Abstract
Objective
To determine longitudinal changes in angle configuration in the eyes of primary angle-closure suspects (PACS) treated by laser peripheral iridotomy (LPI) and in untreated fellow eyes.
Design
Longitudinal cohort study.
Participants
Primary angle-closure suspects aged 50 to 70 years were enrolled in a randomized, controlled clinical trial.
Methods
Each participant was treated by LPI in 1 randomly selected eye, with the fellow eye serving as a control. Angle width was assessed in a masked fashion using gonioscopy and anterior segment optical coherence tomography (AS-OCT) before and at 2 weeks, 6 months, and 18 months after LPI.
Main Outcome Measures
Angle width in degrees was calculated from Shaffer grades assessed under static gonioscopy. Angle configuration was also evaluated using angle opening distance (AOD250, AOD500, AOD750), trabecular-iris space area (TISA500, TISA750), and angle recess area (ARA) measured in AS-OCT images.
Results
No significant difference was found in baseline measures of angle configuration between treated and untreated eyes. At 2 weeks after LPI, the drainage angle on gonioscopy widened from a mean of 13.5° at baseline to a mean of 25.7° in treated eyes, which was also confirmed by significant increases in all AS-OCT angle width measures (P<0.001 for all variables). Between 2 weeks and 18 months after LPI, a significant decrease in angle width was observed over time in treated eyes (P<0.001 for all variables), although the change over the first 5.5 months was not statistically significant for angle width measured under gonioscopy (P = 0.18), AOD250 (P = 0.167) and ARA (P = 0.83). In untreated eyes, angle width consistently decreased across all follow-up visits after LPI, with a more rapid longitudinal decrease compared with treated eyes (P values for all variables ≤0.003). The annual rate of change in angle width was equivalent to 1.2°/year (95% confidence interval [CI], 0.8–1.6) in treated eyes and 1.6°/year (95% CI, 1.3–2.0) in untreated eyes (P<0.001).
Conclusions
Angle width of treated eyes increased markedly after LPI, remained stable for 6 months, and then decreased significantly by 18 months after LPI. Untreated eyes experienced a more consistent and rapid decrease in angle width over the same time period.
Primary angle-closure glaucoma (PACG) predominantly presents in the form of a chronic asymptomatic condition1–3 and is estimated to be responsible for approximately half of binocular glaucoma blindness worldwide.4 A previous population-based study reported that more than 10% of elderly Chinese are asymptomatic suspects at risk of angle-closure.5 A considerable proportion of angle-closure suspects are at risk of progression to primary angle-closure or PACG.6,7 Laser peripheral iridotomy (LPI), a recognized first-line therapy for the treatment of PACG, has been demonstrated to prevent acute attacks of angle-closure in the fellow eyes of patients who have unilateral acute angle-closure.8–10 However, no conclusive evidence has demonstrated that persons with asymptomatic narrow angles on gonioscopy benefit from prophylactic LPI. Previous studies have shown that LPI opens the drainage angle in a majority of primary angle-closure suspects (PACS), whereas angles in a significant minority of eyes remained closed after LPI.8,11 In the context of limited healthcare resources and budgets, the efficacy of prophylactic LPI needs to be demonstrated. Furthermore, the harms (if any) of prophylactic treatment need to be determined.
Since 2008, a large-scale randomized clinical trial12 (the Zhongshan Angle-Closure Prevention [ZAP] trial, trial registration information: http://www.controlled-trials.com/ISRCTN45213099; accessed January 26, 2014) was initiated with the purpose of assessing the efficacy and safety of LPI as a prophylactic measure to prevent the development of acute or chronic primary angle-closure in asymptomatic suspects with gonioscopically evident narrow drainage angles. In the current study, we analyzed the longitudinal changes in anterior chamber angle configuration of PACS eyes in the ZAP cohort. The study design of the ZAP trial (i.e., treating 1 randomly selected eye in each participant with LPI and using the fellow untreated eye as the control) provides a unique opportunity to compare the long-term change of angle configuration in eyes with and without intervention by laser treatment. This will also help evaluate the influence of LPI on the natural history of PACS.
Methods
Ethical approval was obtained from the Ethical Review Board of Sun Yat-sen University and the Ethical Committee of Zhongshan Ophthalmic Center. The study also received institutional review board approval from Moorfields Eye Hospital (via the London School of Hygiene and Tropical Medicine) and Johns Hopkins University Hospital. The study was conducted in accordance with the Tenets of the World Medical Association’s Declaration of Helsinki. Study participants were recruited from a randomized controlled clinical trial, the ZAP trial (trial registration information: http://www.controlled-trials.com/ISRCTN45213099, accessed January 26, 2014). The International Standard Randomized Controlled Trial Number was issued on May 6, 2008.
