Abstract
Introduction
Intestinal epithelial cells represent an important component of innate immunity, with sophisticated responses to inflammatory stimuli. The manner in which intestinal epithelial cell polarity affects responses to inflammatory stimuli is largely unknown. We hypothesized that polarized intestinal epithelial cells exhibit a bidirectional inflammatory response dependent upon the location of the stimulus.
Methods
Caco-2 cells were grown on semi-permeable inserts in a dual-compartment culture system and treated with tumor necrosis factor-α (TNF-α; 100 ng/ml) or serum-free media in the apical or basolateral chamber. Interleukin-8 (IL-8) production in each chamber was measured by enzyme-linked immunosorbent assay. To determine receptor specificity, anti-TNF receptor antibodies were added to the apical or basolateral chamber.
Results
Basolateral stimulation with TNF-α resulted in increased apical and basolateral IL-8 production. Apical TNF-α stimulation resulted in increased apical, but not basolateral IL-8 production. Receptor blockade suggested TNF receptor 1 involvement on both apical and basolateral membranes, while TNF receptor 2 was only active on the apical membrane.
Conclusion
Polarized intestinal epithelial cells respond to TNF-α stimulation with focused, directional secretion of the proinflammatory cytokine IL-8. These findings are important because they suggest that intestinal epithelial cells are capable of organizing their response to inflammatory signals and producing inflammatory mediators in a bidirectional, vectorial fashion.
Keywords: Vectorial, Caco-2, TNF receptor, IL-8, Polarized
Introduction
The intestinal mucosa plays an active role in the response to local and systemic inflammation that occurs in disease states such as trauma, sepsis, inflammatory bowel disease (IBD), lung injury, burn, and infectious diarrhea and has been dubbed a “motor” of inflammation in severe illness.1–5 The intestinal mucosa acts as a barrier to the outside environment and must interact with this environment appropriately, either by exhibiting tolerance or forming an immune response. Mechanisms of interaction between the gut epithelium and luminal contents, including commensal and pathogenic bacteria, are complex and specific to individual types of microbes.6–11 The mechanisms involved in controlling gut based inflammatory response are a critical, but incompletely understood, part of the innate immune system. In this setting, intestinal epithelial cells occupy a unique position in the intestinal mucosal tissue, with potential exposure to stimuli from the luminal contents (apical stimulation) as well as the lamina propria (baso-lateral stimulation). Although not known, it is conceivable that the responses to apical and basolateral stimulation may be distinct.
Tumor necrosis factor-α (TNF-α) is a critical proinflammatory mediator in both acute and chronic stages of intestinal and systemic inflammatory disease states. It is a known activator of the transcription factors nuclear factor kappa B (NF-κB) and activator protein-1 (AP-1), two key modulators of the inflammatory response.12,13 In severe burns, gut-derived TNF-α results in local damage to intestinal mucosa, systemic vascular permeability, and lung injury.14 Anti-TNF-α treatment is an important part of inflammatory bowel disease therapy, resulting in improved remission rates and steroid requirements.15,16 Alteration of TNF-α immune response, however, has been associated with severe septic complications in maintenance therapy and postoperative regimens.17,18 Gut ischemia associated with sepsis and hemorrhage alters mesenteric cytokine profiles as well as intestinal barrier function via TNF-α mechanisms.19 In addition to IBD, there are a host of TNF-α-mediated chronic diseases such as rheumatoid arthritis, psoriasis, heart failure, bronchitis, and colon cancer.12,20
The intestinal epithelial layer plays a key role in gut-mediated inflammation, with capability to receive signals from multiple inputs and generate an inflammatory response. The purpose of this study is to better understand how intestinal epithelial cells actively participate in directing an inflammatory response. We hypothesized that polarized intestinal epithelial cells in culture are capable of generating a bidirectional, vectorial response to an inflammatory signal.
Methods
Materials
Caco-2 cells and Eagle's minimum essential medium with Earle's balanced salt solution and 2 mM L-glutamine (EMEM) were obtained from American Type Culture Collection (Rockville, MD, USA). Sodium pyruvate, non-essential amino acids (NEAA), penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Hyclone Laboratories (Logan, UT, USA). Recombinant human TNF-α, mouse monoclonal anti-human TNF receptor 1 (TNFR1) antibody, mouse monoclonal anti-human TNF receptor 2 (TNFR2) antibody, mouse IgG1 isotype control antibody and enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems (Minneapolis, MN, USA). Culture flasks and Costar Transwells were purchased from Corning, Inc (Corning, NY, USA). EVOM2 epithelial voltohmmeter was purchased from World Precision Instruments (Sarasota, FL, USA). Ninety-six-well plates were purchased from Nunc (Roskilde, Denmark).
