Abstract
It has become clear that the endocannabinoid system is a potent regulator of immune responses, with the cannabinoid receptor 2 (CB2) as the key component due to its high expression by all immune subtypes. CB2 has been shown to regulate immunity by a number of mechanisms including development, migration, proliferation and effector functions. In addition, CB2 has been shown to modulate the function of all immune cell types examined to date. CB2 is a Gi-protein coupled receptor and thus exhibits a complex pharmacology allowing both stimulatory and inhibitory signaling that depends on receptor expression levels, ligand concentration and cell lineage specificities. Here, we discuss both in vitro and in vivo experimental evidence that CB2 is a potent regulator of immune responses making it a prime target for the treatment of inflammatory diseases.
Keywords: Autoimmunity, cannabinoid receptor 2, endocannabinoid, GPCR, immune system, immune suppression
Introduction
Cannabinoids are a group of liophilic and pharmacologically active compounds present in the flowering tops and leaves of the Cannabis sativa plant (marijuana). Cannabis is not only considered one of the oldest known drugs of pleasure in human history; its medicinal uses in controlling pain, convulsion, inflammation and asthma were also known in Central and South Asia from prehistoric times (1, 2). However, its therapeutic value wasn’t scientifically evaluated and reported to the Western world until the early 19th century by Sir William B. O’Shaughnessy, an Irish physician working in Calcutta, India (3). Almost one century later, in 1964, the primary psychoactive component of cannabis was identified as (−)-Δ9-tetrahydrocannabinol (THC) (4). Since then, almost 60 plant-derived cannabinoids have been identified and a plethora of structurally and biologically related compounds have been synthesized, which are termed synthetic cannabinoids (see Table 1 for a list of cannabinoids discussed in this review). However, their molecular mechanism of action remained elusive until Matsuda and colleagues cloned the first receptor for THC, cannabinoid receptor 1 (CB1), from a rat brain cDNA library in 1990 (5). In 1993, Munro and co-workers cloned a second cannabinoid receptor, CB2, from a human promyelocytic leukemia cell line HL60 cDNA library (6). Both receptors are G-protein coupled receptors (GPCR). CB1 is highly expressed in the central nervous system (CNS), and is associated with the psychoactive effects of cannabinoids (5, 7–9). CB1 is also expressed at low levels in peripheral tissues including the heart, testis and immune system (8, 9). CB2 is predominantly expressed by cells of hematopoietic origin and is thought to mediate cannabinoid-induced immune modulation (9). Finally, the identification of endogenous compounds that bind to cannabinoid receptors and their synthesizing and metabolizing enzymes gave rise to the concept of the endocannabinoid system (10–13). Among endocannabinoids, N-arachidonoylethanolamine, also known as anandamide, and 2-arachidonoylglycerol (2-AG) are the best studied. Others include noladine ether, virodhamine and N-arachidonoyl dopamine (14–16). Previous reviews have comprehensively described the complex regulation, signaling, functions and evolution of the endocannabinoid system (1, 17, 18), thus we will discuss the roles of CB2 in immune system regulation.
Table 1.
Commonly used cannabinoid ligands, their receptors and biological functions
| Compounds | CB1 Receptor activity | CB2 Receptor activity | CB2-dependent Functions | Reference |
|---|---|---|---|---|
| Plant cannabinoids
|
||||
| Δ9-THC | Partial agonist Ki: 5.05 nM |
Partial agonist Ki: 3.13 nM |
Suppressed macrophage chemotaxis (in vitro) | 42 |
| Suppressed macrophage costimulation (in vitro) | 25, 53 | |||
| Induced apoptosis in DCs (in vitro) | 48 | |||
| Suppressed immune response against Legionella pneumophila | 61, 62 | |||
| Inhibited anti-tumor immunity | 65 | |||
|
| ||||
| Endocannabinoids
|
||||
| Anandamide | Partial agonist Ki: 89 nM |
Partial agonist Ki: 371 nM |
Inhibited IL-12 and IL-23 production and enhanced IL-10 production by activated microglial cells (in vitro) | 50, 51 |
| 2-AG | Full agonist Ki: 472 nM |
Full agonist Ki: 1400 nM |
Induced chemotaxis in human monocytes, neutrophils, eosinophiles, NK cells, murine DCs, B cells and microglial cells (in vitro) | 33–40 |
| Enhanced proliferation of microglial cells (in vitro) | 47 | |||
|
| ||||
| Synthetic cannabinoids
|
||||
| WIN55212-2 | Agonist Ki: 62.3 nM |
Agonist Ki: 3.3 nM |
Suppressed leukocyte rolling and adhesion to the endothelial cells (in vivo) | 83 |
| CP55940 | Agonist Ki: ~0.6 nM |
Agonist Ki: ~0.7 nM |
Enhanced Ig class switching (in vitro) | 52 |
| Enhanced human B cell proliferation (in vitro) | 46 | |||
| JWH-015 | Ki: 383 nM | CB2-selective agonist Ki: 13. 8 nM |
Induced migration in HL-60 cells, and Inhibited chemotaxis of macrophages, microglial cells and T cells (in vitro) | 38, 43–45 |
| Induced apoptosis in splenocytes and thymocytes (in vitro) | 49 | |||
| Dampened TCR signaling and IL-2 production by T cell (in vitro) | 54 | |||
| Alleviated EAE | 82 | |||
| JWH-133 | Ki: ~ 680 nM | CB2-selective agonist Ki: ~ 3 nM |
Inhibited antigen-specific murine CD4 T cell proliferation and pro-inflammatory cytokine production, (in vitro) | 32 |
| Inhibited IL-12 and IL-23 and enhanced IL-10 production by LPS/IFN-γ-activated microglial cells (in vitro) | 50, 51 | |||
| Alleviated inflammations during HOCl-induced systemic sclerosis, IRBP-induced experimental autoimmune uveoretinitis, and experimental colitis | 73, 76, 78, 79 | |||
| O-2137-2 | Ki: 2700 nM | CB2-selective agonist Ki: 11 nM |
Inhibited murine macrophage chemotaxis to RANTES | 42 |
| GP1a | Ki: 363 nM | CB2-selective agonist Ki: 0.037 nM |
Enhanced neutrophil activation and reduced morbidity during sepsis | 64 |
| HU-308 | Ki: > 10 μM | CB2-selective agonist Ki: ~ 22.7 nM |
Aggravated inflammation in a mice model of DNFB-induced cutaneous contact hypersensitivity | 67, 68 |
| AM1241 | Ki: > 10 μM | CB2-selective antagonist Ki: 658 nM, human |
Suppressed intestinal inflammation in experimental colitis | 78 |
| SR144528 | Ki: 400 nM | CB2-selective antagonist/inverse agonist Ki: 0.6 nM |
Reversed the effects mediated by CB2 | 41 |
| Blocked constitutive activity of CB2 in CB2-transfected CHO cells | 58 | |||
| Attenuated inflammation in DNFB and oxalozone-induced cutaneous contact hypersensitivity models in mice. | 68, 69 | |||
| AM630 | Ki: 5.2 μM | CB2-selective antagonist/inverse agonist Ki: 31.2 nM |
Reversed inhibitory effects of JWH-015 on microglial cell migration to ADP and T cell migration to CXCL-12 | 44, 45 |
| Exacerbated experimental colitis | 78 | |||
| JTE-907 | CB2-selective antagonist/inverse agonist Ki: 35.9nM, human |
Attenuated inflammation during DNFB-induced cutaneous contact hypersensitivity in mice | 68 | |
CB2 is a member of the Gi protein-coupled receptor family (19) and is encoded by the CNR2 gene present on chromosomes 1p36 in humans, 5q36 in rats and 4D3 in mice. Nucleic acid sequence analysis revealed 81% homology between human and rat CNR2 and 90% identity between rat and mouse, emphasizing the evolutionary conserved nature of this protein (20, 21). Structurally, CB2 is comprised of a single polypeptide chain with an extracellular glycosylated N-terminal domain, seven transmembrane α-helices and an intracellular N-terminus. In vitro binding studies indicate that both anandamide and 2-AG bind to CB2 (22). However, anandamide functions as a partial agonist towards CB2, and is poorly detected outside the brain. 2-AG, in contrast, is detected at a very high levels in various mammalian tissues including gut, bone marrow and spleen, and acts as a full agonist towards CB2 demonstrating distinct cannabimimetic effects. Therefore, 2-AG is considered to be the natural primary ligand for CB2 (23). Plant cannabinoids such as THC and cannabinol, and synthetic cannabinoids including WIN55212-2 and CP55940, have also been demonstrated to bind to CB2 (22). However, these cannabinoids can also bind to CB1, which is present on immune cells at low levels (24). Thus cannabinoids that bind both CB1 and CB2 are limited in their usefulness in dissecting the specific functions of CB2. The understanding of CB2-mediated immune-modulation was greatly enhanced with the synthesis of CB2-selective agonists, antagonists and inverse agonists (Table 1). Finally, the generation of CB2−/− mice provided a unique opportunity to study CB2-specific effects in vitro and in vivo (25, 26).
