Abstract
Introduction
Chronic inflammation disrupts dental pulp regeneration by disintegrating the progenitors recruitment process for repair. Bone marrow-derived mesenchymal stem cells (BMMSC) share the common features with dental pulp stem cells (DPSC). The aim of the study was to investigate the migration of BM-MSC towards DPSC, in response to inflammatory chemoattractants. Additionally, our studies also delineated the signaling mechanisms from BMMSC in mediating the proliferation and differentiation of DPSC, in vitro.
Methods
Human DPSC and BM-MSC between passages 2 and 4 were used and were grown in odontogenic differentiation medium. Mineralization was determined by Alizarin Red staining analysis. Migration was assessed using crystal violet staining in cells grown in Boyden chamber transwell inserts. Mineralization potential of DPSC was evaluated using alkaline phosphatase (ALP) activity assay. Real time PCR analysis was performed to assess the gene expression profile of Cxcl 3, 5, 6, 10, 11, 12, 14, 16, SDF-α, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF).
Results
IFN-γ treatment significantly abrogated the differentiation potential of DPSC, as shown using alizarin red and alkaline phosphatase activity analysis. An increase in the migration of BM-MSC was documented when co-cultured with IFN-γ-treated DPSC. RNA expression studies showed an increased in the levels of Cxcl6 and Cxcl12 in BMMSC when co-cultured with IFN-γ-treated DPSC. Additionally, an upregulation of proangiogenic factors, VEGF and FGF were observed in DPSC exposed to IFN-γ.
Conclusion
Our findings indicate that inflamed IFN-γ-treated DPSC release factors (presumably Cxcl6 and 12) that contribute to the homing of MSC. This model might provide a potential research tool for studying MSC-DPSC cross-talk, and for future studies involving recruitment and sustainability of progenitor stem cells sustaining inflammatory cascade to treat pulp inflammation.
Keywords: Dental pulp Stem Cells, Bone marrow-derived Mesenchymal Stem Cells, Regenerative Endodontics, Migration, Interferon- γ, Inflammation
Introduction
Inflammation and regeneration in dental pulp tissue are commonly observed as different processes, hence have been investigated independently. However, recent lines of evidence indicate that these processes are interrelated. The inflammatory cytokines released from the inflammatory mileu communicate with the inflammatory and stem/progenitor cells (1-4). These signaling mechanisms contribute to the mobilization and secretion of a repertoire of soluble factors with demonstrated cytoprotective and anti-inflammatory properties (5-8). Mainly, these factors signal the circulating stem/progenitor cells to migrate towards the injury site and contribute to tissue healing, which characterizes the proliferative and remodeling phases (9-11). Studies have postulated that the alterations in inflammatory signals exacerbate the normal tissue healing and regeneration phases (12-13).
Dental pulp is often submitted to damage or injury, and in most cases, dental pulp stem cells (DPSC) deposit reparative or tertiary dentin in response to the injury (14-16). DPSC are often referred as the undifferentiated mesenchymal stem cells (MSC) residing in the pulp. During the initial steps of inflammation, in pulp and periapical tissues, MSC are demonstrated to be present in inflamed tissues (17-19). The recruitment of MSC to the injury site facilitates the reparative processes. However, prolonged exposure to inflammation impairs stem cell function and the number of MSC, as demonstrated by Fouad & Huang (20).
MSC have the capacity to receive signals from the inflammatory mileu through the surface markers. MSC are documented to express receptors for a large number of cytokines, such as interleukins (IL)-1, IL-4, IL-6, interferon-γ, and tumor necrosis factor (TNF)-α; growth factors, including vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), tissue-like growth factor (TGF)-β, bone morphogenetic proteins (BMPs); and chemokines (21). Conversely, incessant exposure to these cytokines potentially affects the activity of MSC (22) leading to impairment in the immunomodulatory and anti-inflammatory roles of MSC (23). Additionally, long-term exposure of MSC to inflammatory mediators was demonstrated to suppress the differentiation ability of DPSC (20). Albeit these studies confirmed that inflamed-MSC disintegrate dental pulp regeneration, the signals that DPSC emit to cross-talk with MSC and to facilitate the mobilization of MSC to the injury site is not known. Furthermore, the mediators indeterminant for the cross-talk signaling remains unclear. Hence, to improve the reparative regenerative processes, it is critical that we need to understand the biological signals, released by DPSC, that communicate with MSC in response to inflammatory stimuli. Therefore, the aim of this study is to investigate the cross-talk signaling between DPSC and MSC. We hypothesized that IFN-γ-treated DPSC release chemoattractants that facilitate the mobilization of MSC, a process essential for dental pulp tissue regeneration.
