Abstract
Purpose
Neurofibrillary tangles, one of pathological features of Alzheimer’s disease, are produced by the hyperphosphorylation and aggregation of tau protein. This study aimed to investigate the effects of treadmill exercise on PI3K/AKT/mTOR signal transmission, autophagy, and cognitive ability that are involved in the hyperphosphorylation and aggregation of tau protein.
Methods
Experimental animals (NSE/htau23 mice) were divided into non-transgenic control group (Non-Tg-Control; CON; n = 7), transgenic control group (Tg-CON; n = 7), and transgenic exercise group (Tg-Treadmill Exercise; TE; n = 7). The Tg-TE group was subjected to treadmill exercise for 12 weeks. After the treadmill exercise was completed, the cognitive ability was determined by conducting underwater maze tests. Western blot was conducted to determine the phosphorylation status of PI3K/AKT/mTOR proteins and autophagy-related proteins (Beclin-1, p62, LC3-B); hyperphosphorylation and aggregation of tau protein (Ser199/202, Ser404, Thr231, PHF-1); and phosphorylation of GSK-3β, which is involved in the phosphorylation of tau protein in the cerebral cortex of experimental animals.
Results
In the Tg-TE group that was subjected to treadmill exercise for 12 weeks, abnormal mTOR phosphorylation of PI3K/AKT proteins was improved via increased phosphorylation and its activity was inhibited by increased GSK-3β phosphorylation compared with those in the Tg-CON group, which was used as the control group. In addition, the expression of Beclin-1 protein involved in autophagosome formation was increased in the Tg-TE group compared with that in the Tg-CON group, whereas that of p62 protein was reduced in the Tg-TE group compared with that in the Tg-CON group. Autophagy was activated owing to the increased expression of LC3-B that controls the completion of autophagosome formation. The hyperphosphorylation and aggregation (Ser199/202, Ser404, Thr231, PHF-1) of tau protein was found to be reduced in the Tg-TE group compared with that in the Tg-CON group. Furthermore, in the underwater maze test, the Tg-TE group showed a reduced escape time and distance compared with those of the Tg-CON group, suggesting that learning and cognitive ability were improved.
Conclusion
These findings suggest that aerobic exercise such as treadmill exercise might be an effective approach to ameliorate the pathological features (or neurofibrillary tangles) of Alzheimer’s disease.
Keywords: Alzheimer’s disease, treadmaill exercise, tau hyperphosphorylayion, PI3K/AKT/smTOR signaling pathway, autophagy, cognitive function
INTRODUCTION
Alzheimer’s disease is the most common form of dementia, exhibiting memory deterioration and cognitive impairment. This disease is characterized by the presence of amyloid plaque (AP) and neurofibrillary tangles (NFT) [1]. AP are intracellular inclusion bodies composed of amyloid β peptides generated by the degradation of amyloid precursor proteins, while NFTs are aggregates of hyperphosphorylated tau proteins [2].
Tau is a microtubule-binding protein, which functions to facilitate assembly and stabilization of microtubules. Under normal conditions, it maintains homeostasis of phosphorylation and dephosphorylation. It also regulates cytoskeletal stability, which in turn modulates the maintenance of axon morphology [3]. However, hyperphosphorylation of serine and threonine residues of tau protein decreases the binding affinity of tau to microtubules causing aggregation of microtubules, which leads to the formation of paired helical filament (PHF) [4,5]. Intracellular hyperphosphorylation of tau protein is morphologically manifested by the formation of NFT, which is known to induce apoptosis in neuronal cells. This condition is referred to as tauopathy and its clinical characteristics include deterioration of brain function and apoptosis in neuronal cells [6]. Although the exact mechanism, involved in intercellular signaling pathways, that influences the formation of NFTs are not completely clear, mTOR has been reported to be deeply involved in this process [7–9].
mTOR is a sub-signaling protein of Phosphoinositide 3-kinase (PI3K) and Protein kinase B (PKB; also known as AKT) and is expressed ubiquitously in the living body, and two multi-protein complexes named TOR Complex 1 and TOR Complex 2, which are functionally distinct in composition and temperament, form the core of the catalytic domain [10]. These mTOR protein complexes are localized in the center of complex signaling pathways that are activated by intracellular stress or growth factor signals. Moreover, they are involved in regulation of various cellular functions, such as protein synthesis, cell growth, biosynthesis of ribosome and mitochondria, cytoskeletal formation, and autophagy [11,12]. mTOR can undergo self-phosphorylation via its own serine/threonine kinase activities, and regulates the synthesis of other proteins by inhibiting the activation of 4E-BP, an inhibitor protein of mRNA translation process, and through the activation of p70-S6K phosphorylation [13]. Autophagy is a process that sequesters intra-cellular waste by-products, degenerative protein aggregates, and deteriorated organelles in double-membraned vesicle, referred to as autophagosomes, and fuses autophagosomes with lysosome, followed by the degradation by lysosomal proteases [14,15]. The by-products of degradation can be reused for generating energy required for the survival of cells or for building new organelles; thus, autophagy is called a cellular recycling factory [16].
According to recent reports, mTOR was hyperactivated in an Alzheimer’s animal model. This hyperactivity of mTOR was deeply involved in hyperphosphorylation of Aβ and tau [17–20]. In other words, it was reported that hyperactivation of mTOR reduced autophagy and directly contributed to hyperphosphorylation and aggregation of tau proteins [21,22]. Caccamo et al. (2013) reported that, treatment of Alzheimer’s transgenic mice (P301S mice) with rapamycin, an mTOR inhibitor, activated autophagy and decreased hyperphosphorylation of tau proteins. This suggests that inhibition of mTOR expression can increase autophagy and decrease hyperphosphorylation, thus, preventing aggregation of tau proteins. However, mTOR is also engaged in the synthesis of proteins related to learning and memory in the brain [23,24]; hence, if its activity is completely inhibited by treatment with rapamycin, an mTOR inhibitor, it is likely to inhibit the synthesis of proteins related to learning and memory. Thus, appropriately controlling mTOR or avoiding its over-expression is important [22].