An importance for understanding the role of CB2 in immune regulation and its precise molecular mechanism has been increasingly appreciated from a therapeutic perspective. In retrospect, the concept that the endocannabinoid system can be targeted as an anti-inflammatory therapeutic stemmed from a number of in vitro and in vivo animal studies that showed that cannabinoids are capable of inducing immune suppression (27, 28). Several plant and synthetic cannabinoids have been utilized in preclinical and clinical trials to target the endocannabinoid system to treat inflammatory and autoimmune disorders. However, their primary limitation came from the severe psychotic effects produced by these compounds through CB1. Therefore, CB2-selective agonists that do not generate untoward psychotic effects have caught particular pharmacological attention. Pharmaceutical companies are exploring the potency of CB2-selective agonists for the treatment of disease (http://www.pharmoscorp.com). Thus, it is timely to critically evaluate what is known regarding the functions and the mechanisms of action of CB2 in immune suppression.
General immune system overview
The immune system provides defense against pathogens and is segregated into the innate and adaptive immunity arms. Immune cells with innate functions include granulocytes, monocytes/macrophages, dendritic cells (DCs), natural killer (NK) cells and certain subpopulations of B cells. The adaptive immune response, while requiring help from innate cells, consists of B and T lymphocytes. Innate immunity provides a first line of defense against pathogens that are recognized via their expression of invariant molecular patterns. Adaptive immunity takes longer to develop and requires antigen specificity. Upon pathogen encounter, B lymphocytes recognize specific extracellular antigen (on the pathogen surface) by B cell receptors (BCR), undergo clonal expansion and differentiate into plasma cells that secrete antibodies specific for a particular antigen. T cells express T cell receptors (TCR) that recognize antigens as peptides bound to major histocompatibility complex (MHC) molecules expressed on the cell surface of antigen presenting cells (APC). Following antigen recognition, T cells become activated, clonally expand and differentiate into effector cells. The priming of the adaptive immune response largely requires dendritic cells, but the other professional APC macrophages and B cells can also play important roles. APC internalize pathogens following their recognition via germ line encoded cell surface receptors, are directly infected or by phagocytizing infected cells. The pathogens are degraded and the generated peptides are loaded onto MHC molecules that are presented on the cell surface. These processes are termed antigen processing and presentation. MHC class I molecules are expressed by essentially all cells and are recognized by CD8 T cells. MHC class II molecules are expressed by professional APC and are recognized by CD4 T cells. Although their functions overlap, CD8 T cells are cytotoxic leading to the death of pathogen-infected cells. CD4 T cells are less efficient at killing and play essential roles in the inflammatory response. CD4 T cells have two broad functions. The first is to produce cytokines and chemokines that enhance and promote inflammation essential for pathogen clearance. These pro-inflammatory T cells produce a large number of cytokines and have been categorized as Th1 and Th17 cells. Although the cytokines produced by both groups overlap substantially, the hallmark cytokines are IFN-γ production by Th1 cells and IL-17 by Th17 cells. Second, T cells provide “help” to B cells in an antigen-specific manner to facilitate the production of pathogen-specific antibodies. Historically, these B cells were called Th2 because they produce IL-4, an essential cytokine for the production of certain subclasses of immunoglobulins (Ig). However, other cytokines such as IFN-γ also influences the subclass of Ig produced following infection.
Several endogenous factors are known to regulate immune cell development, homeostasis and functions. Although an emerging topic in immunology, strong experimental evidence exists for a role for the endocannabinoid system, in particular CB2, in immune modulation. The CB2 receptor is expressed by all immune cells, but expression levels vary among immune cell populations and activation states. In humans, the highest to lowest CB2-expressing immune cells are: B cells > NK cells > macrophages > polymorphonuclear cells > CD4 T cells > CD8 T cells (8, 9). The finding that CB2 expression changes are dependent on the activation status of different immune cell populations suggests a critical role for CB2 in immune regulation and function (29–32). Indeed, CB2 has been found to play a complex role in regulating migration, proliferation and different effector functions of various immune cells, as discussed below.
CB2 and regulation of immune cell functions: an in vitro perspective
The role of CB2 in regulating immune cell functions has been largely studied using in vitro approaches. Although these approaches are beneficial, they have limitations. We will discuss the strengths and weaknesses of in vitro approaches for the study of CB2-specific immune modulatory functions including effects on migration, proliferation, apoptosis and cytokine production.
The ability of immune cells to migrate throughout the body is critical for their development/maturation as well as for the induction of the immune response during infection. For example, immature B cells migrate from the bone marrow (BM), where hematopoiesis occurs, to the peripheral lymphoid organs to complete their maturation. T cell progenitors migrate from the BM to the thymus. During an infection, granulocytes and macrophages migrate from the blood and within tissues to the site of infection. APC following antigen uptake, migrate to primary lymphoid organs where they encounter T cells to initiate the adaptive immune response. The antigen-specific T cells then migrate into the blood and subsequently into infected tissues to promote pathogen clearance. Plasma cells generated in the spleen and lymph nodes migrate mostly back to the BM where they are long-lived and produce antigen-specific Ig. Chemokines play a central role in regulating immune cell migration/chemotaxis/trafficking by interacting with chemokine receptors on immune cells. Like most chemokine receptors, CB2 is also a Gi-protein coupled receptor (GPCR). Thus a role for CB2 in immune cell migration is of particular interest.