Materials and Methods
Culture of human DPSC
DPSC were obtained from healthy permanent premolars extracted during orthodontic treatment, were generously donated by Dr. Songtao Shi, USC (16). The single cell suspensions were cultured in αMEM (Gibco), supplemented with 20% FBS (Hyclone, UT, USA), 1% Antibiotic-antimycotic (Gibco), Odontogenic medium supplemented with 100μM/ml ascorbic acid, 2mM β-glycerophosphate, and 10mM dexamethasone. DPSC were incubated at 37°C with 5% CO2. DPSC between 3rd and 5th passages were used throughout the study.
Alizarin red staining
DPSC seeded onto 12-well plates (1 × 104 cells per well) were subjected to alizarin red staining at day 14. Briefly, the cells were fixed in 4% paraformaldehyde for 20 min, then stained using alizarin red (Sigma-Aldrich). The phase contrast images were then captured for analysis using EVOS® FL Cell Imaging System.
BrdU Incorporation Assay
For proliferation studies, DPSC were cultured to approximately 50% confluence in 96-well plates (BD Bioscience). At the end of treatment period, cells were starved overnight in low-serum media, followed by an 18-hour pulse with 10 μM 5-bromo-2’-deoxyuridine (BrdU) in EBCM from different time points as well as control media. After the 18-hour pulse, cells were rinsed with PBS and fixed in 70% ethanol with 2M HCl for 10 minutes at room temperature, then rinsed in PBS at least three times. The cell lysates were then measured at excitation: 450 nm and emission: 595 nm using ELISA plate reader (Thermo Scientific, USA). The magnitude of the absorbance for the developed color is proportional to the quantity of BrdU incorporated into DPSC, which serves as a direct indication of cell proliferation.
Transwell Migration Assay
Cultured DPSC were grown on the lower compartment of the 6-well plates, while MSC were grown on the upper compartment Transwell inserts. At 3 days after the initiation of the culture conditions, the upper compartment (8 μm pore size insert) seeded with MSC were placed on to the well by merging the bottom of insert into the medium in the lower compartment. The cells in the Transwell plate were incubated at 37°C and 5% CO2. Seventy two hours after incubation the Transwell insert was carefully removed and the cells that did not migrate through the pores, on the upper side of the filter membranes, were gently removed with a cotton swab. Cells on the lower side of the insert filter were then quickly fixed (using 5% glutaraldehyde, for 10 min) and stained with 1% Crystal Violet for 20 minutes. The time points were averaged for a total quanitification from three independent experiments.
Reverse-Transcription and Real-Time PCR Analysis
DPSC treated with IFN-γ (500 U/ml) were washed and total RNA was isolated with 1 ml TriZol. The isolated RNA was analyzed using a Fisher Scientific NanoDrop 2000 for ng/ul and 260/280 readings used to standardize the samples to 10ng/ul with RNAse free water. A 1 μg of total RNA was used to synthesize complimentary DNA by the high capacity cDNA reverse transcription kit®. The resultant cDNA product was combined with SYBR® green, the SuperScript® one-step rtPCR system with Platinum® Taq kit & GAPDH, VEGF, FGF and ALP primers (Table 1) designed through IDT DNA.
Table 1.
