Abstract
We recently found that sAPPα decreases Aβ generation by directly associating with β-site amyloid precursor protein (APP) converting enzyme 1 (BACE1), thereby modulating APP processing. Because inhibition of BACE1 decreases GSK3β-mediated Alzheimer’s disease (AD)-like tau phosphorylation in AD patient-derived neurons, we determined whether sAPPα also reduces GSK3β-mediated tau phosphorylation. We initially found increased levels of inhibitory phosphorylation of GSK3β in primary neurons from sAPPα over-expressing mice. Further, recombinant human sAPPα evoked the same phenomenon in SH-SY5Y cells. Further, in SH-SY5Y cells overexpressing BACE1, and HeLa cells overexpressing human tau, sAPPα reduced GSK3β activity and tau phosphorylation. Importantly, the reductions in GSK3β activity and tau phosphorylation elicited by sAPPα were prevented by BACE1 but not γ-secretase inhibition. In accord, AD mice overexpressing human sAPPα had less GSK3β activity and tau phosphorylation compared with controls. These results implicate a direct relationship between APP β-processing and GSK3β-mediated tau phosphorylation and further define the central role of sAPPα in APP autoregulation and AD pathogenesis.
Keywords: Alzheimer’s disease, sAPPα, tau, BACE1, GSK3β
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by accumulation in the brain of plaques composed of amyloid-beta (Aβ) proteins and neurofibrillary tangles (NFT) composed of abnormally phosphorylated tau (Hardy and Selkoe, 2002; Selkoe, 2001). Aβ is produced by a proteolytic pathway whereby amyloid precursor protein (APP) is cleaved first by β-site APP-converting enzyme (BACE1) yielding β-carboxy terminal fragment (β-CTF) and a large secreted amino N-terminal fragment called sAPPβ. Finally, β-CTF is further cleaved by a γ-secretase complex generating Aβ peptide (Vassar et al., 1999). On the other hand, under physiological conditions the majority of APP is processed by the non-amyloidogenic pathway mediated by α-secretase cleavage, yielding the C-terminal fragment α-CTF and secretion of the N-terminal fragment sAPPα (Kimberly et al., 2003; Lefort et al., 2012). Found to have neurotrophic and neuroprotective properties, as well as the ability to enhance learning and memory (Copanaki et al., 2010; Corrigan et al., 2012; Gralle et al., 2009), sAPPα is largely considered to have significant therapeutic potential (Mattson, 1997).
The notion that brain accumulation of Aβ is the major influence that promotes the cascade of pathogenic events leading to tau alterations and neuronal death and dysfunction as the final common pathway in AD, has become the leading hypothesis (Hardy and Higgins, 1992). On the other hand, the causal link between amyloidogenic APP processing, and tau alterations in AD are far from fully characterized. Further, autopsy data from AD patients who underwent experimental Aβ immunization revealed a significant decrease in amyloid deposition in certain brain areas but also indicated the presence of additional damage including tau pathology that may be irreversible by attacking only Aβ (Ferrer, 2004; Smith et al., 2003).
Thus we hypothesized there is some direct pathogenic link between not necessarily Aβ plaques themselves and tau, but amyloidogenic APP processing and promotion of tau. Our prior findings indicated that sAPPα can inhibit BACE1 activity and promote nonamyloidogenic processing of APP (Obregon et al., 2012). Recent investigations utilizing inducible pluripotent stem cell-derived neurons from AD patients further suggested that inhibition of BACE1, but not γ-secretase, decreases glycogen synthase kinase-3 (GSK3β)-mediated Alzheimer-like tau phosphorylation as well (Israel et al., 2012). Therefore, amyloidogenic APP processing by BACE1 may be the critical mediator between AD-like amyloidogenic APP processing and tau phosphorylation pathways.
With this in mind, inhibition of the enzyme GSK3, a ubiquitous serine/threonine kinase that regulates an array of fundamental cell processes, is becoming a promising therapeutic strategy in AD for several reasons. First, GKS3β isoform is required for AD-type abnormal hyperphosphorylation of tau (Sereno et al., 2009). Indeed overexpression of GSK3β yields tau hyperphosphorylation and disrupted microtubules in transgenic mice (Lucas et al., 2001). Second, in vitro studies indicate activation of GSK3α promotes amyloidogenic APP processing and Aβ production (Phiel et al., 2003). Third, in vitro and in vivo overexpression of GSK3 has been shown to promote apoptotic neuronal cell death (Beurel and Jope, 2006; Bhat et al., 2000; Hetman et al., 2000; Lucas et al., 2001). Finally, transgenic mice overexpressing GSK3β exhibited impaired spatial memory and long-term potentiation (Hernandez et al., 2002).
The present study was undertaken to further determine the effect of sAPPα on GSK3β-mediated tau phosphorylation. We found sAPPα reduced GSK3β activity, as indicated by the level of inhibitory GSK3β Ser9 phosphorylation in primary neuronal cultures from sAPPα overexpressing PSAPP mice as well as in the human neuroblastoma cell line, SH-SY5Y. In SH-SY5Y cells overexpressing BACE1 (SH-SY5Y/BACE1) and HeLa cells overexpressing human tau (HeLa/tau), sAPPα additionally reduced tau phosphorylation, as shown by phospho-tau (Thr231) and PHF1 tau (Ser396/Ser404) immunodetection. Importantly, the sAPPα-mediated reductions in GSK3β activity and tau phosphorylation were prevented by a specific BACE1 (LY2886721) but not γ-secretase inhibitor (DAPT). Moreover, a transgenic-mouse model of AD (PSAPP) also had lower levels of GSK3β activity and tau phosphorylation when also overexpressing sAPPα. These results bolster the hypothesis of a more direct link between BACE1 amyloidogenic processing and GSK3β-mediated tau phosphorylation, while reducing the importance of Aβ itself, and further define the central role of sAPPα in APP autoregulation and AD pathogenesis.