Cell Culture
Caco-2 cells were grown in flasks at 37°C in 5% CO2 in nutrient media consisting of EMEM supplemented with 10% FBS, 1 mM sodium pyruvate, 0.1 mM NEAA, 100 U/ml penicillin, and 100 mg/ml streptomycin. Cells used for experiments were between passages 5 and 20 and were seeded at a density of 300,000 cells/well onto 24-mm-diameter Transwells permeable inserts with 0.04 μm pores. Cells were grown in supplemented EMEM for 21 days to achieve full differentiation prior to use. Transepithelial electrical resistance (TEER) values were measured in all wells and were adjusted for the area of the membrane (4.5 cm2) and the background resistance of the media and the membrane insert. Only cells with TEER greater than 500Ωcm2 were considered fully differentiated and suitable for use.
Experimental Conditions
Cells were placed in serum-free media for 24 h, then treated with TNF-α at a concentration of 100 ng/ml,19 and added to the apical or basolateral compartment. After 24 h of treatment, supernatants were harvested. In additional experiments, LPS (100 ng/ml) was placed in the apical compartment for the duration of the experiment. In receptor blockade experiments, cells were treated with antibody against TNFR1 (15 μg/ml), TNFR2 (15 μg/ml), or isotype control IgG (30 μg/ml) antibody as described in the results for 24 h prior to addition of TNF-α.
Determination of Supernatant Protein
Interleukin-8 (IL-8) and CD14 protein levels were determined by ELISA according to manufacturer's instructions. Due to unequal volumes in the apical and basolateral compartments, protein concentrations were multiplied by the volume of the respective compartment to normalize amount of protein secreted into each chamber.
Statistical Analysis
When appropriate, results were expressed as mean ± standard error. Statistical analysis was carried out with ANOVA followed by Student–Newman–Keuls test. Statistical analysis was performed using Sigma Plot 11 software (Systat Software, Chicago, IL, USA). A p value of <0.05 was regarded as statistically significant. All experiments were performed at least three times in order to ensure reproducibility.
Results
Vectorial Secretion of IL-8
We first examined the effect of TNF-α treatment on IL-8 production in Caco-2 cells. Basolateral treatment of cells with TNF-α resulted in bidirectional IL-8 production, with significant increases in IL-8 release observed in both apical (Fig. 1a) and basolateral (Fig. 1b) compartments. Apical treatment with TNF-α resulted in release of IL-8 only into the apical compartment (Fig. 1a, b). Because intestinal epithelial cells are constantly exposed to luminal LPS, we next sought to determine if the presence of apical LPS altered the ability of TNF-α to induce directional IL-8 secretion. LPS was added to the apical chamber and cells were treated with TNF-α in apical or basolateral compartments. The presence of apical LPS had no effect on TNF-α-induced vectorial release of IL-8 (Fig. 2a, b).
Fig. 1.
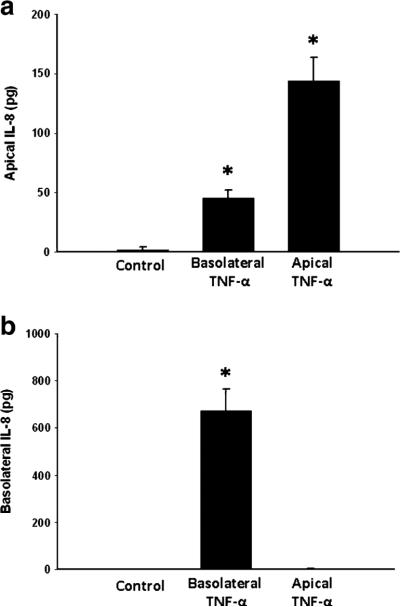
Apical (a) and basolateral (b) IL-8 production in Caco-2 cells after treatment with TNF-α. *p<0.05 vs. control
Fig. 2.
