Abstract
Diisocyanates (dNCOs) used in industrial applications are well known low molecular weight allergens. Occupational exposure is associated with adverse health outcomes including allergic sensitization and occupational asthma. In this study, we report the production and initial characterization of a dNCO-hapten specific murine IgM monoclonal antibody (mAb). Female BALB/c mice were immunized intraperitoneally with 25 µg of 4,4′-methylene diphenyl diisocyanate (MDI)-keyhole limpet hemocyanin. Following six biweekly booster immunizations, splenocytes were recovered and fused to Sp2/0-Ag14 murine myeloma cell line for hybridoma production. Hybridomas were then screened in a solid-phase indirect enzyme-linked immunosorbent assay (ELISA) against 40:1 4,4′-MDI– human serum albumin (HSA). mAb reactivity to dNCO-HSA conjugates and dNCO-HSA spiked human serum were characterized using a sandwich ELISA. One hybridoma produced a multimeric IgM mAb (15D4) that reacted with 4,4′-MDI-HSA. Sandwich ELISA analysis demonstrated comparable reactivity with other occupationally relevant dNCO-HSA adducts, including 2,4-toluene diisocyanate (TDI)-HSA, 2,6-TDI-HSA, and 1,6-hexamethylene diisocyanate (HDI)-HSA, but not other electrophilic chemical HSA conjugates. The limit of quantification (LOQ) of 4,4′-MDI-HSA, 2,4-TDI-HSA, 2,6-TDI-HSA, and 1,6-HDI-HSA sandwich ELISAs were 567.2, 172.7, 184.2, and 403.5 ng/mL (8.67, 2.60, 2.77, and 6.07 pmol/mL), respectively. In contrast, experiments using dNCO-supplemented human sera showed an increase in the detectable limit of the assay. A mAb has been produced that has potential utility for detecting mixed diisocyanate exposures in occupational environments. The mAb may have additional utility in the standardization of specific IgE detection immunoassays as well as chromatographic-mass spectrometric methods to enrich dNCO adducted HSA in the plasma of occupationally exposed workers.
Keywords: diisocyanate, monoclonal antibody, occupational asthma, immunoassay
INTRODUCTION
Diisocyanates (dNCO) are commonly utilized chemicals in the manufacturing sector due to their reactivity with free hydroxyl groups to produce polyurethane polymers. Examples of commercially available products include flexible or rigid foams, elastomers, surface coatings, adhesives, sealants, varnishes, and paints.(1) The two most common dNCOs used in industrial applications include methylene diphenyl diisocyanate (MDI) and toluene diisocyanate (TDI).(1) Hexamethylene diisocyanate (HDI)- and isophorone diisocyanate (IPDI)-based oligomers are also utilized in the automotive industry and autobody repair. In 2010, the annual consumption of dNCO in the United States was 1.9 billion pounds(2) and the National Institute of Occupational Safety and Health (NIOSH) estimates more than 250,000 workers are occupationally exposed to dNCOs.(3)
Diisocyanates are potent sensitizers and are the most commonly reported cause of occupational asthma (OA) in North America.(4,5) Occupational exposure to dNCOs may result in other adverse health outcomes including immune mediated hypersensitivity pneumonitis (HP),(6) reactive airways dysfunction syndrome,(5) and allergic contact dermatitis, as well as irritation of the skin and mucous membranes.(7–11) Currently, NIOSH recognizes worker exposure to liquid, vapor, or aerosol dNCOs as both a respiratory and dermal occupational hazard and the recommended permissible exposure limit (PEL) should not exceed 0.005 part per million for each dNCO.(7)
In spite of the documented health hazards, the allergenic forms of dNCO hapten-protein conjugated products that are produced following occupational exposure remain less clear. These limitations have confounded serodiagnosis and exposure assessment using immunological approaches. dNCOs are electrophiles that react with amines and thiols on proteins.(12) Potential endogenous dNCO adducts have been reported and include glutathione, tubulin, actin, keratin, hemoglobin, and human serum albumin (HSA).(4,13) Recently, binding sites of TDI have been shown to react with the N-terminal amine of HSA, the ε-amino group (ε-NH2) of lysine, and 37 other binding sites on HSA using a high TDI-HSA conjugation ratio (40:1).(12) Although less reactive, similar binding sites have been reported for MDI.(14) Given the abundance of HSA in human serum, these data indicate that dNCO-HSA reaction products may serve as potential serological biomarkers of occupational exposure.
Due to the hazards associated with occupational exposure to dNCOs, there has been great interest in the development of sensitive biomonitoring methodologies for evaluating worker exposure. To date, the availability of antibodies for the serological detection of dNCO-protein adducts has been limited. Polyclonal antibodies (pAbs) against HDI-HSA conjugates have been reported for biomonitoring HDI occupational exposures.(15) Ruwona et al. have developed murine IgM and IgG mAbs with unique specificity for TDI-HSA and other protein adducts.(16,17) More recently, six IgG1 mAbs with specific reactivity for MDI-protein adducts have been reported by Wisnewski and Liu.(18) Although these antibodies have provided potential new tools for the isolation and identification of TDI and MDI target proteins, to our knowledge there are no mAbs that react with either HDI or a combination of other occupationally relevant dNCOs. In this study, we report the production and initial characterization of a murine mAb with broad specificity to the most commonly used dNCOs in occupational environments.
MATERIALS AND METHODS
4,4′-MDI-KLH Antigen Preparation
4,4′-methylene diphenyl diisocyanate (MDI, CAS 101-68-8, Sigma Aldrich, St. Louis, Mo.) was bound to keyhole limpet hemocyanin (KLH). Conjugates used for murine immunizations were prepared in 0.01 M phosphate buffered saline (PBS, pH 7.4) at a KLH (Thermo Fisher Scientific, Rochester, N.Y.) concentration of 0.5 mg/mL. MDI was added to the KLH solution at a molar ratio of 40 4,4′-MDI:1 KLH. Stock solutions (0.03 M) were prepared by dissolving 4,4′-MDI in dry acetone immediately prior to conjugation. The 4,4′-MDI solution (50 µL) was added to 5 mL KLH solution drop wise while vortexing. The conjugate was vortexed for 1 h at room temperature (RT). Conjugates were dialyzed using a 12,144MWCOdialysis membrane (Sigma Aldrich) to remove residual polymerized MDI or methylene dianiline (MDA), an amine generated after MDI hydrolysis. 4,4′-MDI-KLH antigen was stored at 4°C throughout the duration of experiments.