All examinations and interventions were carried out in the Clinical Research Data Collection Center at Zhongshan Ophthalmic Center, a tertiary specialized hospital in Guangzhou, South China. The field procedures for this trial have been reported.12 In brief, 11 991 Guangzhou citizens aged between 50 and 70 years participated in the screening survey from September 2008 to August 2010. Primary angle-closure suspects eligible for this study were defined as those who had 6 or more clock hours of angle circumference in which the posterior/pigmented trabecular meshwork was not visible under static gonioscopy in both eyes, without intraocular pressure (IOP) elevated to >21 mmHg, no peripheral anterior synechiae, no glaucomatous optic neuropathy, and no evidence of anterior segment ischemia from a previous acute IOP increase. All participants underwent a darkroom prone provocative test as part of the enrollment tests. Those who had an IOP increase greater than 15 mmHg above baseline during darkroom prone provocative test were excluded. All eligible participants received LPI in 1 randomly selected eye, with the fellow eye serving as a control. Randomization was carried out using a pre-generated list of random numbers. Each eligible participant was assigned a number according to his/her sequence of entering the study. Randomization numbers and their corresponding eye assignment were generated at the data-monitoring center at Wilmer Eye Institute and sent in sealed envelopes to the clinical data-collection center at Zhongshan Ophthalmic Centre.12
Detailed information on all examinations in the ZAP trial has been reported.12 The limbal anterior chamber depth was assessed under slit-lamp microscopy using a modified van Herick grading scheme.13 The IOP was measured using Goldmann applanation tonometry (Haag-Streit AG, Koeniz, Switzerland). The median of 3 readings for each eye was considered. Ocular biometric measures (i.e., axial length, central anterior chamber depth, and lens thickness) were acquired using ultrasound A-scan (CineScan A/B, Quantel Medical, Bozeman, MT). The width of the anterior chamber angle was assessed using both evaluation under static gonioscopy and quantitative measurements from images acquired by anterior segment optical coherence tomography (AS-OCT).
Gonioscopy
Static gonioscopy was performed using a Goldmann-type, 1-mirror gonioscopic lens (Haag-Streit AG) with low ambient illumination and a 1-mm narrow beam. Gonioscopic examinations were carried out by specialist-trained ophthalmologists (Y.J., S.H.) with high intergrader agreement (weighted kappa values for all variables >0.80).12 Gonioscopy was performed in a standardized dark room with low ambient illumination (<1 lux illumination by digital light meter (measured using the Easy View model EAQ30; Extech Instruments, Inc, Waltham, MA)) at all study visits by an examiner who was masked to the findings collected at other visits. Care was taken to avoid the beam falling on the pupil to prevent alteration of the angle configuration. If trabecular meshwork could not be seen because of marked iris convexity, an “over the hill” view was obtained by slightly tilting the lens toward the trabecular meshwork without causing corneal indentation. Angle width was assessed under static gonioscopy using the Shaffer grading system: The width of anterior chamber angle in each quadrant was estimated as the angle in degrees between a tangent line to the surface of the trabecular meshwork and another tangent line to the peripheral third of the iris, and then was recorded in 5-point categories (Shaffer grades 0 to 4 correspond to 0°, 10°, 20°, 30°, and 40°, respectively). The range of iridotrabecular appositional contact observed under static gonioscopy was recorded. Peripheral iris profile was evaluated under gonioscopy and classified as being regular, steep, plateau, or queer.14
Anterior Segment Optical Coherence Tomography
Horizontal and vertical scans of AS-OCT (Visante, Carl Zeiss Meditec, Dublin, CA) were performed to obtain images of the anterior chamber angle in 4 quadrants. The AS-OCT scans were carried out in the same standardized low ambient illumination at all study visits.