The Human Primer Sequences used for Real-Time PCR
| Gene | Primers |
|---|---|
| GAPDH | For: GGCATCCACTGTGGTCATGAG |
| Rev: TGCACCACCAACTGCTTAGC | |
| VEGF | For: CAAAAACGAAAGCGCAAGAAA |
| Rev: GCGGGCACCAACGTACAC | |
| B-FGF | For: GGCTTCTTCCTGCGCATCCA |
| Rev: GCTCTTAGCAGACATTGGAAGA | |
| Cxcl2 | For: CGCCCAAACCGAAGTCATAG |
| Rev: AGACAAGCTTTCTGCCCATTCT | |
| Cxcl3 | For: TCCCCCATGGTTCAGAAAATC |
| Rev: GGTGCTCCCCTTGTTCAGTATC | |
| Cxcl5 | For: GCATTTCTGTTGCTGTTCACGCTG |
| Rev: CCTCCTTCTGGTTTTTCAGTTTAGC | |
| Cxcl6 | For: TGGGCCTGATCCTTGTTGCGC |
| Rev: GCACCGTTTTTTGTCCATTCTTCAG | |
| Cxcl10 | For: GAACTGTACGCTGTACCTGCA |
| Rev: TTGATGGCCTTCGATTCTGGA | |
| Cxcl11 | For: ATGAGTGTGAAGGGCATGGC |
| Rev: TCACTGCTTTTACCCCAGGG | |
| Cxcl12 | For: TGCCAGAGCCAACGTCAAG |
| Rev: CAGCCGGGCTACAATCTGAA | |
| Cxcl14 | For: CAGGTCGACATGAGGCTCCTGGCGGCCGCG |
| Rev: CGGGGATCCCTATTCTTCGTAGACCCTGCG | |
| Cxcl16 | For: TCTCAAAGAATGTGGACATGC |
| Rev: CAGGGGTGTGGATATCTGAA | |
| SDF-1α | For: GGGGGAATTCCATGAACGCCAA |
| Rev: GGGGTCTAGAGGGCATGGATGAAT |
Statistical Analysis
Comparisons among relative expression levels of genes across the cell lineages were assessed and the experimental values were reported as mean ± standard deviation. The crystal violet positive cells and Alizarin Red nodules were manually quantified with the mean ± standard deviation reported. P<0.05 was considered statistically significant.
Results
IFN-γ treatment impairs DPSC mineralization and differentiation
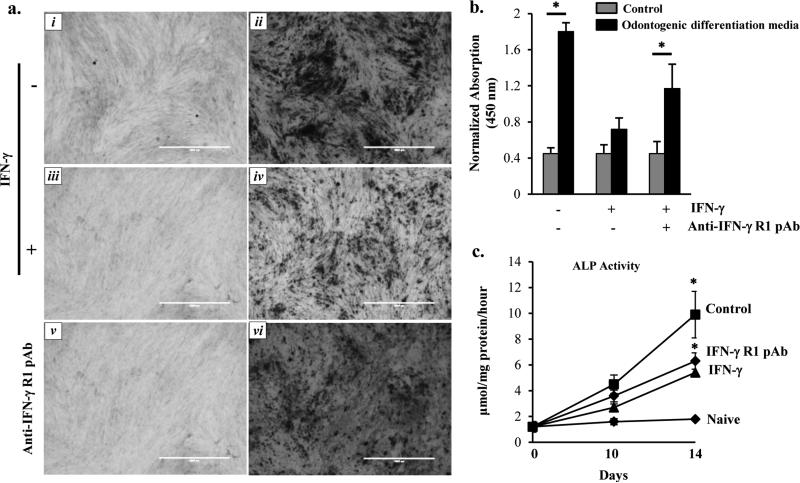
Studies have demonstrated that an early inflammatory reaction is a protective mechanism in pulp cells (12). However, prolonged inflammation deteriorates the mineralization and regenerative potential of pulp (24). We tested whether MSC restore the differentiation potential of DPSC following its exposure to IFN-γ. In order to do that, DPSC from passages 2 and 4 were grown in the odontogenic differentiation medium in the absence or presence of recombinant IFN-γ 500 U/mL (PeproTech, Rocky Hill, NJ). At after 14 days, the cells were analyzed for alizarin red (ALR) staining. DPSC grown in odontogenic differentiation medium containing ascorbic acid, β-glycerophosphate, and dexamethasone showed extensive ALR-positive calcified deposits in control cells. However, IFN-γ treatment markedly reduced the mineralized deposits (Fig. 1a, panel iii and iv, and 1b). To substantiate our findings, DPSC pretreated with IFN-γ-Receptor polyclonal antibody (IFN-γ-R pAb, 200 ng/ml) significantly enhanced the mineralization (Fig. 1a, panels v and vi). To further validate our hypothesis, we also performed alkaline phosphatase (ALP) activity assay. As shown in Fig. 1c, IFN-γ treatment significantly decreased the activity of ALP on day 14, which was ameliorated when pretreated with IFN-γ-R pAb.