Vertebrate Animals
Mice were acclimated for approximately one week prior to 4,4′-MDI-KLH immunizations. The mice were housed in HEPA-filtered ventilated polycarbonate cages on autoclaved hardwood chip bedding. The temperature in the animal facility was maintained between 68 and 72°F and the relative humidity between 36 and 57%. The light/dark cycle was maintained on 12-hour intervals. Mice were provided NIH-31 modified 6% irradiated rodent diet (Harlan Laboratories, Madison, Wisc.) and autoclaved tap water ad libitum. Sentinel mice housed in the animal quarters were free of viral pathogens, parasites, mycoplasmas, Helicobacter spp., and cilia-associated respiratory Bacillus spp. The NIOSH animal facility is an environmentally controlled barrier facility that is fully accredited by the Association for the Assessment and Accreditations of Laboratory Animal Care International. All animal procedures were performed under a NIOSH Animal Care and Use Committee-approved protocol 11-PS-M-006.
Immunization of Female BALB/c Mice
Five female BALB/c mice (The Jackson Laboratory, Bar Harbor, Me.) aged 5–7weeks were immunized intraperitoneally (IP) with a 50:50 (v/v) emulsion of 25 µg 4,4′ -MDI-KLH and TiterMax Gold Adjuvant (TiterMAX USA, Norcross, Ga.). Five subsequent booster IP immunizations containing 25 µg 4,4′-MDI-KLH in sterile PBS were administered at biweekly intervals. The final boost was administered 3 days before the monoclonal antibody (mAb) fusion. Approximately, 100µLof blood was collected from the tail of each mouse 7 days prior to the first immunization (pre-bleed) and 7 days after each subsequent booster immunization (post-bleed) to monitor the development of immune responses.
MDI-antisera Screening ELISA
Pre- and post-immunization mouse sera were screened using an ELISA to determine anti-MDI-HSA titers. Briefly, 96-well Nunc Immuno MaxiSorp microplates (Thermo Fisher Scientific) were coated with 5 µg/mL 40:1 MDI-HSA in carbonate coating buffer (CCB, 60 mM sodium carbonate, 140 mM sodium bicarbonate, pH 9.6) and incubated at RT overnight. Wells were washed three times with PBS containing 0.05% Tween-20 (PBST) and blocked for 1 h at RT with 200 µL PBST containing 3% non-fat dry skim milk powder (SMPBST). Murine immunoglobulin G (IgG) titers were determined by incubating duplicate wells for 1 h at 37°C with 100 µL/well of individual serum 2 fold serially diluted from 1:100 to 1:25,600 (v/v) in SMPBST. Wells were washed and bound murine IgG was detected by incubating wells for 1 h at 37°C with 100 µL/well alkaline phosphatase (AP) conjugated anti-mouse IgG (H+L) (Promega, Madison, Wisc.) diluted 1:5000 (v/v) in SMPBST. Individual wells were washed and developed for 30 min at RT with 100 µL/well AP substrate, 0.5 mg/mL p-nitrophenyl phosphate-containing buffer (Sigma Aldrich). The optical density (OD) was determined spectrophotometrically at 405 nm using an UltraMicroplate Reader, Model ELx800 (Bio-Tek Instruments, Inc., Winooski, Vt.).
Splenocyte Fusion and Hybridoma Screening
Mice were euthanized by CO2 asphyxiation 3 days following the final booster immunization with 25 µg 4,4′ -MDIKLH in sterile PBS. Hybridomas were produced according to standard polyethylene glycol-based cell fusion techniques using SP2/0-AG14 myeloma cells (ATCC# CRL-1581) as previously described.(17)
The supernatant fluid from confluent hybridomas was tested using the previously described indirect serum screening ELISA. Hybridomas that were confirmed by indirect ELISA to produce 4,4′-MDI specific mAb were selected and grown in bulk and then transferred to a 96-well tissue culture plate and cloned twice by limiting dilution. Single positive clones were rescreened using the indirect ELISA, selected, grown in bulk, and purified according to the method of Kent.(19) Purified mAb was utilized in characterization studies. Hybridoma cell lines of individual clones were frozen in 10% dimethyl sulfoxide (DMSO, Sigma Aldrich) and 10% fetal calf serum, stored at −80°C for 2 weeks, and then transferred to a NIOSH liquid nitrogen facility for long-term storage.
Monoclonal Antibody Isotyping and Quantification
An indirect ELISA was used to determine the isotype and quantity of purified mAb. Briefly, 96-well Nunc Immuno MaxiSorpmicroplates (Thermo Fisher Scientific) were coated with either 1 µg/mL AffiniPure goat anti-mouse IgG Fc subclass 1, 2a, 2b, or 3 (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.) in CCB and incubated overnight at RT. For murine IgM, wells were coated with 1 µg/mL AffiniPure goat anti-mouse IgM, µ chain specific polyclonal antibody in CCB, and incubated overnight at RT. Wells were washed with PBST and blocked for 1 h at RT with 200 µL SMPBST. Standard curves for each isotype (IgG1κ, IgG2aκ, IgG2bκ, IgG3λ, and IgMκ; Sigma Aldrich) were prepared in duplicate and ranged from 100–0.6 ng/mL. Duplicate wells containing 100 µL/well purified mAb (1 µg /mL) were twofold serially diluted from 1:250 to 1:128,000 (v/v) in SMPBST and incubated for 1 h at 37°C. Isotype-specific mAbs were included as isotype-specific positive controls. Wells were washed and bound murine IgG isotype mAbs were detected by incubating wells with 100 µL/well AP conjugated anti-mouse IgG (H+L) (Promega, Madison, Wisc.) diluted 1:5000 (v/v) in SMPBST for 1 h at 37°C. For IgM isotype detection, wells were incubated for 1 h at 37°C with 100 µL/well biotin-SP-conjugated AffiniPure goat anti-mouse IgG + IgM (H+L) (Jackson ImmunoResearch Laboratories Inc.) diluted 1:5000 (v/v) in SMPBST. IgM isotype wells were washed and incubated for 1 h at 37°C with 100 µL/well AP conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc.) diluted 1:5000 (v/v) in SMPBST. Following secondary antibody incubations, individual wells of all isotype-specific plates were washed and developed for 30 min at RT with 100 µL/well AP substrate, 0.5 mg/mL p-nitrophenyl phosphate-containing buffer (Sigma Aldrich). The optical density (OD) was determined spectrophotometrically as previously described.