The AS-OCT images were quantitatively assessed using custom software (the Zhongshan Angle Assessment Program15) to measure angle opening distance (AOD), trabecular-iris space area (TISA), and angle recess area (ARA). Image analysis was performed by 3 certified graders who were masked to the intervention assignment and study visit. A set of 200 images from 200 eyes were randomly selected and graded by all 3 graders independently. Good intergrader agreement was shown by high intraclass correlation coefficient (0.77–1.00). The AOD was defined as the length of a line drawn from the anterior surface of the iris to the corneal endothelial perpendicular to the plane of the surface of the trabecular mesh-work at 250 μm, 500 μm, and 750 μm from the scleral spur (AOD250, AOD500, and AOD750, respectively).16 The TISA was defined as the trapezoidal area between anteriorly, AOD500 and AOD750 (TISA500 and TISA750, respectively); posteriorly, a line drawn from the scleral spur perpendicular to the plane of the inner scleral wall to the anterior surface of the iris; superiorly, the inner corneoscleral wall; and inferiorly, the anterior surface of the iris.17,18 The ARA was defined as the triangular area between the anterior iris surface, the inner corneoscleral wall, and the AOD750 line.18 Iris curvature (alternatively named “iris convexity”) was defined as the maximum perpendicular distance between the posterior iris surface and the line connecting the most peripheral and the most central points of iris pigment epithelium relative to the pupillary center. The IT750 was defined as perpendicular iris thickness measured at 750 μm from the scleral spur. Mean pupil diameter of the horizontal and vertical scans acquired in the dark was recorded for each eye. Lens vault, defined as the perpendicular distance between the anterior pole of the crystalline lens and the horizontal line joining the 2 scleral spurs,19 was measured using AS-OCT images.
Statistical Analysis
Baseline measures were compared between the treated and untreated eyes using Wilcoxon signed-rank tests. Angle width–related measures at different visits before and after LPI were compared using 1-way repeated-measures analysis of variance, with inter-visit difference analyzed using Tukey’s method. All statistical analysis was performed using Stata 12 (StataCorp LP, College Station, TX).
Results
A total of 775 participants (135 men and 640 women; age (mean ± standard deviation): 59.4±5.0 years) with data available from all follow-up visits up to 18 months after LPI were included in the current analysis. When comparing eyes treated by LPI with untreated eyes, no significant difference was found in baseline features before LPI, including angle width assessed under static gonioscopy, AS-OCT measures (i.e., AOD250, AOD500, AOD750, TISA500, TISA750, and ARA), limbal anterior chamber depth, iris profile, ocular biometric measures, and IOP (Table 1). Of 889 participants initially recruited, 775 had data available from all 4 visits when the current analysis was carried out. No significant difference was found in baseline demographic and biometric measures between included and excluded participants. The baseline Shaffer grades in the control eyes of subjects who were excluded did not differ significantly from those included. There was a minor but statistically significant difference in baseline Shaffer grades of treated eyes between the excluded and included subjects (excluded vs. included: 1.22±0.56 vs. 1.35±0.60, P = 0.03).
Table 1.
Comparison of Baseline Measures between Treated and Untreated Eyes
| Treated Eyes, Mean (95% CI) | Untreated Eyes, Mean (95% CI) | P Value | |
|---|---|---|---|
| Angle width on gonioscopy (°) | 13.5 (13.1–13.9) | 13.5 (13.1–13.9) | 0.87 |
| IOP (mmHg) | 15.3 (15.1–15.5) | 15.3 (15.1–15.5) | 0.90 |
| van Herick score | |||
| 5% | 3.5* | 3.9* | 0.18 |
| 15% | 30.7* | 31.7* | |
| 25% | 60.9* | 59.4* | |
| 40% | 4.4* | 4.5* | |
| 75% | 0.5* | 0.5* | |
| Iris profile | |||
| Steep | 77.3* | 76.7* | 0.55 |
| Regular | 14.5* | 15.2* | |
| Plateau | 8.2* | 8.1* | |
| Axial length (mm) | 22.49 (22.44, 22.54) | 22.49 (22.44, 22.54) | 0.92 |
| ACD (mm) | 2.21 (2.19, 2.22) | 2.21 (2.19, 2.22) | 0.73 |
| Lens thickness (mm) | 4.87 (4.85, 4.89) | 4.87 (4.85, 4.89) | 0.81 |
| Appositional contact† | 3.20 (2.96, 3.44) | 3.23 (2.98, 3.47) | 0.46 |
| AOD250 (μm) | 59.0 (55.8, 62.3) | 58.0 (54.8, 61.3) | 0.67 |
| AOD500 (μm) | 82.4 (78.8, 86.1) | 79.2 (75.6, 82.8) | 0.21 |
| AOD750 (μm) | 124.2 (119.2, 129.1) | 122.1 (117.3, 126.8) | 0.54 |
| TISA500 (1000 μm2) | 42.5 (40.8, 44.1) | 41.6 (40.0, 43.3) | 0.46 |
| TISA750 (1000 μm2) | 75.3 (72.8, 77.9) | 74.2 (71.8, 76.7) | 0.54 |
| ARA (1000 μm2) | 85.8 (82.5, 89.2) | 85.1 (81.8, 88.5) | 0.77 |
ACD = central anterior chamber depth; AOD = angle opening distance; ARA = angle recess area; CI = confidence interval; IOP = intraocular pressure; TISA = trabecular-iris space area.