Methods
Reagents and antibodies
PHF1 antibody was kindly provided by Dr. Peter Davies (Albert Einstein College of Medicine, Bronx, NY). Additional antibodies were employed against phospho-tau (Thr231, Millipore 1:1000), total tau (1:1000; Cell Signaling), phospho-GSK3β (Ser9) (1:1000; Cell Signaling) and total GSK3β, 1:1000; Cell Signaling). Recombinant sAPPα was generated and purified as previously described (10). Both γ-secretase (N-[N-(3,5-difluorophenacetyl)-1-alanyl]-S-phenylglycine t-butyl ester, DAPT, EMD Biosciences, La Jolla, CA) and BACE1 inhibitors (N-[3-[(4aS,7aS)-2-amino-4,4a,5,7-tetrahydrofuro[3,4-d][1,3]thiazin-7a-yl]-4-fluorophenyl]-5 fluoropyridine-2-carboxamide. LY2886721, APExBIO]) were used at a final concentration 200 nM.
Mice
All mice were housed and maintained in the Morsani College of Medicine Animal Facility at the University of South Florida (USF), and all experiments were conducted in compliance with protocols approved by the USF Institutional Animal Care and Use Committee. Eight-month-old doubly transgenic “Swedish” APPK595N/M596L (APPswe) + PS1ΔE9 B6C3-Tg 85Dbo/J strain (PSAPP mice) were purchased from the Jackson Laboratory (Bar Harbor, ME). Triple transgenic PSAPP/TgsAPPα mice were generated and genotyped as described previously (Rezai-Zadeh et al., 2005).
Primary Neuronal Isolation and Culture
Primary cortical neurons were isolated from E14 embryos from C57BL/6 wild-type mice (Jackson Labs, Bar Harbor, MA). Mouse embryonic brain tissues were mechanically dissociated and cultured as previously described (Zhu et al., 2011). Primary neuronal cells were cultured in suspension in DMEM/F12 (Invitrogen) containing B27 (Invitrogen), 20 ng/mL human epidermal growth factor (hEGF) and 10 ng/mL fibroblast growth factor (FGF) at 37°C in 5% CO2. For differentiation, primary neuronal cells were mechanically dissociated, filtered with a 40 µm cell strainer into single-cell suspensions and plated in 24-well plates (Fisher) at 100,000 cells per well. The cells were incubated at 37°C in DMEM/F12 containing B27, 10% fetal bovine serum and 5% CO2.
Cell lines and cell culture
SH-SY5Y cells and SH-SY5Y cells stably expressing BACE1 were gifts from Dr. Wataru Araki (National Institute of Neuroscience, Tokyo, Japan). HeLa cells stably transfected with wild type 4R0N human tau were a gift from Dr. Chad A. Dickey (University of South Florida, USA). These cells were cultured as described previously (Abisambra et al., 2012; Jinwal et al., 2009; Motoki et al., 2012; Murayama et al., 2006). Cells were plated in 24-well plates at a concentration of 100,000 cells per well and after overnight incubation treated with sAPPα at 0–25 nM for 12 h. SH-SY5Y/BACE1 cells were also treated with sAPPα (25 nM) plus a BACE1 (LY2886721, 200 nM) or γ-secretase inhibitor (DAPT, 200 nM) for 12 h.
Tissue preparation
Mice were euthanized with isoflurane anesthesia and then transcardially perfused with ice-cold phosphate-buffered saline (PBS). Brains were rapidly isolated, and one hemisphere was frozen immediately in liquid nitrogen and stored at −80 °C. For molecular analysis, brain hemispheres were sonicated in RIPA buffer (Cell Signaling Technology, Danvers, MA, USA) and centrifuged at 14,000 rpm for 1 h at 4 °C. Supernatant was transferred to a new tube for Western blot analysis of tau and GSK3β. The other hemisphere was placed in 4% paraformaldehyde for cryostat sectioning. The 25-µm free-floating coronal sections were collected and stored in PBS with 100 mM sodium azide in 24-well plates at 4 °C.
Western blot analysis
Cultured cells were lysed in ice-cold lysis buffer as described previously (Tan et al., 2002). All antibodies were diluted in TBS containing 5% (w/v) nonfat dry milk. Blots were developed using the Luminol reagent (Thermo Fisher Scientific, Waltham, MA). Densitometric analysis was performed as described previously (Rezai-Zadeh et al., 2005) using a FluorS Multiimager with Quantity One software (Bio-Rad, Hercules, CA).