No effective treatment of Alzheimer’s disease is yet available. Although many researchers have attempted to develop pharmacological treatments of Alzheimer’s disease, their efficacy was reported to be temporary or radical treatment was reported to be not feasible. Physical activity, or continued exercise has been gaining much attention as preventive and therapeutic approaches to Alzheimer’s disease. According to the studies that analyzed effects of exercise on degenerative neurological diseases, such as Alzheimer’s disease, exercise contributed to the improvement of cognitive functions and prevention of degenerative neurological disease. These were achieved due to positive changes in brain health, such as increased brain volume, generating neuronal cells and growth factors, and promoting cerebral vasculogenesis [25–27]. Moreover, another study reported that exercise can reduce the expressions of Aβ-42 and hyperphosphorylated tau, which are pathological markers of Alzheimer’s disease [28–30]. Many studies have shown that exercise might reduce AP and NTF, which are the pathological features of Alzheimer’s disease, but studies investigating the effect of exercise on molecular signaling pathways that might reduce the hyperphosphorylation and aggregation of tau protein, or determining whether exercise might control mTOR signaling pathway and autophagy-related proteins are lacking.
Therefore, the present study investigated the effects of long-term treadmill exercise on hyperphosphorylation of tau protein, mTOR signaling pathway, autophagy and cognitive function. For this, we used Alzheimer’s transgenic mice with overexpression of NSE/htau23 fusion gene, generated by recombining human tau23 gene under the regulation of NSE (neuron-specific enolase) promoter.
Methods
Experimental animals
In the present study, tau disease model mice that over-express fusion genes (NSE/htau23 fusion gene) because of the presence of htau23 gene under the control of NSE-gene promoter were used; they were provided by the Korea Food and Drug Administration Laboratory Animal Resources and bred for 18 months at H University Animal Laboratory. During the experimental period, the mice were provided food (Purina rat food-5057), which was produced in accordance with AIN-76A dietary adjustment for experimental animals as recommended by the American Dietetic Association, and food and water were provided ad libitum. The mice were divided into non-transgenic control group (Non-Tg-Control; CON; n = 7), transgenic control group (Tg-CON; n = 7), and transgenic exercise group (Tg-Treadmill Exercise; TE; n = 7). The study protocol was submitted to and approved by the H University Animal Experiment Ethics Committee.
Treadmill exercise
After a prior adaptive training (20 min/day, 15cm/sec, 5 days/week) by using a rodent treadmill (8 Lanes, Daemyung Scientific Co, Ltd, Korea) was completed, NSE/htau23 mice were subjected to exercise of 5 days per week for 12 weeks by modifying the exercise program suggested by Cho et al. [29] in consideration of the exercise performance ability of NSE/htau23 mice. The exercise protocol is shown in <Table 1>.
Table 1.
Exercise protocol
| Duration (wk) | Speed (m/min) | Time (min) | Grade (%) | Frequency (days/wk) |
|---|---|---|---|---|
| 0 | 9 | 20 | 0 | 5 |
| 1~3 | 12 | 30 | 0 | 5 |
| 4~6 | 13.2 | 35 | 0 | 5 |
| 7~9 | 13.2 | 45 | 0 | 5 |
| 10~12 | 14.4 | 50 | 0 | 5 |
Water Maze Test
The water maze test was performed in a laboratory with windows open for ventilation arranged with a table. A cylindrical water tank (1.5 m diameter * 12 cm height) was prepared with water at the temperature of 22°C-25°C and whole milk powder was dissolved to make a target not to be seen. The target (12 cm diameter) was place on the water tank floor about 1 cm below from the water surface. Escape distance and escape latency of an experimental animal was measured and analyzed after 12 weeks of experimental treatment utilizing SMART-CS program (Panlab, Barcelona, Spain), which is the computer program connected on the ceiling right above the water tank. The water maze test was conducted five days a week and mice were made to start from the same place and arrive at the target placed on the same location three times a day. On the last day of 6th, the mice were made to start swimming with the target removed, and two times of arrival at the target place were used as experimental value. Only when mice found the hidden target within 60 seconds, the obtained value was used as experimental result. Each examination was conducted with a minimum of five minute interval.
Brain extraction
The mice were intraperitoneally injected an anesthetic (mixed Rompun/Zoletil, 10 mg/kg in a 2:1 ratio) for inducing anesthesia, and their brain tissues were rapidly extracted; the cerebral cortex was subjected to rapid cooling in liquid nitrogen and stored in a cryogenic freezer at −80°C (Bio-Freezer, Forma Science, USA) until analysis.