Percentage of each category.
Appositional contact: number of clock hours in which appositional iridotrabecular contact was seen under static gonioscopy.
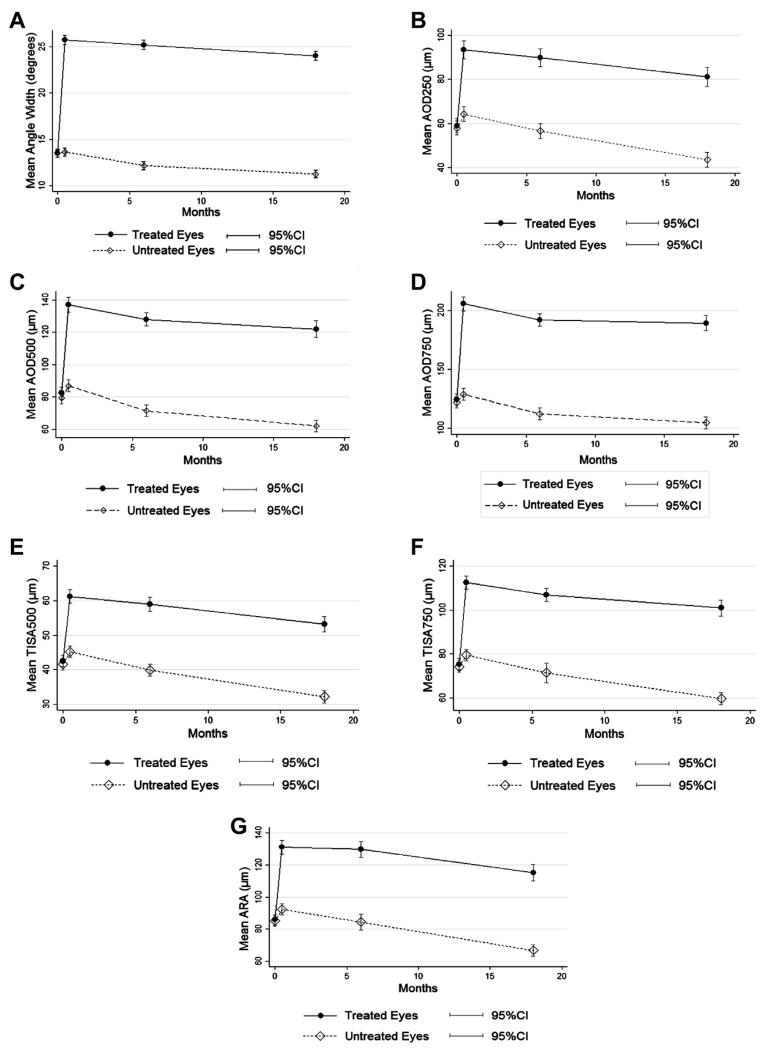
At 2 weeks after LPI, measures from both gonioscopic assessment and AS-OCT showed that the drainage angle width in treated eyes increased markedly compared with baseline (Table 2). For all eyes treated by LPI, the angle width decreased over time except for the time from 2 weeks to 6 months after LPI (Tables 3 and 4, available at www.aaojournal.org; Fig 1).
Table 2.
