Abstract
Purpose
The purpose of this study is to observe the effects of Salicornia herbacea L. powder ingestion on carbohydrate metabolism in STZ-induced diabetic rats.
Methods
To achieve this objective, 35 Sprague-Dawley male rats were raised with feed mixed with Salicornia herbacia L. powder and given specific periods to swim for 5 weeks. There was no significant difference in the insulin increase rate while ingesting Salicornia herbacea L. powder and simultaneously exercising.
Results
Compared to the diabetes mellitus group, HOMA-IR was significantly decreased in the diabetes mellitus + exercise group, diabetes mellitus + Salicornia herbacea group, and the diabetes mellitus + Salicornia herbacea + exercise group. However, changes in blood glucose were significant in each group. Thus, for the result of GLUT-4 and GLUT-2, which are the glycose transporters of the liver and muscle, diabetes mellitus + exercise group, diabetes mellitus + Salicornia herbacea group, and diabetes mellitus + Salicornia herbacea + exercise group showed significantly higher expressions. The glycogen concentration of the liver and muscle was significantly increased in the diabetes mellitus + exercise group, diabetes mellitus + Salicornia herbacea group, and diabetes mellitus + Salicornia herbacea + exercise group.
Conclusion
With the results above, it seems that taking Salicornia herbacea L. powder and exercise will help prevent various diabetic complications. Therefore, the findings of this study could justify Salicornia herbacea L. powder with its basal data of physiological activities and pharmacological components as a type of health functional food.
Keywords: swimming exercise, Salicornia herbacea L., glucose metabolism, streptozotocin
INTRODUCTION
Diabetes mellitus (DM) is known as a disease characterized by the insulin resistance caused by pancreatic functional impairment, and is a disease which cannot regulate the blood glucose or maintain a stable level of blood sugar [1]. As a method to maintain blood sugar levels, it has been suggested that a combination of physical exercise and proper diet for over 1 year can prevent diabetes mellitus [2]. By doing so patients with diabetes mellitus may find that they have a reduction of body weight, waist circumference, and pre-prandial blood glucose levels, resulting in positive diabetes mellitus treatment effects even before starting medical treatment [3,4].
Skeletal muscle primarily uses intra-muscle glycogen as a fuel for physical exercise, but in the case of exercise for long periods of time, glycogen is depleted, while the absorbance and usage of blood glucose and free fatty acid in the liver are increased. Therefore, such changes induce the increase of glucose transporter-4 (GLUT-4), and glucose transporter-2 (GLUT-2) for the synthesis of glycogen. Thus, the combined performance of aerobic and resistance exercise increases GLUT-2 and 4, facilitating the absorbance of glucose assisting in the regulation of blood sugar [5,6]. In addition, the process increases the metabolism of fatty acids, glucose, and skeletal muscle mitochondrial density reportedly resulting in an elevation of insulin sensitivity; regular exercise is suggested as a method for prevention and treatment of diabetes mellitus [7,8]. In addition, the benefits of physical exercise for diabetes suggested by several prior studies, specifically the guidelines of the American Diabetes Association, report that carbohydrates have effects on blood sugar control, not only in terms of amount but in terms of quality, and emphasized the need of individualized therapy and diet according to the quality of the carbohydrate [9]. In particular, the intake of dietary fiber polysaccharides contained in seaweeds, which not only reduces the total energy intake but also improves the parameters of the metabolic syndrome as well [10]. The fibers, also, reduce the postprandial blood glucose level, but due to the issues of selecting fibers and changes made by used food, the needs of further studies about the effectiveness of dietary fibers has been emphasized [11].
Salicornia herbacia L. is a Salicornia genus plant in the family Chenopodiaceae that grows indigenously in salt marshes and salt fields along seashores worldwide. In an old medical book of China, ‘Shennong Bencaojing’ (also The Classic of Herbal Medicine), Salicornia herbacia L. was recorded as Salicornia herbacia L. (Glasswort, Salicornia herbacea) or Saltwort, and in Japanese “Dae Hwa Bon Cho” while it was called as Shincho (God’s Glass) because it was considered a very rare and spiritual herb. Salicornia herbacia L.contains 38.5 g of dietary fiber per 100 g as a functional ingredient, and also contains large quantities of minerals such as choline, betaine, sodium, phosphorus, calcium, potassium and magnesium, so it has been reported that Salicornia herbacia L. could be utilized as a functional food ingredient. [12,13]. When reviewing previous studies on the functionality of Salicornia herbacia L. the betaine contained in Salicornia herbacia L. had reportedly lowered homocysteine, suppressed liver fat, and reduced weight. Also a study on obesity induced rats, had reported that diet adding Salicornia herbacia L. had positive effects on the reduction of leptin in fat and insulin concentrations and improvement on lipid metabolism [14].
When feeding the streptozotocin (STZ) induced diabetic rats with a diet added of 20% Salicornia herbacia L. the result had shown that the diet displayed anti-diabetic effectiveness by reducing blood glucose concentrations and increasing free fatty acids and high-density lipoprotein cholesterol (HDL-C) reportedly [15]. It was also reported that an intensive one time exercise and a Salicornia herbacia L. added diet in STZ-induced diabetic rats was deemed to increase fat oxidation in L-FABP, CPT-1, and cytochrome c oxidase (COx), resulting in improvements of the metabolic syndrome [16]. As the interests in the functionality of food reflects the increase in respect to weight control and prevention, as well as improvement of chronic diseases including metabolic syndrome, the effectiveness has been verified in many studies and their results related to the pharmacological activity of a functional food using the medicinal herbs. Thus the study has intended to verify the effects of the combined treatment of swimming exercise and powdered-Salicornia herbacia L. ingestion on insulin resistance and glucose metabolism in STZ-induced diabetic rats and to present the efficacy of Salicornia herbacia L. as a functional food.
METHODS
Study animal and breeding method
This study used a total of 35, five weeks old Sprague-Dawley male rats (Hyochang Science, Daegu) as its test subjects. The experimental animals were individually bred under specific environmental conditions such as a maintained temperature ranging from 23–25 °C, a relative humidity of ± 60%, and a regulated 12 hour interval cycle of light/dark from 08:00 to 20:00. After STZ-induced diabetes mellitus was confirmed, the rats were divided into 5 groups by a randomized block design: Control group (CON), Diabetes mellitus group (DIM), Diabetes mellitus with Exercise training group (DEX), Diabetes mellitus with Salicornia herbacia L. ingested group (DSH), and Diabetes mellitus and Salicornia herbacia L. ingested with Exercise training group (DSHEX).
Study materials
In order to add the powdered Salicornia herbacia L. to their diets, 100% Salicornia herbacia L. powder being sold by the Farming Association of Korea for the clean areas of the Southwest Coast was purchased and used for this study.