Western blot
The cerebral cortex from the extracted brain tissues was homogenized using homogenization buffer and homogenizer. The homogenate was centrifuged at 4°C and 15,000 rpm for 30 min, and then the total protein amount from the supernatant was measured using the Bradford method. For each sample, a total protein amount of 40 μg was dispensed to the staking gel well available in Mini-Protein II dual-slab apparatus (Bio-Rad Laboratories, Hercules, CA, USA); electrophoresis was conducted at 80 volt for about 2 h until the sample reached the bottom of the gel. PVDF membrane (Amersham, Arlington Heights, IL, USA) and 3M paper wetted in transfer buffer were overlapped one over another and were placed in the Mini transblot module (Bio-Rad Laboratories, Hercules, CA, USA) and subjected to protein transfer at 60 volt for 1 h. After the membrane was blocked for 1 h with 3% BSA solution, the primary antibodies, including p-PI3K (Ab182651), LC3-B (Ab128025), Ser404 (Ab30666, Abcam, MA, USA, dilution: 1:1000), t-PI3K (#4257), p-AKT (#4051), t-AKT (#4685), p-mTor (#2971), t-mTOR (#2983), Beclin-1 (#3783), p62 (#5114), p-GSK-3β (#9323) t-GSK-3β (#9315, Cell signaling Technology, MA, USA, dilution: 1:1000), Ser199/202 (T6819), Thr231 (T7194, Sigma-Aldrich, MO, USA, dilution: 1:1000), PHF-1 (a kind gift from P. Davies, Feinstein Institute for Medical Research, Manhasset, NY, USA, dilution: 1: 1000), β-Actin (sc-4778, Santa Cruz Biotechnology, CA, USA, dilution: 1:1000) were reacted in the blocking solution (5% BSA) in a 1:1,000 concentration for 12 h. On the following day, they were washed 4 times with TBS-T solution for 10 min each. Subsequently, the secondary antibodies (HRP-conjugated goat anti-mouse, Santa Cruz Biotechnology, Santa Cruz, CA, USA; HRP-conjugated goat anti-rabbit, Invitrogen, Carlsbad, CA, USA) corresponding to the primary antibodies were diluted to 1:5,000 with blocking solution, shaken for 90 min, and cleaned 4 times with TBS-T solution for 10 min each. Finally, the membrane was placed into Luminata Forte Western HRP substrate solution (Millipore Corporation, Billerica, MA, USA) for color development for 1 min, and then scanned using an image analysis system (Molecular Imager ChemiDoc XRS System, Bio-Rad); the protein amount was calculated using Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Hercules, CA, USA).
Data processing method
Statistical analysis of the results was performed using SPSS Statistics 18.0 program and descriptive statistics (Mean ± Standard deciation; SD) of all variables were calculated. One-way ANOVA was performed to test differences between groups. When significant differences between groups were observed, the Bonferroni test was used as a post-hoc test. The statistical significance level was set at α= .05.
RESULTS
The effects of 12 weeks of treadmill exercise on PI3K/AKT/mTOR, autophagy-related proteins, hyperphosphorylation of tau protein in the cerebral cortex of NSE/htau23 mice, and the cognitive ability of NSE/htau23 mice are as follows.
Treadmill exercise attenuates impaired PI3K/AKT/mTOR signal transductions in the cerebral cortex of NSE/htau23 mice
The results of the effects of treadmill exercise on PI3K, AKT, and phosphorylation of mTOR in the cerebral cortex of NSE/htau23 mice are shown in <Fig. 1>. The result of one-way ANOVA on p-PI3K/t-PI3K ratio revealed statistically significant difference among the NonTg-CON (1.00%), Tg-CON (0.41 ± 0.03%), and Tg-TE groups (0.76 ± 0.08%) [F(2,18) = 274.739, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed decreased p-PI3K/t-PI3K ratio than the NonTg-CON group (p = .001), p-PI3K/t-PI3K ratio in the Tg-TE group increased than in the Tg-CON group (p = .001). The result of one-way ANOVA on p-AKT/t-AKT ratio revealed statistically significant difference among the NonTg-CON (1.00%), Tg-CON (0.46 ± 0.09%), and Tg-TE groups (0.64 ± 0.11%) [F(2,18) = 80.116, p = .001]. As per the results of a post-hoc test, the Tg-CON group (p = .001) showed decreased p-AKT/t-AKT ratio than the NonTg-CON group (p = .001), p-AKT/t-AKT ratio in the Tg-TE group increased than in the Tg-CON group (p = .002). The result of one-way ANOVA on p-mTOR/t-mTOR ratio revealed statistically significant difference among the NonTg-CON (1.00%), Tg-CON (2.72 ± 0.59%), and Tg-TE groups (1.94 ± 0.38%) [F(2,18) = 31.694, p = .001]. As per the results of a post-hoc test, the Tg-CON group (p = .001) showed increased p-mTOR/t-mTOR ratio than the NonTg-CON group, p-mTOR/t-mTOR ratio in the Tg-TE group (p = .001) decreased than in the Tg-CON group (p = .006).
Fig. 1.
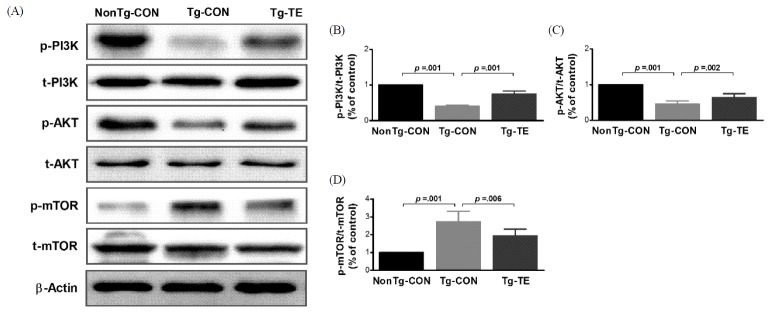
The effects of TE on expression of PI3K/AKT/mTOR proteins in the cerebral cortex of NSE/htau23 mice. (A) Representative western blots of PI3K, AKT and mTOR proteins, (B–D) Densitiometric analysis of the Western blot bands normalized to β-Actin. The data shown in the western blot were means from seven mice brains. β-Actin were probed as an internal control. Bonferroni post hoc test after ANOVA. Values are presented as means ± SD.
