Abstract
Aim
To investigate the prevalence of, and demographic associations with, uncorrected refractive error (URE) in an older British population.
Methods
Data from 4428 participants, aged 48–89 years, who attended an eye examination in the third health check of the European Prospective Investigation into Cancer-Norfolk study and had also undergone an ophthalmic examination were assessed. URE was defined as ≥1 line improvement of visual acuity with pinhole-correction in the better eye in participants with LogMar presenting visual acuity (PVA) <0.3 (PVA <6/12). Refractive error was measured using an autorefractor without cycloplegia. Myopia was defined as spherical equivalent ≤−0.5 dioptre, and hypermetropia ≥0.5 dioptre.
Results
Adjusted to the 2010 midyear British population, the prevalence of URE in this Norfolk population was 1.9% (95% CI 0.6% to 3.1%). Lower self-rated distance vision was correlated with higher prevalence of URE (ptrend<0.001). In a multivariate logistic regression model adjusting for age, gender, retirement status, educational level and social class, independent significant associations with URE were increasing age (ptrend<0.001) and having hypermetropic or myopic refractive error. Wearing distance spectacles was inversely associated with URE (OR 0.34, 95% CI 0.21 to 0.55, p<0.001). There were 3063 people (69.2%) who wore spectacles/contact lenses for distance vision. Spectacle wear differed according to type of refractive error (p<0.001), and use rose with increasing severity of refractive error (ptrend<0.001).
Conclusion
Although refractive error is common, the prevalence of URE was found to be low in this population reflecting a low prevalence of PVA<0.3.
INTRODUCTION
Uncorrected refractive error (URE) has been identified as the most common cause of visual impairment and the second most common cause of blindness worldwide.1 Although easily corrected with spectacles, URE may adversely affect performance at school or the workplace and be associated with impaired quality of life.1 In 2007 there were 158.1 million cases of visual impairment resulting from URE of which 8.7 million were blind.2 URE induced visual impairment was associated with an estimated global economic productivity loss (International $) of I$268.8 billion, after adjustment for country-specific labour force participation and employment rates. Furthermore, URE has been associated with a higher risk of all-cause mortality.3 The prevalence of myopia has been found to be, strongly and positively, correlated with the prevalence of URE.4 There is, therefore, a need for appropriate refractive correction to avoid increases in the prevalence of URE particularly as the prevalence of myopia has been shown to be increasing in certain populations.5
A wide variation in the prevalence of URE worldwide has been reported. In Singapore, prevalence estimates of URE have reached approximately 20%, where URE was associated with increasing age and lower levels of education.6,7 The prevalence of URE in other developed countries including Australia and the USA is lower than reported in Singapore.8,9 In sub-Saharan Africa, URE is a common cause of visual impairment, but blindness due to URE is rare.10 Several studies have investigated URE in the UK and all have indicated that the prevalence of URE was less than the highest reported estimates from East Asia, but several such studies only investigated people aged ≥65 years.11–13 In the 1958 British birth cohort study, reduced uncorrected distance visual acuity (VA) aged 16 years was a poor surrogate for adult myopia status, despite distance VA being an effective way in which to identify myopic and hyperopic refractive error as a teenager.14 Management of refractive error is one of the new priorities of WHO’s Vision 2020 Global Initiative for the Elimination of Blindness, with services for refractive error being targeted at children, the poor and adults aged >50 years.15
The aim of the present study was to determine the prevalence and associations of URE in an older British, mainly Caucasian, population aged 48 years and over.
METHODS
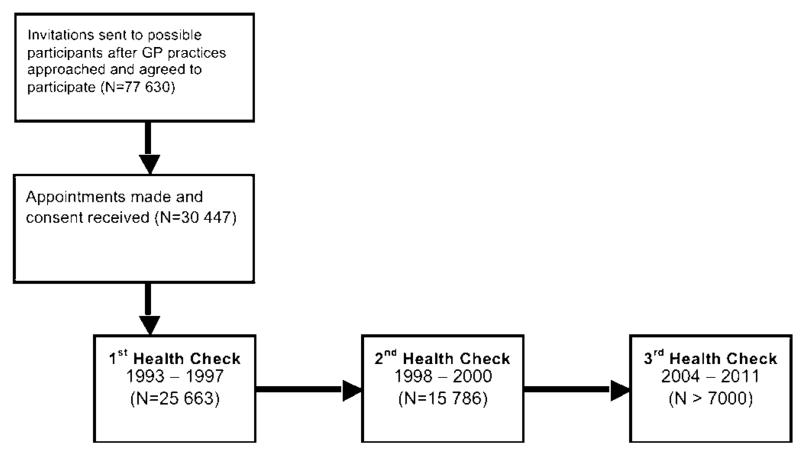
The European Prospective Investigation of Cancer (EPIC) was conceived as a pan-European study of the genetic and environmental determinants of cancer and later expanded to study other health end points in older age.16 Original recruitment in EPIC-Norfolk was via General Practice surgeries in Norwich and surrounding towns and rural regions (figure 1). Initial subjects were representative of the national population with respect to many biometric and clinical characteristics.16 The majority (>85%) participated in the first health check, and the second health check took place 3 years later.
