Abstract
Objective
Large vessel vasculitides (LVV) are a group of autoimmune diseases characterized by injury to and anatomic modifications of large vessels, including the aorta and its branch vessels. Disease etiology is unknown. This study was undertaken to identify antigen targets within affected vessel walls in aortic root, ascending aorta, and aortic arch surgical specimens from patients with LVV, including giant cell arteritis, Takayasu arteritis, and isolated focal aortitis.
Methods
Thoracic aortic aneurysm specimens and autologous blood were acquired from consenting patients who underwent aorta reconstruction procedures. Aorta proteins were extracted from both patients with LVV and age-, race-, and sex-matched disease controls with noninflammatory aneurysms. A total of 108 serum samples from patients with LVV, matched controls, and controls with antinuclear antibodies, different forms of vasculitis, or sepsis were tested.
Results
Evaluation of 108 serum samples and 22 aortic tissue specimens showed that 78% of patients with LVV produced antibodies to 14-3-3 proteins in the aortic wall (93.7% specificity), whereas controls were less likely to do so (6.7% produced antibodies). LVV patient sera contained autoantibody sufficient to immunoprecipitate 14-3-3 protein(s) from aortic lysates. Three of 7 isoforms of 14-3-3 were found to be up-regulated in aorta specimens from patients with LVV, and 2 isoforms (ε and ζ) were found to be antigenic in LVV.
Conclusion
This is the first study to use sterile, snap-frozen thoracic aorta biopsy specimens to identify autoantigens in LVV. Our findings indicate that 78% of patients with LVV have antibody reactivity to 14-3-3 protein(s). The precise role of these antibodies and 14-3-3 proteins in LVV pathogenesis deserves further study.
Large vessel vasculitis (LVV) is characterized by immune-mediated injury of predominantly large vessels. Histopathologic features include mononuclear cell infiltration of the vessel wall that often includes granuloma formation. Within this group of diseases, Takayasu arteritis (TAK) affects younger individuals (younger than 50 years; mean age ~26 years at onset), whereas giant cell arteritis (GCA) is unique to older patients (older than 50 years; mean age ~74 years at onset) (1). Both predominantly affect women. Included within the LVV spectrum is isolated focal aortic disease, which is usually limited to the thoracic aorta. Isolated focal aortic disease is a form of vasculitis within the larger category of single-organ vasculitis (1). Patients with isolated focal aortic disease who do not have histories or features of GCA or TAK at presentation may later develop TAK or GCA, but that is infrequent (2). It has been suggested that GCA (prevalence 1 in 500 in the population older than 50 years) and TAK (annual incidence ~3 per million) may be the same disease with the same etiology, but with phenotypic variations due to immune and substrate senescence that occur with aging (3,4). How and whether isolated focal aortic disease fits into the spectrum of GCA and TAK have not been explored. Limited availability of aorta specimens has been a major deterrent to designing studies that may provide a better understanding of LVV pathogenesis.
TAK and GCA are primarily considered Th1 and Th17 cell–mediated diseases (5,6). In both, certain vascular territories are commonly affected (e.g., aorta and aortic arch vessels) and others mostly spared (e.g., arteries distal to the elbows and knees), begging the question of what might be targeted antigens or shared immune vulnerabilities within affected sites (7). Vascular dendritic cells (DCs) are a component of the resident cell population in muscular arteries (6,8). It has been proposed that resident-sentinel DCs within the adventitia–media border of muscular arteries become activated by unknown antigen(s), leading to the recruitment of CD4+ T cells and release of proinflammatory cytokines (8,9). The inflammatory cascade includes endothelial cell activation, recruitment of macrophages and neutrophils, enhanced production of matrix metalloproteinases, and oxidative products that cause extracellular matrix disruption and reorganization (10,11).
Previous attempts to identify autoantigens and infectious agents in the temporal arteries of patients with GCA have implicated numerous organisms, including parainfluenza virus type 1 (12), herpes simplex virus (13), parvovirus B19 (14), Varicella zoster virus (15), Chlamydia pneumonia (16), and Mycobacterium tuberculosis (17). In a previous study, microbial fragments present in the giant cells of temporal arteries were isolated and found to contain signatures of multiple bacterial species to which patients produced antibodies (18). Other studies have failed to identify suspected microbial agents in temporal arteries. Our study is unique in addressing the issues of autoantigens within sterile, snapfrozen, thoracic aorta specimens.
Within our Heart Vascular Institute, the Center for Aortic Diseases performs thoracic aorta surgeries for >650 patients per year. This has provided opportunities to study specimens from patients with a variety of noninflammatory conditions and LVV. We have explored LVV pathogenesis by asking questions about extractable antigens in the aneurysmal aorta of surgical specimens that may elicit autologous immune responses. The question of antigen reactivity was explored in patients with GCA, TAK, or isolated focal aortic disease, and in disease controls (i.e., patients with thoracic aneurysms who had matrix disorders and no clinical or pathologic evidence of vasculitis). We found that patients with LVV, including GCA, TAK, and isolated focal aortic disease, produce antibodies to select isoforms of 14-3-3 proteins.