Interestingly, CB2 showed a complex regulation pattern with some CB2-ligands promoting and others inhibiting chemotaxis. In addition to ligand type, the differential effects depended upon the cell type examined and the presence of other external stimuli. Using in vitro migration assays, the endocannabinoid 2-AG, was shown to induce the migration of human peripheral blood monocytes, neutrophils, eosinophils, NK cells; and mouse dendritic cells, B cells and microglial cells (33–40). In each case, the effect of 2-AG was reversed by the CB2-selective antagonist/inverse agonist SR144528 (41). These data demonstrate that immune cells migrate in response to 2-AG in a CB2-dependent manner. However, when synthetic CB2 selective-agonists were used to modulate chemotaxis to other chemoattracts they inhibited migration. Pretreatment of mouse macrophages with either O-2137 or JWH-015 resulted in reduced migration to RANTES/CCL5 and CCL2/CCL13, respectively (42, 43). Similarly, in a transwell migration assay, JWH-015-pretreatment suppressed the migration of LPS-activated mouse microglial cells to adenosine diphosphate (ADP) in a dose-dependent manner (44). The reversal of the affect with the CB2-selective antagonist AM630 confirmed a role for CB2. Pretreatment with high doses of JWH-015 also significantly inhibited primary human and Jurkat T cell chemotaxis to CXCL12 (45). However, the interpretation of these in vitro studies needs to be tempered by the finding that at high concentrations, cannabinoid ligands can function via receptor-independent mechanisms (25, 46). This is evidenced by only partial reversal of the inhibitory effect of high doses of JWH-015 on T cell migration by the CB2-selective antagonist AM630 (45).
Taken together, the above studies clearly demonstrate CB2-depdendent immune cell migration in response to 2-AG and that synthetic CB2-agonists, at least in some circumstances, have the capacity to inhibit migration to external stimuli. Whether synthetic CB2 agonists inhibit migration to 2-AG is not known. This latter question is important in regards to the treatment of inflammatory diseases because it is not possible to predict when endogenously administered CB2-selective agonists would inhibit or promote migration. Depending upon the disease state both could be detrimental. In autoimmunity, enhanced migration of cells into tissues would be detrimental, but if inhibited beneficial. The opposite would be true for a bacterial infection.
The ability of immune cells to expand their numbers is crucial for immune cell development, homeostasis and a robust immune response. Hematopoietic stem cells located in the BM continuously undergo self-renewal and differentiation followed by bursts of expansion for the generation of immune cells. In order to generate a protective immune response, T and B cells must undergo clonal expansion to generate sufficient numbers of cells to facilitate pathogen clearance. However, the extent of immune cell proliferation needs to be tightly regulated since over expansion of cells can contribute to chronic inflammation and autoimmunity, while too few immune cells results in immunodeficiency. Although the extent to which CB2 regulates proliferation is still under investigation, evidence does exist for its involvement. Interestingly, as with migration, both positive and negative regulation of proliferation by CB2 has been reported. 2-AG was shown to dose-dependently enhance the proliferation of rat microglial cells in a CB2-dependent manner, as demonstrated using the CB2 antagonist SR144528 (47). In human B cells activated via cross linking of the BCR or CD40 ligation, the synthetic cannabinoid CP55950 caused a dose-dependent increase in proliferation at nanomolar levels of the cannabinoid (46). However, a definitive role for CB2 was not determined. In contrast, our lab has clearly demonstrated the inhibitory effect of CB2 on T cell proliferation using CB2−/− T cells. We found that the CB2-selective agonist JWH-133 significantly suppressed antigen-specific mouse CD4 T cell proliferation through CB2 in vitro (32). These contrasting results suggest that differential outcomes in regards to a role for CB2 in proliferation could be due to differences between ligands, cell types and the activation status of immune cells that regulate CB2 surface expression differently.
Apoptosis or programmed cell death also plays an essential role in development, homeostatic maintenance and the extent of the immune response. Lymphocytes undergoing selection that recognize self-antigens must be eliminated since the expansion of self-reactive T and B cells is known to contribute to autoimmunity. During an immune response to pathogens the inflammatory state is in part kept under control and then ultimately downregulated by extensive cell death of the responding immune cells. The role of CB2 in the regulation of apoptosis is still not completely clear. It has been demonstrated that high doses of THC (binds both CB1 and CB2) induced apoptosis in bone marrow-derived dendritic cells (48). The CB2-selective agonist JWH-015 has also been reported to induce apoptosis in mouse splenocytes and thymocytes, at high doses (49). In both studies, these effects were only partially reversed by the CB2-selective antagonist SR144528. These results suggested that some cannabinoids have the capacity to promote apoptosis in a CB2-dependent manner; however, the involvement of other cannabinoid receptors or receptor-independent mechanisms in this process cannot be ruled out.
Cytokines are a large group of proteins that include interleukins (IL), interferons (IFN) and growth factors that are produced and secreted by immune cells and play key roles in coordinating appropriate immune responses. A number of immune syndromes have been linked to aberrant cytokine production. The literature to date largely supports a role for CB2 in the inhibition of cytokine production. Correa and coworkers activated microglial cells with LPS/IFN-γ in the presence or absence of the CB2-selective agonist JWH-133 or the endocannabinoid anandamide in vitro (50). While they found that both cannabinoids significantly inhibited the production of the pro-inflammatory cytokines IL-12 and IL-23, the CB2 antagonist SR144528 completely reversed the inhibition to JWH-133 and had only a partial effect on anandamide (50). Similar results were obtained with human microglial cells. In a follow-up study, the same group demonstrated that AEA induced a modest upregulation of the anti-inflammatory cytokine IL-10 using the same conditions, which was reversed by the CB2 antagonist (51). Both studies provided evidence that the changes in cytokine production were mediated through ERK1/2 and JNK. In our studies using CD4 T cells, we demonstrated that JWH-133 significantly inhibited the expression of IL-2 and IFN-γ mRNA following antigen stimulation (32). CB2-dependency was confirmed by the lack of inhibition in CB2−/− T cells (32). Thus the limited data available support a role for CB2 in immunosuppression by inhibiting the production of pro-inflammatory cytokines and perhaps by enhancing anti-inflammatory cytokines.
CB2 has been reported to regulate other specific immune cell functions including immunoglobulin class switching in mouse B cells from IgM to IgE following stimulation with anti-CD40 and IL-4 that was enhanced by the nonselective cannabinoid CP55940 with CB2 specificity confirmed by the use of the CB2 antagonist SR144528 (52). In mouse, THC addition to co-cultures of activated macrophages and a T cell hybridoma stimulated by the addition of anti-CD3 lead to a reduction in IL-2 production by the T cells in a dose-dependent manner. When macrophages deficient in CB2 were used, no inhibition of IL-2 production occurred (25, 53). These data indicate that THC has the capacity to suppress co-stimulatory activities of macrophages, at least in vitro. The use of in vitro assays, although limited in biological scope, have clearly shown that immune responses can be modulated by both endogenous and synthetic cannabinoid agonists in a CB2-dependent manner. This was demonstrated using both antagonists and CB2-deficeint mice. However, caution must be used when extrapolating these in vitro data to disease models because depending upon the cannabinoid used, the dosage of the cannabinoid, the cell type and the activation status of the immune cell both enhancing and suppressive activities are possible. Thus much still needs to be learned regarding the complexity of the cannabinoid system in regards to immune regulation. The intracellular signaling events initiated upon CB2-ligand binding are discussed below in an attempt to explain the complex nature of CB2-mediated immune regulation.