Immunohistochemistry
Brain tissues from PSAPP and PSAPP/TgsAPPα mice were fixed in paraformaldehyde for cryostat sectioning. Briefly, we sectioned five coronal sections per region with a 100-µm interval and a thickness of 15-µm for retrosplenial cortex (RSC), entorhinal cortex (EC), and hippocampus (H). Immunohistochemical staining was conducted according to the manufacturer’s protocol using a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) coupled with the diaminobenzidine reaction, except that the biotinylated secondary antibody step was omitted. A biotinylated anti-phospho-tau (Thr231) antibody (1:500, Cell Signaling) was used as a primary antibody. Images at were acquired as digitized tagged-image format files (to retain maximum resolution) using a BX60 bright field microscope with an attached CCD camera system (DP-70, Olympus, Tokyo, Japan), and digital images were routed into a Windows PC for quantitative analyses using SimplePCI software (Hamamatsu Photonics, Hamamatsu, Shizuoka, Japan). We captured images of five 15-µm sections through each anatomic region of interest (RSC, EC, and H) based on anatomical criteria defined by Franklin and Paxinos, and obtained a threshold optical density that discriminated staining from background. Each anatomic region of interest was manually edited to eliminate artifacts. Selection bias was controlled for by analyzing each region of interest in its entirety.
Statistical analysis
All data were normally distributed; therefore, in instances of single mean comparisons, Levene’s test for equality of variances followed by the t-test for independent samples were used to assess significance. In instances of multiple mean comparisons, one-way analysis of variance (ANOVA) was used. Alpha was set at 0.05 for all analyses. The statistical package for the social sciences release IBM SPSS 18.0 (IBM, Armonk, NY) was used for all data analyses.
Results
sAPPα dose-dependently reduces GSK3β activity in primary differentiated neurons and SH-SY5Y cells
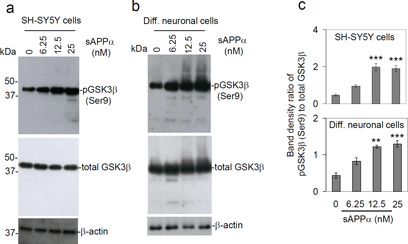
Our previous studies indicate that sAPPα can inhibit BACE1-mediated β-secretase activity and thereby decrease Aβ generation and amyloid pathology in cells and mouse models of AD (Obregon et al., 2012). In addition, Moore et al. (2015) found that BACE1 inhibitors reduced whereas γ-secretase inhibitors increased intracellular tau protein and its phosphorylation. While inhibition of β-secretase may reduce GSK3β-mediated tau phosphorylation (Israel et al., 2012), the effect of sAPPα on this process is not yet known. To determine if sAPPα reduces GSK3β activity, primary differentiated murine neurons and SH-SY5Y human neuroblastoma cells were treated with human sAPPα (0–25 nM) for 12 h and then cell lysates were prepared for Western blot (WB) analysis of inhibitory GSK3β (Ser9) phosphorylation. sAPPα indeed significantly increased GSK3β (Ser9) phosphorylation in a dose-dependent manner in both cell types (Fig. 1), indicating that sAPPα reduces GSK3β activity.
Fig. 1.
Treatment with sAPPα dose-dependently increases GSK3β (Ser9) phosphorylation in SH-SY5Y cells and primary differentiated neuronal cells. (a) Human neuroblastoma (SH-SY5Y) cells and (b) murine primary differentiated neuronal cells (Diff. neuronal cells) were treated with sAPPα at the indicated concentrations for 12 h. Cell lysates were prepared and subjected to Western blotting (WB) analysis with a specific anti-phospho-GSK3β (Ser9) antibody and a total GSK3β antibody. As shown, phosphorylated GSK3β (Ser9) [pGSK3β (Ser9)] was notably elevated following sAPPα treatment in both SH-SY5Y and primary neuronal cells. (c) Densitometry analysis shows the band density ratio of pGSK3β (Ser9) to total GSK3β. pGSK3β (Ser9) and total GSK3β WB results are representative of three independent experiments. A t-test revealed significant difference in the ratio of pGSK3β (Ser9) to total GSK3β for both SH-SY5Y cells and differentiated neuronal cells treated with either 6.25, 12.5 or 25 nM sAPPα compared to control (0 nM). In addition, the levels of pGSK3β (Ser9) and total GSK3β did not differ between control (0 nM sAPPα) and denatured sAPPα (100°C, 30 min, 25 nM, data not shown). **P < 0.05; *** P < 0.001.
sAPPα reduces GSK3β activity and tau phosphorylation in SH-SY5Y/BACE1 and HeLa/tau cells
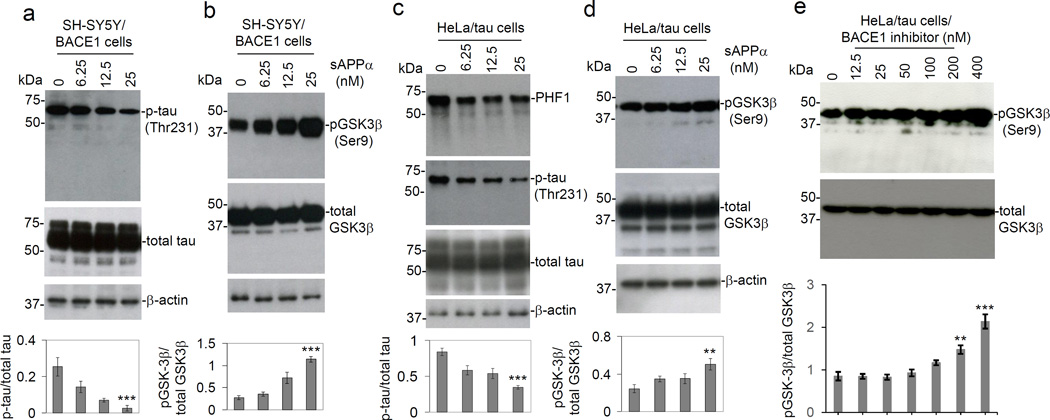
Having shown that sAPPα can reduce GSK3β activity we next set out to determine if sAPPα can also reduce tau phosphorylation. These experiments were performed with SH-SY5Y cells overexpressing BACE1 (SH-SY5Y/BACE1) in order to determine if sAPPα can reduce tau phosphorylation in the context of enhanced β-secretase activity. SH-SY5Y/BACE1 cells were treated with sAPPα (0–25 nM) for 12 h, and then cell lysates were prepared for WB analysis of GSK3β and tau phosphorylation. Application of sAPPα was followed by a significant dose-dependent decrease in tau phosphorylation as indicated by phospho-tau (Thr231) immunoreactivity (Fig. 2a); this response was associated with an increase in GSK3β (Ser9) phosphorylation (Fig. 2b). These findings suggest that sAPPα reduces GSK3β activity and thereby reduces tau phosphorylation, even in the context of enhanced β-secretase activity.