Change in Angle Width 2 Weeks after Laser Peripheral Iridotomy in Treated and Untreated Eyes
|
Treated Eyes, Mean (95% CI)
|
Untreated Eyes, Mean (95% CI)
|
|||
|---|---|---|---|---|
| Change in Angle Width after LPI * | P Value | Change in Angle Width after LPI * | P Value | |
| Angle width (°) | 12.2 (11.7, 12.7) | <0.001 | 0.1 (−0.3, 0.6) | 0.68 |
| AOD250 (μm) | 34.3 (31.3, 37.3) | <0.001 | 6.2 (3.8, 8.5) | <0.001 |
| AOD500 (μm) | 54.7 (51.2, 58.2) | <0.001 | 7.6 (4.9, 10.2) | <0.001 |
| AOD750 (μm) | 81.3 (77.0, 85.7) | <0.001 | 6.7 (3.9, 9.6) | <0.001 |
| TISA500 (×1000 μm2) | 18.7 (17.3, 20.0) | <0.001 | 3.5 (2.6, 4.5) | <0.001 |
| TISA750 (×1000 μm2) | 37.1 (35.0, 39.2) | <0.001 | 5.1 (3.7, 6.5) | <0.001 |
| ARA (×1000 μm2) | 45.2 (42.2, 48.3) | <0.001 | 7.2 (4.9, 9.5) | <0.001 |
AOD = angle opening distance; ARA = angle recess area; CI = confidence interval; LPI = laser peripheral iridotomy; TISA = trabecular-iris space area.
Calculated as the value at 2 weeks after LPI minus the baseline level.
Figure 1.
Changes in angle width over time in treated and untreated eyes. A, Angle width assessed under static gonioscopy. B, AOD250. C, AOD500. D, AOD750. E, TISA500. F, TISA750. G, ARA. AOD = angle opening distance; ARA = angle recess area; CI = confidence interval; TISA = trabecular-iris space area.
In the untreated eyes, a nonsignificant increase in the angle width was seen on gonioscopy at 2 weeks after LPI (mean, 13.5° vs. 13.7°; P = 0.68). All AS-OCT quantitative measures revealed a uniformly significant increase in angle width in treated and untreated eyes at 2 weeks after LPI (Table 2). The angle width decreased over time in the untreated eyes (Table 3, available at www.aaojournal.org). The rate of narrowing of angle width in untreated eyes was comparatively greater (treated vs. untreated: 1.2°/year vs. 1.6°/year, for angle width assessed under static gonioscopy, and P<0.001 for all variables including angle width assessed by Shaffer grades, AOD250, AOD500, AOD750, TISA500, TISA750, and ARA) and more consistent, with nearly all inter-visit differences being statistically significant (Tables 3 and 4, available at www.aaojournal.org).
Iris curvature significantly decreased from 0.354±0.08 mm before LPI to 0.20±0.07 mm 2 weeks after LPI (P<0.001) in treated eyes, and then decreased to 0.19±0.06 mm 6 months post-LPI (2 vs. 6 months post-LPI: P = 0.001). No significant change was found in iris curvature from 6 to 18 months post-LPI (from 0.19±0.06 mm to 0.18±0.07, P = 0.96). Compared with the baseline level, IT750 decreased significantly at 2 weeks after LPI, remained stable until 6 months after LPI, and then increased in treated eyes. No inter-visit difference in peripheral iris thickness was found between 2 weeks and 6 months after LPI (Table 5, available at www.aaojournal.org).
As shown in Table 5 (available at www.aaojournal.org), pupil diameter was found to be significantly smaller at 18 months after LPI in both treated and untreated eyes compared with levels at the other 3 visits (all P values <0.05). No inter-visit difference was found to be statistically significant between pupil diameter measured before LPI and 2 weeks and 6 months after LPI (P values = 0.145–0.999). Compared with the level at 2 weeks and 6 months post-LPI, central anterior chamber depth decreased mildly but significantly in both treated and untreated eyes (P<0.05) at 18 months post-LPI (Table 5, available at www.aaojournal.org).
In treated eyes, mean lens vault increased from 781.6 μm at 2 weeks post-LPI to 821.5 μm at 6 months post-LPI (P<0.001), and then further increased to 837.6 μm at 18 months after LPI (P = 0.012). In eyes without intervention, however, after a statistically significant change in lens vault from 2 weeks to 6 months after LPI (760.8 to 802.0 μm, P<0.001), no significant increase in lens vault over time was found. Compared with controls, eyes treated by LPI had significantly greater lens vault at 18 months post-LPI (treated vs. untreated: 837.6 vs. 802.9 μm, P = 0.008).
Discussion
In this study of longitudinal changes of angle configuration in angle-closure suspects, we observed an overall trend of significant narrowing of the anterior chamber angle over time in both treated and untreated eyes after LPI. The magnitude of decrease in angle width over time was significantly more pronounced in eyes without any intervention. Apart from the increase in angle width due to the elimination of pupillary block, which is an apparent benefit from LPI, the change of angle width after laser treatment also slowed down compared with the untreated eyes.