Western Blot Analysis of Murine IgM
The murine IgM mAb was separated by SDS-PAGE on a 12% polyacrylamide gel at 100 V and transferred to a 0.2 µm nitrocellulose membrane (BioRad, Hercules, Calif.) overnight at 16 V as previously described.(20) The membrane was washed three times with tris buffered saline (TBS, Sigma Aldrich) containing 0.05% Tween 20 (TBST, Fisher Scientific) and then blocked with 3% bovine serum albumin (BSA) in TBST (blocking solution) for 1 h at RT. Confirmation of IgM pentameric structure was determined by incubating membrane lanes 1 and 2 for 1 h at RT with rabbit anti-human IgM J-chain specific antibody (InvivoGen, San Diego, Calif.), a cross-reactive murine J-chain antibody, diluted 1:1000 (v/v) in blocking solution. Lanes 1 and 2 were washed followed by a 1 h incubation at RT with AP-conjugated goat anti-rabbit IgG-Fc (Promega) diluted 1:5000 (v/v) in blocking solution. In contrast, Lanes 3 and 4 were incubated for 1 h with AP-conjugated rabbit anti-mouse IgM µ chain specific antibody (Mabtech, Cincinnati, Ohio) and lanes 5 and 6 were incubated for 1 h at RT with AP-conjugated goat anti-mouse IgM (H+L) (Calbiochem, Billerica, Mass.), diluted 1:5000 (v/v) in blocking solution. Following secondary antibody incubation, the membrane was washed and immunoreactive proteins were visualized following 30 min incubation with One-Step nitroblue tetrazolium and bromocholor-indolyl phosphate (NBT/BCIP) substrate (Promega).
Proteomic Identification of Murine IgM mAb
Murine IgM-specific bands identified in the western blot analysis were excised from imperial stained SDS-PAGE gels prepared in parallel. Excised spots were digested with porcine trypsin (Sigma Aldrich) and analyzed using a nanoACQUITY UPLC coupled to a SYNAPT quadrupole time-of-flight mass spectrometer (Waters, Milford, Mass.) according to previously published methods.(20–22)
Conjugation of dNCOs to Human Serum Albumin
Diisocyanates, including 4,4′-MDI, 2,4-toluene diisocyanate (2,4-TDI), 2,6-toluene diisocyanate (2,6-TDI), and 1,6-hexamethylene diisocyanate (1,6-HDI), and the electrophilic chemicals (EC) 4,4′-methylene bis(phenyl isothiocyanate) (4, 4’-MDIT) and 4,4′-biphenyldicarboxaldehyde (4,4′-BPCA), were prepared in 0.01 M PBS (pH 7.4) and conjugated to 0.5 mg/mL HSA (3.0 × 10−4 M). Stock solutions were prepared by dissolving each dNCO and EC (3.0 × 10−2 M) in dry acetone immediately prior to conjugation. dNCO and EC stock solutions were further diluted in acetone to 1.5 × 10−2 M, 7.5 × 10−3 M, 3.75 × 10−3 M, 7.5 × 10−4 M, and 7.5 × 10−5 M and then conjugated to 0.5 mg/mL HSA for the preparation of 40:1, 20:1, 10:1, 5:1, 1:1, and 0.1:1 molar ratio conjugations, respectively. dNCO and EC-HSA conjugations were prepared by adding 50 µl of each dNCO or EC solution to 5mL HSA solution drop wise while vortexing. The conjugates were then incubated on a vortex for 1 h at RT. After incubation, conjugates were dialyzed using 12,144 MWCO dialysis membrane (Sigma Aldrich) to remove residual polymerized dNCO, EC, or other amines generated following dNCO hydrolysis. Samples were stored at 4°C throughout the duration of experiments. All chemicals used in the conjugation of dNCO or EC to HSA were obtained from Sigma-Aldrich.
dNCO-HSA Murine IgM Sandwich ELISA
Murine IgM mAb 15D4 reactivity to dNCO/EC-HSA conjugates was analyzed by sandwich ELISA. Briefly, Corning high protein binding 96-well plates (Corning, Corning, N.Y.) were coated with 4 µg/mL AffiniPure goat anti-mouse IgM, µ chain specific antibody (Jackson ImmunoResearch Laboratories Inc.) in 0.1Msodium carbonate buffer, pH 9.6 overnight at 4°C. Following overnight incubation, the wells were washed three times with PBST and incubated on a shaker for 1 h at RT with 2 µg/mL mAb 15D4. The wells were blocked with 200 µL/well SMPBST for 1 h at 37°C. Duplicate wells containing 100 µg/mL dNCO-HSA or EC-HSA conjugates at each molar ratio were twofold serially diluted to 98 ng/mL in SMPBST and incubated for 1 h at 37°C. Wells were washed and incubated for 1h at 37°C with biotin-conjugated affinity purified rabbit anti-HSA antibody (Rockland, Gilbertsville, Pa.) diluted 1:5000 (v/v) in SMPBST. Following incubation, the wells were washed and incubated for 1 h at 37°C with AP-conjugated streptavidin (Jackson ImmunoResearch Laboratories Inc.) diluted 1:5000 (v/v) in SMPBST. Binding of murine mAb 15D4 to each dNCO or EC-HSA conjugate was quantified using 0.5 mg/mL p-nitrophenyl phosphate (Sigma Aldrich) in AP substrate. The optical density was determined spectrophotometrically as previously described. The limit of detection (LOD) and limit of quantification (LOQ) for each dNCO were defined as 3 and 10 times the standard deviation of 24 replicate HSA (0.5 µg/mL) control wells.
Detection of dNCO-HSA Conjugates in Supplemented Deidentified Human Serum
Nine de-identified human sera were supplemented with each dNCO-HSA conjugate in proof-of-concept experiments to determine the applications of the sandwich ELISA for the serological detection of dNCO for occupational cohort studies. Briefly, plates were prepared as described in the sandwich ELISA. After blocking, the plates were then incubated with de-identified human serum diluted 1:20 in SMPBST. Duplicate wells were then supplemented with 100 µg/mL 40:1 dNCO-HSA conjugates and twofold serially diluted to 98 ng/mL in SMPBST and incubated for 1 h at 37°C. The remainder of the procedure was performed as described previously.