Diet composition and intake method
The diet composition was prepared by mixing the substituted 5% Salicornia herbacia L. powder based on AIN-76 in comparison to the cellulose of a general diet. For the study, water and the diet were freely given.
Induction of diabetes mellitus
After preliminary breeding for 1 week, 5 week old study animals were fasted for 12 hours prior to the induction of diabetes mellitus, thereafter streptozotocin (Sigma, USA) dissolved in a citrate buffer (pH 4.5) was injected peritoneal (70 mg/kg, bw) [17]. After the intraperitoneal injection, blood samples were collected from the tail vein of the study animals after 12 hours of fasting in order to verify the induction of diabetes mellitus. When their blood glucose was ≥ 180 – 200 ml/dl, it was determined that the diabetes mellitus was induced and the study was carried out.
Exercise method
Exercise was started when the rats reached 6 weeks old and only after confirming the induction of diabetes at 5 weeks. 5 times a week swimming exercise was implemented in a water tank, which had enough secured space for each subject to freely swim (each 30 cm in horizontal and vertical lengths and 80 cm in depth); the water temperature was maintained at 35.0 ± 1.0 °C. The exercises began for 10 minutes at first, with gradual increases each day on the length of time for each exercise. 3 weeks after an adaptation training, the swimming exercise was performed for 60 minutes at each session.
Sample collection
After 12 hours of fasting, under the inhalation anesthesia (Srgivet, Isotec, USA) of lsoflurane (Forane, Aesioa Queen Borough Ltd, UK), blood samples were collected from the abdominal aorta by laparotomy, and were put into a centrifuge (1580MER, Gyrozen, Korea) at 3,000 rpm for 10 minutes. After fractionizing the serum of each group into e-tubes, they were stored at −80 °C (freezer NF-400SF, HFC, Japan) until the analysis. Thereafter, the muscles of the liver and the lower limbs were extracted, separated and quantified, then the activity was suspended in liquid nitrogen and the samples were stored in a −80 °C (freezer NF-400SF, HFC, Japan) until analysis.
Items and method of analysis
Analysis of the blood glucose concentration
The blood glucose concentration was analyzed using an enzyme method quantitative kit (Asan Pharm Co., Ltd.) and was measured by setting the absorbance at 50 nm in a spectrophotometer (Optizen pop, Mecasys, Korea). The outcome of the measured absorbance was calculated by the equation.
Analysis of Insulin Concentration and Homeostasis model assessment of insulin resistance (HOMA-IR)
The blood insulin concentration was measured for absorbance and was quantified after making a Quantitative kit (RayBio, CAT: ELR-Insulin, USA) to react as established in use of the enzyme-linked immunosorbent assay (ELISA) and ELISA (TECAN, Austria).
A homeostasis model assessment of insulin resistance (HOMA-IR) was used to assess insulin resistance [18]. The equation is as below.
Protein expression assay of Glut-2 and 4 in the liver and muscle
For the treatment of muscle tissue and liver tissue, 1 ml of RIPA Buffer (RIPA Buffer, Biosesang, Korea) was added into a sample of 0.1 g and homogenized. After the homogenization was finished, the sample was centrifuged at 12,000 rpm for 10 minutes and the supernatant was separated. Subsequently, 1 μl of the separated supernatant was mixed with 1 ml of 5 × Brad-Ford (Protein Assay, Bio-Rad, USA) to perform protein quantitation of each sample. 200 μl of each mixed sample was placed to an ELISA plate (TECAN, Austria) and the absorbance was measured using a wavelength of 595 nm. Thereafter a 3 × SDS-PAGE sample buffer was mixed to prepare test samples and then were boiled at 100 °C; the boiled samples were then put in ice. For the protein assay, 10 μl of the protein marker (Xpert2 prestained protein marker, Gindepot, USA) and 15 μl of the protein sample were loaded to 10% SDS-PAGE. The mixture was processed by electrophoresis at 80 V to separate the protein. After separation of the protein, using polyvinylidene difluoride membranes (Amersham Hybond, GE Healthcare, USA), they were transferred to the membrane by electroblotting at 100 V for 60 minutes then transferred to the membrane. When the transfer was completed, the skim milk (Skim milk powder, MB cell, Korea) containing blocking buffer (TBST buffer; 50 mM Tris-Hcl; 150 mM NaCl; 0.05%, Hnology Inc., sc-9117, USA) and Glut-4: 1: 200 (Santa Cruz Biotechnology Inc., sc-7938, USA) were diluted according to the respective method and left overnight at 4 °C. Afterward, the samples were washed for 10 minutes 5 times using TBST (5% Tween-20). The secondary antibodies Glut-2: 1: 3000 and Glut-4: 1: 3000 (Goat-anti-rabbit, Santa Cruz Biotechnology, Inc., CA NO sc-2004, USA) both were diluted in accordance with the respective method and left to react for 2 hours. Subsequent to the above, the diluted samples were washed for 10 minutes 5 times, per each time, using TBST (5% Tween-20), thereby the identification of the derived band for the liver and muscle was conducted by phosphorescence of the membrane using the enhanced-chemilumine scene using (RPN 2106, GE Healthcare, USA) kit and then was developed on X-ray film (Ortho-CP-G plus, AGFA, Belgium).
After the development, β-actin was diluted at the ratio of 1:3000 (Santa Cruz biotechnology Inc., sc CA NO. sc-47778, USA), according to the respective method and then left overnight at 4 °C; the samples were washed for 10 minutes 5 times, per each time, using TBST (5% tween-20). Then, the secondary antibodies were diluted and reacted for 2 hours in accordance with the respective method. The membrane was light-emitted using an ECL kit and developed on X-ray film. The outcome of band density was derived as band/β-actin by using the image j (NIH ver. 1.48, USA).
Liver and muscle glycogen concentration analysis
On the day before the experiment, Na2SO4 was added to 700 ml of dWater was heated to dissolve and saturate Na2SO4 into the dWater to make a 30% KOH solution. After filtering the dissolved solution, 300 g KOH was dissolved in the solution and stored in a refrigerator at 4 °C for more than 24 hours and until ready for use. The samples of liver and muscle collected were placed on ice, and liquid nitrogen in the range of 10 to 30 mg was added to the sample of each group for induction. Then, the samples were broken and quantified and put into glass tubes where each 1 ml of 30% KOH was fractionized; thereafter the glass tubes were boiled for 30 minutes. After that, the samples were well mixed using a vortex mixer to ensure they should be well dissolved, 1.5 ml of 95% ethanol was added and vortex mixing was performed. The samples processed as above were stored in a refrigerator at 4 °C for 24 hours.