Fig. 2.
Treatment with sAPPα decreases tau phosphorylation and increases GSK3β (Ser9) phosphorylation in SH-SY5Y/BACE1 cells and HeLa/tau cells. SH-SY5Y/BACE1 cells (a & b) and HeLa/tau cells (c & d) were treated with sAPPα at indicated concentrations for 12 h. Cell lysates were prepared for WB analysis of both total and phosphorylated levels of tau and GSK3β. (a & c) Phosphorylation status of tau was detected by anti-phospho-tau [p-tau (Thr231)] and PHF1 antibodies. Total tau (phosphorylated and non-phosphorylated) was detected by tau-46. (b & d) Phosphorylation status of GSK3β [pGSK3β (Ser9)] was detected by anti-phospho-GSK3β (Ser9) antibody. (e) HeLa/tau cells were treated with BACE1 inhibitor LY2886721 at indicated concentrations (0, 12.5, 25, 50, 100, 200 and 400 nM) for 12 h and then cell lysates were prepared for WB analysis of both total and phosphorylated GSK3β. These results indicated that the GSK3β activity was dependent on BACE1 inhibition. In addition, BACE1 inhibitor dose-dependently induced inhibitory pGSK3β (Ser9) phosphorylation in SH-SY5Y/BACE1 cells (date not shown). Densitometry analysis shows the band density ratio of p-tau (Thr231) to total tau and pGSK3β (Ser9) to total GSK3β as shown below each figure panel. WB results are representative of two independent experiments for pGSK3β (Ser9) and total GSK3β, and three experiments respectively for PHF1, p-tau (Thr231) and total tau. A t-test revealed a significant difference in pGSK3β (Ser9) to total GSK3β and p-tau to total tau ratios for both SH-SY5Y/BACE1 cells and human tau stable transfected Hela cells treated with either 6.25, 12.5 or 25 nM sAPPα compared to control (0 nM). In addition, a significant difference in pGSK3β (Ser9) to total GSK3β ratio was observed for HeLa/tau cells treated with either 200 or 400 nM BACE1 inhibitor compared to control (0 nM) (**P < 0.05; *** P < 0.001). Aβ40, 42 peptides were undetectable by Aβ ELISA of the conditioned media from either cell line with or without sAPPα treatment (data not shown).
To confirm these data, HeLa cells overexpressing human wild-type tau (HeLa/tau) were also treated with sAPPα. Similar to results obtained with SH-SY5Y/BACE1 cells, sAPPα elicited a significant decrease in tau phosphorylation, expressed as both tau (Thr231) phosphorylation as well as PHF1, together with an increase in GSK3β (Ser9) phosphorylation, in these cells (Fig. 2c & d). These results therefore confirm that sAPPα reduces GSK3β activity and tau phosphorylation.
In order to further address the molecular mechanisms for sAPPα inhibition of GSK3β, HeLa/tau cells were treated with the BACE1 inhibitor LY2886721 at indicated concentrations (0, 12.5, 25, 50, 100, 200 and 400 nM) for 12 h and then cell lysates were prepared for WB analysis of both total and phosphorylated GSK3β. pGSK3β (Ser9) levels were promoted following functional inhibition of BACE1 (Fig. 2e), indicating that the GSK3β activity is dependent on BACE1. In addition, we also observed that BACE1 inhibitor dose-dependently induces inhibitory pGSK3β (Ser9) phosphorylation in SH-SYS/BACE1 cells (date not shown).