CB2 signaling
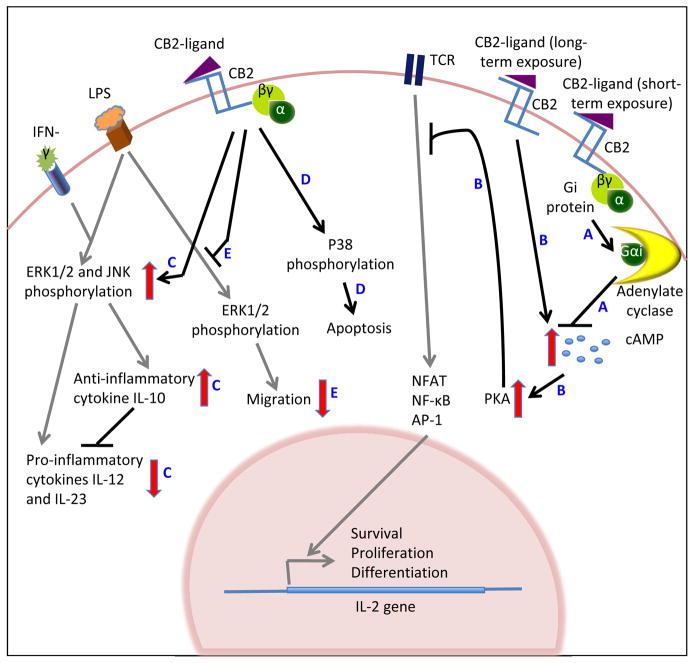
CB2 is known to signal primarily through heterotrimeric Gi-proteins. The complex pharmacology of GPCR has been increasingly appreciated over the years. The most widely studied attributes are the initiation of complex signal cascades and crosstalk with other signaling pathways. Likewise, CB2, upon ligand binding, has been shown to regulate a diverse array of signaling pathways (Figure 1), the most studied of which is adenylate cyclase. The adenylate cyclase pathway plays an important role in immune cell functions. Activation of adenylate cyclase leads to cAMP accumulation in the cell, which in turn activates protein kinase A (PKA). Activated PKA phosphorylates the cAMP response element binding protein, which is a transcription factor that initiates transcription of a number of genes including IL-2. These gene products in turn facilitate survival, proliferation and differentiation of immune cells. Interestingly, CB2 is known to modulate adenylate cyclase activity both positively and negatively depending on the duration of incubation with its ligands. A short-term incubation (30 min) of human CB2-transfected CHO cells with the cannabiniod CP55940, that binds both CB1 and CB2, inhibited forskolin-induced cAMP accumulation (Fig. 1-A), which was reversed by the CB2-selective antagonist/inverse agonist SR144528 (41). However, a prolonged incubation (2–48 h) of Jurkat or primary human T cells with the CB2-selective agonist JWH-015 resulted in a pronounced and long-lasting increase in intracellular cAMP levels (Fig. 1-B) (54). As discussed above, cAMP is known to upregulate IL-2 production by T cells. However, in this study, when T cells were activated by cross-linking the TCR and CD28 following long-term incubation with JWH-015, they found that the JWH-015-mediated increase in cAMP correlated with dampened TCR signaling and decreased IL-2 production (Fig. 1-B) (54). These data demonstrate that the CB2 signaling pathway can crosstalk with and modulate the TCR signaling pathway and likely has the capacity to intersect with others allowing the regulation of diverse cellular responses.
Figure 1.
Signaling pathways induced following CB2 engagement with cannabinoid ligands. Black and red arrows indicate CB2-mediated signaling events, while grey arrows represent other receptor-mediated pathways. CB2 ligation by cannabinoid ligands regulates adenylate cyclase activation (A), which controls PKA activation (B). PKA crosstalks with the TCR signaling pathway attenuating cytokine production (B). CB2 ligation also directly activates MAPK resulting both the positive and negative regulation of cellular functions (C, D). CB2 signaling can also block MPAK signaling induced by other external stimuli (E).
Another major signaling arm that plays a critical role in both immune cell development and immune responses to pathogens is the mitogen-activated protein kinase (MAPK) signaling cascades. These signaling pathways are turned on primarily in response to extracellular stimuli including mitogens, heat, osmotic stresses and cytokines; and regulate a number of immune cell functions including cytokine production, proliferation, apoptosis and migration. There are three major MAPKs: extracellular signal-regulated protein kinases (ERK), p38 MAPK, and c-Jun NH2-terminal kinases (JNK). CB2 has been found to regulate all three MAPK. Like cAMP, there exists evidence for both positive and negative regulation of the different MAPK by CB2. WIN 55,212-2, an agonist for both CB1 and CB2, induced p38 MAPK activation in mantle cell lymphoma cells in a CB2-dependent manner leading to increased apoptosis (Fig. 1-D) (55). Of interest is the finding that normal B cells were unaffected by similar treatments (55). Likewise, anandamide was shown to enhance LPS/IFN-γ-induced activation of ERK1/2 and JNK MAPK in mouse microglial cells through CB2 (50, 51). This in turn lead to a modest increase in IL-10, and inhibited the production of the pro-inflammatory cytokines IL-12 and IL-23 by LPS/IFN-γ-activated microglial cells, as discussed above (Fig. 1-C). In the above studies, the involvement of CB2 was determined using the CB2-selective antagonist SR144528. In contrast, mouse microglial cells when stimulated with LPS in the presence of JWH-015, ERK1/2 phosphorylation as well as migration towards ADP was reduced (44) (Fig. 1-E). Similarly, the long-term incubation of primary human or Jurkat T cells with JWH015 (2–48 h) also abrogated anti-CD3 and anti-CD28-induced MAPK phosphorylation (54).
Apart from the cAMP and MAPK pathways, CB2 has been reported to regulate other signaling molecules including the Rho/Rac and Rap family proteins. These are small GTP-binding proteins that switch between an inactive (GDP-bound) and active (GTP-bound) form. The active proteins bind to downstream effector molecules, which in turn regulate a number of cellular events including cytoskeletal rearrangement, cell polarization, migration and cell cycle progression. In regards to migration, the CB2-selective agonist AM1241 was shown to activate Rac1 activation in a pertussis toxin-dependent manner, which is consistent with its role in promoting the chemotaxis of human hematopoietic stem cells in vitro (56). Using a neutrophil-like human promyelocytic HL-60 cell line it was shown that the CB2-selective agonist JWH-015 induced chemotaxis that was associated with inhibition of RhoA-GTP and activation of Rac-1, Rac-2 and Cdc42 (38). Interestingly, in human neutrophils, JWH-015 alone did not induce migration or the activation of RhoA or Rac1/2, but was able to inhibit the activation of RhoA in response to N-formyl-L-metionyl-L-leucyl-L-phenyl-alanine (38). However, CB2-specificity was not determined in these studies (38). Although these data provide mechanistic insight into how CB2 regulates migration, much still needs to be learned regarding how CB2 signaling impacts the activation of GTPases.
One emerging theme as to how CB2 can regulate such a vast array of cellular processes is crosstalk with receptor-mediated signaling pathways. Furthermore, an additional layer of complexity is that different CB2 ligands can initiate distinct intracellular signaling events in the same cellular environment. Shoemaker and colleagues investigated signaling mediated by the endocannabinoids 2-AG and noladin ether and the synthetic agonist CP55940 in human CB2 receptor-transfected CHO cells (57). They found that 2-AG most potently activated ERK-MAPK, but noladin ether and CP55940 inhibited adenylate cyclase more efficiently. While the CB2 antagonist AM630 inhibited the above signaling events, it had no effect on 2-AG-induced transient intracellular Ca2+ release (57). Interestingly, the CB2 antagonist SR144528 was able to partially inhibit the calcium flux (57). Several explanations for these differential outcomes include differences in receptor ligand affinity and occupancy sites (57). Adding another level of complexity, Bouaboula and colleagues also using CB2-transfected CHO cells, demonstrated that CB2 possessed a high constitutive activity that was blocked by SR144528 functioning as an inverse agonist (58). To fully understand how CB2 signaling impacts its many biological functions, knowledge gaps in the regulation of CB2 surface expression and mechanism of CB2 activation and desensitization still need to be filled.