The 24 hours stored samples were centrifuged for 30 minutes at 3000 rpm and the supernatants were discarded. After adding 3 ml of dWater to each sample and mixing well using the vortex mixer, each sample was fractionized by 15 μl into an empty glass tube where 350 μl of dWater was already dispensed, again mixing well using a vortex mixer. Then 0.5 ml of 5% phenol (dWater 475 ml + phenol 25 ml) was added and mixed using a vortex mixer for good color development. Finally, 2.5 ml of H2SO4 was added and mixed well using a vortex mixer. The absorbance was analyzed by 490 nm, using a spectrophotometer (Optizen pop, Mecasys, Korea) and it was calculated using an showing formula.
Data process method
For data of the study results, a statistics program for Windows SPSS/PC + 21.0 was used. All experimental results were expressed as means and standard errors, and a one-way ANOVA was performed to verify the significance of each group. For items resulting in a significant difference, a post hoc test was performed by the least significant different (LSD) method, and a statistically significant difference was set as p < 0.05.
RESULTS
Changes in the content of plasma insulin
Changes in plasma insulin concentration are as shown in Fig. 1A. It was 0.09 ± 0.0025 in the CON group, 0.12 ± 0.0028 in DIM group, 0.12 ± 0.0028 in DEX group, 0.11 ± 0.0025 in DSH group, and 0.11 ± 0.0025 in DSHEX group and did not present any statistically significant inter-group differences. However, the DIM group, DEX group, DSH group and DSHEX group resulted in higher insulin concentrations than the CON group.
Fig. 1.
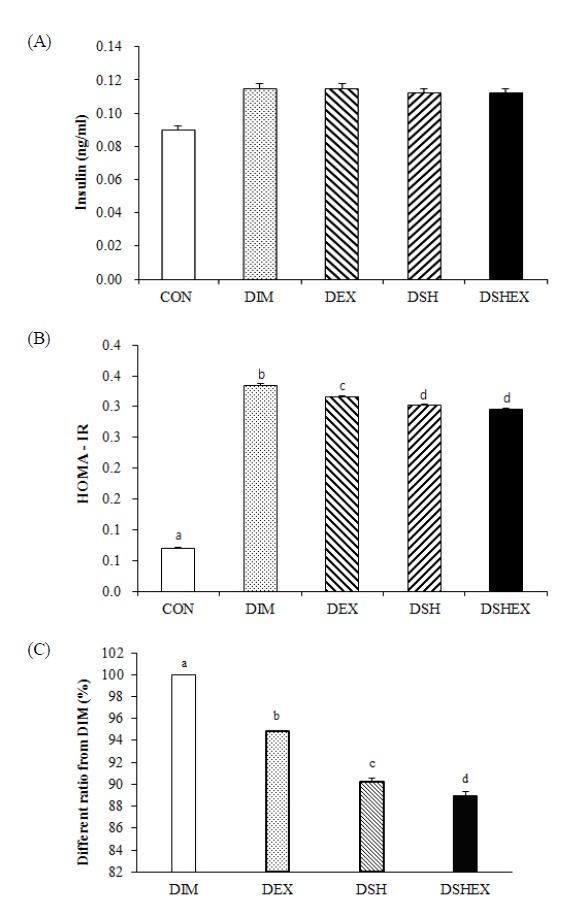
Difference of insulin concentrations and HOMA-IR and HOMA-IR ratio from DIM. (A) insulin concentrations in blood serum, (B) HOMA-IR level, (C) HOMA-IR ratio from DIM. CON: normal control group; DIM: diabetes mellitus group; DEX: diabetes mellitus with exercise training group; DSH: diabetes mellitus with Salicornia herbacea ingested group; DSHEX: diabetes mellitus and Salicornia herbacea ingested with exercise training group; different letter means significance at p < .05, respectively.
Changes in HOMA-IR
Changes in HOMA-IR are as shown in Fig. 1B. In this study, when identifying the changes in HOMA-IR by using the plasma insulin concentrations and glucose concentrations, it was higher and statistically significant, presenting at 0.09 ± 0.0025 in the CON group, 0.12 ± 0.0028 in DIM group, 0.12 ± 0.0028 in DEX group, 0.11 ± 0.0025 in the DSH group, and 0.11 ± 0.0025 in DSHEX group (p < 0.05) Fig. 1B. However, when comparing the percentage of the DIM group and other respective groups in the HOMA-IR index, except for the CON group, it was 100 ± 0.00 in the DMI group, 94.8 ± 0.08 in the DEX group, 90.2 ± 0.34 in the DSH group, and 88.9 ± 0.44 in the DSHEX group, presenting statistically significant lower inter-group differences. In particular, the DSHEX group had shown significantly lower results revealing a combination of Salicornia herbacia L. Powder added diet and exercise had reduced the insulin resistance (p < 0.05) Fig. 1C.
Changes in blood glucose concentrations
For the changes in blood glucose concentrations, the outcomes were 73.1 ± 1.69 in the CON group, 293.0 ± 0.98 in the DIM group, 278.6 ± 1.20 in the DEX group, 266.0 ± 0.86 in the DSH group, and 0.11 ± 0.0025 in the DSHEX group, presenting there were statistically significant inter-group differences (p < 0.05) Fig. 2A. In addition, the outcomes of the percentage for each group, except the CON group, compared to 100 ± 0.00 in the DIM group, were 95.0 ± 0.41 in the DEX group, 90.7 ± 0.29 in the DSH group, and 89.5 ± 0.19 in the DSHEX group, which were statistically lower, and in particular, as the DSHEX group had presented the lowest percentage so that it indicated the exercise and Salicornia herbacia L. Powder-added diet intake had improved the blood glucose concentration in association with diabetes mellitus (p < 0.05) Fig. 2B. Nevertheless, with the experiment for 4 weeks only, the combination of Salicornia herbacia L. Powder-added diet intake and exercise had reduced the blood glucose concentration but could not reduce the significant differences from the CON group (p < 0.05) Fig. 2A.
Fig. 2.