SAPPα-mediated GSK3β inactivation and reduction in tau phosphorylation were not prevented by γ-secretase inhibition in SH-SY5Y /BACE1 cells
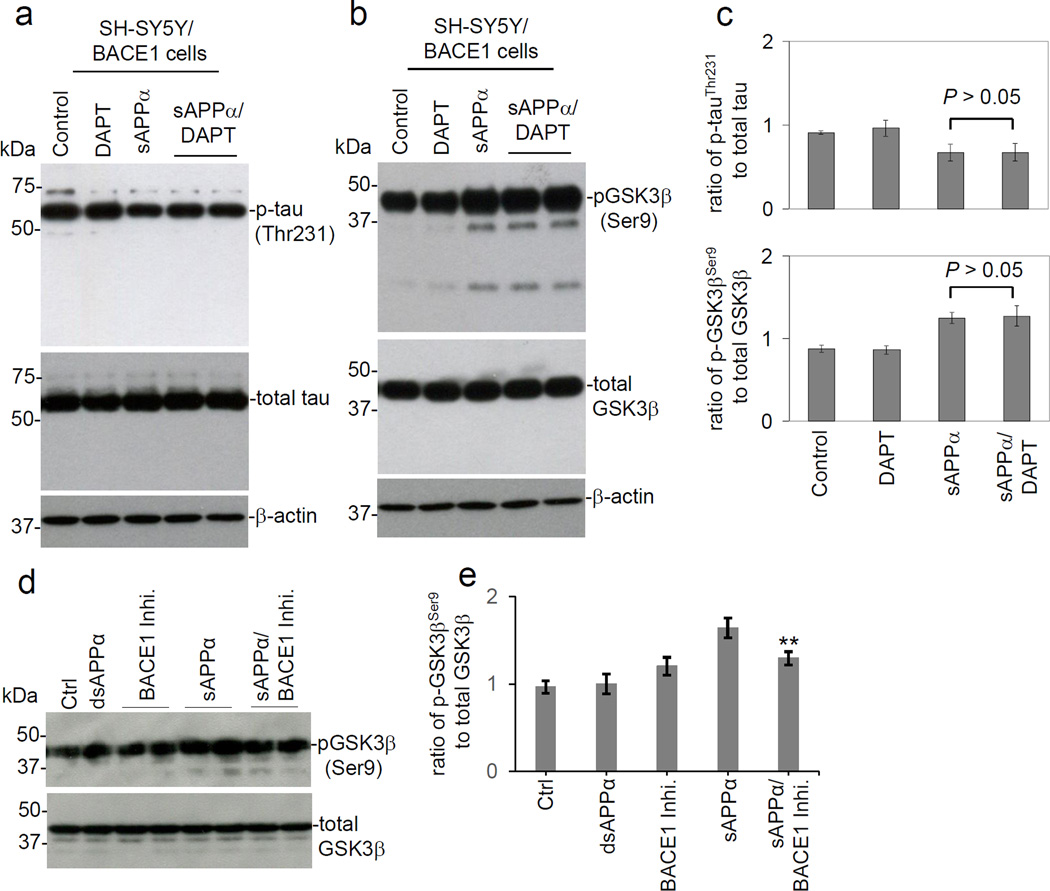
Because sAPPα can reduce β-secretase activity (Obregon et al., 2012), sAPPα-mediated reduction in tau phosphorylation might be mediated by reduced levels of Aβ. To exclude contributions of Aβ in the observed reduction in tau phosphorylation, SH-SY5Y/BACE1 cells were treated with the γ-secretase inhibitor DAPT in the presence of sAPPα. The results show that γ-secretase inhibition did not prevent sAPPα-elicited changes in GSK3β (Ser9) or tau phosphorylation (Fig. 3a, b & c), indicating that the effects of sAPPα on GSK3β and tau phosphorylation was independent of γ-secretase and, thus, of Aβ. Most importantly, functional inhibition of BACE1 using a specific inhibitor attenuated sAPPα-mediated induction of pGSK3β (Ser9) phosphorylation in SH-SY5Y cells. This result suggests that sAPPα causes GSK3β inhibitory phosphorylation likely through BACE1 (Fig. 3d & e).
Fig. 3.
The effects of sAPPα on GSK3β activity and tau phosphorylation are independent of γ-secretase. SH-SY5Y/BACE1 cells were treated with sAPPα (25 nM) with or without γ-secretase inhibitor DAPT (200 nM) for 12 h and then total and phosphorylated levels of tau and GSK3β were analyzed by WB. (a) Phosphorylation status of tau was detected by anti-phospho-tau (Thr231) antibody [p-tau (Thr231)]. Total tau (phosphorylated and non-phosphorylated) was detected by tau-46. (b) Phosphorylation status of GSK3β was detected by an anti-phospho-GSK3β (Ser9) antibody [p-GSK3β (Ser9)]. (c) Densitometry analysis shows the band density ratio of p-tau (Thr231) to total tau (upper panel) and ratio of p-GSK3β (Ser9) to total GSK3β (lower panel). (d) In order to further investigate if sAPPα mediated GSK3β (Ser9) inhibitory phosphorylation could be via BACE1 inhibition, SH-SY5Y cells were treated with sAPPα (25 nM), the heat-denatured sAPPα (dsAPPα, 25 nM) or PBS (Control, Ctrl) in the presence or absence of BACE1 inhibitor (BACE1 Inhi.) at 200 nM for 12 h. Total and phosphorylated levels GSK3β were analyzed by WB. (e) Densitometry analysis shows the band density ratio of p-GSK3β (Ser9) to total GSK3β (**P < 0.05). These WB results are representative of three independent experiments. A t-test revealed no statistically significant difference between sAPPα and sAPPα/DAPT in either the ratio of p-tau (Thr231) to total tau or the ratio of pGSK3β (Ser9) to total GSK3β. Aβ40, 42 peptides were undetectable by Aβ ELISA of the conditioned media from SH-SYS/BACE1 cells (data not shown).