Biological effects of CB2: In vivo studies
Taken together, the in vitro and signaling studies provide a general theme that CB2 is an important regulator of biological processes essential to immune responses. However, in vitro studies focusing on single cell types are only a generalized model for the complex in vivo immune responses that require multiple immune cell types, numerous activation stimuli and a multitude of cytokine/chemokines. Thus based on in vitro experimentation alone, it is difficult to predict when and how targeting of CB2 would suppress or enhance immune responses in vivo. The study of CB2 in both healthy and diseased animals is beginning to provide some clues as to the types of cells and diseases that can be efficiently and safely targeted via their CB2 receptors. These studies have been aided by the generation of CB2−/− mice. Currently, two CB2−/− mice strains are available. The first was generated by Buckley and coworkers (25) and the second by Deltagen Inc. CB2−/− mice are viable and breed normally. In vivo, CB2−/− mice were shown to have a significant reduction in marginal zone B, splenic memory CD4 T, intestinal intraepithelial NK and NK T cells, suggesting a critical role for CB2 in their development and/or maintenance (59). However, the mechanism by which CB2 regulates the development/maintenance of these cells is not understood. One potential mechanism as suggested by in vitro studies is adhesion and migration, which were recently demonstrated to play a role in the retention of immature B cells in BM sinusoids (60). These studies demonstrate that in unchallenged animals, CB2 has specific effects on immune cells that could be further manifested during disease processes.
Some of the first modern data linking CB2 to immune regulation was the emergence of evidence that marijuana users were more susceptible to infections (27). This link has now been more solidly established using mouse models of human infection. Legionella pneumophila, the bacterium causing legionnaires’ disease in humans, is also known to infect mice, and leads to an immediate increase in serum IFN-γ and IL-12, and the subsequent development of a robust Th1 type response (61). A robust Th1 response is important for the survival of mice after a secondary infection with lethal doses of L. pneumophila. In vivo administration of Δ9-THC prior to the primary infection was found to suppress serum levels of IFN-γ and IL-12 and the production of IFN-γ by T cells stimulated in vitro in an antigen non-specific manner (61, 62). In addition, THC administration led to increased mortality following secondary challenge with L. pneumophila at lethal doses (62). The i.v. administration of CB1 or CB2 selective antagonists partially reversed the inhibitory effect of THC on cytokine production, suggesting roles for both receptors (61). Of importance, L. pneumophila infection was one of the first models to demonstrate that CB2 is capable of suppressing Th1 type immune responses (62).
Sepsis is a systemic illness that occurs when bacteria enter the blood stream. The subsequent bacteremia can lead to organ failure and death that at least in part, are thought to be mediated by the exaggerated production of cytokines, chemokines and other inflammatory mediators. To evaluate whether CB2 would positively or negatively influence sepsis, the cecal ligation puncture (CLP) model was used whereby the cecum was punctured with a needle causing peritonitis and rapid translocation of bacteria into the blood stream. In a severe model of sepsis, where the cecum was perforated twice (through and through), CB2−/− mice had significantly lower bacterial loads and a significantly higher survival rate as compared to WT mice (63). This was correlated with lower levels of both the anti-inflammatory cytokine IL-10 and the pro-inflammatory cytokine IL-6 and the chemokine MIP-2. These data suggest that CB2 restricts the immune response required for bacterial clearance allowing for the development of a detrimental inflammatory response. In contrast, when the same model was used causing a milder sepsis (one puncture), CB2−/− mice had both a higher bacterial burden and mortality rate compared to controls, as well as increased levels of pro-inflammatory cytokines and chemokines (64). This model suggests that CB2 enhances the immune response leading to bacterial clearance. Although the reasons for the contrasting results are not known, the CLP model highlights the difficulties in designing experiments to determine how CB2 regulates immune responses. Since CLP models involve many immune cell types, in such a complex system with a global deficiency in CB2, it is not possible to determine which immune and inflammatory parameters are positively and which are negatively regulated by CB2.
Role for CB2 in inflammatory and autoimmune diseases
Although some studies reported enhancement of immune cell functions by CB2, several others suggested its immunosuppressive potential. CB2-mediated immune suppression has also been evidenced in tumor studies, where Δ9-THC inhibited anti-tumor immunity in mice by a CB2-receptor-mediated mechanism by upregulating the anti-inflammatory cytokines IL-10 and TGF-β (65). Therefore, to explore whether CB2 could be a potential anti-inflammatory therapeutic target, its role has been vastly studied in different animal models of inflammatory and autoimmune diseases.
Allergic contact dermatitis (ACD) is a hypersensitivity of the skin to specific allergens. The pathogenesis is caused by inflated immune reactions to the allergen involving multiple immune cells (66). A commonly used mouse model for this condition is the 2,4-dinitro-1-fluorobenzene (DNFB)-induced cutaneous contact hypersensitivity model, where mice are repeatedly challenged with DNFB on the ear following an initial sensitization on the shaved abdomen. Ear swelling is measured as a clinical symptom. Using this model, a complex involvement of CB2 was found in ACD. CB2−/− mice or CB2 blocking in WT mice by subcutaneous administration of the CB2-antagonist SR144528 30 min before and after DNFB challenge demonstrated significant increased ear swelling compared to WT or vehicle treatment groups (67). This suggested that CB2 limits the immune reaction against DNFB thereby alleviating the symptoms of the cutaneous contact hypersensitivity. In contrast, in the same model, subcutaneous or topical application of the CB2-selective agonist HU-308 significantly increased ear swelling as compared to vehicle treatment (67). In addition, the CB2-selective antagonists/inverse agonists JTE-907 and SR144528, when administered orally prior to and post-challenge with DNFB, attenuated ear swelling (68). Furthermore, in a similar oxalozone-induced allergic contact hypersensitivity model, topically administered SR144528 (immediately after challenge) was also found to attenuate ear swelling (69). These cumulative results indicate that CB2 can both exaggerate and block inflammatory responses in ACD. These contradictory results, like sepsis, are difficult to explain and again emphasize the difficulties in understanding CB2-mediated regulation of complex immune responses involving many immune cells and multiple pro- and anti-inflammatory parameters.
Systemic sclerosis (SSc) is a connective tissue disorder, characterized by vascular damage, collagen deposition and extensive fibrosis in the skin and visceral organs. Uncontrolled immune responses are thought to contribute to disease initiation. The production of autoantibodies, including antibodies against centromere and DNA topoisomerase-I, is commonly found in patients (70, 71). In mice, repeated subcutaneous administration of the reactive oxygen species generating agent hypochlorite (HOCl) induces SSc that mimics human symptoms (72). In this model, CB2−/− mice had more severe disease showing significantly increased dermal thickness, greater skin and lung damage, higher collagen deposition in the skin and the lung, and anti-DNA topoisomerase-1 antibody production in the sera as compared to WT mice (73). Targeting CB2 with the CB2-selective agonist JWH-133 significantly attenuated clinical disease (73). These results suggested that CB2 restricts exaggerated immune responses that lead to tissue damage and that administration of exogenous CB2-selective agonists could be beneficial for the treatment of SSc.