PATIENTS AND METHODS
Patients
The study included patients undergoing thoracic aorta reconstruction for aneurysms caused by LVV and noninflammatory conditions, such as matrix disorders (e.g., Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, bicuspid aortic valves, and chronic hypertension) that served as controls for LVV. Controls were age-, race-, and sex-matched to LVV patients, and are referred to herein as matched controls. We analyzed aorta specimens from 11 patients with LVV and 11 matched controls. For Western blot and immunoprecipitation studies, additional serum samples were obtained from patients with aortitis who were undergoing thoracic aortic reconstruction (n = 23), matched controls with noninflammatory aortic aneurysm (n = 23), and controls with vasculitis or other diseases (n = 41) (Table 1). For enzyme-linked immunosorbent assays (ELISAs), sera were obtained from 23 patients with LVV, 26 matched controls, 48 disease controls, and 11 healthy controls (Table 1). Serum samples from patients who did not undergo thoracic surgical procedures (other than patients with LVV and matched controls) are referred to as “serum-only controls.” Frozen and paraffin-embedded human tissue sections of hepatocellular carcinoma and adjacent nontumor tissues were obtained from the Cleveland Clinic Central Biorepository. The study (IRB#10-1128) was approved by the Institutional Review Board of the Cleveland Clinic.
Table 1.
List of sera tested on aortic lysates*
| Serum | Western blot analysis, no. of samples (no. with 30-kd positive reactivity) |
ELISA, no. of samples |
No. of patients receiving prednisone |
|---|---|---|---|
| LVV | 23 (18) | 23 | 6† |
| Matched controls‡ | 23 (2) | 26 | 0 |
| Sera-only controls | |||
| GPA | 15 (2) | 20 | 3 |
| EGPA | 5 (0) | – | 3 |
| SLE | 4 (0) | 10 | 1 |
| IgAV | 5 (0) | 6 | 1 |
| ANA | 8 (0) | 8 | 0 |
| Sepsis | 4 (0) | 4 | 0 |
| Healthy | – | 11 | 0 |
ELISA = enzyme-linked immunosorbent assay; GPA = granulomatosis with polyangiitis (Wegener’s); EGPA = eosinophilic granulomatosis with polyangiitis (Churg-Strauss); SLE = systemic lupus erythematosus; IgAV = IgA vasculitis (Henoch-Schönlein); ANA = antinuclear antibody.
Of all patients with large vessel vasculitis (LVV) tested, 6 were receiving prednisone treatment of 2–55 mg/day (1 received 2 mg/day, 1 received 5 mg/day, 1 received 20 mg/kg/day, 2 received 30 mg/kg/day, and 1 received 55 mg/day). Prednisone dosages of <30 mg/day did not affect autoantigen detection, as measured by 30-kd reactivity on aortic lysates in Western blots. One of the serum samples from the patients receiving 30 mg/day and the serum sample from the patient receiving 55 mg/day were negative.
Matched for age, sex, and race.
Reagents
All chemicals were purchased from Sigma unless stated otherwise. Antibodies were purchased from the following companies. Isoform-specific anti–14-3-3 antibodies were from Cell Signaling Technology, pan–14-3-3 antibody was from Santa Cruz Biotechnology, anti-CD3 and smooth muscle actin (SMA) antibodies were from Sigma-Aldrich, and anti-human IgG and protein G–Sepharose were from Pierce (Thermo Fisher Scientific). ELISA plates and Ultra TMB were from Millipore and Pierce (Thermo Fisher Scientific), respectively.
Western blotting and immunoprecipitation
Immediately after the aorta excision, tissue was snap-frozen in liquid nitrogen and transferred to −80°C freezers for storage. While frozen, small samples of tissue were cut and then lysed with radioimmunoprecipitation assay buffer (plus protease and phosphatase inhibitors) on ice using a tissue homogenizer with a stainless steel rotor. The lysate was then centrifuged at 10,000 revolutions per minute for 20 minutes at 4°C. Equal amounts of proteins were loaded onto 10% gel and later transferred to a PVDF membrane using a semi-dry transfer apparatus. Membranes were then blocked with milk and incubated with diluted sera (1:50) overnight at 4°C on a rotary shaker. After washing with phosphate buffered saline containing 0.1% Tween (PBST), membranes were incubated with mouse anti-human IgG for 1 hour at room temperature followed by horseradish peroxidase (HRP)–conjugated anti-mouse IgG for 30 minutes, as per standard Western blot procedure. Membranes were then developed using enhanced chemiluminescence (ECL; Amersham). For immunoprecipitation of antigens from aorta specimens, tissue lysates (250 µg) were incubated with either 2 µg of commercially available 14-3-3 antibodies or 20 µl of sera overnight at 4°C. For serum IgG immunoprecipitation, 12 µg of purified protein was incubated with 100 µl of serum for 4 hours at 4°C. Washed and equilibrated protein G–Sepharose beads were added and incubated for 2 hours at 4°C. Beads were then spun down, washed with lysis buffer, and boiled with sample buffer containing β-mercaptoethanol. Eluates were analyzed by Western blotting or mass spectrometry as needed.