Figure 1.
Timeline of the European Prospective Investigation of Cancer-Norfolk Study. Examination of vision was only conducted in the third health check (2006–2010).
Fully initiated in 2006, the third health check aimed to assess objectively physical, cognitive and ocular characteristics of approximately 7000 participants now aged between 48 and 89 years. The third health examination was reviewed and approved by the East Norfolk and Waverney NHS Research Governance Committee (2005EC07L) and the Norfolk Research Ethics Committee (05/Q0101/191). The work was undertaken in accordance with the principles of the Declaration of Helsinki.
Clinical examination
Refractive error was measured once in each eye using an autorefractor (Humphrey model 500, Humphrey Instruments, San Leandro, California, USA). Cycloplegic eye drops were not used. Participants with bilateral pseudophakia or aphakia and those who had undergone refractive surgery were excluded. Refractive error, expressed in dioptres, was recorded in terms of spherical equivalent, equal to half of the cylinder power added to the spherical power. Refractive error was categorised into six groups.17
Presenting visual acuity (PVA) was measured using a LogMar chart at four metres from the subject who wore their usual distance correction. Among those with a PVA <0.3 (equivalent Snellen PVA<6/12), those who improved ≥1 line with a pinhole-corrected refraction in their better eye were classified as having URE.
Questionnaire methods
All of the demographic components were recorded at the third health check. Level of education attained was categorised as follows: degree or equivalent, A-level or equivalent, O-level or equivalent, and less than O-level or no qualifications. Social class was classified according to the Registrar General’s occupation-based system, and dichotomised into manual and non-manual. Participants were asked the following questions:
Do you wear spectacles/contact lenses (CLs)?
If yes, for what reason? (Distance/Reading/Distance and reading)
How good is your eyesight for seeing things at a distance, like recognising a friend across the street (using spectacles or corrective lens if you usually wear them)? Five responses were possible (Excellent/Very good/Good/Fair/Poor). The lowest two categories (fair and poor) were combined due to low numbers in the poor category.
Statistical methods
Differences between two or more categorical variables were assessed using the χ2 test. Equality of mean between two groups of an unpaired normally distributed variable was assessed using the unpaired t test. Trends across categories were assessed using Cuzick’s non-parametric test for trend. Univariate logistic regression was performed to estimate the OR of having URE (with 95% CI) in one exposure group compared with another. Multivariate logistic regression was utilised to determine independent associations of URE after adjustment for several covariates. The prevalence of URE was adjusted by age and gender using direct standardisation with the age and gender distribution of the Mid-2010 population estimates of the UK as the reference population. Statistical analysis was performed using Stata SE V.10.1 for Macintosh (Stata Corporation). All p values determined were two-tailed. The level of statistical significance was taken as p<0.05.
RESULTS
Compared with the subjects participating in the first health check, subjects with data available on URE (and covariates) were younger (p<0.001), more likely to be female subjects (p=0.01), and had a higher educational attainment (p<0.001) and higher (more professional) social class (p<0.001). Similar associations were found between subjects recruited for the third health check that had available or unavailable data on URE (and covariates). Increasing age (OR 1.24 per decade, 95% CI 1.13 to 1.36, ptrend<0.001) and decreasing education level (OR 0.90, 95% CI 0.84 to 0.97, ptrend =0.005) were associated with having unavailable data. Sex was not associated with having unavailable data (OR female = 0.88, 95% CI 0.75 to 1.04, p=0.102).
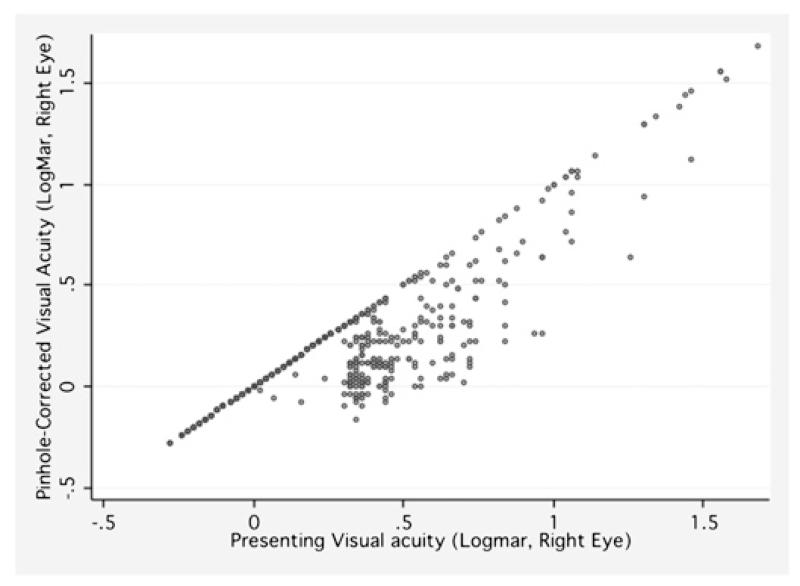
From the 4428 participants with available data in the third health check, and after exclusion of n=153 with (bilateral pseudophakia, aphakia or previous refractive surgery), mean age was 68.4±8.0 years (range 48–89 years) and 2452 (55.4%) were female subjects. Nearly half of participants had hypermetropia (2189; 49.4%) with 1230 (27.8%) having myopia. Mean SE was 0.15±2.3 dioptre. There were 146 people (3.3%) with PVA<0.3 in their better eye, and 743 (16.8%) in the worse eye. A scatter plot between PVA and pinhole-corrected VA reveals only a modest improvement in VA in this population (figure 2).