RESULTS
Hybridoma Screening and Antibody Characterization
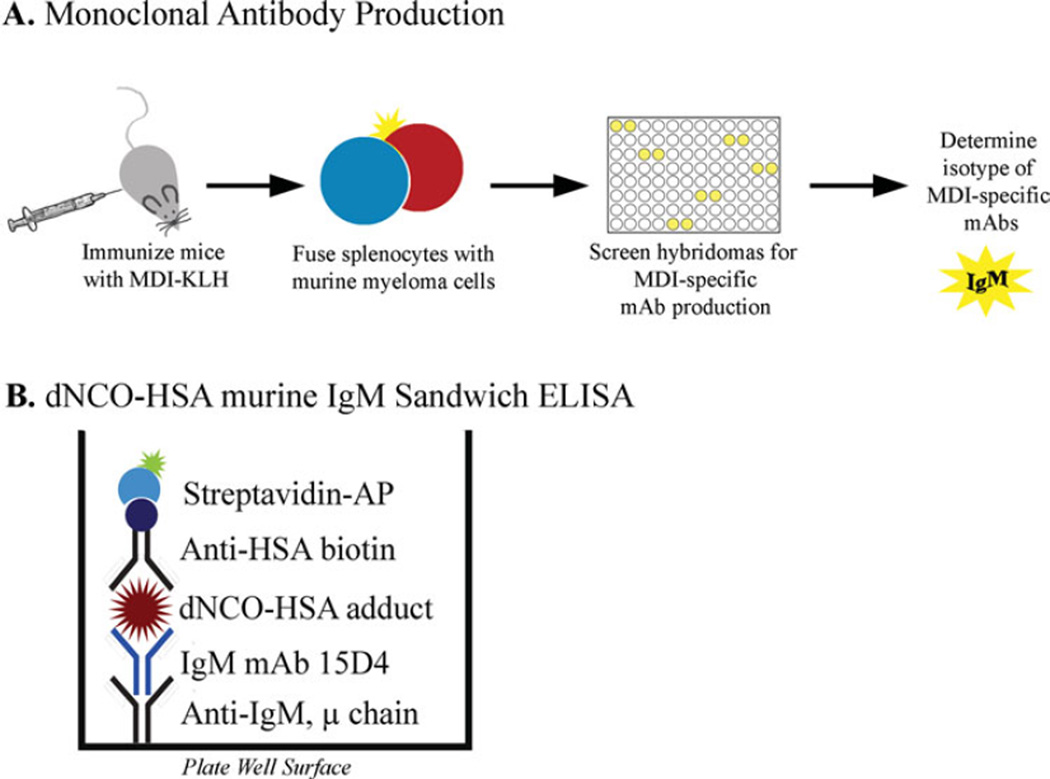
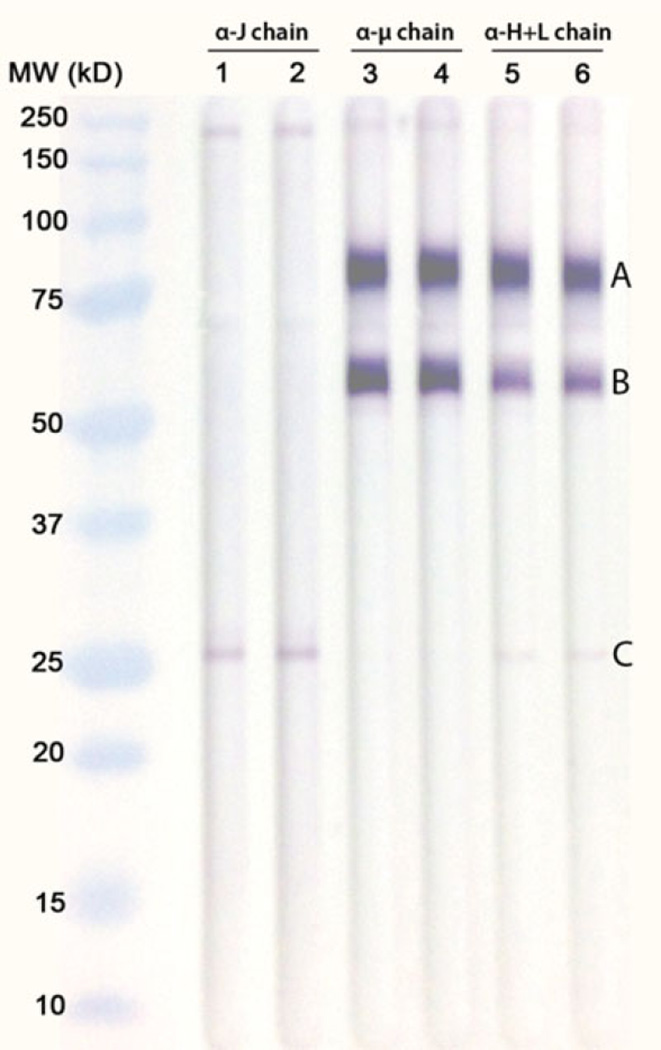
Figure 1A outlines the methodology used for the production of MDI-specific mAbs. The splenocyte-myeloma cell fusion resulted in one hybridoma that secreted a 4,4′-MDI specific mAb. Isotyping experiments revealed the hybridoma to produce a murine IgM isotype mAb (mAb 15D4). The isotype of mAb 15D4 was further confirmed in nanoUPLC-MS/MS and Western blot analysis (Figure 2). Figure 2 shows an immunoblot of IgM heavy (78 kDa) and light (25 kDa) chains as well as a truncated heavy chain localized at ~55 kDa separated on a denaturing gel. Constant and variable regions were identified in both the 78 kDa heavy chain and the 25 kDa light chain in the nanoUPLC-MS/MS proteomic analysis. Proteomic analysis of the ~55 kDa truncated heavy chain resulted in the identification of a constant region but not a variable region (Figure 2). A truncated murine IgM heavy chain has been previously reported for an IgM producing hybridoma.(23) mAb 15D4 does not appear to be a pentamer as a murine Jchain (~15kD) could not be identified in either the proteomic or Western blot analysis (Figure 2). Native PAGE analysis of mAb 15D4 suggested that the mAb was multimeric with up to six subunits (data not shown).
FIGURE 1.
Schematic of the experimental design: (A) Flow diagram illustrating the methods used to produce the dNCO-specific IgM mAb 15D4; (B) Representation of an ELISA plate well from the murine IgM sandwich ELISA used to detect dNCO adducted has. (color figure available online)
FIGURE 2.