Treadmill exercise improves impaired autophagy in the cerebral cortex of NSE/htau23 mice
The results of the effects of treadmill exercise on the expression of Beclin-1, p62 and LC3-B in the cerebral cortex of NSE/htau23 mice are shown in <Fig. 2>. The results of one-way ANOVA for Beclin-1 protein expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (44.72 ± 11.06%), and Tg-TE groups (68.71 ± 4.51%) [F(2,18) = 113.111, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed decreased Beclin-1 protein expression than the NonTg-CON group (p = .001), Beclin-1 protein expression in the Tg-TE group increased than in the Tg-CON group (p = .001). The results of one-way ANOVA for p62 protein expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (271.33 ± 49.41%), and Tg-TE groups (123.25 ± 14.09%) [F(2,18) = 68.709, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased p62 protein expression than the NonTg-CON group (p = .001), p62 protein expression in the Tg-TE group decreased than in the Tg-CON group (p = .001). The results of one-way ANOVA for LC3-B protein expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (49.59 ± 7.79%), and Tg-TE groups (68.93 ± 4.10%) [F(2,18) = 175.421, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased LC3-B protein expression than the NonTg-CON group (p = .001), LC3-B protein expression in the Tg-TE group decreased than in the Tg-CON group (p = .001).
Fig. 2.
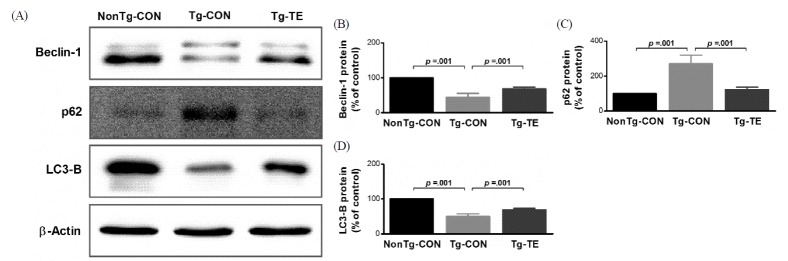
The effects of TE on expression of autophagy-related proteins in the cerebral cortex of NSE/htau23 mice. (A) Representative western blots of Beclin-1, p62 and LC3-B proteins, (B–D) Densitiometric analysis of the Western blot bands normalized to β-Actin. The data shown in the western blot were means from seven mice brains. β-Actin were probed as an internal control. Bonferroni post hoc test after ANOVA. Values are presented as means ± SD.
Treadmill exercise increases phosphorylation of GSK-3β proteins in the cerebral cortex of NSE/htau23 mice
The results of the effects of treadmill exercise on the phosphorylation of GSK-3β in the cerebral cortex of NSE/htau23 mice are shown in <Fig. 3>. The result of one-way ANOVA on p-GSK3β/t-GSK3β ratio revealed statistically significant difference among the NonTg-CON (1.00%), Tg-CON (0.56 ± 0.08%), and Tg-TE groups (0.92 ± 0.03%) [F(2,18) = 171.381, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed decreased p-GSK3β/t-GSK3 β ratio than the NonTg-CON group (p = .001), p-GSK3β/t-GSK3β ratio in the Tg-TE group increased than in the Tg-CON group (p = .001).
Fig. 3.
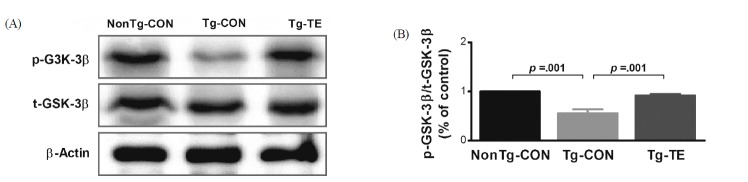
The effects of TE on expression of GSK-3β protein in the cerebral cortex of NSE/htau23 mice. (A) Representative western blots of GSK3-β protein, (B) Densitiometric analysis of the western blot bands normalized to β-Actin. The data shown in the Western blot were means from seven mice brains. β-Actin were probed as an internal control. Bonferroni post hoc test after ANOVA. Values are presented as means ± SD.
Treadmill exercise suppresses hyperphosphorylation of tau proteins in the cerebral cortex of Tg-NSE/htau23 mice
The results of the effects of treadmill exercise on the phosphorylation of tau protein in the cerebral cortex of NSE/htau23 mice are shown in <Fig. 4>. The results of one-way ANOVA for Ser199/202 expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (233.47 ± 89.60%), and Tg-TE groups (143.17 ± 50.25%) [F(2,18) = 9.231, p = .002]. As per the results of a post-hoc test, the Tg-CON group showed increased Ser199/202 expression than the NonTg-CON group (p = .002), Ser199/202 expression in the Tg-TE group decreased than in the Tg-CON group (p = .032). The results of one-way ANOVA for Ser404 expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (160.97 ± 12.52%), and Tg-TE groups (111.39 ± 19.83%) [F(2,18) = 40.112, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased Ser404 expression than the NonTg-CON group (p = .001), Ser404 expression in the Tg-TE group decreased than in the Tg-CON group (p = .001). The results of one-way ANOVA for Thr231 expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (297.26 ± 49.91%), and Tg-TE groups (174.40 ± 11.97%) [F(2,18) = 79.103, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased Thr231 expression than the NonTg-CON group (p = .001), Thr231 expression in the Tg-TE group decreased than in the Tg-CON group (p = .001). The results of one-way ANOVA for PHF-1 expression show statistically significant differences among the NonTg-CON (100%), Tg-CON (321.49 ± 71.86%), and Tg-TE groups (205.02 ± 39.86%) [F(2,18) = 38.176, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased PHF-1 expression than the NonTg-CON group (p = .001), PHF-1 expression in the Tg-TE group decreased than in the Tg-CON group (p = .001).