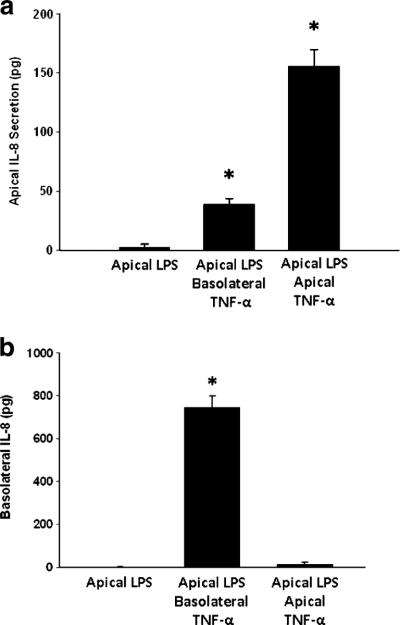
Apical (a) and basolateral (b) IL-8 production in Caco-2 cells after treatment with TNF-α in the presence of apical LPS (100 ng/ml). *p<0.05 vs. control
CD14 Secretion
Previous studies indicate that Caco-2 cells secrete CD14, a protein associated with LPS binding.21 In order to determine if the effects of TNF-α were a generalized, non-specific response, we examined the effect of TNF-α treatment on apical and basolateral release of CD14. Treatment with TNF-α had no effect on the release of CD14 in either apical or basolateral compartments (Fig. 3a, b).
Fig. 3.
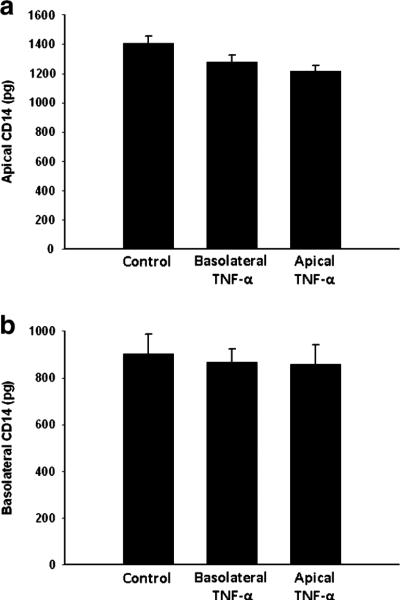
Apical (a) and basolateral (b) CD14 levels in Caco-2 cells after treatment with TNF-α
Transepithelial Electrical Resistance
TEER values were measured during each of the experimental conditions. TEER remained stable throughout the experiment, suggesting that bidirectional IL-8 production was not the result of lost barrier function (Fig. 4).
Fig. 4.
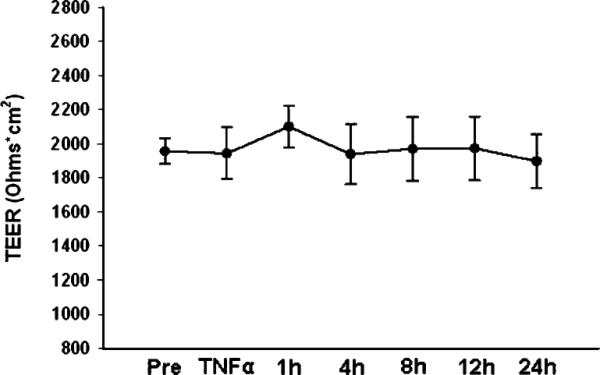
Transepithelial electrical resistance of Caco-2 cells during treatment
TNF Receptor Blockade
In order to determine if there was differential receptor utilization for the observed vectorial secretion of IL-8 induced by TNF-α, cells were treated with neutralizing antibodies to TNFR1 or TNFR2 in either apical or basolateral compartments prior to treatment with TNF-α. Apical blockade of either TNFR1 or TNFR2 resulted in significantly decreased secretion of IL-8 (Fig. 5a, b). Combined blockade of both TNFR1 and TNFR2 reduced IL-8 release in a similar capacity as singular receptor blockade. Blockade of basolateral TNFR1 and TNFR2, either individually or combined, had no effect on apical IL-8 release (Fig. 6a). In contrast, only blockade of basolateral TNFR1, and not TNFR2, resulted in significantly reduced basolateral IL-8 release (Fig. 6b). Basolateral blockade of both TNFR1 and TNFR2 reduced IL-8 release in a similar fashion as blockade of TNFR1 alone (Fig. 6b).
Fig. 5.