Western blot analysis of murine IgM mAb 15D4. Molecular weight (MW) markers: Bio-Rad Precision Plus Protein Standards, Lanes 1–2: mAb 15D4 probed with rabbit anti-human IgM J-chain specific antibody; Lanes 3–4: mAb 15D4 probed with AP-conjugated rabbit anti-mouse IgM µ chain specific antibody; Lanes 5–6: mAb 15D4 probed with AP-conjugated goat antimouse IgM (H+L). Murine IgM mAb bands were then excised from corresponding Imperial protein stained SDS-PAGE gels and identified using mass spectrometry. (a) Mass spectrometric protein characterization of mAb 15D4 (UniProtKB/Swiss-Prot accession number) include (A) Ig mu chain C region Mus musculus (P01872) and Ig heavy chain V region Mus musculus (P06330), (B) Ig mu chain C region Mus musculus (P01872), and (C) Ig kappa chain C region Mus musculus (P01837), Ig kappa chain V VI region Mus musculus (P04942), Ig kappa chain C region Mus musculus (P01837), and Ig kappa chain V VI region Mus musculus (P04941). (color figure available online)
Quantification of 4,4′-MDI-HSA Conjugates
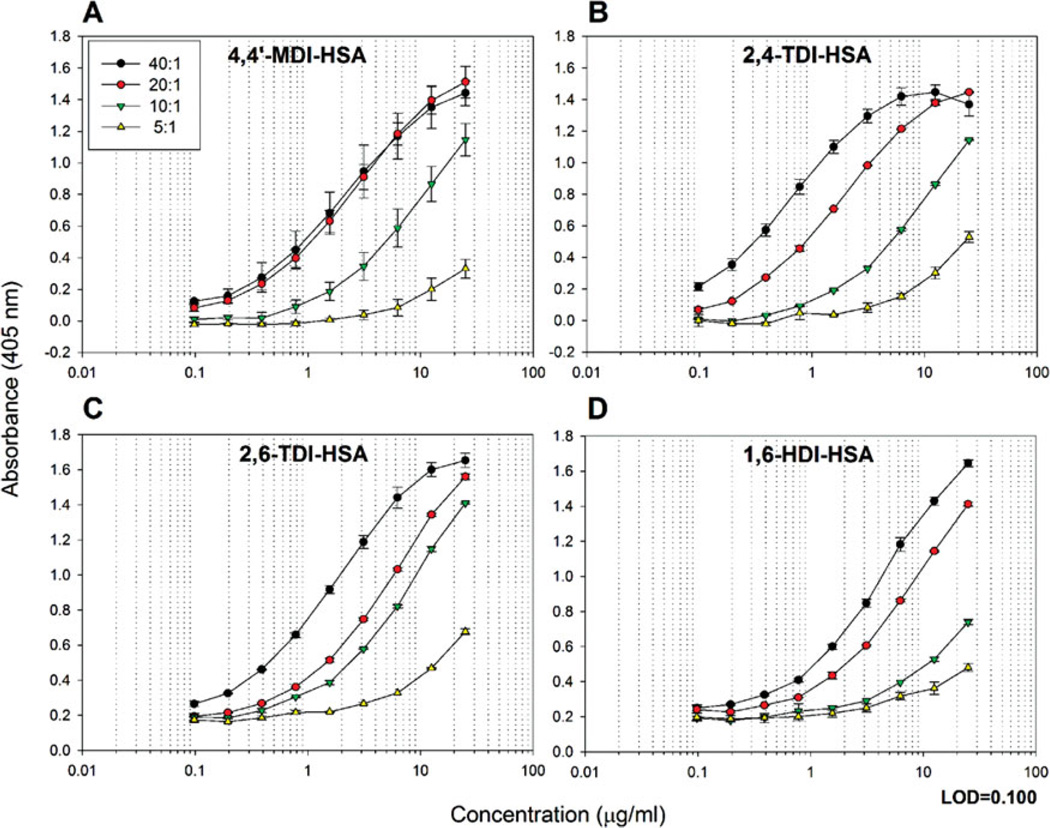
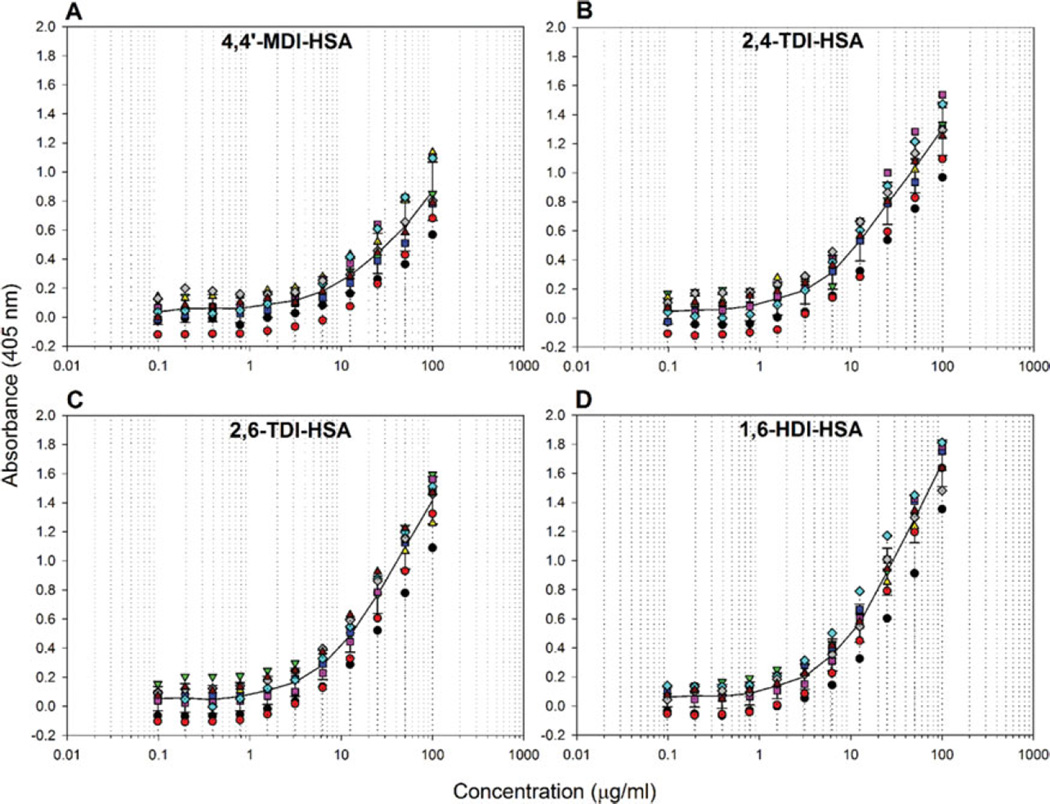
A sandwich ELISA was developed to determine the reactivity of mAb 15D4 to 4,4′-MDI-HSA conjugates (Figure 1B). 4,4′-MDI molar ratios including 40:1, 20:1, 10:1, 5:1, 1:1, and 0.1:1 were conjugated to HSA and tested in the sandwich ELISA. mAb 15D4 reacted with the 40:1, 20:1, 10:1, and 5:1 4,4′-MDI-HSA conjugates in a concentration-dependent manner (Figure 3A). At a 20:1 4,4′-MDI-HSA molar ratio, all HSA was haptenated by at least 1 MDI molecule. No mAb 15D4 reactivity was observed in samples at either the 1:1 or 0.1:1 4,4′-MDI-HSA conjugation ratios (data not shown). The LOQ for the 40:1 4,4′-MDI-HSA sandwich ELISA assays was 567.2 ng/mL (8.67 pmol/mL) (Table I).
FIGURE 3.
Binding of IgM mAb 15D4 to other occupationally relevant diisocyanates. (A) ELISA results of IgM mAb 15D4 binding different molar ratios of 4,4′-MDI-HSA, (B) 2,4-TDI-HSA, (C) 2,6-TDI-HSA, and (D) 1,6-HDI-HSA. The results of panel A represent the mean OD405 values corrected for HSA background of duplicate ELISA assays containing 2 well replicates. The results of panels B–D represent the mean OD405 values corrected for HSA background of 2 ELISA well repeats. Background controls using 0.5 µg/mL HSA were examined in parallel. (color figure available online)
TABLE I.