Figure 1. Conditioned Media collected from MSC preserve DPSC mineralization and differentiation.
(a) Alizarin Red staining of the 14-day cultures with images captured for differentiation analysis at 40X. The panels are labelled as follows; (i) naïve, (ii) Odontogenic medium alone, (iii) Odontogenic medium + IFN-γ, (iv) Odontogenic medium + IFN-γ + CM MSC, (v) naïve + anti-IFN-γ R pAb, and (vi) Odontogenic differentiation media + anti-IFN-γ-R-pAb. (b) Quantitative analysis of the absorption of ALR at 450 nm, spectrophotometrically. (c) Alkaline phosphatase (ALP) activity of DPSC following treatment with IFN-γ in the absence or presence of IFN-γ-R pAb at day 0, 10, and 14. The data shown are the representative of three independent experiments. * indicates p<0.05.
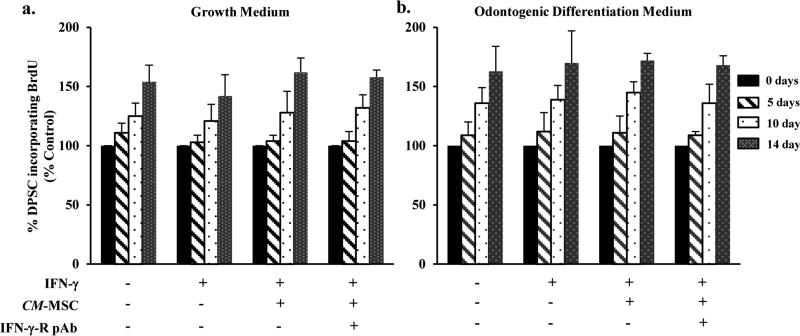
Proliferation of DPSC remain unaltered upon exposure to IFN-γ
To investigate whether IFN-γ treatment altered the proliferation potential of DPSC, we performed nonradioactive BrdU incorporation assay. DPSC challenged with IFN-γ for 0, 5, 10, and 14 days were labeled with BrdU (3 μg/ml). While DPSC exhibited an increase in the proliferation, time dependently, we failed to observe a significant difference when treated without or with IFN- γ (Fig. 2a and 2b). To examine whether MSC contribute to the proliferation potential of DPSC, we cultured DPSC with the conditioned media collected from MSC (CMMSC). Our findings suggest that treatment with CM-MSC showed no significant increase in the rate of proliferation when compared to cells treated IFN-γ alone.
Figure 2. IFN-γ does not alter DPSC proliferation.
BrdU incorporation assay of DPSC treated with IFN-γ in the absence (a) or presence (b) of odontogenic differentiation media. The incorporation of BrdU was measured spectrophotometrically at absorbance 490 nm at DPSC at different time points (5, 7, and 14 days). Note: No significant difference between cells treated with IFN-γ alone or along with CM-MSC.
IFN-γ-induced upregulation of angiogenic signaling factors in DPSC, enhances MSC migration
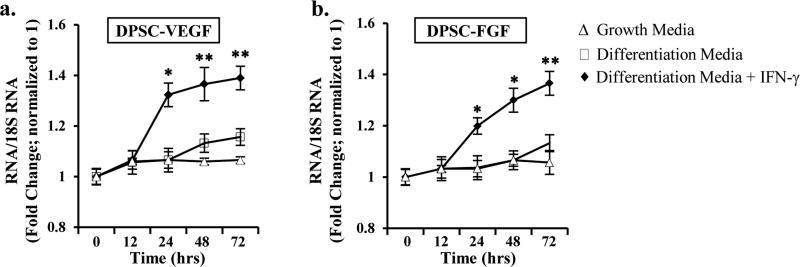
To determine the mechanisms by which IFN-γ treated DPSCs, communicate with MSC, we examined the expression of VEGF and FGF in DPSC either in the absence or presence of IFN-γ. As shown in Fig. 3, DPSC treated with IFN-γ for varying time points (0, 12, 24, 48, and 72 hours) showed an upregulation of both VEGF (Fig. 3a) and FGF RNA (Fig. 3b), with a significant increase observed at after 24 hours. Furthermore, to examine whether IFN-γ-mediated increase in angiogenic signaling factors in DPSC attract MSC, we performed Transwell migration assay.