Fig. 4.
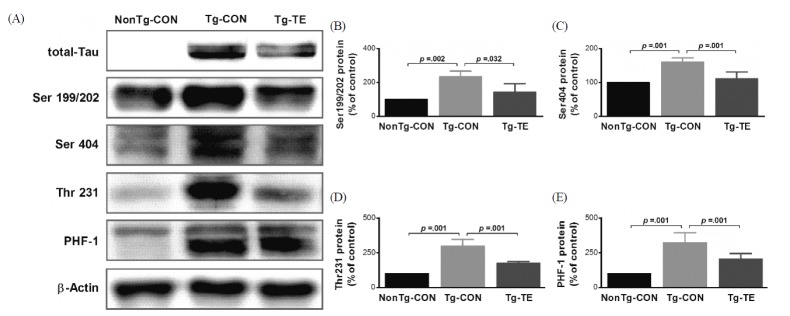
The effects of TE on expression of p-Tau proteins in the cerebral cortex of NSE/htau23 mice. (A) Representative western blots of p-Tau proteins, (B–E) Densitiometric analysis of the Western blot bands normalized to β-Actin. The data shown in the western blot were means from seven mice brains. β-Actin were probed as an internal control. Bonferroni post hoc test after ANOVA. Values are presented as means ± SD.
Treadmill exercise improves cognitive decline in the Tg-NSE/htau23 mice
The results of the effects of treadmill exercise on the cognitive ability of NSE/htau23 mice are shown in <Fig. 5>. The results of one-way ANOVA for escape latency show statistically significant differences among the NonTg-CON (26.63 ± 8.71), Tg-CON (54.77 ± 4.01), and Tg-TE groups (31.86 ± 6.91) [F(2,18) = 33.695, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased escape latency than the NonTg-CON group (p = .001), escape latency in the Tg-TE group decreased than in the Tg-CON group (p = .001). The results of one-way ANOVA for escape distance show statistically significant differences among the NonTg-CON (565.81 ± 78.17), Tg-CON (1203.20 ± 189.09), and Tg-TE groups (559.29 ± 98.05) [F(2,18) = 55.815, p = .001]. As per the results of a post-hoc test, the Tg-CON group showed increased escape distance than the NonTg-CON group (p = .001), escape distance in the Tg-TE group decreased than in the Tg-CON group (p = .001).
Fig. 5.
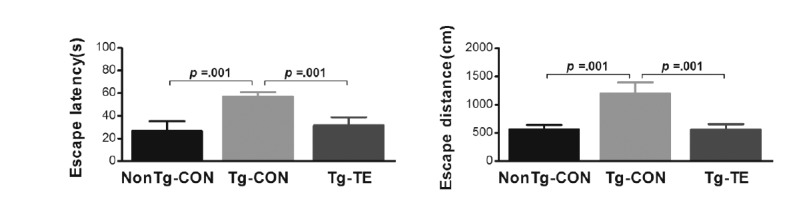
Treadmill exercise improve cognitive performance of the water maze test. Patterns of (A) escape latency and (B) escape distance to cross to the former platform location in the water maze. Bonferroni post hoc test after ANOVA. Values are presented as mean ± SD.
DISCUSSION
This study aimed to investigate the effects of 12-week treadmill exercise on mTOR signaling pathway, autophagy-related proteins, hyperphosphorylation of tau protein, and cognitive ability of NSE/htau23 transgenic mice over-expressing tau protein.
First, the results for PI3K/AKT/mTOR signaling suggested that the expression level of phosphorylated p85 part of PI3K was reduced in the Tg-CON group compared with that in the non-Tg-CON group, whereas its expression level was increased in the Tg-TE group. The expression level of phosphorylated AKT was reduced in the Tg-CON group compared with that in the NonTg-CON group, but its expression level was found to be increased in the Tg-TE group that was subjected to treadmill exercise. In general, PI3K/AKT signaling pathway is known to respond to trophic factors, metabolic signals, and environmental stress and to regulate survival, growth, differentiation, and homeostatic function [31]. AKT is transported from the cytoplasm to cell membrane by PI3K signaling, leading to the phosphorylation of threonine-308 and serine-473 [32]. Since AKT that is known to induce apoptosis in various disease conditions, including diabetes and degenerative brain diseases, is known to play an important role in pathogenic mechanisms [33], the increased AKT activity in the Tg-TE group is considered to suggest an increased survival and protective effect of neurons as well as inhibition of the activity of GSK-3β. The expression level of phosphorylated mTOR was found to be increased in the Tg-CON group compared with that in NonTg-CON group. In addition, the Tg-TE group showed reduced expression level of phosphorylated mTOR compared with that in the Tg-CON group. A noteworthy finding was that, although the activities of PI3K and AKT need to be increased for ensuring an increased activity of mTOR, the Tg-CON group showed an increased activity of mTOR without an increased activity of PI3K and AKT. Villamil-Ortiz & Cardona-Gomez [34] reported that the expression of mTOR and AKT were increased in a 3xTG mouse model aged 18 months; this finding is contradictory to the results of this study with regard to AKT. This could be attributed to the fact that phosphorylation of tau protein is increased depending on the age of transgenic mice with Alzheimer’s disease, or that the genetic features and the activity of mTOR might be controlled by many factors such as 5’AMP-activated protein kinsas (AMPK), tuberous sclerosis complex (TSC1/TSC2) and phospholipase D (PLD1) as well as PI3K/AKT signaling pathway. Further studies are warranted in this regard to clarify this issue [35].