The finding of widening of the drainage angle immediately after LPI is not surprising. Despite various studies suggesting the possible existence of non–pupil block mechanisms of angle-closure,8,20–22 pupil block is still recognized as a major factor in the development of primary angle-closure. In our study, iris curvature measured in AS-OCT images significantly decreased after LPI (P<0.001). This finding was in accordance with a previous study reporting immediate change in angle width of 176 PACS eyes after LPI,23 in which AS-OCT scans showed significant widening of the drainage angle along with reduced iris curvature at 1 week after LPI. In contrast to the findings of a previous study that showed a minor insignificant increase in iris curvature at 18 months compared with immediately after LPI (0.15±0.05 mm vs. 0.16±0.06 mm, P = 0.334),24 our analysis revealed a slight but significant decrease in iris curvature from 2 weeks to 6 months post-LPI (P = 0.001). From 6 months post-LPI, the convexity of the iris stabilized; no further significant change in iris curvature was found up to 18 months post-LPI (P = 0.96). This result from a relatively large LPI-treated PACS cohort provides further evidence to the theory that non–pupil block mechanisms also play a considerable role in causing primary angle-closure, especially in east Asians. By using the definition of posterior/pigmented trabecular meshwork not visible under static gonioscopy in at least 2 quadrants, approximately 25% of all eyes had persistent angle-closure after LPI. Over the period of 2 weeks to 18 months post-LPI, this proportion increased in both treated and untreated eyes over time. If a more stringent definition of angle-closure (i.e., eyes in which posterior/pigmented trabecular meshwork was not visible under static gonioscopy in at least 3 quadrants) was used, the proportions of eyes with persistent angle-closure at follow-up visits in the current study were all relatively lower than the proportion reported from a previous study carried out in the same geographic area.11,22
We unexpectedly found a small but significant increase in AS-OCT angle measures in eyes with no intervention at 2 weeks after LPI (Table 2, Fig 1), compared with the baseline. These significant inter-visit differences in AS-OCT measures cannot be readily explained by variations in the quantitative analysis of AS-OCT images, because the changes are unidirectional (i.e., increase from the baseline visit to 2 weeks after LPI), and the graders who had high intergrader and intragrader reproducibility were masked to both the time of image acquisition and the randomization result. It is possible that systemic absorption of pilocarpine administered to the treated eye before LPI and the increased amount of light shed on the fundus of the treated eye after LPI might result in a certain change in pupil diameter or iris configuration, which then widens the drainage angle. However, no significant difference was found in pupil diameter before LPI and 2 weeks after LPI. Of note, peripheral iris (measured as IT750, iris thickness at 750 μm from the scleral spur) was significantly thinner at 2 weeks after LPI compared with the baseline level and increased back to baseline level at 18 months in the treated eyes but no further significant change in peripheral iris thickness 2 weeks and 6 months post-LPI (Table 5, available at www.aaojournal.org). There was also a minor 0.13° increase in angle width calculated from Shaffer grades, in untreated eyes between 2 weeks and 6 months post-LPI, although the change was statistically insignificant. This suggests that Shaffer grading under gonioscopy is not as quantitative and sensitive as measurements from AS-OCT images. Despite the wide acceptance of gonioscopy as the “gold standard” in morphologic observation and detection of pathologies, the highly reproducible AS-OCT–derived measurements17 may be more appropriate for determining small changes in quantitative analyses.
The slow decline in angle width after LPI in the PACS cohort in our study is in accordance with findings of a previous report on PACS over a similar follow-up duration, albeit in a smaller sample.24 By observing AS-OCT scans in 32 PACS eyes, Lee et al24 also found an immediate increase in AOD750 and ARA after LPI and a slow reduction over time up to 18 months after treatment. They suggested that decreased angle width could be due to increased lens vault (also a measure from quantitative analysis of AS-OCT images, defined as the perpendicular distance between the anterior pole of the crystalline lens and the line connecting the left and right scleral spurs on an AS-OCT scan25). In this study, the direction of change in angle width after the initial post-LPI increase was not consistent. Compared with the angle width 2 weeks after treatment, values of some variables were even slightly increased at 6 months (Fig 1), although no statistically significant differences were found (Table 4, available at www.aaojournal.org). However, by 18 months post-LPI, all angle width parameters had demonstrated a decrease relative to the 2-week time-point. In agreement with findings from previous studies, we also found an overall trend of increase in lens vault over time in the eyes treated by LPI and the fellow untreated eyes. Accordingly, in both treated and untreated eyes, central anterior chamber depth was found to be significantly smaller at 18 months after LPI compared with other follow-up visits (Table 5, available at www.aaojournal.org). These suggest that, apart from aging, there might be an additional effect from LPI on the bulk of the crystalline lens, especially the contour of its anterior surface.