Purification of proteins
Glutathione S-transferase–tagged constructs of 14-3-3 isoforms (generated in the laboratory of Michael Yaffe, MD, PhD [Massachusetts Institute of Technology, Cambridge, MA]) were purchased from Addgene under a material transfer agreement. Constructs were used to transform BL21 Escherichia coli for protein expression. Aetailed protocol of protein purification is published elsewhere (19). Briefly, cells were grown in 100 ml Luria broth for 16 hours at 37°C followed by inoculation in 1 liter of terrific broth. Protein expression was induced using 1 mM IPTG for 48 hours followed by cell harvest and lysis using sonication. Protein was then purified using glutathione Sepharose beads according to the recommendations of the manufacturer (Amersham Biosciences).
Mass spectroscopy (MS)
Protein identification experiments were performed by tryptic digestion followed by liquid chromatography tandem MS (LC-MS/MS). For these experiments, the sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) bands were excised from the Coomassie-stained gels, washed, reduced, and alkylated prior to in-gel digestion with trypsin (20). The chromatographic separation of the protein digest was performed using a Dionex Ultimate 3000 high-performance liquid chromatography system (Thermo Scientific) equipped with an Acclaim PepMap RSLC reverse-phase nanocolumn (75 µm × 15 cm, C18, 2 µm, 100Å) (Thermo Fisher Scientific). Peptides were eluted using a 0.1% formic/95% acetonitrile linear gradient. The mass spectra were recorded on a Thermo LTQ Orbitrap Elite (Thermo Electron Corporation) and operated in a data-dependent manner for protein identification. The LC-MS/MS data were searched against the human reference sequence using the Mascot program (Matrix Science). Positive protein identifications required the presence of at least 2 matching peptides with Mascot ion scores of >40.
ELISA
We used control aorta lysate (or bovine serum albumin [BSA] as a control) to detect antiaorta antibodies in serum samples. One hundred micrograms of either aorta lysate or BSA diluted in PBST was allowed to coat each well of Immobilon-P ELISA plates overnight at 4°C on a rotary shaker. Coated plates were then washed twice with PBST buffer and blocked with 5% milk for 1 hour followed by washing and addition of sera at 50 µg/ml in 1% BSA for 2 hours at room temperature. Followed by washes with PBST, plates were then treated with primary and HRP-conjugated secondary antibody, as described above for the Western blotting procedure. After secondary antibody removal, plates were washed twice with PBST, and Ultra TMB was added according to the recommendations of the manufacturer. Color development was read at 650 nm.
Immunohistochemistry
Paraffin-embedded aorta sections (5-µ thick) were obtained from the Pathology Tissue Center at the Cleveland Clinic. Sections were then immunostained with pan–14-3-3 antibody (1:100), human sera (1:200), SMA (1:100), CD3 (1:100), or hematoxylin and eosin at the immunohistochemistry core facility at Lerner Research Institute.
Statistical analysis
Data were analyzed using Student’s t-test for 2-tailed distribution of 2 samples with equal variance. P values less than 0.05 were considered significant.
RESULTS
Presence of autoantibodies in LVV sera
To test for the presence of and to quantify autoantibodies in patient sera, we developed an ELISA-based colorimetric assay. We placed 100 µg/ml of common aortic lysate or BSA on a 96-well plate, and used individual serum samples in each well for testing. We screened a total of 108 serum samples, including 23 samples from LVV patients and 26 samples from matched controls with noninflammatory aortic aneurysm, 48 samples from patients with inflammatory diseases including other forms of vasculitis, and 11 samples from healthy controls (Table 1). First, we observed much higher binding of sera IgG to aortic lysate than to BSA, as measured by absorbance at 650 nm. Second, sera from either LVV or disease controls did not show any significant absorbance difference in BSA-coated wells (see Supplementary Figure 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). Third, LVV sera showed higher absorbance compared to sera from disease controls and healthy controls. This indicated that the LVV sera had IgG antibodies against unknown aortic protein(s) (Figure 1A), and this difference was found to be highly significant (P < 0.001).
Figure 1.
High antibody titers against aorta protein in sera from patients with large vessel vasculitis (LVV). A, Presence of anti-IgG in sera from healthy individuals, patients with systemic lupus erythematosus (SLE), patients with antinuclear antibody (ANA), patients with IgA vasculitis (Henoch-Schönlein) (HSP), patients with granulomatosis with polyangiitis (Wegener’s) (GPA), patients with LVV, and matched controls. A noninflamed aorta lysate was used to test for anti-IgG by enzyme-linked immunosorbent assay. Bound antibodies were detected using antihuman IgG as described in Patients and Methods. The difference between patients with LVV and matched controls and between patients with LVV and healthy controls was significant (P = 0.001). Values are absorbance (Abs) at 650 nm normalized to values for bovine serum albumin (BSA) to indicate specificity to aorta protein. Each data point represents a single subject; horizontal lines show the mean. B, Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of aortic lysates from tissue from matched controls (n = 7) and LVV patients (n = 8), resolved on 10% SDS-PAGE gel. After transfer, PVDF membranes were first blotted with control serum and later stripped for reprobing with LVV serum. Representative gels are shown for each serum blot. Two nonvasculitis serum controls (procalcitonin positive and ANA positive) were also screened for reactivity. C, Gel used for mass spectrometry analysis. In-gel trypsin digestion and mass spectroscopic analysis of the 30-kd region on protein gel identified 14-3-3 to be the most abundant protein in this region, particularly in LVV samples. C1 and C2 indicate aorta lysates from 2 separate matched controls, whereas L1 and L2 indicate aorta lysates from 2 separate patients with LVV.