sAPPα inhibits GSK3β-elicited tau phosphorylation in vivo
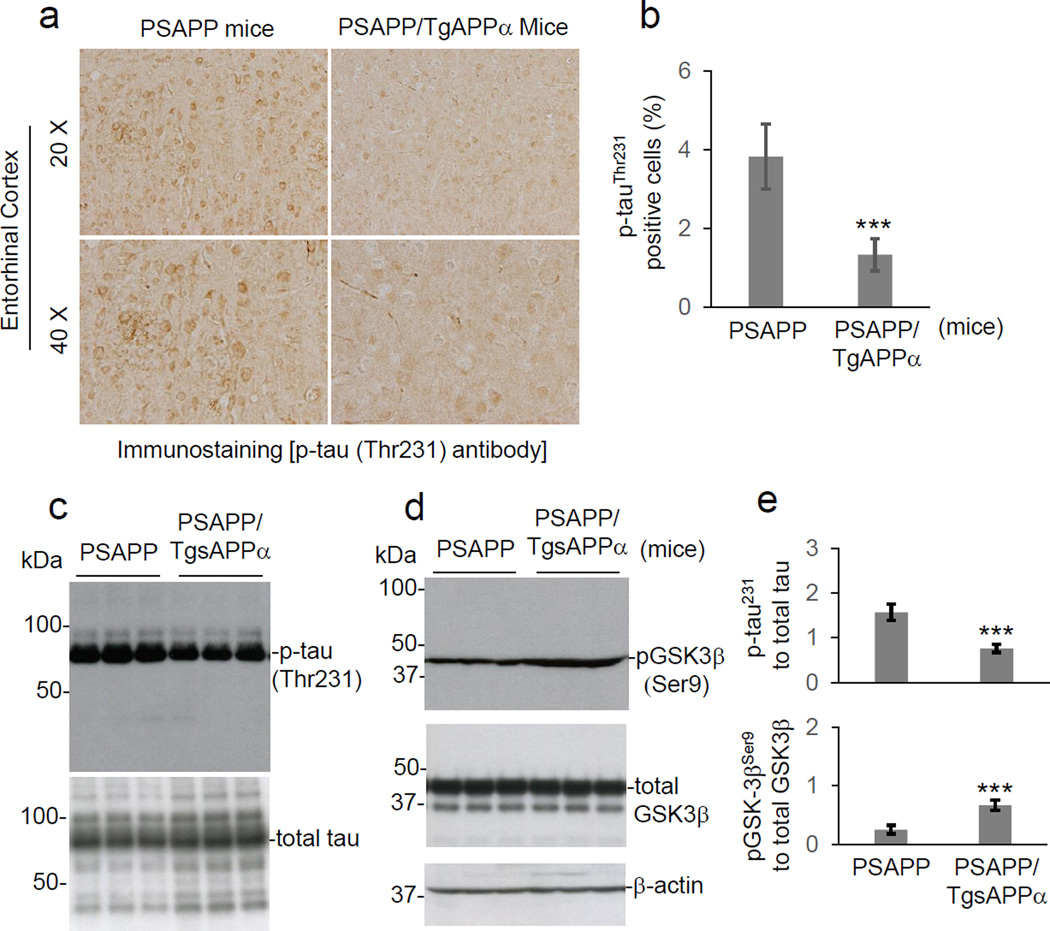
To further determine if sAPPα reduces GSK3β activity and tau phosphorylation in vivo, we employed a transgenic mouse model of AD that exhibits tau hyperphosphorylation (PSAPP). This mouse line was crossed with a transgenic line overexpressing sAPPα (TgsAPPα), creating triple transgenic (PSAPP/TgsAPPα) mice along with littermate controls. The mice were sacrificed at 9 months of age, and brain slices were prepared for analysis of tau (Thr231) phosphorylation by immunohistochemistry. Brain homogenates were additionally prepared for analysis of GSK3β and tau phosphorylation by western blotting. Compared with their PSAPP littermate controls, PSAPP/TgsAPPα mice displayed reduced levels of tau (Thr231) phosphorylation (Fig. 4a, b, c & e) and elevated levels of GSK3β (Ser9) phosphorylation (Fig. 4d & e), implying that sAPPα reduces GSK3β activity and thereby reduces tau phosphorylation in vivo.
Fig. 4.
Overexpression of sAPPα in PSAPP mice attenuates abnormal tau phosphorylation and GSK3β activation. Mouse brain tissue sections and homogenates were prepared from 9-month-old PSAPP and PSAPP/TgsAPPα mice (n = 5 females per group). (a) Mouse brain sections from both groups were stained with anti-phospho-tau (Thr231) [p-tau (Thr231)] antibody. (b) Percentages of anti-p-tau (Th231) antibody positive cells [p-tau (Th231) positive area/total area; mean ± S.D.] at 20 × magnification (15–20 fields per section) were calculated by quantitative image analysis for mouse brain entorhinal cortical (EC) region as indicated (***P < 0.001). Mouse brain homogenates were subjected to WB analysis with antibodies against p-tau (Thr231), or total tau (c) or with antibodies against phospho- or total-GSK3β (d). Phosphorylation status of GSK3β was detected by anti-phospho-GSK3β (Ser9) [pGSK3β (ser9)] antibody. β-actin was used as an internal reference control. (e) Densitometry analysis shows the band density ratios of p-tau (Thr231) to total tau (upper panel) and pGSK-3β (Ser9) to total GSK3β (lower panel). A t-test revealed significant differences in the ratios of p-tau (Thr231) to total tau and pGSK3β (Ser9) to total GSK3β between PSAPP/TgsAPPα and PSAPP mice (***P < 0.001). Similar results from both immunochemistry staining and WB analyses were also obtained with PHF-1 antibody (data not shown). There are no notable differences in p-tau (Th231) and PHF-1 antibody staining between PSAPP and PSAPP/TgsAPPα mice for retrosplenila cortex (RSC) and hippocampus (H) (data not shown).