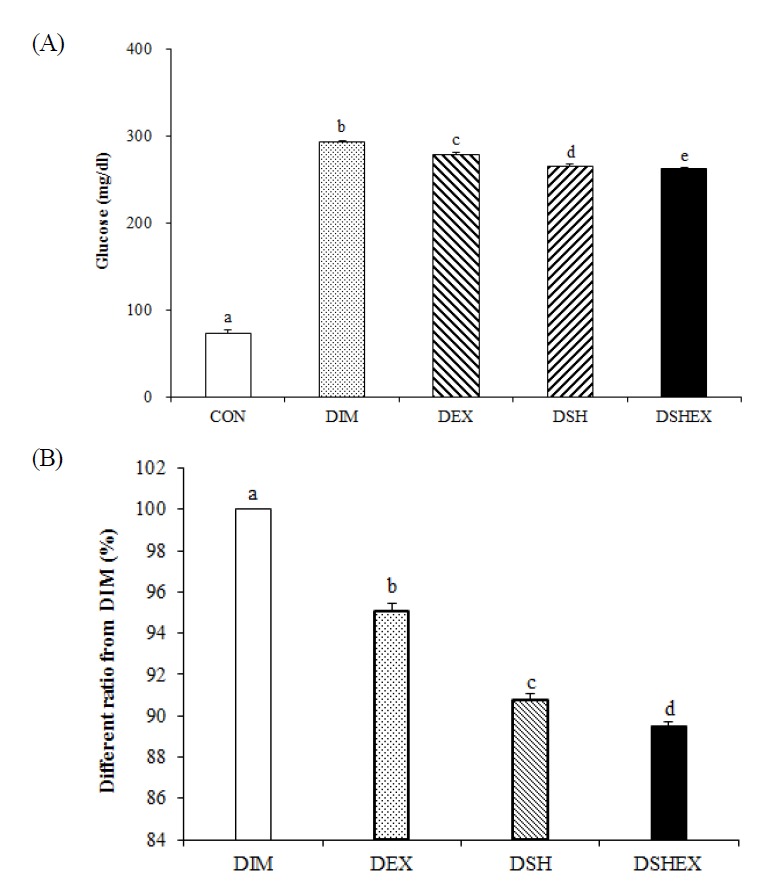
Difference of blood glucose concentrations and blood glucose concentrations ratio from DIM. (A) glucose concentrations in blood serum, (B) blood glucose concentrations ratio from DIM. CON: normal control group; DIM: diabetes mellitus group; DEX: diabetes mellitus with exercise training group; DSH: diabetes mellitus with Salicornia herbacea ingested group; DSHEX: diabetes mellitus and Salicornia herbacea ingested with exercise training group; different letter means significance at p < .05, respectively.
Changes in Glut-2 protein expression
When studying the changes in Glut-2 protein expressions in the liver, compared to 0.79 ± 0.0025 in the CON group, it was significantly lower in the DIM group as 0.69 ± 0.0028, whereas the DEX group, the DSH group and the DSHEX group had shown statistically significant higher expression levels as 1.06 ± 0.0085, 0.98 ± 0.0135 and 1.08 ± 0.0118, respectively (p < 0.05) Fig. 3A, B. In addition, the DEX group and the DSHEX group did not show significant inter-group differences between them as 1.06 ± 0.0085 and 1.08 ± 0.0118 respectively, but they had shown high expression levels of the protein compared to other groups. When comparing the ratio of protein expression levels, compared to 100 ± 0.00 in the DIM group, it had presented significantly higher proportion with the outcome values that were 153.5 ± 1.22 in the DEX group, 141.0 ± 1.94 in the DSH group, and 156.4 ± 1.69 in the DSHEX group (p < 0.05) Fig. 3C.
Fig. 3.
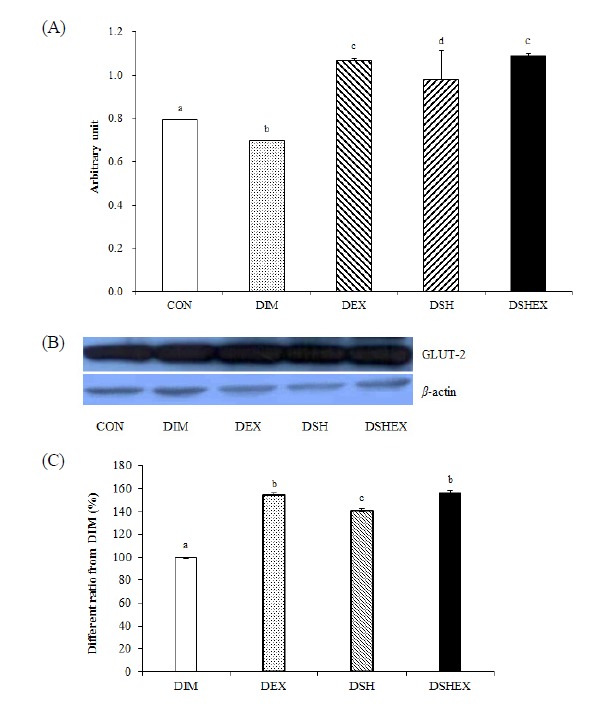
Difference of Glut-2 protein expressions and Glut-2 protein expressions image and Glut-2 protein expressions ratio from DIM. (A) Glut-2 protein expressions in liver, (B) Glut-2 protein expressions of image, (C) Glut-2 protein expressions ratio from DIM. CON: normal control group; DIM: diabetes mellitus group; DEX: diabetes mellitus with exercise training group; DSH: diabetes mellitus with Salicornia herbacea ingested group; DSHEX: diabetes mellitus and Salicornia herbacea ingested with exercise training group; different letter means significance at p < .05, respectively.
Changes in Glut-4 protein expression
The results of gastrocnemius muscle protein expressions did not show any significant differences in the DIM group as 0.68 ± 0.014 compared to 0.76 ± 0.012 in the CON group, but the DEX group, DSH group, and DSHEX group had shown statistically significant higher expression levels as 1.48 ± 0.055, 1.24 ± 0.112 and 1.54 ± 0.051, respectively (p < 0.05) Fig. 4 A, B. In addition, when reviewing the proportions compared with the DMI group, the DEX group, DSH group and DSHEX group had shown significantly higher proportions as 218.8 ± 8.20, 182.9 ± 16.52 and 226.4 ± 3.38 compared to 100 ± 0.00 in the DIM group, in particular, the DEX group and DSHEX group had shown significantly higher differences (p < 0.05) Fig. 4C. Therefore, as the results of this study, the combination of exercise and the Salicornia herbacia L.-added diet is likely to increase the glucose transport capacity of muscle so as to increase the synthesis of glycogen.
Fig. 4.