Figure 2.
Scatter plot of presenting visual acuity and pinhole-corrected visual acuity in the European Prospective Investigation of Cancer-Norfolk Eye Study.
Prevalence estimates of URE
Crude prevalence of URE in this population was 1.8% (95% CI 1.4% to 2.2%). Mean age was higher in people with URE compared with those without URE (73.5 vs 68.3 years, p<0.001). Adjusted to the 2010 British population, the age and sex-adjusted prevalence of URE was 1.9 (95% CI 0.6-3.1)%. The age-adjusted prevalence of URE in men and women was 1.8% (95% CI 0.6% to 3.1%) and 1.9% (95% CI 0.7% to 3.2%), respectively.
Using different definitions for URE the prevalence estimates were repeated. In participants with a PVA<0.3, a ≥1 line improvement following pinhole-correction in either eye (worse eye with URE) resulted in a prevalence of URE of 9.5% (95% CI 8.6% to 10.3%). If a ≥2 line improvement following pinhole-correction was required for the diagnosis of URE, the prevalence estimates for URE were 1.3% (95% CI 1.0% to 1.6%) and 8.0% (95% CI 7.2% to 8.8%) in the better or worse eye, respectively.
Univariate and multivariate associations with URE
The prevalence of URE was higher with increasing age (ptrend<0.001) (table 1). Unsurprisingly, spherical refractive error (either myopia or hypermetropia) was associated with the prevalence of URE. Wearing spectacles for distance vision was inversely associated with having URE. Educational status and social class were not found to have any significant association with URE. We performed a multivariate logistic regression analysis of URE, adjusting for all of the variables examined in the univariate analyses (table 2). Increasing age was strongly associated with URE following adjustment for covariates, whereas wearing distance spectacles was strongly and inversely associated with URE. The association between being retired and URE was attenuated in the multivariate model.
Table 1.
Prevalence of and univariate associations with URE in the EPIC-Norfolk Eye Study (n=4428)
| Variable | Number | Number with URE | URE % (95% CI) | Crude OR | p Value |
|---|---|---|---|---|---|
| Total | 4428 | 80 | 1.8 | (1.4 to 2.2) | |
| Sex | 0.60 | ||||
| Male | 1975 | 38 | 1.9 (1.3 to 2.5) | 1 (referent) | |
| Female | 2453 | 42 | 1.8 (1.2 to 2.3) | 0.89 (0.57 to 1.38) | |
| Age | <0.001 (trend) | ||||
| 50–59 | 1008 | 11 | 1.1 (0.4 to 1.7) | 1 (referent) | |
| 60–69 | 1917 | 20 | 1.0 (0.5 to 1.5) | 0.96 (0.47 to 2.00) | |
| 70–79 | 1255 | 37 | 2.9 (2.0 to 3.9) | 2.75 (1.40 to 5.42) | |
| 80+ | 248 | 12 | 4.8 (2.1 to 7.5) | 4.61 (2.01 to 10.57) | |
| Education level | 0.64 (trend) | ||||
| No qualifications | 1131 | 24 | 2.1 (1.3 to 3.0) | 1 (referent) | |
| O-level | 523 | 7 | 1.3 (0.4 to 2.3) | 0.63 (0.27 to 1.46) | |
| A-level | 1957 | 34 | 1.7 (1.2 to 2.3) | 0.82 (0.48 to 1.38) | |
| Tertiary | 817 | 15 | 1.8 (0.9 to 2.8) | 0.86 (0.45 to 1.65) | |
| Social class | |||||
| Non-manual | 2966 | 53 | 1.8 (1.3 to 2.3) | 1 (referent) | 0.89 |
| Manual | 1462 | 27 | 1.8 (1.2 to 2.5) | 1.03 (0.65 to 1.65) | |
| Retirement status | 0.09 | ||||
| Not-retired | 1017 | 12 | 1.2 (0.5 to 1.8) | 1 (referent) | |
| Retired | 3411 | 68 | 2.0 (1.5 to 2.5) | 1.70 (0.92 to 3.16) | |
| Have distance spectacles | 0.001 | ||||
| No | 1364 | 38 | 2.8 (1.9 to 3.7) | 1 (referent) | |
| Yes | 3064 | 42 | 1.4 (1.0 to 1.8) | 0.48 (0.31 to 0.76) | |
| Type of refractive error | 0.05 | ||||
| Myopia | 1230 | 18 | 1.5 (0.8 to 2.1) | 1.23 (0.59 to 2.57) | 0.75 (trend) |
| Emmetropia | 1009 | 12 | 1.2 (0.5 to 1.9) | 1 (referent) | |
| Hypermetropia | 2189 | 50 | 2.3 (1.7 to 2.9) | 1.94 (1.03 to 3.67) |
EPIC, European Prospective Investigation of Cancer; URE, uncorrected refractive error.