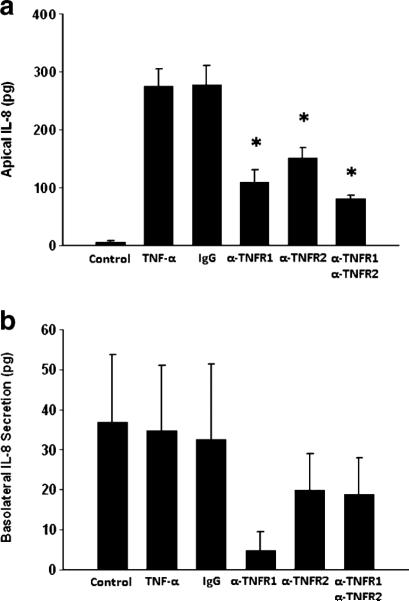
IL-8 production in Caco-2 cells after treatment with TNF-α in the presence of antibodies to TNFR1 or TNFR2 in the apical chamber. α-TNFR1 antibody to TNF-α receptor 1 (15 μg/ml), α-TNFR2 antibody to TNF-α receptor 2 (15 μg/ml), IgG isotype control antibody (30 μg/ml). *p<0.05 vs. TNF-α alone
Fig. 6.
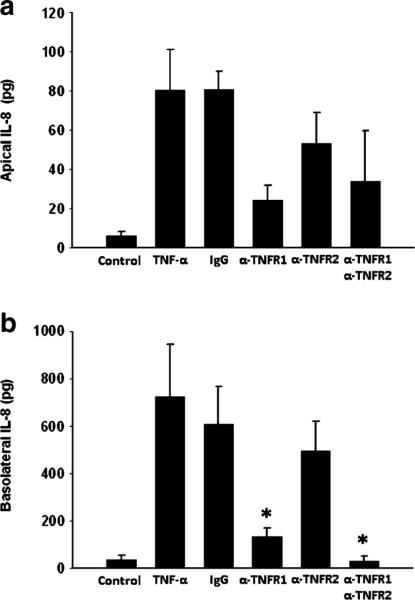
IL-8 production in Caco-2 cells after treatment with TNF-α in the presence of antibodies to TNFR1 or TNFR2 in the basolateral chamber. α-TNFR1 antibody to TNF-α receptor 1 (15 μg/ml), α-TNFR2 antibody to TNF-α receptor 2 (15 μg/ml), IgG non-specific antibody (30 μg/ml). *p<0.05 vs. TNF-α alone
Discussion
In the present study, our data demonstrate that TNF-α-induced IL-8 production in differentiated Caco-2 intestinal epithelia cells is vectorial in nature, with the predominant response directed toward the direction of the stimulus. In addition, both apical and basolateral IL-8 secretion appears to be mediated primarily by TNFR1, while apical secretion appears to involve both TNFR1 and TNFR2. This is important because of the unique arrangement of the intestinal epithelium as part of the innate immune system. Intestinal epithelial cells are sandwiched between the antigen rich contents of the gut lumen and the largest lymphoid organ of the body, the gut-associated lymphoid tissue. Our data suggest that intestinal epithelial cells are capable of responding to proinflammatory stimuli in a nuanced fashion, with directed, vectorial secretion, rather than simply in a binary “on or off” manner.
Bidirectional, vectorial secretion, similar to that seen in the current study, has been observed in other epithelial cells. In a combined in vitro and ex vivo model of ophthalmologic inflammation, IL-6 and IL-8 were secreted in a vectorial manner after treatment with IL-1β. Retinal pigment epithelial (RPE) cells were used, including both ARPE-19 cell cultures and donor RPE cells cultures grown in a dual chamber system. Similar to intestinal epithelial cells, the RPE forms an important barrier layer between body compartments and is also associated with both acute and chronic inflammatory disorders.22 In another study using a model of differential cytokine expression in testicular inflammation and spermatogenesis, primary culture Sertoli cell cultures were shown to exhibit bidirectional, vectorial secretion of IL-1β and IL-6 following treatment with microbial antigens.23 Other examples of vectorial secretion show interesting interactions between an inflammatory or other signal, with a specific directional cytokine or other cellular response.24–29
In this study, we used differentiated, polarized Caco-2 cells as a model of human intestinal epithelium in a dual-chambered culture system. Caco-2 cells were chosen because, when allowed to fully differentiate, they express characteristics similar to mature enterocytes. In dual-chambered systems, Caco-2 cells spontaneously organize into a polarized monolayer with expression of apical tight junctions as evidenced by formation of domes on microscopy.30 Caco-2 cells also have the ability to transport ions in a vectorial manner, one of the crucial functions of in vivo enterocytes. This cell line also develops an apical brush border with associated brush border enzymes such as lactase, sucrase, dipeptidylpeptidase, aminopeptidase, and alkaline phosphatase.30–32 Thus, this cell line provides an ex vivo reductionist model with several relevant in vivo characteristics.