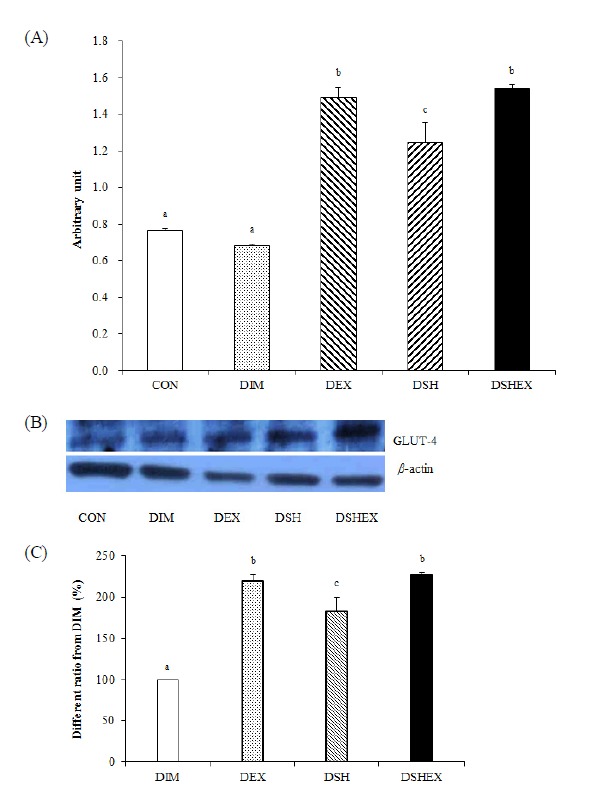
Difference of Glut-4 protein expressions and Glut-4 protein expressions image and Glut-4 protein expressions ratio from DIM. (A) Glut-4 protein expressions in gastrocnemius, (B) Glut-4 protein expressions of image, (C) Glut-4 protein expressions ratio from DIM. CON: normal control group; DIM: diabetes mellitus group; DEX: diabetes mellitus with exercise training group; DSH: diabetes mellitus with Salicornia herbacea ingested group; DSHEX: diabetes mellitus and Salicornia herbacea ingested with exercise training group; different letter means significance at p < .05, respectively.
Changes in the liver and gastrocnemius muscle glycogen concentrations
Changes in the liver and gastrocnemius muscle glycogen concentrations were as follows. Compared to the glycogen concentration of the CON group 147.8 ± 0.61, the DIM group had shown a significantly low content as 143.6 ± 0.41, whereas the DEX group, DSH group, and DSHEX group had shown statistically significant higher contents as 155.9 ± 0.89, 154.6 ± 0.90 and 158.6 ± 0.31 (p < 0.05) Fig. 5A. When comparing the concentration ratio of liver glycogen, the DEX group, DSH group and DSHEX group had shown significantly high results as 108.4 ± 0.62, 107.6 ± 0.62 and 110.4 ± 0.21 respectively, compared to 100 ± 0.00 in the DIM group, and in particular, when comparing each group, the DSHEX group had shown significantly high contents (p < 0.05) Fig. 5B.
Fig. 5.
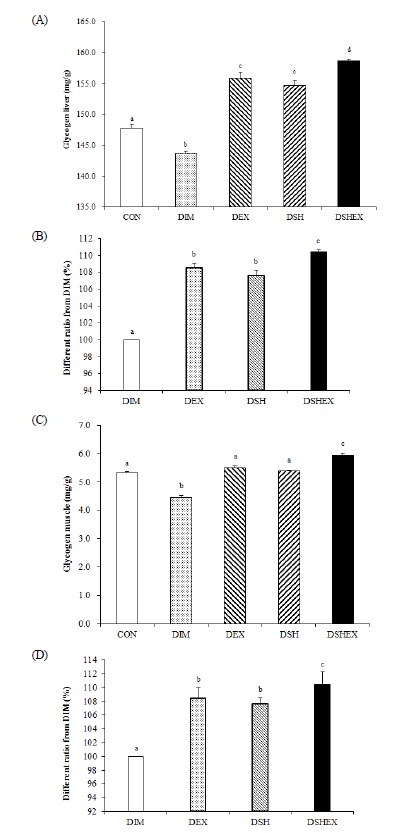
Difference of liver and gastrocnemius muscle glycogen concentrations and liver and gastrocnemius muscle glycogen concentrations ratio from DIM. A: glycogen concentrations in liver, B: liver glycogen concentrations ratio from DIM, C: glycogen concentrations in gastrocnemius muscle, D: gastrocnemius muscle concentrations ratio from DIM. CON: normal control group; DIM: diabetes mellitus group; DEX: diabetes mellitus with exercise training group; DSH: diabetes mellitus with Salicornia herbacea ingested group; DSHEX: diabetes mellitus and Salicornia herbacea ingested with exercise training group; different letter means significance at p < .05, respectively.
When studying the glycogen content of the muscle, compared to the glycogen concentration of the CON group 5.31 ± 0.048, the DIM group had shown significantly a low concentration as 4.45 ± 0.070 (p < 0.05) Fig. 5C, whereas the DEX group and DSH group were 5.50 ± 0.065 and 5.38 ± 0.04 respectively and they did not show any significant differences. However, the DSHEX group as 5.93 ± 0.084 had presented statistically significant high concentration compared to each group (p < 0.05) Fig. 5C. The results of concentration ratio comparisons of the DIM group with other groups, except the CON group, had shown that the DEX group, DSH group and DSHEX group presented significantly high concentration ratios as 123.5 ± 1.46, 120.8 ± 0.92 and 133.3 ± 1.89, and the DSHEX group in particular had shown a significantly high concentration compared to each group (p < 0.05) Fig. 5D. From the results, it was concluded that the induction of diabetes mellitus had reduced the amount of glycogen storage of the liver and muscle, but the combination of exercise and the Salicornia herbacia L.-added diet was likely to increase the amount of glycogen storage of liver and muscle.
DISCUSSION
The interests in exercise and health functional foods are increasing for the promotion and maintenance of health, and the demands are becoming increased just as much as the interest. In particular, the preference of functional foods ingestible with physical exercise shows a trend of steady increase. To this end, the study aimed to find out the effects of a 4-week swimming exercise and Salicornia herbacia L. powder-added diet combination on the metabolism of carbohydrate in STZ-induced diabetic rats.
STZ has been reported as a substance being used widely in diabetic model animal studies that induces selective beta cell destruction of Langerhans’ cells in the pancreas, which produces insulin [19,20]. A STZ-induced pancreas destroys β-cells and has been reported as resulting in chronic hyperglycemia subsequent to changes in the insulin secretion and blood glucose concentrations so that they cause a reduction of insulin sensitivity for glucose uptake [21]. Insulin is a hormone being secreted from the pancreas and it is an important metabolic hormone, which facilitates sugar uptake among multiple functions of the body. It is suggested that its dysfunction accompanies diabetes and is associated insulin resistance [22]. In a study about insulin, patients with type 2 diabetes had shown high concentrations of insulin and HOMA-IR [23]. The results of this study did not show significant inter-group differences in serum insulin levels, but HOMA-IR had shown statistically significant high levels in the DIM group, DEX group, DSH group and DSHEX group compared to the CON group and the result of the HOMA-IR ratio had shown a significantly lower ratio in each group compared to the DIM group. From such results, it was considered that STZ had induced selective destructions of β-cells of Langerhans’ cells in the pancreas producing insulin, in connection to the increases of insulin and HOMA-IR in the DIM group, DEX group, DSH group and DSHEX group except the CON group, and they were consistent to the results of previous studies. When reviewing the results obtained by comparing HOMA-IR ratios, the DEX group, DSH group, and DSHEX group had shown significantly low ratios of HOMA-IR compared to the DIM group. Such results exhibited that there have been reports on exercise to reduce HOMA-IR by purposely decreasing blood glucose and insulin concentrations, nevertheless, the physiologic active substance contained in medicinal herbs are being used in treating the complications of diabetes as an alternative to glucose. It has also been reported that it reduces HOMA-IR and decreases insulin, but the bioactive substances, which contains medicinal plants, blood glucose and insulin decreased during omit findings movements that used in diabetes complications has recommended [24,25]. The medicinal plant, Salicornia herbacia L. used in this study contains a large amount of water-soluble dietary fiber [26], and the combination of water-soluble dietary fiber intake and physical activity has been reported as largely involved in the effects of insulin resistance reduction [27]. Therefore, the combination of Salicornia herbacia L. powder intake and physical exercise was considered to control the increased amount of insulin, which would result in an elevation of actions to save insulin and consequently it was likely to reduce insulin and HOMA-IR. Such mechanisms are considered as having positive anti-diabetic effects.