Figure 3. IFN-γ-induced upregulation of VEGF and FGF in DPSC.
Real time PCR analysis of (a) VEGF, and (b) FGF RNA showing an upregulation upon IFN-γ treatment at 0, 12, 24, 48, and 72h. The Ct values are normalized to 18S RNA. The results shown are representatives of four independent experiments. * indicates p<0.05, and ** indicates p<0.001.
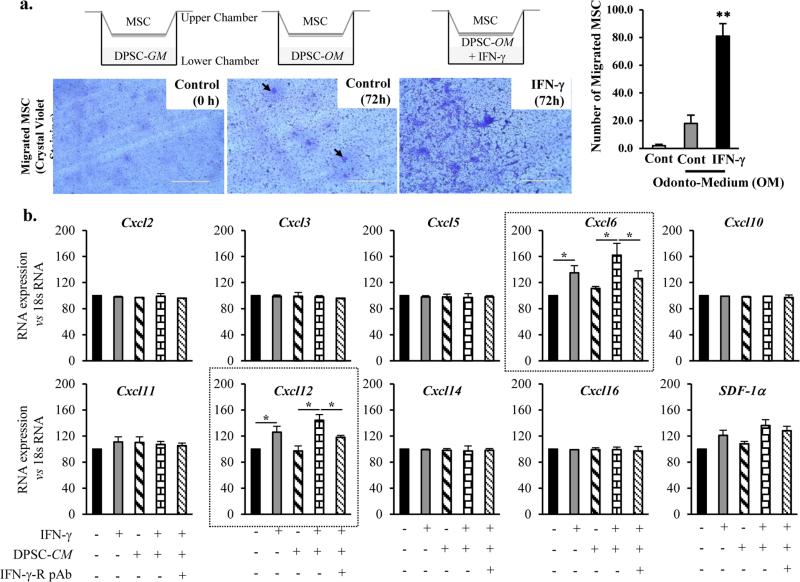
DPSC (0.5 × 105 cells) grown on the lower chamber in the presence of odontogenic differentiation medium (OM) were treated with IFN-γ (500 U/Ml). At 48 hours, the upper chamber was plated with MSC (0.2 × 104 cells). At 72 hours after incubation, the upper chamber was removed and the lower portion of the insert filter was employed for Crystal violet staining analysis. The number of cells stained was counted to determine the number of migrated MSC (Fig. 4a). An approximately 3-fold increase in the number of crystal violet-stained MSC (39.7 ± 7) were observed in conditions where DPSC were treated with IFN-γ, whereas the number of crystal violet-stained MSC were significantly less in DPSC treated with media alone (13.6 ± 2). Furthermore, we also examined the factors that are essential for the homing of MSC and that are crucial to rescue the effects of IFN-γ on DPSC. In order to do this, we performed RNA expression analysis of the members of Cxcl family (Cxcl2, 3, 5, 6, 10, 11, 12, 14, 16, and SDF-1α). These studies revealed an upregulation of Cxcl6 and Cxcl12 upon treatment with IFN-γ and/or the conditioned medium collected from DPSC culture (DPSC-CM) (Fig. 4b).
Figure 4. IFN-γ-treated DPSC mediates MSC migration.
(a) Transwell migration assay showing an increase in the crystal violet staining of the upper chamber (transwell insert). Increase in the number of crystal violet staining depicts the number of migratory cells. Arrows indicates the migrated cells. The images shown are the representatives of five independent experiments. ** indicates p<0.001. Real time PCR analysis showing the differential expression of Cxcl members of the family including Cxcl2, 3, 5, 6, 10, 11, 12, 14, 16, and SDF-1α. Note a significant increase in the RNA levels of Cxcl6 and Cxcl12 in the presence of IFN-γ and/or conditioned media from DPSC (DPSC-CM).