Autophagy is a degradative system that is induced in response to various intracellular stresses (nutrient depletion, growth factor deficiency, endoplasmic reticulum stress, etc.). When autophagy is controlled abnormally, it might be highly associated with neurodegenerative diseases, diabetes, and cardiovascular diseases [36,37]. With regard to autophagy, Beclin-1 was thought to be engaged in controlling the onset of autophagosome formation, p62 is an adapter related to autophagosome formation, and LC3B controls the completion of autophagosome formation [38,39]. Therefore, this study determined the effects of treadmill exercise on Beclin-1, p62 and LC3-B, which are related to autophagy.
The results of this study showed that the expression of Beclin-1 was reduced in the Tg-CON group; this finding was consistent with those of previous studies showing that the expression of Beclin-1 was reduced with aging [40] and that the expression of Beclin-1 was reduced in the brains of patients with Alzheimer’s disease [41]. In other words, when the phosphorylation and aggregation of tau protein are increased, autophagy is abnormally controlled, thereby reducing the activity of Beclin-1, which, in turn, might inhibit autophagy. However, treadmill exercise was found to increase the expression of Beclin-1, which increased autophagy formation. The p62 protein was over-expressed in the Tg-CON group, and its expression was reduced by treadmill exercise. Because p62 protein has an ubiquitin-associated domain (UBA), it acts as an adapter to combine with and transport protein aggregates or organelles in the cells to be removed [42]. The p62 protein expression is increased when oxidative stress is increased, and it is essential for efficient autophagy process. However, when p62 is over-expressed, the activity of autophagy is known to be rather inhibited, and thus the expression of p62 needs to be maintained at an appropriate level [43,44]. Various findings with regard to p62 protein expression and treadmill exercise have been reported. Odochi et al. [45] reported that, in P301S transgenic mice that were subjected to long-term (chronic) treadmill exercise, there was no statistically significant difference in spinal cord, cortex and hippocampus areas. He et al. [46] also reported that exercise might induce autophagy in the brain; however, in their study, exercise was performed one time, and BCL2 AAA mice and wild-type mice were used. Thus, directly comparing their results with those of the present study is difficult. In addition, Marques-Aleixo et al. [47] reported that, compared between normal mice subjected to treadmill exercise and voluntary wheel running and the control group, there was no statistically significant difference in the expression of p62 in the cerebral cortex and hindbrain areas. Therefore, the results of this study suggest that, although autophagy is inhibited by over-expression of p62 protein in NSE/htau23 mice, the autophagy process of p62 protein was normally induced via a treadmill exercise performed for 12 weeks with progressive loading. Lastly, LC3-B is known to control the completion stage of autophagosome formation; its expression was found to be reduced in the Tg-CON group compared with that in the NonTg-CON group, whereas its expression was increased in the Tg-TE group. The increase of LC3 II expression after treadmill exercise was consistent with the findings of previous studies showing that exercise might induce autophagy [46,47]. In other words, the abnormal autophagy in NSE/htau23 transgenic mice was found to be ameliorated by treadmill exercise.
The expression of phosphorylated GSK-3β, which affects the hyper-phosphorylation of tau protein in the cerebral cortex of NSE/htau23 transgenic mice, was found to be reduced in the Tg-CON group compared with that in the NonTg-CON group, whereas it was found to be increased in the Tg-TE group compared with that in Tg-CON group. GSK-3β is known to be located in the downstream region of AKT kinase and is phosphorylated by AKT kinase, leading to a reduced activity. [48]. This study also showed that treadmill exercise might induce the phosphorylation of PI3K and AKT and reduce the activity of GSK-3β, because of which the hyper-phosphorylation of tau protein was reduced. These findings are consistent with those of a study showing that, in transgenic mice with Alzheimer’s disease, treadmill exercise for 3 months increased the phosphorylation of GSK-3β and reduced the phosphorylation of tau protein [30]. Furthermore, Chen & Russo-Neustadt [49] reported that the expression of brain-derived neurotrophic factor (BDNF) was increased through exercise and such an increased BDNF expression might be highly associated with an increased activity of PI3K and AKT signaling pathways.
This study intended to analyze the effects of treadmill exercise on the hyperphosphorylation of PHF-1, Ser404, Ser202, Thr231, and the cognitive ability in the cerebral cortex of NSE/htau23 transgenic mice. The phosphorylation of Ser404, Ser202, Thr231 and PHF-1, the phosphorylated residues of tau protein, was found to be increased in the Tg-CON group compared with that in the NonTg-CON group, whereas it was reduced in the Tg-TE group after the completion of the treadmill exercise. These findings are consistent with those of previous studies showing that, when transgenic mice with Alzheimer’s disease were subjected to treadmill exercise, the phosphorylation of Ser404, Ser202 and Thr231 as phosphorylated residues of tau protein was reduced [30,50]. Furthermore, the analysis of cognitive ability in the underwater maze tests revealed that the Tg-CON group had an increased time and distance to locate the circular escape bar compared with the NonTg-CON, showing a decreased learning and memory capacity. However, the Tg-TE group that underwent treadmill exercise had a reduced time and distance to locate the circular escape bar, suggesting an enhanced cognitive ability through treadmill exercise. These findings are consistent with those of previous studies showing that treadmill exercise might be effective in improving deteriorated spatial learning and memory in transgenic mice with Alzheimer’s disease [30,51,52]. The disposition of Amyloid-β or inflammatory response and neuronal cell death due to hyperphosphorylation of tau protein is known to be the major cause of decreased cognitive ability in Alzheimer’s disease [53]. Treadmill exercise maintained a balance between the process of phosphorylation and dephosphorylation of tau protein, stabilized the microtubules, and ameliorated the abnormal autophagy phenomenon. Consequently, physical activities such as treadmill exercise were found to inhibit the hyperphosphorylation of tau protein as one of the pathological features of Alzheimer’s disease, thereby improving the cognitive ability [54,55].