Hapten-protein interactions and binding to IgM mAb 15D4 A
| Chemical Name & Structure |
Hapten-Protein | ELISA Readings (OD405nm) |
Comparison to MDI (%) |
Limit of Quantification (ng/ml HSA) |
|---|---|---|---|---|
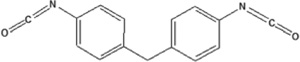
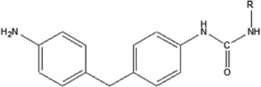
| 4,4′methylene diphenyl diisocyanate | 4,4′MDI-HSA | 1.100±0.013 | — | 567.2 |
 |
 |
|||
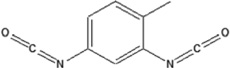
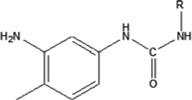
| 2,4-toluene diisocyanate | 2,4-TDI-HSA | 1.85 ± 0.057 | 168.8% | 172.7 |
 |
 |
|||
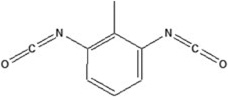
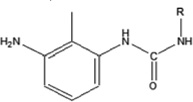
| 2,6-toluene diisocyanate | 2,6-TDI-HSA | 1.724 ± 0.079 | 156.7% | 184.2 |
 |
 |
|||
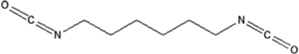
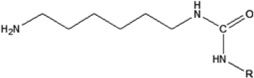
| 1,6-hexamethylene diisocyanate | 1,6-HDI-HSA | 1.666 ± 0.019 | 151.5% | 403.5 |
 |
 |
|||
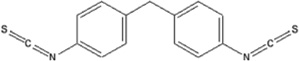
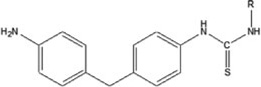
| 4,4′methylenebis(phenyl isothiocyanate) | 4,4′MDIT-HSA | 0.167 ± 0.087 | 15.1% | NA |
 |
 |
|||
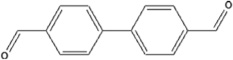
| 4,4′biphenyldicarboxaldehyde | 4,4′BPCA-HSA | 0.049 ± 0.043 | 4.4% | NA |
 |
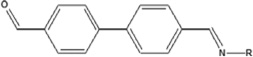 |
The results represent the mean OD405 values of each 40:1 hapten-HSA corrected for 0.5 µg/mL HSA background of 2 ELISA well repeats. Background controls using 0.5 µg/ml HSA were examined in parallel. Limit of quantification was determined by calculating the concentration of dNCO-HSA that corresponded to 10x the standard deviation of sandwich ELISA 0.5 µg/mL HSA control absorbance values. Chemical structures and hapten-protein interactions are illustrated along with each chemical hapten abbreviation.
mAb 15D4 Binding to Other dNCOs
mAb 15D4 binding specificity was also tested against other occupationally relevant dNCO-HSA conjugates. Figure 3 shows mAb 15D4 reactivity to the aromatic dNCO-HSA adducts, 2,4-TDI-HSA (Figure 3B), and 2,6-TDI-HSA (Figure 3C) as well as the aliphatic dNCO, 1,6-HDI-HSA (Figure 3D). mAb 15D4 reacted to all 3 additional dNCOs (Figure 3B–D, Table I). Compared to 4,4′-MDI-HSA, mAb 15D4 reactivity was over 50% greater for 2,4-TDI-HSA, 2,6-TDI-HSA, and 1,6-HDI-HSA at 25 µg/mL 40:1 dNCO-HSA (Table I). A decrease in reactivity at a 20:1 molar ratio for both TDI-HSA adducts as well as 1,6-HDI-HSA was also observed (Figure 3B and 3C). The LOQ for the dNCO-HSA sandwich ELISA assays for 40:1 2,4-TDI-HSA, 2,6-TDI-HSA, and 1,6-HDIHSA were 172.7, 184.2, and 403.5 ng/mL (2.60, 2.77, and 6.07 pmol/mL), respectively (Table I).
mAb 15D4 Reactivity to Other Electrophilic Chemicals
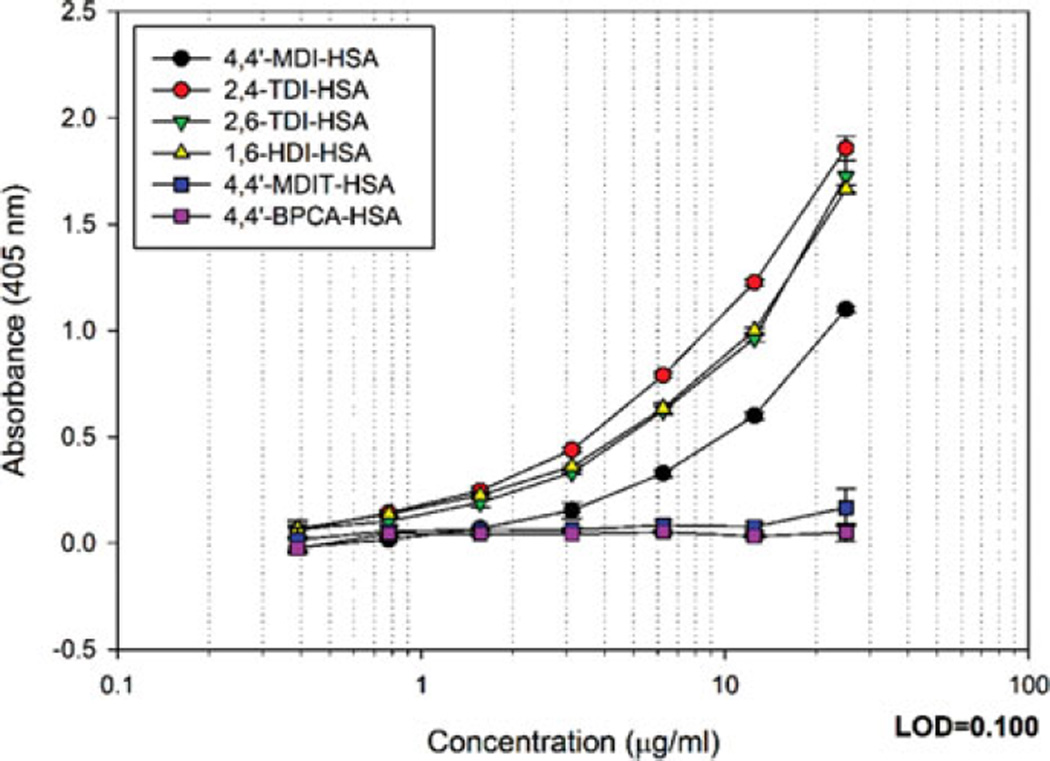
Due to the structural similarities with 4,4′-MDI-HSA, 4,4′-MDIT (aromatic diisothiocyanate), and 4,4′-BPCA (aromatic aldehyde) were selected to further characterize the binding determinants of mAb 15D4. 4,4′-MDIT haptenated HSA differed from 4,4′-MDI-HSA in that the urea linkage is changed to a thiourea (substituting a carbonyl; C=O for a thioketone; C=S) (Table I). In contrast, the aromatic ring of the 4,4′-BPCA hapten is linked to the HSA by an imine group, shortening the distance between HSA and the aromatic ring of the adduct through the removal of the carbonyl moiety (C=O). mAb 15D4 reactivity was only 15.1% for 4,4′-MDIT-HSA and 4.4% 4,4′-BPCA-HSA compared to 4,4′-MDI-HSA (Figure 4, Table I). These data further suggest that the major binding determinant of mAb 15D4 is the dNCO urea linkage onto the protein.