Table 2.
Multivariate logistic regression model of URE in EPIC-Norfolk Eye Study (N=4428)
| Variable | Multivariate OR (95% CI) | p Value |
|---|---|---|
| Sex | ||
| Male | 1 (referent) | – |
| Female | 1.04 (0.66 to 1.65) | 0.85 |
| Age | ||
| 50–59 | 1 (referent) | |
| 60–69 | 0.96 (0.43 to 2.18) | 0.93 |
| 70–79 | 2.86 (1.24 to 6.56) | 0.01 |
| 80+ | 5.19 (1.97 to 13.69) | <0.001 |
| p Trend | <0.001 | |
| Education level | ||
| No qualifications | 1 (referent) | – |
| O-level | 0.99 (0.57 to 1.72) | 0.98 |
| A-level | 0.86 (0.38 to 2.05) | 0.73 |
| Tertiary | 1.19 (0.58 to 2.42) | 0.63 |
| p Trend | 0.77 | |
| Social class | ||
| Non-manual | 1 (referent) | – |
| Manual | 0.84 (0.51 to 1.39) | 0.50 |
| Retirement status | ||
| Not-retired | 1 (referent) | |
| Retired | 1.00 (0.47 to 2.13) | 0.99 |
| Have distance spectacles | ||
| No | 1 (referent) | – |
| Yes | 0.34 (0.21 to 0.55) | <0.001 |
| Type of refractive error | ||
| Myopia | 2.24 (1.04 to 4.86) | 0.04 |
| Emmetropia | 1 (referent) | – |
| Hypermetropia | 2.81 (1.44 to 5.45) | 0.02 |
All variables adjusted for other variables in the table.
EPIC, European Prospective Investigation of Cancer; URE, uncorrected refractive error.
Self-reported distance vision
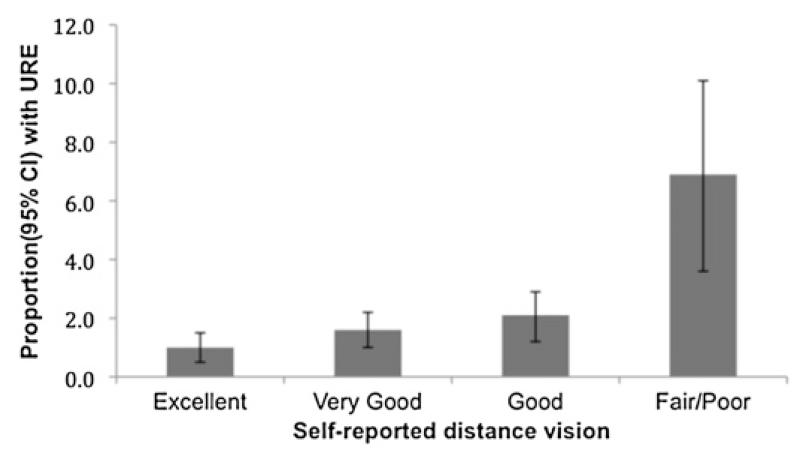
Distribution of self-reported distance vision was as follows: excellent (1315 people; 29.7%), very good (1807; 40.8%), good (1073; 24.2%) and fair/poor (233; 5.3%). URE was associated with a lower score in self-reported distance vision (ptrend<0.001) (figure 2). Compared with excellent self-reported distance vision, the ORs for having URE using the following categories were: 1.63 for ‘very good’ (95% CI 0.85 to 3.16, p=0.143); 2.10 for ‘good’ (95% CI 1.05 to 4.18, p=0.035); and 7.39 for ‘fair/poor’ (95% CI 3.50 to 15.58 p<0.001) (figure 3).
Figure 3.
Uncorrected refractive error and self-reported distance vision in the European Prospective Investigation of Cancer-Norfolk Eye Study.