Autoimmune uveitis is a human autoimmune disease of the eye that can cause significant vision loss leading to blindness if untreated. Its pathogenesis includes a prominent immune axis (74, 75). Experimental autoimmune uveoretinitis is its animal model and disease is induced by immunization with ocular antigens such as interphotoreceptor retinoid binding protein (IRBP) peptide. Using this model, Xu and colleagues studied the effects of administering the CB2-selective agonist JWH-133 daily for 7 or 15 days post-immunization in modulating the severity of disease. JWH-133 significantly suppressed leukocyte rolling in the retinal venules, cellular infiltration in the eyes as well as clinical symptoms (76). In addition, in vitro stimulation of splenocyte and lymph node cells from the JWH-133-treated animals with IRBP peptide resulted in reduced proliferation and cytokine production (76). These data are supportive of an immunosuppressive role for CB2 in the context of IRBP-induced autoimmune uveoretinitis in mice.
Inflammatory bowel diseases including ulcerative colitis and Crohn’s disease are chronic intestinal disorders, where the epithelium lining the gut lumen and the mucosa is severely damaged. Besides genetic contributions, inflammatory responses play a critical role in the establishment of disease pathogenesis (77). Experimental colitis is induced in animals by intrarectal or intracolonic administration of a variety of chemicals including trinitrobenzene-sulfonic acid (TNBS), oxalozone or allyl isothiocyanate, or oral administration of dextran sodium sulfate (DSS) in the drinking water. In the TNBS-induced mouse model of colitis, intraperitoneal administration of the CB2-selective agonists JWH-133 or AM1241 given just before or after colitis induction, resulted in a significant reduction in macroscopic damage, as determined by the presence of ulcers, adhesions, shortening of the colon, hemorrhage, fecal blood and diarrhea, as well as microscopic damage as assessed by histology of colon sections (78). Myeloperoxidase activity in the colon, which is indicative of the extent of neutrophil accumulation was also markedly reduced (78). These inhibitory effects were absent in CB2−/− mice and disease was aggravated by administration of the CB2-selective antagonist/inverse agonist AM630 (78). JWH-133 was also reported to suppress macroscopic and microscopic damage in the DSS and allyl isothiocyanate-induced models of colitis (79). Collectively, these studies indicate that CB2 dampens detrimental inflammation leading to tissue damage in colitis.
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system characterized by sensory and motor weaknesses, tremor and ataxia. Although the complex etiology of MS is not completely understood multiple immune cells including T cells, macrophages and B cells are thought to contribute to disease pathogenesis by inducing autoimmunity against CNS antigens that results in the formation of inflammatory lesions, demyelination and neuronal damage (80). Although little is known regarding a role for CB2 in MS, CB2 gene expression was found to be elevated in the peripheral blood of primary progressive MS patients as compared to controls (81). More convincing evidence has been generated using animal models of MS including experimental autoimmune encephalomyelitis (EAE) and Theiler’s murine encephalomyelitis virus (TMEV).
In the TMEV infection model, administration of the CB2 selective agonist JWH-015 or the CB1/CB2 agonist WIN55212-2 after disease establishment significantly inhibited microglia activation and CD4 T cell accumulation in the spinal cord with a concomitant improvement in motor function and spinal cord remyelination (82). In myelin oligodendrocyte glycoprotein-induced EAE, WIN55212-2 inhibited leukocyte rolling and adhesion in the cerebral microcirculation, which was reversed by a CB2, but not a CB1, antagonist (83). The blockage of immune cells entering the CNS is one mechanism whereby CB2 could reduce the symptoms of EAE (83).
However, the interpretation of the pharmacological use of cannabinoids can be complex since CB2-selective agonists and antagonists are not specific for CB2. Therefore, it is possible that at high doses, they bind to and function through other cannabinoid receptors or in a receptor independent manner. For example, in vivo administration of the CB2-selective agonists JWH-133 (Ki CB2 = 3 nM, CB1 = 680 nM) and RWJ400065 (Ki CB2 = 10 nM, CB1 = 600 nM) were reported to reduce spasticity associated with EAE in mice (84). However, in the same model, another CB2-selective agonist JWH056, that had lower affinity for CB1 (Ki CB2 = 32 nM, CB1 = 8770 nM) than JWH-133 or RWJ400065 could not suppress spasticity and interestingly, RWJ400065-mediated inhibition in spasticity was completely absent in CB1−/− mice (85). These data strongly suggested that at high concentrations, CB2-selective agonists might produce suppressive effects through receptors other than CB2, and highlight the importance of using CB2−/− mice for definitive determination of CB2-mediated effects. Of note, our laboratory was the first to demonstrate the immunosuppressive effect of CB2 on encephalitogenic T cells during EAE using CB2−/− mice (32). This was achieved by comparing EAE induced by myelin-specific T cells with or without CB2. We found that EAE disease severity, the number of encephalitogenic T cells in the CNS and IFN-γ production within the CNS were significantly increased when EAE was induced with CB2−/− T cells (32). The more severe disease was linked to hyperproliferation and increased cytokine production of CB2−/− encephalitogenic T cells. Interestingly, in this same report, the Baker laboratory demonstrated that THC inhibition of EAE was mediated by CB1 expressed on neurons (32). Although many cell types are involved in the induction and manifestation of EAE, this was the first study to identify the cellular targets of cannabinoids in the treatment of autoimmune disease.
Collectively, the knowledge gained from a number of vivo disease studies, indicates that CB2 expressed by immune cells is a good therapeutic target for the treatment of autoimmune diseases where suppression of immune responses is the goal. However, in infectious disease and cancer, immune suppression could be detrimental. Since both endogenous and synthetic cannabinoids bind to multiple cannabinoid receptors with differing affinities, their biological outcomes cannot be accurately predicted. Thus, more careful and comprehensive studies are needed if we are to understand which immune cells are positively or negatively regulated by CB2 and to gain the ability to channel CB2-mediated effects in a specific direction.
Concluding remarks
CB2, a component of the endocannabinoid system primarily expressed by immune cells, clearly participates in immune homeostasis and responses to pathogens. Since the discovery of CB2, the complexity of its regulation of immune responses has been revealed. Both in vitro and in vivo studies indicate that CB2 is capable of suppressing immune responses, which raised the possibility that CB2-selective agonists could potentially be used as anti-inflammatory therapeutics. However, CB2 in certain models was shown to induce or aggravate inflammatory responses accompanied by CB2-selective antagonists/inverse agonists alleviating inflammation. Thus a clear understanding of the factors and molecular mechanisms that influence these contrasting outcomes is still forthcoming. It is possible that various cellular components of the immune system are regulated differentially by CB2 and that different inflammatory microenvironments shape the CB2-mediated responses in distinct ways. The complexity of CB2-mediated immune regulation is evident from signaling studies, where CB2 was found to regulate a wide array of signaling events in different cell types. In addition, its ability to extensively crosstalk with other receptor-mediated signaling pathways adds to its complexity. Additional challenges in interpreting the effects of CB2 in immune responses include a poor understanding of the regulation of its surface expression, activation and desensitization. Moreover, it is likely that individual CB2-selective agonists would evoke distinct signaling events leading to varied biological effects. Therefore, although targeting of CB2 clinically is a tempting approach for the treatment of specific inflammatory disorders, thorough investigation of lead compounds using both in vivo and in vitro approaches should be conducted. Finally, due to the pharmacology of the CB2 receptor, finding the correct dosage will be critical for the safe and efficacious targeting of CB2 for the treatment of human diseases.