The gut mucosa is somewhat unique in that it receives constant exposure to a high level of endotoxin in the intestinal lumen. In experiments designed to determine if the presence of apical LPS altered TNF-α-induced IL-8 production, we found no evidence to suggest that apical LPS regulates this response. These data suggest that Caco-2 cells are appropriately resistant to LPS stimulation, a classic characteristic of intestinal epithelial cells.33 Additionally, it showed that presence of an apical stimulus did not change the vectorial secretion signaled by treatment with TNF-α.
TEER measurements are traditionally measured as a correlate to level of differentiation, tight junction integrity,34 and monolayer permeability.35 Our experimental conditions did not significantly alter TEER levels from baseline, suggesting that leakage or diffusion of TNF-α or IL-8 is not an alternate explanation for our results. The effects of TNF-α on mediator release was not a global, generalized effect as there was no effect of TNF-α on CD14 release.
TNF-α exerts its effects via two specific cell membrane bound receptors, TNFR1 or TNFR2. Our data show differential TNF-α receptor expression on Caco-2 cells. Apical TNF-α signaling appears to take place via both TNFR1 and TNFR2 receptors, while basolateral TNF-α signaling appears to take place primarily via TNFR1. TNFR1 is commonly found in most tissues, where TNFR2 is typically found in immune cells and is more strictly regulated.36–38 Upon ligand binding, TNFR1 interacts with many complex intracellular signaling factors, including TNF receptor-associated death domain protein and TNF receptor-associated factors (TRAFs), TRAF1 and TRAF2, resulting in potent induction of NF-κB and AP-1 gene expression. TNFR1 also signals apoptosis via its Fas-associated death domain protein.39–41 TNFR2 appears to modulate inflammation via TRAF2 and contains no death domain. TNFR2 also influences TNFR1-related mechanisms, where it can temper or intensify responses. Also, TNFR2 appears to be more prominent in chronic disease states.42 It is clear that differential signaling through one or both of these receptors influences the character of inflammatory response to TNF-α.
The proinflammatory chemokine, IL-8, is crucial to the intestinal response to injury and systemic inflammation. IL-8 is regulated by the transcription factor NF-κB43 and is produced by many cell types including macrophages, endothelial cells, fibroblasts, and various epithelial cell types.44–46 In the intestinal mucosa, IL-8 has been shown to be a potent stimulator of neutrophil recruitment to the lamina propria.47 Though additional signals may be required,48 IL-8 has repeatedly been demonstrated to participate in migration of neutrophils across the intestinal epithelium in response to acute inflammation.49–51 Both inflammatory disorders of the intestine and hypoperfusion due to ischemia or shock may result in damage to the intestinal mucosa. Intestinal restitution, the in vivo response to a mucosal injury, consists of three stages: de-differentiation, migration, and re-differentiation of intestinal epithelial cells. Together, this process leads to healing of mucosal lesions.52 IL-8 has been shown to play a significant role in all three stages of this process via the CXCR1 receptor.53,54 Human intestinal microvascular cells treated with IL-8 show increased chemotaxis, proliferation, and tube formation via the CXCR2 receptor.55
Vectorial secretion of IL-8 may be an important factor in one or more of the above functions. Apical IL-8 released after an apical stimulus may improve the chemotactic gradient and facilitate neutrophil transepithelial migration. Alternatively, a basolateral stimulus resulting in bidirectional IL-8 secretion could result in neutrophil recruitment to the lamina propria and improved epithelial restitution luminally.
Conclusion
In conclusion, our data demonstrated that differentiated, polarized Caco-2 cells respond to TNF-α by vectorially secreting IL-8. This response appeared to be mediated by differential TNF-α receptor expression: TNFR1 basolaterally and TNFR1 and TNFR2 apically. Our findings provide important clues to the mechanism by which the intestinal epithelium regulates local inflammation.
Biography
Discussant
Dr. Edward E. Whang (Boston, MA): Good job with the studies and very clear presentation. TNF-stimulated IL-8 secretion in this cell line is a well-known phenomenon. Your identification of directional asymmetry to this process is intriguing. I will ask you to address issues related to validity and biological significance of these findings.
First, you have suggested but not actually shown differential distribution of TNF receptor subtypes. Have you done these studies?