Spectacle use
Three thousand and sixty-three people (69.2%) wore spectacles/CLs for distance vision. Of people with myopia, 1065 (86.7%) wore distance spectacles/CLs. Of people with hypermetropia, 75.2% wore distance spectacles/CLs, and of people with emmetropia 34.8% wore distance spectacles/CLs. All people with severe refractive error (high myopia or high hypermetropia) wore distance spectacles/CLs. Spectacle/CL use differed according to refractive error (p<0.001), and increased according to severity of refractive error (ptrend <0.001). Self-reported distance vision was associated with spectacle/CL wear. Compared with people with ‘excellent’ self-reported distance vision, the OR for wearing distance spectacles for people with ‘very good’ self-reported distance vision was 1.56 (95% CI 1.35 to 1.82, p<0.001); for ‘good’ self-reported distance vision was 1.94 (95% CI 1.62 to 2.31, p<0.001); and for ‘fair/poor’ self-reported distance vision was 3.37 (95% CI 2.34 to 4.85, p<0.001).
We assessed univariate and multivariate associations of wearing distance spectacles/CLs: age (per 10 years) (OR 1.24, 95% CI 1.14 to 1.35, p<0.001), female sex (OR 1.15, 95% CI 1.01 to 1.31, p=0.034), manual social class (OR 0.86, 95% CI 0.75 to 0.98, p=0.029) and retirement (OR 1.45, 95% CI 1.25 to 1.69, p<0.001) were significant on univariate analysis. Increasing education (ptrend=0.093) was not statistically significant. On multivariate analysis (controlling for all five variables), independent associations with wearing distance spectacles were age (per 10 years) (OR 1.20, 95% CI 1.08 to 1.32, p<0.001); female sex (OR 1.19, 95% 1.04 to 1.35, p=0.025); increasing education level (OR 1.07, 95% CI 1.00 to 1.14, ptrend =0.033); and retirement (OR 1.31, 95% CI 1.03 to 1.46, p=0.020). Manual social class (OR 0.93, 95% CI 0.80 to 1.07 p=0.293) was not significant in this multivariate model.
DISCUSSION
Prevalence estimates of URE from the present study Norfolk population need to be interpreted cautiously. In EPIC-Norfolk, initial sampling was performed through general practices, and despite relatively good population coverage and a socioeconomic profile similar to that of the UK general population, characteristics of people attending general practices may have differed from people who never attend.14 This is especially likely for outcomes obtained from the third health check as the returning participants were younger, had higher levels of education and social class and were generally healthier than non-attendees from all those who attended the baseline survey. Additionally, people who have severe visual impairment may have been less likely to have attended either baseline or follow-up examination, although visual data on non-responders were not collected (table 3).
Table 3.
Spectacle wearing according to refractive status in the EPIC-Norfolk Eye Study
| Total (N=4428) |
No distance spectacles (N=1365; 30.8%) |
Distance spectacles (N=3063; 69.2%) |
||||
|---|---|---|---|---|---|---|
| Refractive error | N | % | N | % | N | % |
| Emmetropia | 1009 | 22.8 | 658 | 65.2 | 351 | 34.8 |
| Low hypermetropia | 582 | 13.1 | 313 | 53.9 | 269 | 46.1 |
| Moderate hypermetropia | 1590 | 35.9 | 229 | 14.4 | 1361 | 85.6 |
| High hypermetropia | 17 | 0.4 | 0 | 0.0 | 17 | 100.0 |
| Low myopia | 322 | 7.3 | 118 | 36.7 | 204 | 63.4 |
| Moderate myopia | 809 1 | 8.3 | 46 | 5.7 | 763 | 94.3 |
| High myopia | 99 | 2.2 | 0 | 0.0 | 99 | 100.0 |
High myopia, −6.00 D or less; Moderate myopia, −5.99 D to −1.00 D; Low myopia, −0.99 D to −0.50 D; Emmetropia, −0.49 D to +0.49 D; Low hypermetropia, +0.50 D to +0.99 D; Moderate hypermetropia, +1.00 D to +5.99 D; High hypermetropia, +6.00 D or more.
D, Dioptre; EPIC, European Prospective Investigation of Cancer.
In this predominantly white British population, the prevalence of URE was found to be low and was associated with increasing age and inversely associated with distance spectacle/CL use. Our finding of a low prevalence of URE reflects the consistent with a low prevalence of reduced vision (PVA<0.3) in this study population. Despite the majority of our study population having a refractive error (hypermetropia or myopia), the majority of participants with refractive error did not have PVA<0.3 and were suitably corrected. Comparison of studies of URE has been hampered by use of different definitions for URE, which vary in the level of VA used to classify visual impairment, the degree of improvement in VA with correction required for a diagnosis of URE or in defining URE according to unilateral or bilateral impairment.18 Nevertheless, the prevalence estimate in the present study is among the lowest published, although it should be noted that the list of studies presented is not exhaustive (table 4).
Table 4.