CONCLUSION
In conclusion, this study revealed that treadmill exercise reduced the activity of GSK-3β, which might lead to the hyperphosphorylation of tau protein, by increasing the phosphorylation of PI3K/AKT in the cerebral cortex of NSE/htau23 transgenic mice. Such a signaling pathway was found to reduce the hyperphosphorylation and aggregation (Ser199/202, Ser404, Thr231, PHF-1) of tau protein. In addition, abnormal autophagy was found to be improved via the reduced expression level of mTOR that controls the activity of autophagy. In other words, these neuropathic improvements were found to improve cognitive deterioration, as one of the major clinical features of Alzheimer’s disease. These findings suggest that regular exercise can be used as a preventive and therapeutic treatment for improving Alzheimer’s disease.
REFERENCES
- 1.Katzman R, Saitoh T. Advances in Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1991;5(3):278–286. [PubMed] [Google Scholar]
- 2.Ittner LM, Gotz J. Amyloid-beta and tau--a toxic pas de deux in Alzheimer’s disease. Nature reviews Neuroscience. 2011;12(2):65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- 3.Tschampa HJ, Schulz-Schaeffer W, Wiltfang J, Poser S, Otto M, Neumann M, Kretzschmar HA. Decreased CSF amyloid beta42 and normal tau levels in dementia with Lewy bodies. Neurology. 2001;56(4):576. doi: 10.1212/wnl.56.4.576. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal K, Grundke-Iqbal I, Smith AJ, George L, Tung YC, Zaidi T. Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(14):5646–5650. doi: 10.1073/pnas.86.14.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science (New York, NY) 1991;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 6.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annual review of neuroscience. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 7.Harris H, Rubinsztein DC. Control of autophagy as a therapy for neurodegenerative disease. Nature reviews Neurology. 2012;8(2):108–117. doi: 10.1038/nrneurol.2011.200. [DOI] [PubMed] [Google Scholar]
- 8.Oddo S. The role of mTOR signaling in Alzheimer disease. Frontiers in bioscience (Scholar edition) 2012;4:941–952. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, Branca RM, Lehtio J, Guan Z, Filipcik P, Xu S, Winblad B, Pei JJ. Mammalian Target of Rapamycin (mTor) Mediates Tau Protein Dyshomeostasis: IMPLICATION FOR ALZHEIMER DISEASE. The Journal of biological chemistry. 2013;288(22):15556–15570. doi: 10.1074/jbc.M112.435123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskar PT, Hay N. The two TORCs and Akt. Developmental cell. 2007;12(4):487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Dazert E, Hall MN. mTOR signaling in disease. Current opinion in cell biology. 2011;23(6):744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnett PE, Barrow RK, Cohen NA, Snyder SH, Sabatini DM. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(4):1432–1437. doi: 10.1073/pnas.95.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferraro E, Cecconi F. Autophagic and apoptotic response to stress signals in mammalian cells. Archives of biochemistry and biophysics. 2007;462(2):210–219. doi: 10.1016/j.abb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Rami A. Review: autophagy in neurodegeneration: firefighter and/or incendiarist? Neuropathology and applied neurobiology. 2009;35(5):449–461. doi: 10.1111/j.1365-2990.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- 16.Levine B, Yuan J. Autophagy in cell death: an innocent convict? The Journal of clinical investigation. 2005;115(10):2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.An WL, Cowburn RF, Li L, Braak H, Alafuzoff I, Iqbal K, Iqbal IG, Winblad B, Pei JJ. Up-regulation of phosphorylated/activated p70 S6 kinase and its relationship to neurofibrillary pathology in Alzheimer’s disease. The American journal of pathology. 2003;163(2):591–607. doi: 10.1016/S0002-9440(10)63687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin RJ, Moloney A, Kelliher M, Johnston JA, Ravid R, Dockery P, O’Connor R, O’Neill C. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. Journal of neurochemistry. 2005;93(1):105–117. doi: 10.1111/j.1471-4159.2004.02949.x. [DOI] [PubMed] [Google Scholar]
- 19.Pei JJ, Bjorkdahl C, Zhang H, Zhou X, Winblad B. p70 S6 kinase and tau in Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2008;14(4):385–392. doi: 10.3233/jad-2008-14405. [DOI] [PubMed] [Google Scholar]
- 20.Pei JJ, Hugon J. mTOR-dependent signalling in Alzheimer’s disease. Journal of cellular and molecular medicine. 2008;12(6B):2525–2532. doi: 10.1111/j.1582-4934.2008.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meske V, Albert F, Ohm TG. Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3-dependent phosphorylation of Tau. The Journal of biological chemistry. 2008;283(1):100–109. doi: 10.1074/jbc.M704292200. [DOI] [PubMed] [Google Scholar]
- 22.Caccamo A, Maldonado MA, Majumder S, Medina DX, Holbein W, Magri A, Oddo S. Naturally secreted amyloid-beta increases mammalian target of rapamycin (mTOR) activity via a PRAS40-mediated mechanism. The Journal of biological chemistry. 2011;286(11):8924–8932. doi: 10.1074/jbc.M110.180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nature medicine. 2008;14(8):843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nature neuroscience. 2009;12(9):1152–1158. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- 25.Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behavioural brain research. 2001;120(1):87–95. doi: 10.1016/s0166-4328(00)00364-8. [DOI] [PubMed] [Google Scholar]