Second, Caco-2 cells are commonly used to model small intestinal epithelium, but they are colon cancer cells, after all. Are you aware whether normal small bowel enterocytes express TNF receptors and whether they respond to TNF by secreting IL-8? How would you validate that the directionality of TNF-stimulated IL-8 secretion you have reported today exists in normal intestine?
Finally, let us assume you were to figure out the detailed mechanisms responsible for this directional phenomenon. What would you do with that information? What would be the potential biological or clinical significance of this information?
Closing Discussant
Dr. Dennis Sonnier: Thank you very much for your comments and insightful questions. I think that looking at this in an ex vivo setting or in other cell lines would be helpful.
In addition, the IBD literature contains data regarding expression of various inflammatory markers in the stool, and they use this to track disease. This suggests that findings similar to ours make occur in the in vivo setting. IBD patients also show considerable responses to anti-TNF receptor therapy. The presence and function of these receptors on the apical membrane in tissue specimens and animal models is something else we plan on looking at as well.
What do we plan to do with this? Transferring this into a mouse model after we work out some of the cellular mechanisms will be going to be very important for studying various causes of intestinal inflammation as well as possible use in assessment of clinical severity of intestinal inflammation after trauma.
Regarding your questions about receptor expression, I agree that we have not demonstrated receptor expression during these studies. We have performed some initial confocal microscopy studies to help determine receptor expression.
Discussant
Dr. Michael Sarr (Rochester, MN): There are several other enterocyte-like cell models, such as RIE and IEC-6 cells. And they are more from younger animals. Have you thought about looking at those cell lines as well?
Closing Discussant
Dr. Dennis Sonnier: Thank you for your comments and questions. We have not yet looked at those specific cell lines, but these and other cell lines should be considered. We used Caco-2 cells in the current experiments because of their known ability to polarize when differentiated on Transwells.
Discussant
Dr. Michael Sarr (Rochester, MN): Why would a cell secrete something into the lumen when it is polarized. We have been struggling with that, looking at the effects of some hormones that are secreted into the lumen that have an effect. Why does this cell line secrete IL-8 into the lumen?
Closing Discussant
Dr. Dennis Sonnier: Dr. Sarr, that is a great question. By way of pure speculation, I think luminal secretion of cytokines may be a way of autocrine or paracrine control of inflammation. Upstream, intestinal epithelial cells can control a response downstream by the mediators that they secrete into the lumen in a way that cannot be achieved by secreting basolaterally into the bloodstream.
Additional effects of IL-8 specifically related to cell restitution or angiogenesis could also be important. In other words, mucosal healing, I think, could also be affected by mediators from cells upstream.
Discussant
Dr. Carlos Chan (Montreal, Canada): I have one quick question about the slide that you showed regarding LPS treatment on the apical side. As far as I understand, the Caco-2 cells do not express Toll-like receptor 4, which is the receptor for the LPS, although you show that CD14 is expressed. So it may not respond. So have you thought of using other cell lines that you have that expresses Toll-like receptor 4?
Closing Discussant
Dr. Dennis Sonnier: Thank you for your comments and questions. Some investigators have demonstrated TLR4 expression in Caco 2 cells, at least under some conditions, but they generally are hyporesponsive to LPS due to lack of MD2 and other proteins related to LPS signaling.
Discussant
Dr. Carlos Chan (Montreal, Canada): Actually, there are some papers that show there is no receptor and some papers show there is a receptor. Have you shown on your study that these cells that you have actually have Toll-like receptor 4?
Closing Discussant
Dr. Dennis Sonnier: I have not demonstrated that myself, but unpublished data from previous residents in our lab indicate that Caco-2 cells do respond to LPS stimulation if MD2 is added to the treatments.
I would like to thank the Society for the privilege of presenting our data.