Definition and prevalence of URE in adult population-based studies
| Study name, location | N | Age (years) |
Definition of URE | Prevalence, % (95% CI) |
|---|---|---|---|---|
| Australia, Melbourne19 | 4735 | 40+ | Improvement of ≥1 line of VA chart (ETDRS) with refraction in better eye | 9.8 (NS) |
| Australia, Blue Mountains20 | 3654 | 49–97 | Improvement in ≥2 lines on the logMAR chart in subjects with PVA 6/9 or worse after refraction in better eye | 10.2 (NS) |
| India, Andhra Pradesh21 | 3203 | 15–50 | PVA<6/12 and improving to ≥6/12 with pinhole in better eye | 2.7 (2.1 to 3.2)* |
| Singapore, Singapore Malay population6 | 3280 | 40–80 | Improvement of ≥0.2 logMAR (2 lines equivalent) in best corrected VA compared with PVA in the better eye | 18.3 (16.7 to 20.0)* |
| Singapore, Tanjong Pagar (Chinese population)7 | 1232 | 40-79 | Improvement of ≥0.2 logMAR (2 lines equivalent) in best corrected VA compared with PVA in the better eye | 17.3 (15.0 to 19.5)* |
| Taiwan, Shihpai Study22 | 1361 | 65+ | Correctable visual impairment was defined as PVA <6/12 that improved to ≥6/12 after refractive correction in better eye | 9.5 (7.97 to 11.13) |
| Timor Leste, National23 | 2014 | 40+ | Uncorrected and undercorrected refractive error was defined as PVA <6/18, but ≥6/18 in better eye with pinhole, in absence of any significant findings on eye examination | 10.9% (NS) (0.3% undercorrected+ 10.6% uncorrected) |
| UK, National Diet and Nutrition Survey11 | 1362 | 65+ | Improvement in uncorrected VA or PVA (best reading included) by equivalent of ≥1 lines of Snellen VA chart with pinhole | 21.2 |
| UK, MRC Trial of Assessment and Management of Older People in Community12 | 14 403 | 75+ | PVA <6/18 that improves with pinhole to ≥6/18 in better eye | 3.2 (2.6 to 3.8) |
| USA, Arizona (Latino population)24 | 4509 | 40+ | Among those with refractive error (see below) those who on refraction achieved ≥2 lines (modified ETDRS distance chart) improvement in both eyes | 18.6 (NS) |
| USA, California (Latino population)8 | 6129 | 40+ | Improvement of ≥2 lines (modified ETDRS chart) in VA in better seeing eye after refraction of the same eye | 15.1 (NS) |
Age and gender-adjusted estimates.
ETDRS, early treatment diabetic retinopathy study; NS, not significant; PVA, presenting visual acuity; URE, uncorrected refractive error; VA, visual acuity.
The prevalence estimates of URE in the present study are lower than previous estimates from the UK. Between 1994 and 1995, 1362 community and institutionalised adults aged ≥65 years participated in the National Diet and Nutrition Survey. Prevalence of presenting visual impairment (VA<6/18) was 14.3% and one in five participants in the sample (21.2%) were able to improve their vision by one or more lines on the Glasgow acuity chart with a pinhole.11 In the Medical Research Council (MRC) Trial of Assessment and Management of Older People in the Community, which investigated 14 403 people aged ≥75 years, there were 3.2% whose PVA improved with a pinhole to better than 6/18 also using the Glasgow acuity chart.12 In a North London population aged ≥65 years, 9% of people improved their VA to 0.3 or better (LogMar) following refraction.13 In EPIC-Norfolk, the prevalence of URE in subjects aged ≥65 or 75 years was 2.4% (95% CI 1.8% to 3.0%) and 4.0% (95% CI 2.8% to 5.2%), respectively. The age-restricted results in EPIC-Norfolk were similar to those reported in the MRC study; however, the MRC study used a stricter VA cut-off.
In order to provide refractive services it is necessary to identify people with poor vision that can be improved with correction (spectacles, CLs or refractive surgery), dispense the refractive correction and ensure follow-up.15 During a refractive optical assessment there is an opportunity for an assessment of any potential cataract, the prevalence of which, in addition to URE, increases with advancing age. Improvement in VA with refraction (pinhole or otherwise) may be attributed to ‘index’ myopia, where myopia is attributed to development of incident cataract,25 and presence of cataract has been associated with URE.7 Unfortunately, data relating to the prevalence of lens opacity were not available in the present study.
Elucidating the factors placing an individual at increased risk of URE is complex. Schneider and colleagues have identified social factors (including socioeconomic status, isolation, education), treatment/service factors (rural domicile, access among minority groups, access to health insurance) and individual factors (including psychological factors) to be associated with URE.18 In another study of individuals ≥65 years of age attending the accident and emergency departments in the UK, more than half had not seen their optician in the previous 2 years and cost, mobility and the perception of ‘attendance at an optician not being necessary’ were reasons put forward for non-attendance.26 In the present study, it was found that wearing of spectacles was strongly and inversely associated with URE; this finding being intuitive, and reported previously.6,19,22 Manual social class (univariate analysis only) and lower educational attainment level (multivariate analysis only) were associated with reduced odds of wearing spectacles/CLs for distance vision. Interestingly, we did not find education or social class to be associated with URE in the present study. In other studies, however, lower levels of education8,27,28 and lower income8 have been identified as being associated with a greater degree of URE. The possible reasons why 35% of emmetropes wore spectacles as assessed through questionnaire may include differences among ownership and use of spectacles, measurement error, and longitudinal changes in refraction.