There may be other explanations for the longitudinal decrease in angle width after the initial increase after LPI. Inflammatory processes in the iris might result in changes in the position of the ciliary processes, which may have some effect on the drainage angle configuration. However, it is difficult to test this assumption in the current study, because AS-OCT does not provide clear visualization of anatomic structures behind the iris. Further evidence is needed from observation using ultrasound biomicroscopy to test this hypothesis. Another hypothesis is that the late decrease in angle width might be related to shrinkage of the iridotomy, which may compromise aqueous humor flow through it. Although data on iridotomy size were not available in the current study, the curvature of the peripheral iris may serve as a surrogate measure, because it reflects the pressure gradient between the posterior and anterior chambers. However, we observed that iris curvature decreased slightly but significantly from 0.20 mm to 0.18 mm (P = 0.001) over the 18 months, rather than increasing the forward bowing of the peripheral iris, which might be expected with a lesser-functioning iridotomy.
In conclusion, laser peripheral iridotomy resulted in a marked immediate increase in angle width in PACS, with clear evidence of the elimination of pupil block. However, after several months of stabilization from 2 weeks to 6 months post-LPI, drainage angles narrowed significantly over time. A more pronounced decrease in angle width was observed in the untreated fellow eyes of the same cohort of PACS.
Supplementary Material
Acknowledgments
Supported by Fight for Sight (grant no. 1655) (United Kingdom) and the Sun Yat-sen University 5010 Project Fund (grant no. 2007033) (China). Y.J. receives additional support from the British Council for Prevention of Blindness PhD Scholarship and UCL Overseas Research Scholarship. A.P.K. is supported by a Wellcome Trust Clinical Research Fellowship (grant no. 094791/Z/10/Z). P.J.F. receives additional support from the NIHR Biomedical Research Centre at Moorfields Eye Hospital, London, United Kingdom (NIHR-BRC2 009; Moorfields/UCL-IOO), and the Richard Desmond Charitable Foundation (via Fight for Sight UK). The sponsors or funding organizations had no roles in the design or conduct of this research.
Abbreviations and Acronyms
- AOD
angle opening distance
- ARA
angle recess area
- AS-OCT
anterior segment optical coherence tomography
- CI
confidence interval
- IOP
intraocular pressure
- IT750
iris thickness measured at 750 μm from the scleral spur
- LPI
laser peripheral iridotomy
- PACG
primary angle-closure glaucoma
- PACS
primary angle-closure suspects
- TISA
trabecular-iris space area
- ZAP
Zhongshan Angle-Closure Prevention.
Footnotes
Financial Disclosure(s):
The author(s) have no proprietary or commercial interest in any materials discussed in this article.
Supplemental material is available at www.aaojournal.org.