Discussion
The mechanisms of amyloid plaque and NFT accumulation in the brains of AD patients have remained under intense investigation in hopes of preventing or slowing the progression of this disease (Huang and Mucke, 2012). While amyloid plaques and NFTs are known to be composed of Aβ and hyperphosphorylated tau proteins, respectively, little is known about the precise mechanisms that lead to their accumulation or their roles in generating the symptoms of dementia. Our recent studies suggest that sAPPα, secreted upon α-secretase cleavage of APP, can reduce β-secretase activity, thereby reducing Aβ generation and cerebral amyloidosis in cells and mouse models of AD (Obregon et al., 2012). Since reduced β-secretase activity can also reduce the levels of GSK3β-mediated tau phosphorylation (Israel et al., 2012), the present study was undertaken to determine if sAPPα can also impact this process. In primary cultures of cortical neurons and in the SH-SY5Y cell line, sAPPα increased inhibitory GSK3β (Ser9) phosphorylation indicative of decreased GSK3β activity. In additional studies utilizing SH-SY5Y cells overexpressing BACE1 (SH-SY5Y/BACE 1), as well as HeLa cells overexpressing human tau (HeLa/tau), sAPPα increased GSK3β (Ser9) phosphorylation while reducing tau phosphorylation, as indicated by phospho-tau (Thr231) and PHF1 immunoreactivity. These results suggest that sAPPα is a very potent endogenous regulator since it reduces GSK3β activity and thereby reduces tau phosphorylation, even in the context of enhanced BACE1 activity. This is important because removal of pre-formed plaques in humans has met with little clinical efficaciousness, pointing to a need to intervene earlier at the APP processing step. It is not known whether sAPPα inhibition of GSK3β, and thus tau hyperphosphorylation, is a distinct and separate phenomenon from sAPPα inhibition of BACE1 is yet to be explored. These two processes may or may not occur simultaneously in the disease progression.
Additionally, sAPPα-mediated reduction of tau phosphorylation was not altered by γ-secretase inhibition, confirming and extending previous studies showing that γ-secretase inhibition does not alter GSK3β-mediated tau phosphorylation in pluripotent stem cell-derived neurons from AD patients (Israel et al., 2012). This result indicates that the effects of sAPPα are not mediated by an impact on production of Aβ or the APP intracellular domain (AICD) liberated by γ-secretase. However, β-secretase inhibition attenuated sAPPα-induced inhibitory GSK-3β (Ser9) phosphorylation, indicating that the effect of sAPPα on GSK3β activity is mediated by BACE1. Together, these findings suggest that there is a direct link between APP β-secretase processing and GSK3β-mediated tau phosphorylation pathways. While the link between these pathways remains to be determined, β-CTF is one attractive candidate because levels of this product correspond with axonopathies in mouse models of AD overexpressing APP (Leroy et al., 2007; Wong et al., 2002; Zheng and Koo, 2006).
Of particular importance is our finding that mouse models of AD overexpressing sAPPα (PSAPP/TgsAPPα) display greater GSK3β (Ser9) phosphorylation and less tau phosphorylation than littermate controls (PSAPP). Therefore, sAPPα appears to reduce GSK3β activity and thereby reduce tau phosphorylation in vivo as well as in vitro. These results correspond with those from our previous study showing that PSAPP/TgsAPPα mice exhibit reductions in Aβ levels, β-CTF production, cerebral amyloidosis, and APP/BACE1 interaction compared to PSAPP littermate controls. Taken together, these results indicate that sAPPα can reduce β-secretase activity in vitro and in vivo, thereby reducing amyloidogenic APP processing, tau phosphorylation and potentially NFT formation, the major hallmarks of AD. Because sAPPα interaction with BACE1 may precede NFT and Aβ generation in AD patients, development of selective BACE1 inhibitors, molecular agents able to restore or enhance BACE1 inhibition by sAPPα or sAPPα mimetics could hold therapeutic value in preventing or slowing the progression of AD.
Acknowledgments
This work was supported by the NIH/NCCIH (R01AT007411, J.T.) and NIH/NIA (R01AG032432, J.T.); R21AG033215 and P01AG012411 (S.W.B.). This work was also supported by the National Natural Science Foundation of China (Grant No. 81200987; No. 81470058, D.J.). S.W.B. receives royalties from Sigma-Aldrich resulting from the sales of a prokaryotic source of sAPPα. We would like to thank Dr. Chad Dickey for helpful discussion and also Dr. Peter Davies (Albert Einstein University, Bronx, NY) for kindly providing PHF1 antibody.
Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- AICD
APP intracellular domain
- APP
amyloid precursor protein
- sAPPα
soluble amyloid precursor protein alpha
- BACE1
β-site APP converting enzyme 1
- β-CTF
β-carboxy terminal fragment
- DAPT
N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-Butyl Ester
- GSK3β
glycogen synthase kinase 3 beta
- NFT
neurofibrillary tangles
- PHF1
paired helical filament 1
- α-secretase
alpha-secretase
- β-secretase
beta-secretase
- γ-secretase
gamma-secretase
Footnotes
Author contributions
J.D and A.H. performed the experiments including WB, IH and ELISA, assisted in the design of the study, analyzed the data and drafted the manuscript. D.O., S.W.B., B.G., Y.J.W., H.H. and D.S. assisted in the design of the study, manuscript composition and editing. J.T. designed and supervised the study, analyzed the data, assisted in the composition and editing of the manuscript. All the authors discussed the results and commented on the final version of the manuscript.