- 26.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 27.Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(5):1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bayod S, Del Valle J, Canudas AM, Lalanza JF, Sanchez-Roige S, Camins A, Escorihuela RM, Pallas M. Long-term treadmill exercise induces neuroprotective molecular changes in rat brain. Journal of applied physiology (Bethesda, Md : 1985) 2011;111(5):1380–1390. doi: 10.1152/japplphysiol.00425.2011. [DOI] [PubMed] [Google Scholar]
- 29.Cho JY, Um HS, Kang EB, Cho IH, Kim CH, Cho JS, Hwang DY. The combination of exercise training and alpha-lipoic acid treatment has therapeutic effects on the pathogenic phenotypes of Alzheimer’s disease in NSE/APPsw-transgenic mice. International journal of molecular medicine. 2010;25(3):337–346. doi: 10.3892/ijmm_00000350. [DOI] [PubMed] [Google Scholar]
- 30.Um HS, Kang EB, Koo JH, Kim HT, Jin L, Kim EJ, Yang CH, An GY, Cho IH, Cho JY. Treadmill exercise represses neuronal cell death in an aged transgenic mouse model of Alzheimer’s disease. Neuroscience research. 2011;69(2):161–173. doi: 10.1016/j.neures.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 31.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochimica et biophysica acta. 2007;1773(8):1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nature reviews Molecular cell biology. 2001;2(10):760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 33.Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Medicinal research reviews. 2002;22(4):373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- 34.Villamil-Ortiz JG, Cardona-Gomez GP. Comparative analysis of autophagy and tauopathy related markers in cerebral ischemia and Alzheimer’s disease animal models. Frontiers in aging neuroscience. 2015;7:84. doi: 10.3389/fnagi.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caron E, Ghosh S, Matsuoka Y, Ashton-Beaucage D, Therrien M, Lemieux S, Perreault C, Roux PP, Kitano H. A comprehensive map of the mTOR signaling network. Molecular systems biology. 2010;6:453. doi: 10.1038/msb.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annual review of genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao Y, Klionsky DJ. Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell research. 2007;17(10):839–849. doi: 10.1038/cr.2007.78. [DOI] [PubMed] [Google Scholar]
- 39.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Molecular biology of the cell. 2008;19(5):2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-Coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. The Journal of clinical investigation. 2008;118(6):2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rohn TT, Wirawan E, Brown RJ, Harris JR, Masliah E, Vandenabeele P. Depletion of Beclin-1 due to proteolytic cleavage by caspases in the Alzheimer’s disease brain. Neurobiology of disease. 2011;43(1):68–78. doi: 10.1016/j.nbd.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zatloukal K, Stumptner C, Fuchsbichler A, Heid H, Schnoelzer M, Kenner L, Kleinert R, Prinz M, Aguzzi A, Denk H. p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases. The American journal of pathology. 2002;160(1):255–263. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, Stenmark H, Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. The Journal of cell biology. 2005;171(4):603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng YT, Shahnazari S, Brech A, Lamark T, Johansen T, Brumell JH. The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. Journal of immunology (Baltimore, Md : 1950) 2009;183(9):5909–5916. doi: 10.4049/jimmunol.0900441. [DOI] [PubMed] [Google Scholar]
- 45.Ohia-Nwoko O, Montazari S, Lau YS, Eriksen JL. Long-term treadmill exercise attenuates tau pathology in P301S tau transgenic mice. Molecular neurodegeneration. 2014;9:54. doi: 10.1186/1750-1326-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He C, Sumpter R, Jr, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8(10):1548–1551. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marques-Aleixo I, Santos-Alves E, Balca MM, Rizo-Roca D, Moreira PI, Oliveira PJ, Magalhaes J, Ascensao A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto(mito)phagy markers. Neuroscience. 2015;301:480–495. doi: 10.1016/j.neuroscience.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 48.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Progress in neurobiology. 2001;65(4):391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 49.Wolf DM, Vazirani VV, Arkin AP. Diversity in times of adversity: probabilistic strategies in microbial survival games. Journal of theoretical biology. 2005;234(2):227–253. doi: 10.1016/j.jtbi.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. Journal of neuroscience research. 2009;87(11):2561–2570. doi: 10.1002/jnr.22075. [DOI] [PubMed] [Google Scholar]
- 51.Mirochnic S, Wolf S, Staufenbiel M, Kempermann G. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19(10):1008–1018. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- 52.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biological psychiatry. 2006;60(12):1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 53.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;97(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 54.Liu HL, Zhao G, Zhang H, Shi LD. Long-term treadmill exercise inhibits the progression of Alzheimer’s disease-like neuropathology in the hippocampus of APP/PS1 transgenic mice. Behavioural brain research. 2013;1(256):261–272. doi: 10.1016/j.bbr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Sun L, Liu SY, Zhou XW, Wang XC, Liu R, Wang Q, Wang JZ. Inhibition of protein phosphatase 2A- and protein phosphatase 1-induced tau hyperphosphorylation and impairment of spatial memory retention in rats. Neuroscience. 2003;118(4):1175–82. doi: 10.1016/s0306-4522(02)00697-8. [DOI] [PubMed] [Google Scholar]