Reaction of LVV sera with 30-kd aortic proteins
We studied 22 frozen sterile aorta samples (11 from LVV patients and 11 from matched controls). We observed that both control and LVV aorta lysates reacted with LVV sera as detected by binding of IgG-specific antibody. In addition to light and heavy chains of IgG, 78% of sera from patients with LVV also showed a third band of positive reactivity at ~30 kd when tested on aortic lysates (Figure 1B and Table 1) (see Supplementary Figures 2 and 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). This 30-kd band of positive reactivity was absent in 89% of serum samples from matched controls and 80% of serum samples from patients with granulomatosis with polyangiitis (Wegener’s) (GPA), and was completely absent in sera from other controls, such as patients with SLE, eosinophilic granulomatosis with polyangiitis (Churg-Strauss), IgA vasculitis (Henoch-Schönlein), or sepsis, and in sera from antinuclear antibody (ANA)–positive patients. There was only 6.7% positive reactivity in the control set (93.7% specificity).
Representative results comparing 30-kd binding in matched controls, serum-only controls, and patients with LVV serum from on the same aortic lysates are shown in Figure 1B. Four of the patients with LVV who provided aorta tissue samples were receiving prednisone (2–55 mg/day). We did not observe any difference in the detection of 30-kd aorta protein lysate reactivity in prednisone-treated patients (<30 mg/day) versus untreated patients. However, aortic proteins from 1 LVV patient receiving high-dose prednisone (55 mg/day) and 1 LVV patient receiving 30 mg/day, did not display 30-kd protein binding to LVV sera. No specific reactivity was observed when IgM-, IgE-, or IgG4-specific antibodies were screened (data not shown).
The 14-3-3 protein is the most abundant protein in the 30-kd region
To identify the possible antigen eliciting the immune response in LVV, we decided to identify proteins present in the 30-kd region. We used a gel parallel to the one used to study immunoreactivity (Figure 1C) and subjected it to in-gel trypsin digestion of the 30-kd region followed by MS analysis. A total of 33 proteins were identified in this region of 30 kd wherein, based on ion intensities, 14-3-3 was most abundant in LVV lysates (see Supplementary Table 1, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). Of the 7 mammalian isoforms known in the 14-3-3 protein family, 6 (β, ε, η, γ, τ, and ζ) were identified in the aortic lysates. Other major proteins identified in the 30-kd region included actin, apolipoprotein, different isoforms of annexins (annexin I and annexin II), calponins, and chloride channel proteins. Annexins and other cytoskeletal proteins have previously been studied in GCA, but none has been shown to be autoantigenic (21,22). Therefore, we investigated 14-3-3 proteins to determine autoantigenicity in LVV.
Presence of anti–14-3-3 antibodies in LVV sera
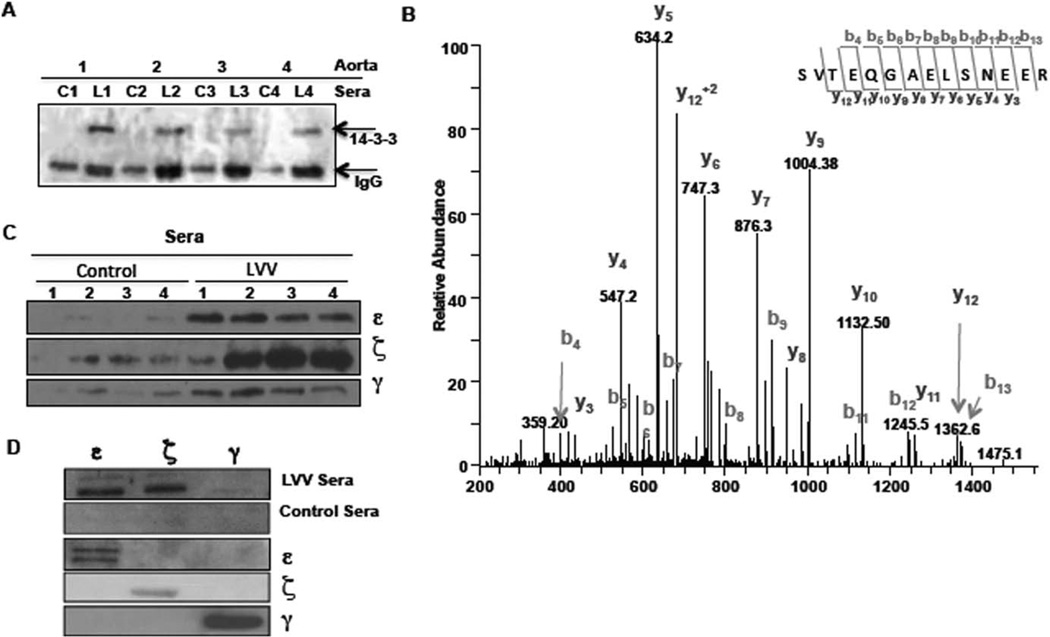
To confirm the antigenicity of 14-3-3 protein(s), we tested for the presence of anti–14-3-3 antibodies in sera. We performed 3 independent experiments with 8 aorta lysates (4 from controls and 4 from patients with LVV) and 12 serum samples (6 from controls and 6 from patients with LVV) for the antigen pulldown assays. As shown in Figure 2A, LVV (n = 4) or matched control (n = 4) sera were used to pull down antigen from the 4 LVV aortic lysates, and eluates were probed for the presence of 14-3-3. Using both Western blotting and MS, we observed successful 14-3-3 immunoprecipitation from the tissue (LVV or matched control) lysates when LVV sera were used (Figures 2A and B) (see Supplementary Table 2 and Supplementary Figure 3, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). This indicated that sera from all 6 patients with LVV (3 with GCA, 2 with isolated focal aortic disease, and 1 with TAK) had anti-14-3-3 antibodies, whereas similar antibody reactivity was not found in matched controls.