Financial disclosures
The authors declare no competing interests.
Data and materials availability: A materials transfer agreement is required for transfer of materials.
References
- Abisambra JF, Jinwal UK, Suntharalingam A, Arulselvam K, Brady S, Cockman M, Jin Y, Zhang B, Dickey CA. DnaJA1 antagonizes constitutive Hsp70-mediated stabilization of tau. J Mol Biol. 2012;421:653–661. doi: 10.1016/j.jmb.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, Lee CM. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copanaki E, Chang S, Vlachos A, Tschape JA, Muller UC, Kogel D, Deller T. sAPPalpha antagonizes dendritic degeneration and neuron death triggered by proteasomal stress. Mol Cell Neurosci. 2010;44:386–393. doi: 10.1016/j.mcn.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Vink R, Blumbergs PC, Masters CL, Cappai R, van den Heuvel C. sAPPalpha rescues deficits in amyloid precursor protein knockout mice following focal traumatic brain injury. J Neurochem. 2012;122:208–220. doi: 10.1111/j.1471-4159.2012.07761.x. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Stress kinases involved in tau phosphorylation in Alzheimer's disease, tauopathies and APP transgenic mice. Neurotox Res. 2004;6:469–475. doi: 10.1007/BF03033283. [DOI] [PubMed] [Google Scholar]
- Gralle M, Botelho MG, Wouters FS. Neuroprotective secreted amyloid precursor protein acts by disrupting amyloid precursor protein dimers. J Biol Chem. 2009;284:15016–15025. doi: 10.1074/jbc.M808755200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Hetman M, Cavanaugh JE, Kimelman D, Xia Z. Role of glycogen synthase kinase-3beta in neuronal apoptosis induced by trophic withdrawal. J Neurosci. 2000;20:2567–2574. doi: 10.1523/JNEUROSCI.20-07-02567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, Carson CT, Laurent LC, Marsala M, Gage FH, Remes AM, Koo EH, Goldstein LS. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinwal UK, Miyata Y, Koren J, 3rd, Jones JR, Trotter JH, Chang L, O'Leary J, Morgan D, Lee DC, Shults CL, Rousaki A, Weeber EJ, Zuiderweg ER, Gestwicki JE, Dickey CA. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Gamma-secretase is a membrane protein complex comprised of presenilin, nicastrin, Aph-1, and Pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefort R, Pozueta J, Shelanski M. Cross-linking of cell surface amyloid precursor protein leads to increased beta-amyloid peptide production in hippocampal neurons: implications for Alzheimer's disease. J Neurosci. 2012;32:10674–10685. doi: 10.1523/JNEUROSCI.6473-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy K, Bretteville A, Schindowski K, Gilissen E, Authelet M, De Decker R, Yilmaz Z, Buee L, Brion JP. Early axonopathy preceding neurofibrillary tangles in mutant tau transgenic mice. Am J Pathol. 2007;171:976–992. doi: 10.2353/ajpath.2007.070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. Embo J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Moore S, Evans LD, Andersson T, Portelius E, Smith J, Dias TB, Saurat N, McGlade A, Kirwan P, Blennow K, Hardy J, Zetterberg H, Livesey FJ. APP metabolism regulates tau proteostasis in human cerebral cortex neurons. Cell Rep. 2015;11(5):689–696. doi: 10.1016/j.celrep.2015.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoki K, Kume H, Oda A, Tamaoka A, Hosaka A, Kametani F, Araki W. Neuronal beta-amyloid generation is independent of lipid raft association of beta-secretase BACE1: analysis with a palmitoylation-deficient mutant. Brain Behav. 2012;2:270–282. doi: 10.1002/brb3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama KS, Kametani F, Saito S, Kume H, Akiyama H, Araki W. Reticulons RTN3 and RTN4-B/C interact with BACE1 and inhibit its ability to produce amyloid beta-protein. Eur J Neurosci. 2006;24:1237–1244. doi: 10.1111/j.1460-9568.2006.05005.x. [DOI] [PubMed] [Google Scholar]
- Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-alpha modulates beta-secretase activity and amyloid-beta generation. Nat Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, Agullo JM, Perez M, Avila J, Guardia-Laguarta C, Clarimon J, Lleo A, Gomez-Isla T. A novel GSK-3beta inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–367. doi: 10.1016/j.nbd.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Smith C, Graham DI, Murray LS, Nicoll JA. Tau immunohistochemistry in acute brain injury. Neuropathol Appl Neurobiol. 2003;29:496–502. doi: 10.1046/j.1365-2990.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Wong PC, Cai H, Borchelt DR, Price DL. Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci. 2002;5:633–639. doi: 10.1038/nn0702-633. [DOI] [PubMed] [Google Scholar]
- Zheng H, Koo EH. The amyloid precursor protein: beyond amyloid. Mol Neurodegener. 2006;1:5. doi: 10.1186/1750-1326-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hou H, Rezai-Zadeh K, Giunta B, Ruscin A, Gemma C, Jin J, Dragicevic N, Bradshaw P, Rasool S, Glabe CG, Ehrhart J, Bickford P, Mori T, Obregon D, Town T, Tan J. CD45 deficiency drives amyloid-beta peptide oligomers and neuronal loss in Alzheimer's disease mice. J Neurosci. 2011;31:1355–1365. doi: 10.1523/JNEUROSCI.3268-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]