URE was associated with worse scores in self-reported distance vision (ptrend <0.001). Although the measure of self--reported distance vision was relatively crude and not validated, answers to the questions correlated strongly with prevalence of URE. Various other methods of assessing quality of life in URE have been used.18 In the Singapore Malay Eye Study, which investigated people aged between 40 and 80 years, Lamoureux and co-workers reported that uncorrected myopia was associated with poorer overall visual function using the VF-11 scale.29 In addition, Lamoureux and colleagues found that myopic URE adversely affected specific activities including reading street signs, recognising friends and watching television, following adjustment for age, gender, education, ocular conditions and non-ocular comorbidity.29
The strengths of our study include a large sample size, and inclusion of both subjective and objective assessment of distance vision and its relationship with spectacle/CL use. There were several limitations associated with our study, including that demographic characteristics of participants undertaking visual testing were significantly different from those who did not. Notably, subjects participating in the third health check were more likely to have higher educational levels and social class and younger age than non-participants, all of which are associated with a myopic refraction in subjects without cataract.27 In addition, ethnic differences exist in the prevalence of refractive error30 and these associations from a predominantly white population may not be generalisable to other ethnic groups. Last, no participants in EPIC-Norfolk were resident in nursing homes which might have led to an underestimation of URE prevalence since such residents are more likely to have higher prevalence of URE than those living elsewhere.31 Nevertheless, within this cohort it was possible to examine the major demographic factors associated with URE.
In conclusion, the prevalence of URE in this relatively older, British and mainly Caucasian, population was low. URE was found to be associated with advancing age, reduced wearing of spectacles/CLs for distance vision and poorer self-reported distance vision. Lower levels of education and lower social class were not identified as being associated with URE in this Norfolk population.
Acknowledgments
Funding EPIC was funded by The Medical Research Council, UK (G0401527), Cancer Research UK (C864/A8257) and Research into Ageing, UK (262). Professor Foster has received additional support from The Richard Desmond Charitable Trust (via Fight for Sight) and the Department for Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital and The UCL Institute of Ophthalmology for a specialist Biomedical Research Centre for Ophthalmology.
Footnotes
Competing interests None.
Ethics approval Approval provided by the East Norfolk and Waverney NHS Research Governance Committee (2005EC07L) and the Norfolk Research Ethics Committee (05/Q0101/191).
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Resnikoff S, Pascolini D, Mariotti SP, et al. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008;86:63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith TS, Frick KD, Holden BA, et al. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ. 2009;87:431–7. doi: 10.2471/BLT.08.055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karpa MJ, Mitchell P, Beath K, et al. Direct and indirect effects of visual impairment on mortality risk in older persons. Arch Ophthalmol. 2009;127:1347–53. doi: 10.1001/archophthalmol.2009.240. [DOI] [PubMed] [Google Scholar]
- 4.McCarty CA. Uncorrected refractive error. Br J Ophthalmol. 2006;90:521–2. doi: 10.1136/bjo.2006.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127:1632–9. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 6.Rosman M, Wong TY, Tay WT, et al. Prevalence and risk factors of undercorrected refractive errors among Singaporean Malay adults: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2009;50:3621–8. doi: 10.1167/iovs.08-2788. [DOI] [PubMed] [Google Scholar]
- 7.Saw SM, Foster PJ, Gazzard G, et al. Undercorrected refractive error in Singaporean Chinese adults: the Tanjong Pagar survey. Ophthalmology. 2004;111:2168–74. doi: 10.1016/j.ophtha.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 8.Varma R, Wang MY, Ying-Lai M, et al. The prevalence and risk indicators of uncorrected refractive error and unmet refractive need in Latinos: the Los AngelesLatino Eye Study. Invest Ophthalmol Vis Sci. 2008;49:5264–73. doi: 10.1167/iovs.08-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foran S, Rose K, Wang JJ, et al. Correctable visual impairment in an older population: the blue mountains eye study. Am J Ophthalmol. 2002;134:712–19. doi: 10.1016/s0002-9394(02)01673-2. [DOI] [PubMed] [Google Scholar]