FIGURE 4.
Binding of IgM mAb 15D4 to other isocyanate-like compounds. ELISA results of IgM mAb 15D4 binding 40:1 molar ratios of 4,4′-MDI-HSA, 2,4-TDI-HSA, 2,6-TDI-HSA, 1,6-HDI-HSA, 4,4′-MDIT-HSA, and 4,4′-BPCA-HSA. The results represent the mean OD405 values corrected for HSA background of 2 ELISA well repeats. Background controls using 0.5 µg/mL HSA were examined in parallel. (color figure available online)
Detection of dNCO-HSA Supplemented Human Serum
The utility of the sandwich ELISA for the detection of dNCO protein adducts was examined by supplementing deidentified human serum (n−9) with 2 fold serial dilutions of 100 µg/mL 40:1 dNCOs-HSA. In preliminary experiments, dilutions ranging from neat, 1:5, 1:10, and 1:20 were empirically tested to determine the optimal dilution for the sandwich ELISA. Non-specific binding of human serum proteins to mAb 15D4 was observed in all controls for each tested dilution. Background interference was reduced at 1:20 and this dilution was selected for the experiments (data not shown). Figure 5 shows the reactivity of mAb 15D4 dNCO-HSA to each serum sample. At 25 µg/mL, mAb binding to adducted HSA in these assays was 30.5%, 57.6%, 46.4%, and 56.1% less compared to that observed in the initial sandwich ELISA results for 4,4′-MDI-HSA, 2,4-TDI-HSA, 2,6-TDIHSA, and 1,6-HDI-HSA, respectively. The LOD for the immunoassays ranged from <0.1 to 11.5 µg/mL in non-atopic (tIgE <100 kU/L) sera (n = 4) and 1.9 to 52.5 µg/mL in atopic (tIgE >100 kU/L) sera (n = 5) compared to the LOD in the initial sandwich ELISA which was <0.1 µg/mL. These data demonstrate that serum proteins may interfere with the binding of the mAb 15D4 to dNCO adducted HSA in this immunoassay.
FIGURE 5.
Binding of IgM mAb 15D4 to diisocyanate-HSA conjugates in deidentified human sera. ELISA results of IgM mAb 15D4 binding 40:1 molar ratios of 4,4′-MDI-HSA, 2,4-TDI-HSA, 2,6-TDI-HSA, and 1,6-HDI-HSA in deidentified human sera diluted 1:20. The results represent the mean OD405 values corrected for 0.5 µg/mL HSA background of duplicate ELISA assays containing 2 well replicates. Background controls using deidentified human sera diluted 1:20 were examined in parallel. (color figure available online)
DISCUSSION
Methods to assess dNCO occupational exposure have included the detection of dNCO specific serum immunoglobulins or signature dNCO biomarkers using chromatographic methods. The quantification of dNCO-derived (di-) amines from hydrolyzed clinical samples, such as urine, is a common approach for biomonitoring worker exposures.(24,25) However, toxicological kinetic studies of workers who received an inhaled dNCO challenge have shown that the excretion half-life of dNCOs ranges between 2.5 and 24 hours.(25) As a result, the detection of isocyanate metabolites in hydrolyzed urine samples at the end of an 8-hour work shift may not be an accurate assessment of worker exposure.(25) In contrast, animal studies have shown that [14C]-dNCO (presumably adducted to proteins or other biomolecules) is available systemically following exposure.(24) Greatest amounts of [14C]-dNCO were localized in the respiratory and gastrointestinal tracts as well as the blood.(24) Kennedy and Brown(26) have shown that dNCO forms adducts with blood and plasma proteins, in particular hemoglobin and HSA.(27–29) As a result of the longer half-lives of dNCO-protein adducts, researchers have focused on these detectable biomarkers for assessing dNCO occupational exposure.(29)
Immunoassays for the detection of dNCO-protein adducts is an important development to quantify occupationally relevant dNCOs. In the past 3 years, significant advances in the production of dNCO-protein adduct reactive antibodies have been reported. We have reported 29 TDI-HSA specific IgG isotype mAbs(17) as well as 10 TDI-protein adduct specific IgM mAbs.(16) Recently, Wisnewski and Liu have produced 6 MDI-protein adduct specific IgG1 isotype mAbs.(18) pAbs have also been produced for 1,6-HDI(15,30) and TDI.(31) Although the specificity and application of these antibodies in immunoassays have been established, their utility in detecting dNCO-protein adducts in human serum or other clinical samples has not been fully evaluated. The utility of these dNCO-specific antibodies for biomonitoring occupational dNCO exposure is further confounded by worker exposures to combinations of dNCOs. In autobody painting operations, HDI and TDI, or more commonly, HDI and IPDI, may be used concurrently.(25,32,33) In this study, we produced an IgM mAb with broad specificity to occupationally relevant dNCO-HSA adducts, including 1,6-HDI, 2,4-TDI, 2,6-TDI, and 4,4′-MDI.
In preliminary characterization studies, a sandwich ELISA was developed andmAb15D4was shown to broadly react with each dNCO adducted HSA. The LOQ of the sandwich ELISA ranged between 173 ng/mL (2.60 pmol/mL) for 40:1 2,4-TDIHSA to 567 ng/mL (8.67 pmol/mL) for 40:1 4,4′-MDI-HSA. Reactivity with other occupationally relevant dNCOs, such as IPDI and 1,5-naphthalene diisocyanate (NDI) was not determined. In addition, occupational exposure to dNCO oligomers has been previously reported, however, higher oligomers of dNCO species, such as the prominent MDI trimer, were not evaluated in this study. The mAb 15D4 assay results were comparable to the 1,6 HDI-HSA pAb sandwich ELISA reported by Lemus et al.(15) Although the mAb 15D4 sandwich ELISA was sufficient to demonstrate reactivity to dNCO-HSA adducts, its application in detecting adducts in human serum required further evaluation.