Discussion
During inflammatory episodes, dental pulp is more sensitive to changes in tissue pressure and requires an active drainage system to eliminate excess fluid and macromolecular substances. This effective system plays a crucial role in the repair and wound healing processes (25). The pulpal healing potential is associated with the ability of dental pulp cells to secrete growth factors, including angiogenic factors (e.g. VEGF. FGF, platelet-derived growth factor (PDGF), tissue-like growth factor (TGF), epidermal growth factor (EGF), bone morphogenetic proteins (BMPs) (22, 26). These different factors can influence either sides of a virtual balance, i.e. favor the persistence of inflammation on one side or the wound healing on the other. Accumulation of inflammatory cells at the site of injury also releases large number of cytokines (e.g. IL-1, IL-4, IL-6, TNF-α, IFN-γ) and alter the mechanical properties via cytoskeletal reorganization (27).
Bone marrow-derived MSC are commonly reported to be seen in peripheral blood, in the circulatory system (28). MSC express several surface markers that demonstrate their ability to interact with the immune system, hematopoietic cells, and immune cells. Furthermore, MSC were shown to have the capacity to receive signals from the inflammatory mileu (22) and respond to some of these signals. MSC are demonstrated to mobilize to the site and play an immunomodulatory role to alleviate the inflammatory challenges. MSC are often utilized in clinical application in humans for the treatment of graft-versus-host-disease (GVHD) (29, 30). However, during dental pulp inflammation, whether DPSC attract MSC to the injury or inflamed site is not known.
In our study, we examined the mechanisms by which inflamed-DPSC attract the mobilization of MSC. As observed in our findings, long term exposure to IFN-γ significantly abrogated the mineralization and differentiation potential of DPSC. Blocking the signaling mechanisms pertaining to IFN-γ using IFN-γ R pAb validated our findings that IFN-γ plays a significant role in the deterioration of the differentiation and mineralization potential of DPSC. Additionally, our findings also reveal that IFN-γ affects only the mineralization and/or differentiation potential of DPSC, while the proliferation of DPSC remains unaltered. These observations suggest that although DPSC failed to show any changes in the proliferation, it is likely that they undergo phenotypic alterations with an increased angiogenic potential.
Using Transwell migration assay, we observed an increase in the migration of MSC when exposed to the IFN-γ-treated DPSC. In parallel, an upregulation of Cxcl6 and Cxcl12 RNA levels in response to IFN-γ and/or DPSC-CM treatment suggests the possibility that these factors might play a possible role in inducing the migration of MSC. However, treatment with the conditioned medium obtained from MSC culture (for 14 days) restored the mineralization and differentiation potential of DPSC. These findings suggest that MSC disseminate soluble factors, which may plan as an important component of the repair mechanisms and healing processes following pulp injury. Therefore, our studies are to implicate that inhibiting or blocking the growth factors may potentially be a therapeutic target for dental tissue pulp regeneration.
In summary, within the limitation of this study, it is imperative that growth factors or immune factors produced in the early inflammatory reactions may attract MSC to the site. Recruitment or migration of MSC may serve as an important mechanism for host defense, wound healing, and tissue repair.
ACKNOLEDGEMENTS
This study is supported by Grant NIH/NIDCR grant (DE019514-SBA) to SA and American Association of Endodontists Foundation (AAEF) to SA and PS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors deny any conflicts of interest related to this study.