Figure 2.
Presence of anti–14-3-3 antibodies in sera from patients with large vessel vasculitis (LVV). A, Presence of anti–14-3-3 antibodies in sera from 4 matched controls (C1–C4) and 4 patients with LVV (L1–L4). Sera were used to pull down antigen(s) from 4 LVV aorta lysates followed by probing for 14-3-3 (~30 kd). The lower band shows IgG light chain (~25 kd). B, Mass spectrometry (MS) analysis of parallel gel of autologous sera pulldowns of 2 control (C1 and C2) and 2 LVV (L1 and L2) aorta lysates. Proteins identified by MS are listed in Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract. An example of MS/MS spectra from the in-gel tryptic digestion of samples derived from L1 is shown. This spectrum was matched to the 14-3-3 ζ/δ–derived peptide (28) SVTEQGAELSNEER (41). Several sequence-specific ions were identified in this spectrum, including 10 C-terminal–derived fragments (labeled y ions) and 10 N-terminal–derived fragments (labeled b ions). C, Presence of isoform-specific anti–14-3-3 IgG in LVV sera. Twelve micrograms of purified 14-3-3 protein ε, ζ, or γ was added to the LVV (n = 4) or matched control (n = 4) sera, followed by protein G–Sepharose pulldown. D, Immunoreactivity of 14-3-3 protein ε, ζ, and γ isoforms. Purified proteins (5 µg of ε, ζ, and γ) were electrophoresed and blotted with either LVV or matched control serum sample. Membranes were then probed with isoform-specific antibodies to confirm the presence and purity of respective proteins.
In the detailed MS analysis, of a total of 7 isoforms of 14-3-3, only 3 isoforms (ζ, ε, and γ) were detected in the eluates in 2 independent experiments (see Supplementary Table 2, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). To more specifically identify the antigenic isoform of 14-3-3, we used purified 14-3-3 protein ε, ζ, and γ. Figure 2C shows that anti–14-3-3 antibodies in LVV sera were primarily targeted against the isoforms ε (4 of 4 LVV samples) and ζ (3 of 4 LVV samples). Anti–14-3-3 protein γ antibodies were marginally increased in LVV sera compared to matched control sera. To further strengthen our results, we probed the 3 LVV and 3 control sera against purified protein using Western blotting. As shown in Figure 2D, LVV sera strongly reacted with purified ε and ζ isoforms, but not with γ isoform. However, each isoform-specific antibody confirmed the presence of respective protein on the membrane, thereby strengthening the specificity of LVV sera immunoreactivity with specific 14-3-3 protein isoforms.
Since 14-3-3 has also been reported to be an autoantigen in hepatocellular carcinoma (23), we tested LVV serum reactivity with 3 hepatocellular carcinoma tissues as well as their adjacent nontumor tissue (normal) lysates. Unlike aorta, liver lysates from either hepatocellular carcinoma or normal tissue samples showed variable immunoreactivity with LVV sera (see Supplementary Figure 4A, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). When tested for the expression of the 14-3-3 protein ζ isoform, hepatocellular carcinoma tissue lysates (versus nontumor liver tissue) did not show a statistically significant increase. A marginal increase in sinusoidal staining of pan–14-3-3 was observed in sections of hepatocellular carcinoma tissue compared to nontumor liver tissue (see Supplementary Figure 4B, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract).
Increased expression of 14-3-3 in the aorta in LVV patients
To understand the role of 14-3-3 in LVV, we studied the expression profile of total 14-3-3 and of the 7 individual isoforms of 14-3-3 in 10 LVV and 10 control aortas. Total 14-3-3 was significantly up-regulated in LVV aorta lysates compared to control aorta lysates (P = 0.003). While most of the isoforms were up-regulated in the aorta in patients with LVV, only 3 of the 7 isoforms showed statistically significant up-regulation of expression (γ, P = 0.014; τ, P = 0.005; and β, P = 0.0006) (Figure 3A) (see Supplementary Figure 5, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract).
Figure 3.
Expression and localization of 14-3-3 protein in human aorta. A, Significant increase in total 14-3-3 protein (pan–14-3-3) and in the γ (g), τ (t), and β (b) isoforms in aorta lysates from patients with large vessel vasculitis (LVV) compared to controls. Aorta tissue lysates from controls and LVV patients were evaluated for various isoforms of 14-3-3 family members. Expression of total 14-3-3 and individual isoforms was studied by Western blotting, and band intensity (shown on the y-axis) was quantified using ImageJ software. Each data point represents a single subject; horizontal lines show the mean. B, Staining of tissue sections from LVV or control aorta with anti–pan-14-3-3 antibody. In control aorta (a and b), 14-3-3 was weakly expressed in smooth muscle cells (M) in the media. In LVV aorta (c and d), 14-3-3 was strongly expressed in the inflammatory cells surrounding necrotic area (N), as well as in smooth muscle cells. I = intimal side; O = adventitial side. C, Staining of LVV aorta with CD3 and smooth muscle actin (SMA), showing colocalization of 14-3-3 proteins with T lymphocytes and smooth muscle cells, respectively. D, Similar patterns of activity in tissue sections from LVV aorta immunostained with pan–14-3-3 antibody or LVV sera. Arrows indicate the region stained with both 14-3-3 and sera IgG.