Symptoms of diabetes induced by the increase of blood glucose concentration are such as eating too much, polyuria and drinking too much. Such symptoms are caused by abnormal carbohydrate, protein, and lipid metabolism and result in the increase of blood sugar as induced by the lack of insulin secretion or increase of insulin resistance [28]. However, exercise is effective in reducing blood glucose concentrations in patients with type 2 diabetes [29]. In addition, as the blood glucose concentration and the level of glycated hemoglobin were reduced in STZ-induced rats dosed with 1 g/kg/day of a medicinal herb so that the use of such medicinal herbs are reported as effective for hypoglycemic action as well as for the treatment of anti-diabetic anemia [30].
The results of this study presented that there were statistically significant inter-group differences, and the result of the glucose concentration ratio comparison exhibited in the DEX group, DSH group, and DSHEX group with statistically lower results, compared to the DIM group. Such results show that Salicornia herbacea L. as a well-known medicinal herb, contains approximately 7.3 g of dietary fiber as per 100 g and known to have excellent effects in the blood sugar control. A study supporting the efficacy of Salicornia herbacea L. presented the Salicornia herbacia L.-added diet had reduced body weight as well as the blood glucose. Another study presented that administration of Salicornia herbacia L. extract for 6 weeks in db/db mice had inhibited pancreatic lipase thus reducing the blood lipid and glucose concentration [31,32]. However, since it had been reported that exercise had positive effects on prevention and improvement of various metabolic disorders-induced complications [33], the reduction of the blood glucose concentration indicated by the results of this study are considered as resulted from the inhibition of the blood glucose absorption by the polysaccharide dietary fibers contained in Salicornia herbacea L. and from the possible increase in consumption of glucose by exercise. Therefore the combination of Salicornia herbacia L. powder-added diet intake and physical exercise in diabetes mellitus is considered to have positive effects on the control of blood glucose by reducing the blood glucose concentration.
The main role of the liver glycogen is to circulating glucose to maintain normal blood glucose levels. The accumulation and degradation of glycogen in the muscles and in the liver is regulated by the interaction of glycogen-phosphorylase and glycogen-synthase [34], and the Glut-2 and 4 are increased and absorb glucose for glycogen synthesis [35]. Insulin resistance and type 2 diabetes suggest that diabetes mellitus reduces the protein expression of Glut-4, a glucose transporter chain in skeletal muscles which is led to inhibition of glycogen synthesis [36]. However, regular exercise reduces fasting glucose concentration, insulin resistance and glycated hemoglobin and plays important roles in improvement of type 2 diabetes, hypertension or metabolic syndrome. On the other hand, when fed the diabetes induced rats with the medicinal herb and skipping exercise, it has been reported to induce activity of AMPK and increase the protein expression of Glut-4, resulted in the increase of glycogen synthesis by absorption of the plasma glucose [37,38].
When reviewing the results from comparisons of protein expressions of Glut-2 and 4, changes in the glycogen concentrations and the concentration ratio of each item in the liver and muscle in this study, for Glut-2 as a glucose transporter chain in liver, DEX group, DSH group, and DSHEX group had shown significantly high expression ratios compared to the DIM group. In particular, the DEX group and DSHEX group showed significant increases in protein expression compared to each group. For Glut-4 as a as a glucose transporter chain in muscle, DEX group, DSH group and DSHEX group had shown statistically significant high expression ratio, compared to the DIM group, whereas the DEX group and DSHEX group showed significant increases in protein expression compared to each group and results of Glut-2. Regarding to the protein expression in the glucose transporter, when studying the results related to liver glycogen, the DIM group had shown a significantly low concentration, whereas the DEX group, DSH group and DSHEX group had shown significantly high concentrations. In particular, the results of the muscle glycogen, DEX group, DSH group, and DSHEX group had shown significantly high concentrations compared to the DIM group, and the DSHEX group showed statistically significant high concentrations compared to each group. Such results were obtained because the glucose metabolism by insulin secretion were incurred mainly in the muscle and liver, and in particular muscular contractions during exercise increased the serum glucose absorption in connection to the facilitation of glucose absorption, while the blood glucose level was maintained by using glycogen degradation, gluconeo-genesis and free fatty acid as alternative fuel [39,40]. A previous study supporting the aforementioned findings had reported the increases of glucose concentration as well as PPAR-γ, PGC-1α and Glut-4 in the diabetes-induced rats were induced by exercises [41]. Meanwhile, it was suggested that because Halophytes were ingested after passing through the secondary reduction inside the plant itself, and such intake induced over-expression of Glut-2 and 4 increased the glycogen concentration [42]. This indicated that a large amount of water-soluble dietary fiber contained in Salicornia herbacia L. was recently identified as also being contained in a large amount in Helianthus tuberosus. Helianthus tuberosus passes through the digestive tract without being digested, so it delays the rise of the blood glucose but helps the bowel activity to be smooth, helps the excretion of bile acid thereby lowering of the blood lipid levels [43]. Thus, as the results of this study, it was identified that PI3K in liver and muscle facilitates Akt and AMPK during exercise to increase expression of Glut-2 and 4, and likely inhibited glucose absorption. It has also been considered that the activated Akt would have been involved in the expression of Glut-2, and inhibited the glucose degradation and gluconeogenesis. In addition, has been considered the combination of exercise and Salicornia herbacia L. powder-added diet would contribute in the increase of Glut-2 and 4 as well as glycogen concentration as a large amount of water-soluble dietary fibers contained in Salicornia herbacia L. had delayed the elevation of blood glucose by digestion. Also, as the combination of exercise and Salicornia herbacia L. powder-added diet intake had been involved in the metabolism activation of Glut-2 and 4 as well as glucose content and then induced over-expression of Glut-2 and 4 and increased the concentration of glycogen, it implicated the improvement potential of anti-diabetic disorders, impaired glucose tolerance (IGT), and insulin resistance. To this end, by conducting studies in the future on the physiological actions of the phytochemicals contained in Salicornia herbacia L., the stability and the value as a functional food should be verified and its potential as supplementary food should be presented.
