384. doi: 10.1002/1097-4652(200009)184:3<380::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Hamel FG, Upward JL, Siford GL, Duckworth WC. Inhibition of proteasome activity by selected amino acids. Metabolism. 2003;52:810–814. doi: 10.1016/s0026-0495(03)00094-5. [DOI] [PubMed] [Google Scholar]
- 33.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Ishida A, Yamazaki M, Abe H. Leucine suppress myofibrillar proteolysis by down-regulating ubiquitin-proteasome pathway in chick skeletal muscles. Biochem Biophys Res Commun. 2005;336:660–666. doi: 10.1016/j.bbrc.2005.08.138. [DOI] [PubMed] [Google Scholar]
- 35.Wang N, Campbell LE, Miller CM, Proud CG. Amino acid availability regulates p70S6 kinase and multiple translation factors. Biochem J. 1998;334(1):261–267. doi: 10.1042/bj3340261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshizawa F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem Biophys Res Commun. 2004;313:417–422. doi: 10.1016/j.bbrc.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathsway: insights from genetic models. Skeletal muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Disease Models & Mechanisms. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci. 1990;79:457–466. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- 40.Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabilism. 1995;44:424–429. doi: 10.1016/0026-0495(95)90047-0. [DOI] [PubMed] [Google Scholar]