Footnotes
Presented at the Society for Surgery of the Alimentary Tract, New Orleans, Louisiana, May 2010
References
- 1.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26(1):85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 2.Diebel LN, Liberati DM. Intestinal epithelial cells mediate lung injury after ethanol exposure and hypoxic insult. J Trauma. 2009;67(2):296–302. doi: 10.1097/TA.0b013e3181ae99d0. [DOI] [PubMed] [Google Scholar]
- 3.Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28(4):384–93. doi: 10.1097/shk.0b013e31805569df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz A, Franklin GA, Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38(12):1336–45. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22(4):382–9. doi: 10.1007/s002689900401. [DOI] [PubMed] [Google Scholar]
- 6.Alverdy JC, Chang EB. The re-emerging role of the intestinal microflora in critical illness and inflammation: why the gut hypothesis of sepsis syndrome will not go away. J Leukoc Biol. 2008;83(3):461–6. doi: 10.1189/jlb.0607372. [DOI] [PubMed] [Google Scholar]
- 7.McCormick BA, et al. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160(1):455–66. [PubMed] [Google Scholar]
- 8.Neal MD, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. J Immunol. 2006;176(5):3070–9. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 9.Reilly N, et al. Probiotics potentiate IL-6 production in IL-1beta-treated Caco-2 cells through a heat shock-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1169–79. doi: 10.1152/ajpregu.00770.2006. [DOI] [PubMed] [Google Scholar]
- 10.Wehkamp J, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72(10):5750–8. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, et al. Recognition of host immune activation by Pseudomonas aeruginosa. Science. 2005;309(5735):774–7. doi: 10.1126/science.1112422. [DOI] [PubMed] [Google Scholar]
- 12.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214(2):149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 13.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10(1):45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 14.Spies M, et al. Role of TNF-alpha in gut mucosal changes after severe burn. Am J Physiol Gastrointest Liver Physiol. 2002;283(3):G703–8. doi: 10.1152/ajpgi.00149.2001. [DOI] [PubMed] [Google Scholar]
- 15.Colombel JF, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362(15):1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 16.Owczarek D, et al. Biological therapy of inflammatory bowel disease. Pol Arch Med Wewn. 2009;119(1–2):84–8. [PubMed] [Google Scholar]
- 17.Appau KA, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn's patients. J Gastrointest Surg. 2008;12(10):1738–44. doi: 10.1007/s11605-008-0646-0. [DOI] [PubMed] [Google Scholar]
- 18.Marehbian J, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastroenterol. 2009;104(10):2524–33. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 19.Tamion F, et al. Gut ischemia and mesenteric synthesis of inflammatory cytokines after hemorrhagic or endotoxic shock. Am J Physiol. 1997;273(2 Pt 1):G314–21. doi: 10.1152/ajpgi.1997.273.2.G314. [DOI] [PubMed] [Google Scholar]
- 20.Onizawa M, et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G850–9. doi: 10.1152/ajpgi.00071.2008. [DOI] [PubMed] [Google Scholar]
- 21.Bocker U, et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int J Colorectal Dis. 2003;18(1):25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 22.Holtkamp GM, et al. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol. 1998;112(1):34–43. doi: 10.1046/j.1365-2249.1998.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cudicini C, et al. Vectorial production of interleukin 1 and interleukin 6 by rat Sertoli cells cultured in a dual culture compartment system. Endocrinology. 1997;138(7):2863–70. doi: 10.1210/endo.138.7.5289. [DOI] [PubMed] [Google Scholar]
- 24.Kruger S, et al. Interleukin-8 secretion of cortical tubular epithelial cells is directed to the basolateral environment and is not enhanced by apical exposure to Escherichia coli. Infect Immun. 2000;68(1):328–34. doi: 10.1128/iai.68.1.328-334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panigrahi P, et al. Escherichia coli transcytosis in a Caco-2 cell model: implications in neonatal necrotizing enterocolitis. Pediatr Res. 1996;40(3):415–21. doi: 10.1203/00006450-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Nasreen N, et al. Polar production of interleukin-8 by mesothelial cells promotes the transmesothelial migration of neutrophils: role of intercellular adhesion molecule-1. J Infect Dis. 2001;183(11):1638–45. doi: 10.1086/320700. [DOI] [PubMed] [Google Scholar]
- 27.Sitaraman SV, et al. Neutrophil–epithelial crosstalk at the intestinal lumenal surface mediated by reciprocal secretion of adenosine and IL-6. J Clin Invest. 2001;107(7):861–9. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vreugdenhil AC, et al. Lipopolysaccharide-binding protein is vectorially secreted and transported by cultured intestinal epithelial cells and is present in the intestinal mucus of mice. J Immunol. 2000;165(8):4561–6. doi: 10.4049/jimmunol.165.8.4561. [DOI] [PubMed] [Google Scholar]