- 10.Sherwin JC, Lewallen S, Courtright P. Blindness and visual impairment due to uncorrected refractive error in sub-Saharan Africa: review of recent population-based studies. Br J Ophthalmol. 2012 doi: 10.1136/bjophthalmol-2011-300426. Published Online First 8 February 2012. doi:10.1136/bjophthalmol-2011-300426. [DOI] [PubMed] [Google Scholar]
- 11.van der Pols JC, Bates CJ, McGraw PV, et al. Visual acuity measurements in a national sample of British elderly people. Br J Ophthalmol. 2000;84:165–70. doi: 10.1136/bjo.84.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans JR, Fletcher AE, Wormald RP. Causes of visual impairment in people aged 75 years and older in Britain: an add-on study to the MRC trial of assessment and management of older people in the community. Br J Ophthalmol. 2004;88:365–70. doi: 10.1136/bjo.2003.019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reidy A, Minassian DC, Vafidis G, et al. Prevalence of serious eye disease and visual impairment in a north London population: population based, cross sectional study. BMJ. 1998;316:1643–6. doi: 10.1136/bmj.316.7145.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cumberland PM, Peckham CS, Rahi JS. Inferring myopia over the lifecourse from uncorrected distance visual acuity in childhood. Br J Ophthalmol. 2007;91:151–3. doi: 10.1136/bjo.2006.102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006-2011. World Health Organization (WHO); Geneva: 2007. [Google Scholar]
- 16.Day N, Oakes S, Luben R, et al. EPIC-Norfolk: study design and characteristics of the cohort. European prospective investigation of cancer. Br J cancer. 1999;80(Suppl 1):95–103. [PubMed] [Google Scholar]
- 17.Foster PJ, Broadway DC, Hayat S, et al. Refractive error, axial length and anterior chamber depth of the eye in British adults: the EPIC-Norfolk eye study. Br J Ophthalmol. 2010;94:827–30. doi: 10.1136/bjo.2009.163899. [DOI] [PubMed] [Google Scholar]
- 18.Schneider J, Leeder SR, Gopinath B, et al. Frequency, course, and impact of correctable visual impairment (uncorrected refractive error) Surv Ophthalmol. 2010;55:539–60. doi: 10.1016/j.survophthal.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Liou HL, McCarty CA, Jin CL, et al. Prevalence and predictors of undercorrected refractive errors in the Victorian population. Am J Ophthalmol. 1999;127:590–6. doi: 10.1016/s0002-9394(98)00449-8. [DOI] [PubMed] [Google Scholar]
- 20.Thiagalingam S, Cumming RG, Mitchell P. Factors associated with undercorrected refractive errors in an older population: the Blue Mountains Eye Study. Br J Ophthalmol. 2002;86:1041–5. doi: 10.1136/bjo.86.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmamula S, Keeffe JE, Rao GN. Uncorrected refractive errors, presbyopia and spectacle coverage: results from a rapid assessment of refractive error survey. Ophthalmic Epidemiol. 2009;16:269–74. [PubMed] [Google Scholar]
- 22.Kuang TM, Tsai SY, Hsu WM, et al. Correctable visual impairment in an elderly Chinese population in Taiwan: the Shihpai eye study. Invest Ophthalmol Vis Sci. 2007;48:1032–7. doi: 10.1167/iovs.06-0616. [DOI] [PubMed] [Google Scholar]
- 23.Ramke J, du Toit R, Palagyi A, et al. Correction of refractive error and presbyopia in Timor-Leste. Br J Ophthalmol. 2007;91:860–6. doi: 10.1136/bjo.2006.110502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uribe JA, Swenor BK, Munoz BE, et al. Uncorrected refractive error in a Latino population: proyecto VER. Ophthalmology. 2011;118:805–11. doi: 10.1016/j.ophtha.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dandona R, Dandona L. Refractive error blindness. Bull World Health Organ. 2001;79:237–43. [PMC free article] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Dorward NL, Wormald RP, et al. ’Correctable undetected visual acuity deficit’ in patients aged 65 and over attending an accident and emergency department. Br J Ophthalmol. 1993;77:293–6. doi: 10.1136/bjo.77.5.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourne RR, Dineen BP, Ali SM, et al. Prevalence of refractive error in Bangladeshi adults: results of the national blindness and low vision survey of Bangladesh. Ophthalmology. 2004;111:1150–60. doi: 10.1016/j.ophtha.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Fotouhi A, Hashemi H, Raissi B, et al. Uncorrected refractive errors and spectacle utilisation rate in Tehran: the unmet need. Br J Ophthalmol. 2006;90:534–7. doi: 10.1136/bjo.2005.088344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamoureux EL, Saw SM, Thumboo J, et al. The impact of corrected and uncorrected refractive error on visual functioning: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2009;50:2614–20. doi: 10.1167/iovs.08-2164. [DOI] [PubMed] [Google Scholar]
- 30.Kleinstein RN, Jones LA, Hullett S, et al. Refractive error and ethnicity in children. Arch Ophthalmol. 2003;121:1141–7. doi: 10.1001/archopht.121.8.1141. [DOI] [PubMed] [Google Scholar]
- 31.Rifaat R, Kivela SL. Visual acuity and refractive errors in the elderly in nursing homes. Z Gerontol. 1989;22:315–20. [PubMed] [Google Scholar]