Altered 14-3-3 localization in the aorta in LVV patients
Immunostaining of the paraffin-embedded sections of control aorta with pan–4-3-3 antibody revealed faint positive staining of smooth muscle cells (SMCs) and cells around the vasa vasorum of the adventitia. In contrast, aorta samples from LVV patients exhibited inflammatory infiltrates, intimal hyperplasia, medial degeneration, and adventitial thickening. In the samples from LVV patients, the laminar arrangement of the SMCs was modified by focal degeneration and necrosis. Pan–14-3-3 antibody strongly stained areas of necrosis, infiltrating chronic inflammatory cells (CD3+) and SMCs (SMA stained) as observed by stain colocalization (Figures 3B and C). Endothelial cells showed faint staining with 14-3-3. In the adventitia, 14-3-3 was strongly expressed in the vaso vasora and neovascularized region of media and adventitia. Similar patterns but with more intense staining were observed when serum from LVV patients was used for staining the autoantigen. Significant colocalization was observed between sera and 14-3-3 antibody in these sections. Figure 3D shows a representative tissue section from a GCA patient, in which infiltrating mononuclear cells were stained with both 14-3-3 and diluted serum antibodies.
DISCUSSION
This is the first study to use aorta specimens to identify autoantigens in patients with LVV. Our findings indicate that of 23 serum samples from LVV patients, 78% produced antibodies to aortic protein(s), and the antigen target belonged to the family of 14-3-3 proteins. In comparison, patients with noninflammatory aortic diseases, patients who produced ANAs, patients who had a variety of autoimmune inflammatory conditions, and patients with sepsis were usually 14-3-3 antibody negative. Our study required development of a prototypic ELISA-based assay to quantitate the presence of autoantibodies in the sera, which showed that antiaortic antibodies in sera from LVV patients can be quantified. Our results showed that total 14-3-3 as well as 3 of its isoforms are significantly increased in LVV aorta tissues, and 2 of its isoforms were found to be antigenic. Furthermore, redistribution of 14-3-3 within the LVV aorta tissue was observed and found to associate primarily with smooth muscle and inflammatory cells. Importantly, 14-3-3 localization correlated well with diluted serum staining for autoreactivity. Our data do not allow concluding whether 14-3-3 antigenicity is an important element in the early stages of pathogenesis of LVV or is a late feature in the cycle of tissue injury and repair. Evidence favoring the former possibility is the absence or low levels of anti–14-3-3 antibodies in control patients with noninflammatory aortic aneurysm.
The 14-3-3 proteins are a family of adaptor molecules known to interact with more than 300 serine or threonine phosphorylated proteins (24). Both expression and function of each of the 7 isoforms (α/β, δ/ζ, η, τ, ε, γ, and σ) are under strict regulatory control (24–26). Increased expression of 14-3-3 compared to that in healthy controls has been demonstrated in patients with rheumatoid arthritis, patients with multiple sclerosis, and patients with endometriosis (27–29). Both gain and loss of 14-3-3 functions can have significant impact on cellular health. The 14-3-3 protein has also been reported to be antigenic in patients with toxoplasmosis, echinococcosis, or angiostrongylus (23,30–32). For each of these examples it is not clear whether enhanced expression of 14-3-3 or immune responses to 1 or more of its isoforms are critical elements in pathogenesis or secondary epiphenomena (23,28,33). Factors such as isoform switching, heterodimerization, posttranslational modification, and altered localization can contribute to the altered functions and antigenicity of 14-3-3 (34).
We observed increased expression of several isoforms, relocalization, and an antigenic role of 14-3-3 in aorta tissue from patients with LVV. It is not known if up-regulation of the 14-3-3 isoforms (γ, β, and τ) in disease has any effect on antigenicity. Our preliminary results in LVV suggest that expression and antigenicity of 14-3-3 isoforms may be independent events. Specificity of LVV serum immunoreactivity to 14-3-3 is further strengthened by the observation of variable reactivity with liver lysates, suggesting that a distinct antigen may be at play. Interestingly, liver tissues stained with pan–14-3-3 showed increased staining, especially in the hepatic sinusoids of hepatocellular carcinoma (see Supplementary Figure 4B, available on the Arthritis & Rheumatology web site at http://onlinelibrary.wiley.com/doi/10.1002/art.39130/abstract). Since sinusoidal regions are rich in immune cells, including DCs and Kupffer cells, increased 14-3-3 staining in these areas is similar to what we observed in the mononuclear infiltrate of LVV sections.