- 29.Zeillemaker AM, et al. Polarized secretion of interleukin-8 by human mesothelial cells: a role in neutrophil migration. Immunology. 1995;84(2):227–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Chantret I, et al. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988;48(7):1936–42. [PubMed] [Google Scholar]
- 31.Grasset E, Bernabeu J, Pinto M. Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol. 1985;248(5 Pt 1):C410–8. doi: 10.1152/ajpcell.1985.248.5.C410. [DOI] [PubMed] [Google Scholar]
- 32.Grasset E, et al. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247(3 Pt 1):C260–7. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 33.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174(8):4453–60. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 34.Duizer E, et al. Absorption enhancement, structural changes in tight junctions and cytotoxicity caused by palmitoyl carnitine in Caco-2 and IEC-18 cells. J Pharmacol Exp Ther. 1998;287(1):395–402. [PubMed] [Google Scholar]
- 35.Li N, et al. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14(7):401–8. doi: 10.1016/s0955-2863(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 36.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–7. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 37.Ferrero E, et al. Roles of tumor necrosis factor p55 and p75 receptors in TNF-alpha-induced vascular permeability. Am J Physiol Cell Physiol. 2001;281(4):C1173–9. doi: 10.1152/ajpcell.2001.281.4.C1173. [DOI] [PubMed] [Google Scholar]
- 38.Orlinick JR, Chao MV. TNF-related ligands and their receptors. Cell Signal. 1998;10(8):543–51. doi: 10.1016/s0898-6568(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 39.Edelblum KL, et al. TNFR1 promotes tumor necrosis factor-mediated mouse colon epithelial cell survival through RAF activation of NF-kappaB. J Biol Chem. 2008;283(43):29485–94. doi: 10.1074/jbc.M801269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heyninck K, Beyaert R. Crosstalk between NF-kappaB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol Cell Biol Res Commun. 2001;4(5):259–65. doi: 10.1006/mcbr.2001.0295. [DOI] [PubMed] [Google Scholar]
- 41.Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43(6):856–60. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holtmann MH, Neurath MF. Differential TNF-signaling in chronic inflammatory disorders. Curr Mol Med. 2004;4(4):439–44. doi: 10.2174/1566524043360636. [DOI] [PubMed] [Google Scholar]
- 43.Vallee S, et al. Cytokine-induced upregulation of NF-kappaB, IL-8, and ICAM-1 is dependent on colonic cell polarity: implication for PKCdelta. Exp Cell Res. 2004;297(1):165–85. doi: 10.1016/j.yexcr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Gross V, et al. Regulation of interleukin-8 production in a human colon epithelial cell line (HT-29). Gastroenterology. 1995;108(3):653–61. doi: 10.1016/0016-5085(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 45.Schroder JM, Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol. 1989;142(1):244–51. [PubMed] [Google Scholar]
- 46.Strieter RM, et al. Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem. 1989;264(18):10621–6. [PubMed] [Google Scholar]
- 47.Kucharzik T, et al. Acute induction of human IL-8 production by intestinal epithelium triggers neutrophil infiltration without mucosal injury. Gut. 2005;54(11):1565–72. doi: 10.1136/gut.2004.061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick BA. Bacterial-induced hepoxilin A3 secretion as a pro-inflammatory mediator. FEBS J. 2007;274(14):3513–8. doi: 10.1111/j.1742-4658.2007.05911.x. [DOI] [PubMed] [Google Scholar]
- 49.Blake KM, et al. Neutrophils migrate across intestinal epithelium using beta2 integrin (CD11b/CD18)-independent mechanisms. Clin Exp Immunol. 2004;136(2):262–8. doi: 10.1111/j.1365-2249.2004.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JM, et al. Nuclear factor-kappa B activation pathway in intestinal epithelial cells is a major regulator of chemokine gene expression and neutrophil migration induced by Bacteroides fragilis enterotoxin. Clin Exp Immunol. 2002;130(1):59–66. doi: 10.1046/j.1365-2249.2002.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kucharzik T, Williams IR. Neutrophil migration across the intestinal epithelial barrier—summary of in vitro data and description of a new transgenic mouse model with doxycyclineinducible interleukin-8 expression in intestinal epithelial cells. Pathobiology. 2002;70(3):143–9. doi: 10.1159/000068146. [DOI] [PubMed] [Google Scholar]
- 52.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31(8 Suppl):S532–7. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 53.Maheshwari A, et al. Effects of interleukin-8 on the developing human intestine. Cytokine. 2002;20(6):256–67. doi: 10.1006/cyto.2002.1996. [DOI] [PubMed] [Google Scholar]
- 54.Sturm A, et al. CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine. 2005;29(1):42–8. doi: 10.1016/j.cyto.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Heidemann J, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278(10):8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]