Acknowledgments
We thank Dr. C.C. Hillard for helpful discussions on cannabinoid biology, Dr. A. Ray for suggestions on the manuscript and L. Gaspard for administrative assistance.
References
- 1.Salzet M, Breton C, Bisogno T, Di Marzo V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur J Biochem. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- 2.Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 3.O’Shaughnessy WB. On the preparations of the Indian hemp, or Gunjah (Cannabis Indica); Their effects on the animal system in health, and their utility in the treatment of tetnus and other convulsive diseases. Trans Med Phy Soc, Bengal. 1839;71:421–426. [PMC free article] [PubMed] [Google Scholar]
- 4.Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of Hashish. J Amer Chem Soc. 1964;86:1646–1647. [Google Scholar]
- 5.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 6.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 7.Gerard C, Mollereau C, Vassart G, Parmentier M. Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res. 1990;18:7142. doi: 10.1093/nar/18.23.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- 9.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 10.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 11.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Itoh K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 13.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti JM, Walker P, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2004;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 17.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 18.Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- 19.Glass M, Northup JK. Agonist selective regulation of G proteins by cannabinoid CB(1) and CB(2) receptors. Mol Pharmacol. 1999;56:1362–1369. doi: 10.1124/mol.56.6.1362. [DOI] [PubMed] [Google Scholar]
- 20.Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2002;292:886–894. [PubMed] [Google Scholar]
- 21.Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessegue B, Bonnin-Cabanne O, Le Fur G, Caput D, Ferrara P. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta. 1996;1307:132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- 22.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- 23.Sugiura T, Waku K. Cannabinoid receptors and their endogenous ligands. J Biochem. 2002;132:7–12. doi: 10.1093/oxfordjournals.jbchem.a003200. [DOI] [PubMed] [Google Scholar]
- 24.Kaminski NE, Abood ME, Kessler FK, Martin BR, Schatz AR. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol Pharmacol. 1992;42:736–742. [PMC free article] [PubMed] [Google Scholar]
- 25.Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur J Pharmacol. 2002;396:141–149. doi: 10.1016/s0014-2999(00)00211-9. [DOI] [PubMed] [Google Scholar]
- 26.Buckley NE. The peripheral cannabinoid receptor knockout mice: an update. Br J Pharmacol. 2008;153:309–318. doi: 10.1038/sj.bjp.0707527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein TW, Friedman H, Specter S. Marijuana, immunity and infection. J Neuroimmunol. 1998;83:102–115. doi: 10.1016/s0165-5728(97)00226-9. [DOI] [PubMed] [Google Scholar]
- 28.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat Rev Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 29.Carayon P, Marchand J, Dussossoy D, Derocq JM, Jbilo O, Bord A, Bouaboula M, Galiegue S, Mondiere P, Penarier G, Fur GL, Defrance T, Casellas P. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood. 1998;92:3605–3615. [PubMed] [Google Scholar]
- 30.Carlisle SJ, Marciano-Cabral F, Staab A, Ludwick C, Cabral GA. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int Immunopharmacol. 2002;2:69–82. doi: 10.1016/s1567-5769(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay S, Das S, Williams EA, Moore D, Jones JD, Zahm DS, Ndengele MM, Lechner AJ, Howlett AC. Lipopolysaccharide and cyclic AMP regulation of CB(2) cannabinoid receptor levels in rat brain and mouse RAW 264. 7 macrophages. J Neuroimmunol. 2006;181:82–92. doi: 10.1016/j.jneuroim.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng Y, Carrie EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB(1) on neurons and CB(2) on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- 33.Jorda MA, Verbakel SE, Valk PJ, Vankan-Berkhoudt YV, Maccarrone M, Finazzi-Agro A, Lowenberg B, Delwel R. Hematopoietic cells expressing the peripheral cannabinoid receptor migrate in response to the endocannabinoid 2-arachidonoylglycerol. Blood. 2002;99:2786–2793. doi: 10.1182/blood.v99.8.2786. [DOI] [PubMed] [Google Scholar]
- 34.Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J Biol Chem. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- 36.Maestroni J. The endogenous cannabinoid 2-arachidonoyl glycerol as in vivo chemoattractant for dendritic cells and adjuvant for Th1 response to a soluble protein. Faseb J. 2004;18:1914–1916. doi: 10.1096/fj.04-2190fje. [DOI] [PubMed] [Google Scholar]
- 37.Oka S, Ikeda S, Kishimoto S, Gokoh M, Yanagimoto S, Waku K, Sugiura T. 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, induces the migration of EoL-1 human eosinophilic leukemia cells and human peripheral blood eosinophils. J Leukoc Biol. 2004;76:1002–1009. doi: 10.1189/jlb.0404252. [DOI] [PubMed] [Google Scholar]
- 38.Kurihara R, Tohyama T, Matsusaka S, Naruse H, Kinoshita E, Tsujioka T, Katsumata Y, Yamamura H. Effects of peripheral cannabinoid receptor ligands on motility and polarization in neutrophil-like HL60 cells and human neutrophils. J Biol Chem. 2006;281:12908–12918. doi: 10.1074/jbc.M510871200. [DOI] [PubMed] [Google Scholar]
- 39.Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–223. doi: 10.1093/jb/mvi021. [DOI] [PubMed] [Google Scholar]
- 40.Tanikawa T, Kurohane K, Imai Y. Induction of preferential chemotaxis of unstimulated B-lymphocytes by 2-arachidonoylglycerol in immunized mice. Microbiol Immunol. 2007;51:1013–1019. doi: 10.1111/j.1348-0421.2007.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 42.Raborn ES, Marciano-Cabral F, Buckley NE, Martin BR, Cabral GA. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J Neuroimmune Pharmacol. 2008;3:117–129. doi: 10.1007/s11481-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294:H1145–1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- 44.Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25–39. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosh S, Preet A, Groopman JE, Ganju RK. Cannabinoid receptor CB2 modulates the CXCL12/CXCR4-mediated chemotaxis of T lymphocytes. Mol Immunol. 2006;43:2169–2179. doi: 10.1016/j.molimm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Derocq JM, Segui M, Marchand J, Le Fur G, Casellas P. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 1995;369:177–182. doi: 10.1016/0014-5793(95)00746-v. [DOI] [PubMed] [Google Scholar]
- 47.Carrier EJ, Kearn CS, Barkmeier AJ, Breese W, Yang K, Nithipatikom, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999. [DOI] [PubMed] [Google Scholar]
- 48.Do Y, McKallip RJ, Nagarkatti M, Nagarkatti PS. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-kappaB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J Immunol. 2004;173:2373–2382. doi: 10.4049/jimmunol.173.4.2373. [DOI] [PubMed] [Google Scholar]