The finding of anti–14-3-3 in 78% of the sera from LVV patients tested in this study and its infrequent presence among disease controls (6.7%) suggests that these antibodies may be relevant to both pathogenesis and diagnosis of LVV. A limitation of this study is the low numbers of controls tested within this cohort of patients with rare diseases. However, the strengths of the study included being able to gather unique samples of snap-frozen aortic aneurysms, comparing them to highly matched noninflammatory aneurysm controls, and including control serum samples from patients with other chronic inflammatory and autoimmune diseases. While our findings will need to be confirmed, they do raise important questions for further studies that may address whether targeted 14-3-3 isoforms are native or modified antigens, whether there is a relevant T cell immune response to 14-3-3, and whether circulating antigen concentrations and/or antibodies to these proteins may be useful surrogates for diagnosis and monitoring disease activity.
Our findings also touch on the unresolved question of whether clinical similarities between GCA, TAK, and isolated focal aortic disease reflect commonalities in pathogenesis, with modified phenotypes being a product of age-related substrate and immune senescence. The 14-3-3 protein in the human aorta is an autoantigen in LVV. Its expression and localization are altered in the aortas of patients with aortitis compared to matched controls.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to all of the members of the Clinical Research Unit at the Cleveland Clinic who participated in the collection and storage of specimens. We also thank Denise Hatala at Lerner Research Institute image core services for help with immunohistochemical analysis of aorta sections.
Supported by the Vasculitis Foundation (grant VF1301), the Clinical and Translational Science Collaborative of Cleveland (CTSC-Cleveland grant T53250), the American Heart Association (Scientist Development grant 23080025 to Dr. Chakravarti), and the Konigsberg Family Fund for Vasculitis Research (grant to Dr. Hoffman). The acquisition of the Orbitrap Elite instrument was made possible by the NIH Shared Instrumentation Grant Program (grant NIH-1S10-RR-031537-01).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Chakravarti had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Chakravarti, Gupta, Scholtz, Svensson, Stuehr, Hoffman.
Acquisition of data Chakravarti, Gupta, Swain, Willard, Scholtz, Roselli, Pettersson, Johnston, Soltesz, Yamashita, Daly, Hoffman.
Analysis and interpretation of data. Chakravarti, Gupta, Willard, Scholtz, Soltesz, Yamashita, Hoffman.
REFERENCES
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Rojo-Leyva F, Ratliff NB, Cosgrove DM, III, Hoffman GS. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum. 2000;43:901–907. doi: 10.1002/1529-0131(200004)43:4<901::AID-ANR23>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore) 2009;88:221–226. doi: 10.1097/MD.0b013e3181af70c1. [DOI] [PubMed] [Google Scholar]
- 4.Grayson PC, Maksimowicz-McKinnon K, Clark TM, Tomasson G, Cuthbertson D, Carette S, et al. Distribution of arterial lesions in Takayasu’s arteritis and giant cell arteritis. Ann Rheumatic Dis. 2012;71:1329–1334. doi: 10.1136/annrheumdis-2011-200795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visvanathan S, Rahman MU, Hoffman GS, Xu S, Garcia-Martinez A, Segarra M, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis—a prospective longitudinal study. Rheumatology (Oxford) 2011;50:2061–2070. doi: 10.1093/rheumatology/ker163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121:906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman GS. Determinants of vessel targeting in vasculitis. Ann N Y Acad Sci. 2005;1051:332–339. doi: 10.1196/annals.1361.075. [DOI] [PubMed] [Google Scholar]
- 8.Weyand CM, Ma-Krupa W, Pryshchep O, Groschel S, Bernardino R, Goronzy JJ. Vascular dendritic cells in giant cell arteritis. Ann N Y Acad Sci. 2005;1062:195–208. doi: 10.1196/annals.1358.023. [DOI] [PubMed] [Google Scholar]
- 9.Provinciali M, Cardelli M, Marchegiani F, Pierpaoli E. Impact of cellular senescence in aging and cancer. Curr Pharm Des. 2013;19:1699–1709. [PubMed] [Google Scholar]
- 10.Rodriguez-Pla A, Martinez-Murillo F, Savino PJ, Eagle RC, Jr, Seo P, Soloski MJ. MMP-12, a novel matrix metalloproteinase associated with giant cell arteritis. Rheumatology (Oxford) 2009;48:1460–1461. doi: 10.1093/rheumatology/kep271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Pla A, Bosch-Gil JA, Rossello-Urgell J, Huguet-Redecilla P, Stone JH, Vilardell-Tarres M. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–269. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- 12.Duhaut P, Bosshard S, Calvet A, Pinede L, Demolombe-Rague S, Dumontet C, et al. Groupe de Recherche sur l’Arterite a Cellules Geantes. Giant cell arteritis, polymyalgia rheumatica, and viral hypotheses: a multicenter, prospective case-control study. J Rheumatol. 1999;26:361–369. [PubMed] [Google Scholar]