References
- 1.Németh K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemeda H, Jakob M, Ludwig AK, et al. Interferon-g and tumor necrosis factor-a differentially affect cytokine expression and migration properties of mesenchymal stem cells. Stem Cells Dev. 2010;19:693–706. doi: 10.1089/scd.2009.0365. [DOI] [PubMed] [Google Scholar]
- 3.Ip JE, Wu Y, Huang J, et al. Mesenchymal stem cells use integrin b1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang H, Kim HJ, Chang EJ, et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 2009;16:1332–1343. doi: 10.1038/cdd.2009.74. [DOI] [PubMed] [Google Scholar]
- 5.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 6.Matthay MA, Goolaerts A, Howard JP, et al. Mesenchymal stem cells for acute lung injury: preclinical evidence. Crit Care Med. 2010;38:S569–S573. doi: 10.1097/CCM.0b013e3181f1ff1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W, Ren G, Huang Y, et al. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cárdenes N, Cáceres E, Romagnoli M, et al. Mesenchymal stem cells: a promising therapy for the acute respiratory distress syndrome. Respiration. 2013;85:267–278. doi: 10.1159/000347072. [DOI] [PubMed] [Google Scholar]
- 9.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Zhu F, Zhang M, et al. Stromal cell-derived factor-1 enhances wound healing through recruiting bone marrow-derived mesenchymal stem cells to the wound area and promoting neovascularization. Cells Tissues Organs. 2013;197:103–113. doi: 10.1159/000342921. [DOI] [PubMed] [Google Scholar]
- 11.Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 12.Saika S, Ikeda K, Yamanaka O, et al. Loss of tumor necrosis factor alpha potentiates transforming growth factor beta-mediated pathogenic tissue response during wound healing. Am J Pathol. 2006;168:1848–1860. doi: 10.2353/ajpath.2006.050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinozaki M, Okada Y, Kitano A, et al. Impaired cutaneous wound healing with excess granulation tissue formation in TNFalpha-null mice. Arch Dermatol Res. 2009;301:531–537. doi: 10.1007/s00403-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 14.Cooper PR, Takahashi Y, Graham LW, et al. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. 2010;38:687–697. doi: 10.1016/j.jdent.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi S, Robey P, Gronthos S. Comparison of human dental pulp and bone marrow stromal cells by cDNA microarray analysis. Bone. 2001;29:532–39. doi: 10.1016/s8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg M, Farges JC, Lacerda-Pinheiro S, et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res. 2008;58:137–47. doi: 10.1016/j.phrs.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alongi DJ, Yamaza T, Song Y, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regenerative Medicine. 2010;5:617–31. doi: 10.2217/rme.10.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao J, Al Shahrani M, Al-Habib M, et al. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod. 2011;37:1217–24. doi: 10.1016/j.joen.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fouad AF, Huang GT. Inflammation and immunological responses. In: Ingle J, Bakland LK, Baumgartner JC, editors. Ingle's endodontics Hamilton. 6th ed. BC Decker Inc; Ontario:Canada: 2008. pp. 343–75. [Google Scholar]
- 21.Docheva D, Haasters F, Schieker M. Mesenchymal stem cells and their cell surface receptors. Curr Rheum Rev. 2008;4:155–160. [Google Scholar]
- 22.Tran-Hung L, Laurent P, Camps J, et al. Quantification of angiogenic growth factors released by human dental cells after injury. Arch Oral Biol. 2008;53:9–13. doi: 10.1016/j.archoralbio.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–61. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 24.Min KS, Kwon YY, Lee HJ, et al. Effects of proinflammatory cytokines on the expression of mineralization markers and heme oxygenase-1 in human pulp cells. J Endod. 2006;32:39–43. doi: 10.1016/j.joen.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Boyle M, Chun C, Strojny C, Narayanan R, Bartholomew A, Sundivakkam P, Alapati S. Chronic inflammation and angiogenic signaling axis impairs differentiation of dental pulp stem cells. PLoS One. 2014;9:e113419. doi: 10.1371/journal.pone.0113419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006;85:819–23. doi: 10.1177/154405910608500908. [DOI] [PubMed] [Google Scholar]
- 27.Jones TD, Naimipour H, Sun S, Cho M, Alapati SB. Mechanical Changes in Human Dental Pulp Stem Cells during Early Odontogenic Differentiation. J Endod. 2015 Jan;41(1):50–5. doi: 10.1016/j.joen.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joensuu K, Paatero I, Alm JJ, et al. Interaction between marrow-derived human mesenchymal stem cells and peripheral blood mononuclear cells in endothelial cell differentiation. Scand J Surg. 2011;100:216–22. doi: 10.1177/145749691110000314. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 30.Kebriaei P, Robinson S. Mesenchymal stem cell therapy in the treatment of acute and chronic graft versus host disease. Front Oncol. 2011;4:1–16. doi: 10.3389/fonc.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]