CONCLUSIONS
From the results of this study, It was considered that the low content of blood glucose in each of the diabetes induced groups, except the control group, would be increased to a high level and the blood glucose would be maintained at high levels due to secretion of insulin induced by the combination of exercise and Salicornia herbacia L. powder added diet, while inhibiting the increase of HOMA-IR caused by functional deterioration of insulin. Also, as the results for the glucose transporters Glut-2 and 4 as well as glycogen concentration, it was considered that the combination of exercise and Salicornia herbacia L. powder added diet would increase the transport capacity so as to increase the glycogen synthesis and storage in liver and muscle. It was considered that such results were obtained due to the actions by regular exercise and physiologic functional ingredients of Salicornia herbacia L. and as the results of this study exhibited positive outcomes for Salicornia herbacia L., it was considered that the value of Salicornia herbacia L. would be verified through various studies according to the physiological activity and pharmacological ingredients of Salicornia herbacia L. based on such positive outcomes.
REFERENCES
- 1.Bergman M, Chetrit A, Roth J, Dankner R. Dysglycemia and long-term mortality: observations from the Israel study of glucose intolerance, obesity and hypertension. Diabetes Metab Res Rev. 2014 doi: 10.1002/dmrr.2618. [DOI] [PubMed] [Google Scholar]
- 2.Alharbi M, Gallagher R, Kirkness A, Sibbritt D, Tofler G. Long-term outcomes from Healthy Eating and Exercise Lifestyle Program for overweight people with heart disease and diabetes. Eur J Cardiovasc Nurs. 2014 doi: 10.1177/1474515114557222. pii: 1474515114557222. [DOI] [PubMed] [Google Scholar]
- 3.Inouye J, Matsuura C, Li D, Castro R, Leake A. Lifestyle intervention for filipino americans at risk for diabetes. J Community Health Nurs. 2014;31(4):225–37. doi: 10.1080/07370016.2014.926674. [DOI] [PubMed] [Google Scholar]
- 4.Kwon MJ, Park JH. The effect of therapeutic lifestyle change on cardiovascular disease and mortality in diabetic patients. The Journal of Korean Diabetes. 2014;15(3):129–33. [Google Scholar]
- 5.Kim SH. Effect of exercise on glycose metabolism. The Journal of Korean Diabetes. 2011;12(1):21–4. [Google Scholar]
- 6.Jeong IG, Oh MJ, Jang MN, Koh YS, Kyle DB, David N, Vic BE. Effects of Dietary Caloric Restriction and Exercise on GLUT 2 in Liver and GLUT-4 and VAMP-2 in Muscle Tissue of Diabetic Rats. Journal of Exercise Nutrition & Biochemistry. 2009;13(1):1–7. [Google Scholar]
- 7.Marcinko K, Steinberg GR. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp Physiol. 2014 doi: 10.1113/expphysiol.2014.082255. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Jekal YS, Jeon JY. Epidemiology of physical activity participation and type 2 diabetes in Korea. The Journal of Korean Diabetes. 2011;12(1):12–20. [Google Scholar]
- 9.Noh MY. Quality of carbohydrate and diabetes mellitus. 2014;15(2):104–9. [Google Scholar]
- 10.Kondo K, Ishikado A, Morino K, Nishio Y, Ugi S, Kajiwara S, Kurihara M, Iwakawa H, Nakao K, Uesaki S, Shigeta Y, Imanaka H, Yoshizaki T, Sekine O, Makino T, Maegawa H, King GL, Kashiwagi A. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: a pilot study. Nutr Res. 2014;34(6):491–8. doi: 10.1016/j.nutres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins AL, Morgan LM, Bishop J, Jovanovski E, Vuksan V. Randomized Clinical Trial in Healthy Individuals on the Effect of Viscous Fiber Blend on Glucose Tolerance When Incorporated in Capsules or into the Carbohydrate or Fat Component of the Meal. J Am Coll Nutr. 2014;10:1–6. doi: 10.1080/07315724.2014.905762. [DOI] [PubMed] [Google Scholar]
- 12.Jeong JH, Kim YS. Functional and material content of the antioxidant to Salicornia herbacea L. Journal of Plant Biotechnology. 2008;0:154. [Google Scholar]
- 13.Jung SH, Park KU, Kim JY, Park CK, Choi KS, Seo KI. Biological activities of crude polysaccharids and crude saponins from Salicornia herbacea. The Korean of Food Preservation. 2009;16(1):109–14. [Google Scholar]
- 14.Lee YK, Kang SB. The Effects of Gamioryung-san Extracts on Streptozotocin-induced Diabetic Nephropathy Rats. Journal of Korean oriental internal medicine. 2012;33(4):367–86. [Google Scholar]
- 15.Kim MW. Effects of Salicornia Herbacea L. Supplementation on Antioxidative Enzyme Activities in Streptozotocin-Induced Diabetic Rats. The Korean Nutrition Society. 2008;41(7):583–93. [Google Scholar]
- 16.Seo HB, Lee IH, Jeon BD, Kwon TD, Ryu S. Acute exercise with Salicornia herbacia L. powder ingestion increases Lipids metabolism in STZ-induced diabetic rats. Journal of Exercise Nutrition & Biochemistry. 2012;16(4):167–75. [Google Scholar]
- 17.Zhao L, Liu X, Xie L, Gao H, Lin D. 1H NMR-based metabonomic analysis of metabolic changes in streptozotocin-induced diabetic rats. Analytical Sciences. 2010;26:1277–82. doi: 10.2116/analsci.26.1277. [DOI] [PubMed] [Google Scholar]
- 18.Al-Gayyar MM, Alyoussef A, Hamdan AM, Abbas A, Darweish MM, El-Hawwary AA. Cod liver oil ameliorates sodium nitrite-induced insulin resistance and degradation of rat hepatic glycogen through inhibition of cAMP/PKA pathway. Life Sci. 2015;120:13–21. doi: 10.1016/j.lfs.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Chung HC, Kim IY, Kim SR, Son JM, Lee DW, Song SH, Seong EY, Kwak IS, Lee SB. Effects of lipoxygenase inhibitor on diabetic nephropathy in rats: Decreasing proteinuria and preserving renal function. The Korean Journal of Nephrology. 2011;30:452–58. [Google Scholar]