- 13.Powers JF, Bedri S, Hussein S, Salomon RN, Tischler AS. High prevalence of herpes simplex virus DNA in temporal arteritis biopsy specimens. Am J Clin Pathol. 2005;123:261–264. doi: 10.1309/2996tt2ctltkn0kt. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel SE, Espy M, Erdman DD, Bjornsson J, Smith TF, Hunder GG. The role of parvovirus B19 in the pathogenesis of giant cell arteritis: a preliminary evaluation. Arthritis Rheum. 1999;42:1255–1258. doi: 10.1002/1529-0131(199906)42:6<1255::AID-ANR23>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell BM, Font RL. Detection of varicella zoster virus DNA in some patients with giant cell arteritis. Invest Ophthalmol Vis Sci. 2001;42:2572–2577. [PubMed] [Google Scholar]
- 16.Wagner AD, Gerard HC, Fresemann T, Schmidt WA, Gromnica-Ihle E, Hudson AP, et al. Detection of Chlamydia pneumoniae in giant cell vasculitis and correlation with the topographic arrangement of tissue-infiltrating dendritic cells. Arthritis Rheum. 2000;43:1543–1551. doi: 10.1002/1529-0131(200007)43:7<1543::AID-ANR19>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Pantell RH, Goodman BW., Jr Takayasu’s arteritis: the relationship with tuberculosis. Pediatrics. 1981;67:84–88. [PubMed] [Google Scholar]
- 18.Gordon LK, Goldman M, Sandusky H, Ziv N, Hoffman GS, Goodglick T, et al. Identification of candidate microbial sequences from inflammatory lesion of giant cell arteritis. Clin Immunol. 2004;111:286–296. doi: 10.1016/j.clim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarti R, Aulak KS, Fox PL, Stuehr DJ. GAPDH regulates cellular heme insertion into inducible nitric oxide synthase. Proc Natl Acad Sci U S A. 2010;107:18004–18009. doi: 10.1073/pnas.1008133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinter M. Protein sequencing and identification using tandem mass spectrometry. New York: John Wiley & Sons; 2000. [Google Scholar]
- 21.Nadkarni S, Dalli J, Hollywood J, Mason JC, Dasgupta B, Perretti M. Investigational analysis reveals a potential role for neutrophils in giant-cell arteritis disease progression. Circ Res. 2014;114:242–248. doi: 10.1161/CIRCRESAHA.114.301374. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta B, Duke O, Kyle V, Macfarlane DG, Hazleman BL, Panayi GS. Antibodies to intermediate filaments in polymyalgia rheumatica and giant cell arteritis: a sequential study. Ann Rheum Dis. 1987;46:746–749. doi: 10.1136/ard.46.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Liu X, Ren P, Li J, Chai Y, Zheng SJ, et al. A cancer-related protein 14-3-3ζ is a potential tumor-associated antigen in immunodiagnosis of hepatocellular carcinoma. Tumour Biol. 2014;35:4247–4256. doi: 10.1007/s13277-013-1555-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison DK. The 14-3-3 proteins: integrators of diverse signaling cues that impact cell fate and cancer development. Trends Cell Biol. 2009;19:16–23. doi: 10.1016/j.tcb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu HM, Loo YM, Horner SM, Zornetzer GA, Katze MG, Gale M., Jr The mitochondrial targeting chaperone 14-3-3ε regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe. 2012;11:528–537. doi: 10.1016/j.chom.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shandala T, Woodcock JM, Ng Y, Biggs L, Skoulakis EM, Brooks DA, et al. Drosophila 14-3-3ε has a crucial role in antimicrobial peptide secretion and innate immunity. J Cell Sci. 2011;124:2165–2174. doi: 10.1242/jcs.080598. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Meyerkord CL, Du Y, Khuri FR, Fu H. 14-3-3 proteins as potential therapeutic targets. Semin Cell Dev Biol. 2011;22:705–712. doi: 10.1016/j.semcdb.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilani RT, Maksymowych WP, Aitken A, Boire G, St-Pierre Y, Li Y, et al. Detection of high levels of 2 specific isoforms of 14-3-3 proteins in synovial fluid from patients with joint inflammation. J Rheumatol. 2007;34:1650–1657. [PubMed] [Google Scholar]
- 29.Hatzipetros I, Gocze P, Koszegi T, Jaray A, Szereday L, Polgar B, et al. Investigating the clinical potential for 14-3-3 zeta protein to serve as a biomarker for epithelial ovarian cancer. J Ovarian Res. 2013;6:79. doi: 10.1186/1757-2215-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siles-Lucas M, Merli M, Mackenstedt U, Gottstein B. The Echinococcus multilocularis 14-3-3 protein protects mice against primary but not secondary alveolar echinococcosis. Vaccine. 2003;21:431–439. doi: 10.1016/s0264-410x(02)00517-0. [DOI] [PubMed] [Google Scholar]
- 31.Meng M, He S, Zhao G, Bai Y, Zhou H, Cong H, et al. Evaluation of protective immune responses induced by DNA vaccines encoding Toxoplasma gondii surface antigen 1 (SAG1) and 14-3-3 protein in BALB/c mice. Parasit Vectors. 2012;5:273. doi: 10.1186/1756-3305-5-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morassutti AL, Levert K, Perelygin A, da Silva AJ, Wilkins P, Graeff-Teixeira C. The 31-kDa antigen of Angiostrongylus cantonensis comprises distinct antigenic glycoproteins. Vector Borne Zoonotic Dis. 2012;12:961–968. doi: 10.1089/vbz.2011.0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermeking H. The 14-3-3 cancer connection. Nat Rev Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- 34.Obsil T, Obsilova V. Structural basis of 14-3-3 protein functions. Semin Cell Dev Biol. 2011;22:663–672. doi: 10.1016/j.semcdb.2011.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.