- 49.Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: potential role for CB2-selective ligands as immunosuppressive agents. Clin Immunol. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Correa F, Docagne F, Mestre L, Clemente D, Hernangomez M, Loria F, Guaza C. A role for CB2 receptors in anandamide signalling pathways involved in the regulation of IL-12 and IL-23 in microglial cells. Biochem Pharmacol. 2009;77:86–100. doi: 10.1016/j.bcp.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Correa F, Hernangomez M, Mestre L, Loria F, Spagnolo A, Docagne F, Di Marzo V, Guaza C. Anandamide enhances IL-10 production in activated microglia by targeting CB(2) receptors: roles of ERK1/2, JNK, and NF-kappaB. Glia. 2010;58:135–147. doi: 10.1002/glia.20907. [DOI] [PubMed] [Google Scholar]
- 52.Agudelo M, Newton C, Widen R, Sherwood T, Nong L, Friedman H, Klein TW. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol. 2008;3:35–42. doi: 10.1007/s11481-007-9088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chuchawankul S, Shima M, Buckley NE, Hartmann CB, McCoy KL. Role of cannabinoid receptors in inhibiting macrophage costimulatory activity. Int Immunopharmacol. 2004;4:265–278. doi: 10.1016/j.intimp.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Borner C, Smida M, Hollt V, Schraven B, Kraus J. Cannabinoid receptor type 1- and 2-mediated increase in cyclic AMP inhibits T cell receptor-triggered signaling. J Biol Chem. 2009;284:35450–35460. doi: 10.1074/jbc.M109.006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gustafsson K, Christensson B, Sander B, Flygare J. Cannabinoid receptor-mediated apoptosis induced by R(+)-methanandamide and Win55,212-2 is associated with ceramide accumulation and p38 activation in mantle cell lymphoma. Mol Pharmacol. 2006;70:1612–1620. doi: 10.1124/mol.106.025981. [DOI] [PubMed] [Google Scholar]
- 56.Jiang S, Alberich-Jorda M, Zagozdzon R, Parmar K, Fu Y, Mauch P, Banu N, Makriyannis A, Tenen DG, Avraham S, Groopman JE, Avraham HK. Cannabinoid receptor 2 and its agonists mediate hematopoiesis and hematopoietic stem and progenitor cell mobilization. Blood. 2011;117:827–838. doi: 10.1182/blood-2010-01-265082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Shoemaker JL, Ruckle MB, Mayeux PR, Prather L. Agonist-directed trafficking of response by endocannabinoids acting at CB2 receptors. J Pharmacol Exp Ther. 2005;315:828–838. doi: 10.1124/jpet.105.089474. [DOI] [PubMed] [Google Scholar]
- 58.Bouaboula M, Desnoyer N, Carayon P, Combes T, Casellas P. Gi protein modulation induced by a selective inverse agonist for the peripheral cannabinoid receptor CB2: implication for intracellular signalization cross-regulation. Mol Pharmacol. 1999;55:473–480. [PubMed] [Google Scholar]
- 59.Ziring D, Wei B, Velazquez P, Schrage M, Buckley NE, Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- 60.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klein TW, Newton CA, Nakachi N, Friedman H. Delta 9-tetrahydrocannabinol treatment suppresses immunity and early IFN-gamma, IL-12, and IL-12 receptor beta 2 responses to Legionella pneumophila infection. J Immunol. 2000;164:6461–6466. doi: 10.4049/jimmunol.164.12.6461. [DOI] [PubMed] [Google Scholar]
- 62.Newton CA, Klein TW, Friedman H. Secondary immunity to Legionella pneumophila and Th1 activity are suppressed by delta-9-tetrahydrocannabinol injection. Infect Immun. 1994;62:4015–4020. doi: 10.1128/iai.62.9.4015-4020.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Csoka B, Nemeth ZH, Mukhopadhyay P, Spolarics Z, Rajesh M, Federici S, Deitch EA, Batkai S, Pacher P, Hasko G. CB2 cannabinoid receptors contribute to bacterial invasion and mortality in polymicrobial sepsis. PLoS One. 2009;4:e6409. doi: 10.1371/journal.pone.0006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tschop J, Kasten KR, Nogueiras R, Goetzman HS, Cave CM, England LG, Dattilo J, Lentsch AB, Tschop MH, Caldwell CC. The cannabinoid receptor 2 is critical for the host response to sepsis. J Immunol. 2009;183:499–505. doi: 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu LX, Sharma S, Stolina M, Gardner B, Roth MD, Tashkin DP, Dubinett SM. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J Immunol. 2000;165:373–380. doi: 10.4049/jimmunol.165.1.373. [DOI] [PubMed] [Google Scholar]
- 66.Vocanson M, Hennino A, Rozieres A, Poyet G, Nicolas JF. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009;64:1699–1714. doi: 10.1111/j.1398-9995.2009.02082.x. [DOI] [PubMed] [Google Scholar]
- 67.Karsak M, Gaffal E, Date R, Wang-Eckhardt L, Rehnelt J, Petrosino S, Starowicz K, Steuder R, Schlicker E, Cravatt B, Mechoulam R, Buettner R, Werner S, Di Marzo V, Tuting T, Zimmer A. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 68.Ueda Y, Miyagawa N, Matsui T, Kaya T, Iwamura H. Involvement of cannabinoid CB(2) receptor-mediated response and efficacy of cannabinoid CB(2) receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur J Pharmacol. 2005;520:164–171. doi: 10.1016/j.ejphar.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 69.Oka S, Wakui J, Gokoh M, Kishimoto S, Sugiura T. Suppression by WIN55212-2, a cannabinoid receptor agonist, of inflammatory reactions in mouse ear: Interference with the actions of an endogenous ligand, 2-arachidonoylglycerol. Eur J Pharmacol. 2006;538:154–162. doi: 10.1016/j.ejphar.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 70.Bunn CC, Black CM. Systemic sclerosis: an autoantibody mosaic. Clin Exp Immunol. 1999;117:207–208. doi: 10.1046/j.1365-2249.1999.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nihtyanova SI, Denton CP. Autoantibodies as predictive tools in systemic sclerosis. Nat Rev Rheumatol. 2010;6:112–116. doi: 10.1038/nrrheum.2009.238. [DOI] [PubMed] [Google Scholar]
- 72.Servettaz A, Goulvestre C, Kavian N, Nicco C, Guilpain P, Chereau C, Vuiblet V, Guillevin L, Mouthon L, Weill B, Batteux F. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J Immunol. 2009;182:5855–5864. doi: 10.4049/jimmunol.0803705. [DOI] [PubMed] [Google Scholar]
- 73.Servettaz A, Kavian N, Nicco C, Deveaux V, Chereau C, Wang A, Zimmer A, Lotersztajn S, Weill B, Batteux F. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. Am J Pathol. 2010;177:187–196. doi: 10.2353/ajpath.2010.090763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caspi RR. A look at autoimmunity and inflammation in the eye. J Clin Invest. 2010;120:3073–3083. doi: 10.1172/JCI42440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nussenblatt RB, Gery I. Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory disease. J Autoimmun. 1996;9:575–585. doi: 10.1006/jaut.1996.0077. [DOI] [PubMed] [Google Scholar]
- 76.Xu H, Cheng CL, Chen M, Manivannan A, Cabay L, Pertwee RG, Coutts A, Forrester JV. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J Leukoc Biol. 2007;82:532–541. doi: 10.1189/jlb.0307159. [DOI] [PubMed] [Google Scholar]
- 77.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G364–371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- 80.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 81.Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA, Constantinescu CS. Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci. 2009;287:212–215. doi: 10.1016/j.jns.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 82.Arevalo-Martin A, Vela JM, Molina-Holgado E, Borrell J, Guaza C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J Neurosci. 2003;23:2511–2516. doi: 10.1523/JNEUROSCI.23-07-02511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler MW, Tuma RF. Win 55212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- 84.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- 85.Pryce G, Baker D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br J Pharmacol. 2007;150:519–525. doi: 10.1038/sj.bjp.0707003. [DOI] [PMC free article] [PubMed] [Google Scholar]