- 20.Wang J, Lv C, Xie T, Ouyang J. The variance of peripheral blood lymphocyte subsets of streptozotocin-induced diabetic mice after bone marrow transplantation. International Journal of Clinical and Experimental Medicine. 2015;8(3):4115–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Badalzadeh R, Layeghzadeh N, Alihemmati A, Mohammadi M. Beneficial effect of troxerutin on diabetes-induced vascular damages in rat aorta: histopathological alterations and antioxidation mechanism. International Journal of Endocrinology and Metabolism. 2015;13(2):e25969. doi: 10.5812/ijem.25969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E. Medicinal chemistry of the anti-diabetic effects of momordica charantia: active constituents and modes of actions. Open Med Chem J. 2011;5(2):70–7. doi: 10.2174/1874104501105010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalić K, Jotić A, Rajković N, Singh S, Stošić L, Popović L, Lukić L, Miličić T, Seferović JP, Maćešić M, Stanarčić J, Čivčić M, Kadić I, Lalić NM. Altered Daytime Fluctuation Pattern of Plasminogen Activator Inhibitor 1 in Type 2 Diabetes Patients with Coronary Artery Disease: A Strong Association with Persistently Elevated Plasma Insulin, Increased Insulin Resistance, and Abdominal Obesity. Int J Endocrinol. 2015;2015:390185. doi: 10.1155/2015/390185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han JY, Lee MG, Sung SC. Effects of combined training of rope skipping and walking on body composition, physical fitness, blood lipids, and insulin resistance in middle-aged women. Korean Journal of Sport Science. 2009;20(2):199–211. [Google Scholar]
- 25.Hamid K, Alqahtani A, Kim MS, Cho JL, Cui PH, Li CG, Groundwater PW, Li GQ. Tetracyclic triterpenoids in herbal medicines and their activities in diabetes and its complications. Current Topics in Medicinal Chemistry. 2015 Jun 19; doi: 10.2174/1568026615666150619141940. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Kim MH, Hong GJ. Qualities of soybean Dasik with added saltwort (Salicornia herbacea L.) powder. The Korean Society of Food Culture. 2011;26(5):501–5. [Google Scholar]
- 27.Breneman CB, Tucker L. Dietary fibre consumption and insulin resistance-the role of body fat and physical activity. Br J Nutr. 2013;110(2):375–83. doi: 10.1017/S0007114512004953. [DOI] [PubMed] [Google Scholar]
- 28.Oh TW, Kang SY, Kim KH, Song MY, Park YK. Anti-diabetic effect of medicinal plants used for lower wasting-thirst in streptozotocin-induced diabetic rats. Kor J Herbology. 2013;28(5):53–60. [Google Scholar]
- 29.Chimkode SM, Kumaran SD, Kanhere VV, Shivanna R. Effect of yoga on blood glucose levels in patients with type 2 diabetes mellitus. J Clin Diagn Res. 2015;9(4):CC01–3. doi: 10.7860/JCDR/2015/12666.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitak TY, Wasser SP, Nevo E, Sybirna NO. The Effect of the Medicinal Mushrooms Agaricus brasiliensis and Ganoderma lucidum (Higher Basidiomycetes) on the Erythron System in Normal and Streptozotocin-Induced Diabetic Rats. Int J Med Mushrooms. 2015;17(3):277–86. doi: 10.1615/intjmedmushrooms.v17.i3.70. [DOI] [PubMed] [Google Scholar]
- 31.Bang MA, Kim HA, Cho YJ. Hypoglycemic and Antioxidant Effect of Dietary Hamcho Powder in Streptozotocin-induced Diabetic Rats. Journal of the Korean society of food science and nutrition. 2002;31(5):840–6. [Google Scholar]
- 32.Hwang JY, Lee SK, Jo JR, Kim ME, So HA, Cho CW, Seo YW, Kim JI. Hypolipidemic effect of Salicornia herbacea in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2007;1(4):371–5. doi: 10.4162/nrp.2007.1.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56(3):836–48. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Docsa T, Marics B, Nemeth J, Huse C, Somsak L, Gergely P, Peitl B. Insulin sensitivity is modified by a glycogen phosphorylase inhibitor: glucopyranosylidene-spiro-thiohydantoin in streptozotocin-induced diabetic rats. Curr Top Med Chem. 2015 Jun 21; doi: 10.2174/1568026615666150622091407. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Seo H, Koo BM, Choi S, Kim W, Ryu S. The effects of needles powder intake and swimming exercise on energy metabolic substrate in rats. The Korean Society of Living Environmental System. 2015;22(2):296–304. [Google Scholar]
- 36.Moreno P, Acitores A, Gutiérrez-Rojas I, Nuche-Berenguer B, El Assar M, Rodriguez-Mañas L, Gomis R, Valverde I, Visa M, Malaisse WJ, Novials A, González N, Villanueva-Peñacarrillo ML. Amylin effect in extrapancreatic tissues participating in glucose homeostasis, in normal, insulin-resistant and type 2 diabetic state. Peptides. 2011;32(10):2077–85. doi: 10.1016/j.peptides.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Lin X, Zhang X, Guo J, Roberts CK, McKenzie S, Wu WC, Liu S, Song Y. Effects of Exercise Training on Cardiorespiratory Fitness and Biomarkers of Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2015;4(7) doi: 10.1161/JAHA.115.002014. pii: e002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkhalidy H, Moore W, Zhang Y, McMillan R, Wang A, Ali M, Suh KS, Zhen W, Cheng Z, Jia Z, Hulver M, Liu D. Small Molecule Kaempferol Promotes Insulin Sensitivity and Preserved Pancreatic β-Cell Mass in Middle-Aged Obese Diabetic Mice. J Diabetes Res. 2015;2015:532984. doi: 10.1155/2015/532984. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Choi CS. Insulin resistance of mechanisms and new treatment target. Korean Society for Biochemistry and Molecular Biology. 2011;31(1):86–92. [Google Scholar]
- 40.Kim KS, Park SW. Exercise and type 2 diabetes: ACSM and ADA joint position statement. The Journal of Korean Diabetes. 2012;13(2):61–8. [Google Scholar]
- 41.Ha TG, Kim JC. The effects of endurance training combined with rosiglitazone on the expression of PPARs, PGC-1 α, GLUT-4 and p-AMPK- α2 in the skeletal muscle of diabetic induced-rats. Journal of Exercise Nutrition & Biochemistry. 2009;13(2):131–140. [Google Scholar]
- 42.Seo HB, Nam JO, Song YJ, Kwon TD, Yeo Y, Ryu S. Study of the effect of diet supplemented with powdered Salicornia herbacea L. on glucose transporter and antioxidant capacities in rats. Exercise Nutrition & Biochemistry. 2012;16(2):85–92. [Google Scholar]
- 43.Kim SH. Jerusalem artichoke and inulin. The Journal of Korean Diabetes. 2014;15(4):227–31. [Google Scholar]