References
- 1.Foster PJ, Oen FT, Machin D, et al. The prevalence of glaucoma in Chinese residents of Singapore: a cross-sectional population survey of the Tanjong Pagar district. Arch Ophthalmol. 2000;118:1105–11. doi: 10.1001/archopht.118.8.1105. [DOI] [PubMed] [Google Scholar]
- 2.Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia. A population-based survey in Hovsgol province, northern Mongolia. Arch Ophthalmol. 1996;114:1235–41. doi: 10.1001/archopht.1996.01100140435011. [DOI] [PubMed] [Google Scholar]
- 3.Congdon N, Wang F, Tielsch JM. Issues in the epidemiology and population-based screening of primary angle-closure glaucoma. Surv Ophthalmol. 1992;36:411–23. doi: 10.1016/s0039-6257(05)80022-0. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He M, Foster PJ, Ge J, et al. Prevalence and clinical characteristics of glaucoma in adult Chinese: a population-based study in Liwan District, Guangzhou. Invest Ophthalmol Vis Sci. 2006;47:2782–8. doi: 10.1167/iovs.06-0051. [DOI] [PubMed] [Google Scholar]
- 6.Thomas R, George R, Parikh R, et al. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003;87:450–4. doi: 10.1136/bjo.87.4.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilensky JT, Kaufman PL, Frohlichstein D, et al. Follow-up of angle-closure glaucoma suspects. Am J Ophthalmol. 1993;115:338–46. doi: 10.1016/s0002-9394(14)73585-8. [DOI] [PubMed] [Google Scholar]
- 8.Nolan WP, Foster PJ, Devereux JG, et al. YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol. 2000;84:1255–9. doi: 10.1136/bjo.84.11.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang LP, Aung T, Chew PT. Acute primary angle closure in an Asian population: long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology. 2000;107:2092–6. doi: 10.1016/s0161-6420(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed Q, Fahey DK, Manners RM. Angle closure in fellow eye with prophylactic pilocarpine treatment [letter] Br J Ophthalmol. 2001;85:1263. doi: 10.1136/bjo.85.10.1260b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in primary angle-closure suspects: biometric and gonioscopic outcomes: the Liwan Eye Study. Ophthalmology. 2007;114:494–500. doi: 10.1016/j.ophtha.2006.06.053. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Friedman DS, He M, et al. Design and methodology of a randomized controlled trial of laser iridotomy for the prevention of angle closure in southern China: the Zhongshan Angle Closure Prevention trial. Ophthalmic Epidemiol. 2010;17:321–32. doi: 10.3109/09286586.2010.508353. [DOI] [PubMed] [Google Scholar]
- 13.Foster PJ, Devereux JG, Alsbirk PH, et al. Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol. 2000;84:186–92. doi: 10.1136/bjo.84.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spaeth GL, Aruajo S, Azuara A. Comparison of the configuration of the human anterior chamber angle, as determined by the Spaeth gonioscopic grading system and ultrasound biomicroscopy. Trans Am Ophthalmol Soc. 1995;93:337–47. [PMC free article] [PubMed] [Google Scholar]
- 15.Console JW, Sakata LM, Aung T, et al. Quantitative analysis of anterior segment optical coherence tomography images: the Zhongshan Angle Assessment Program. Br J Ophthalmol. 2008;92:1612–6. doi: 10.1136/bjo.2007.129932. [DOI] [PubMed] [Google Scholar]
- 16.Pavlin CJ, Harasiewicz K, Foster FS. Ultrasound biomicroscopy of anterior segment structures in normal and glaucomatous eyes. Am J Ophthalmol. 1992;113:381–9. doi: 10.1016/s0002-9394(14)76159-8. [DOI] [PubMed] [Google Scholar]
- 17.Radhakrishnan S, See J, Smith SD, et al. Reproducibility of anterior chamber angle measurements obtained with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:3683–8. doi: 10.1167/iovs.06-1120. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005;123:1053–9. doi: 10.1001/archopht.123.8.1053. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa H, Liebmann JM, Ritch R. Quantitative assessment of the anterior segment using ultrasound biomicroscopy. Curr Opin Ophthalmol. 2000;11:133–9. doi: 10.1097/00055735-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Thomas R, Arun T, Muliyil J, George R. Outcome of laser peripheral iridotomy in chronic primary angle closure glaucoma. Ophthalmic Surg Lasers. 1999;30:547–53. [PubMed] [Google Scholar]
- 21.Pavlin CJ, Ritch R, Foster FS. Ultrasound biomicroscopy in plateau iris syndrome. Am J Ophthalmol. 1992;113:390–5. doi: 10.1016/s0002-9394(14)76160-4. [DOI] [PubMed] [Google Scholar]
- 22.He M, Friedman DS, Ge J, et al. Laser peripheral iridotomy in eyes with narrow drainage angles: ultrasound biomicroscopy outcomes. The Liwan Eye Study. Ophthalmology. 2007;114:1513–9. doi: 10.1016/j.ophtha.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 23.How AC, Baskaran M, Kumar RS, et al. Changes in anterior segment morphology after laser peripheral iridotomy: an anterior segment optical coherence tomography study. Ophthalmology. 2012;119:1383–7. doi: 10.1016/j.ophtha.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Lee KS, Sung KR, Shon K, et al. Longitudinal changes in anterior segment parameters after laser peripheral iridotomy assessed by anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:3166–70. doi: 10.1167/iovs.13-11630. [DOI] [PubMed] [Google Scholar]
- 25.Nongpiur ME, He M, Amerasinghe N, et al. Lens vault, thickness, and position in Chinese subjects with angle closure. Ophthalmology. 2011;118:474–9. doi: 10.1016/j.ophtha.2010.07.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.