In experiments using de-identified human serum supplemented with dNCOs, 4,4′-MDI, 2,4-TDI, 2,6-TDI, and 1,6-HDI adducted HSA were detected in a concentration-dependent manner. However, the detectable limit of the immunoassay appeared to be confounded by non-specific human serum protein binding to solid phase IgM mAb 15D4 as reported values were outside the range of dNCO plasma concentrations reported in workers (8-hour shift).(28,34) Previous studies have reported non-specific binding of HSA and dNCO-HSA conjugates with serum immunoglobulins including IgE(35) and IgG.(36) Nonspecific binding of murine IgM to other human proteins and lipid antigens has also been reported.(37,38) Glee and colleagues have also identified a unique set of peptide ligands that bind murine, rabbit, and human pentameric and monomeric IgM via proposed determinants within the heavy chain constant regions.(38) Certain motifs including WIS/PS/QXDW and a C-terminal aspartate-tryptophan residue set were commonly associated with peptide sequences that bound murine IgM.(38) To overcome non-specific binding interactions, human serum was diluted 1:20 and also pre-incubated with 50 µg/mL of control murine IgM. Human serum dilutions improved assay performance; however, inhibition with a control murine IgM mAb did not improve non-specific binding interactions (data not shown). Future studies are required to examine the inhibitory role of preincubating IgM mAb 15D4 with peptide ligands that bind murine IgM antibodies. Identification of a suitable peptide ligand may improve the overall utility of the sandwich assay for the serological detection of dNCO-protein adducts.
The reactivity of mAb 15D4 to occupationally relevant dNCOs was determined in mAb binding studies. mAb reactivity to 2,4-TDI-HSA, 2,6-TDI-HSA, and 1,6-HDI was greatest, whereas reduced binding to 4,4′-MDI was observed in both sandwich ELISAs. Characterization of the binding determinants of mAb 15D4 was evaluated by testing other electrophilic chemical HSA conjugates with similar structures to occupationally relevant dNCOs. In this series of experiments, 4,4′-MDIT (aromatic diisothiocyanate) and 4,4′-BPCA (aromatic aldehyde) were selected because of the structural similarities with 4,4′-MDI-HSA.
However, 4,4′-MDIT-HSA differs from 4,4′-MDI-HSA in that the urea linkage is changed to a thiourea (substituting a carbonyl; C−O for a thioketone; C−S). In contrast, the aromatic ring of the 4,4′-BPCA-HSA is linked to HSA by an imine group, resulting in the removal of the carbonyl moiety (C−O) and shortening the distance between the hapten aromatic ring and the protein. mAb 15D4 showed little to no reactivity with either 4,4′-BPCA-HSA or 4,4′-MDIT-HSA. These data suggest that the major mAb binding determinant revolves around the urea linkage between the dNCO and the protein. These findings are of additional importance as isothiocyanates are commonly found in the environment and cruciferous vegetables.(36)
Although mAb 15D4 reactivity to dNCO-HSA adducts was the focus of the current study, the conjugation of dNCOs to other proteins associated with respiratory and dermal exposures has also been reported.(4) dNCOs may conjugate to an assortment of host bronchial epithelial proteins following respiratory exposures.(31,39) In addition to albumin, trans-1,2-dihydrobenzene-1,2-diol dehydrogenase, actin, ciliary tubulin, keratin 18, and glucose-regulated proteins have been identified in human/animal bronchalveolar lavage and biopsy samples.(30,40) In contrast, keratin was one of the major proteins associated with 1,6-HDI adduction following dermal exposures.(30)
In the present study, mAb 15D4 reactivity to other protein dNCO-conjugates was not evaluated; however, reactivity to 40:1 4,4′-MDI-keratin was identified in preliminary mAb screening studies (data not shown). These data demonstrate the potential utility of mAb 15D4 for the enrichment of other dNCO protein adducts in human or animal studies of dNCO respiratory or dermal exposure. Furthermore, Mhike and colleagues have recently shown the potential utility of mAb 15D4 for the characterization and standardization of dNCO protein adducts used to detect specific antibody in exposed worker sera.(41)
Preliminary studies by Luna and colleagues have recently demonstrated application ofmAb15D4 to concentrate and partially purify MDI-HSA to decrease the LOD in a liquid chromatography tandem mass spectrometry method (LC/MS/MS) to quantify 4,4′-MDI signature peptide biomarkers.(42) Using this LC/MS/MS approach, the MDI adducted K(MDA)VPQVS TPTLVEVSR peptide was the most frequently quantified adduct and accounted for 10.3% of total MDI adducted HSA (0.1 mg/mL HSA digested). The results of this preliminary analysis were comparable in sensitivity to Sabbioni’s method for detection of MDI-lysine amino acids adducts following pronase digestion;(43) however, the detection of dNCO-HSA conjugates in workers exposed to dNCO requires further validation and is the focus of future research efforts.
CONCLUSION
Amurine IgM mAb has been produced and characterization studies demonstrated broad reactivity to dNCOs that cause occupational allergic sensitization and OA. The specificity of the antibody for both aromatic and aliphatic dNCOs, but not isothiocyanates, suggests utility of the mAb for biomonitoring in mixed dNCO occupational environments, although reactivity to IPDI, a commonly used dNCO in the autobody industry, was not tested. This mAb may also have utility in the enrichment of dNCO-protein adducts in LC/MS/MS-based approaches to assess signature peptide biomarkers following worker exposure to occupational dNCOs.
ACKNOWLEDGMENTS
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. This study was supported in part by an interagency agreement with NIEHS IAA# AES 12007-00100000. This article is not subject to US copyright law.
Footnotes
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at http://www.tandfonline.com/page/terms-and-conditions
The authors declare no conflict of interest.
REFERENCE
- 1.Allport DC, Gilbert DS, Outterside SM. MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide. West Sussex, UK: John Wiley & Sons, Ltd.; 2003. [Google Scholar]
- 2.American Chemistry Council (ACS) American Chemistry Council Center for the Polyurethanes Industry. Washington, D.C.: 2011. 2010 End-Use Market Survey on the Polyurethanes Industry in the U.S., Canada, and Mexico. [Google Scholar]
- 3.National Institute for Occupational Safety and Health (NIOSH) CDC/NIOSH Alert. DHHS; 1996. Preventing asthma and death from diisocyanate exposure; pp. 1–12. [Google Scholar]
- 4.Redlich CA, Karol MH. Diisocyanate asthma: Clinical aspects and immunopathogenesis. Int. Immunopharmacol. 2002;2(2–3):213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- 5.Tarlo SM, Liss GM, Dias C, Banks DE. Assessment of the relationship between isocyanate exposure levels and occupational asthma. Am. J. Ind.Med. 1997;32(5):517–521. doi: 10.1002/(sici)1097-0274(199711)32:5<517::aid-ajim12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Baur X. Hypersensitivity pneumonitis (extrinsic allergic alveolitis) induced by isocyanates. J. Allergy Clin. Immunol. 1995;95(5 Pt 1):1004–1010. doi: 10.1016/s0091-6749(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Occupational Safety and Health (NIOSH) CDC/NIOSH Alert. DHHS; 2006. Preventing Asthma and Death from MDI Exposure During Spray-on Truck Bed Liner and Related Applications; pp. 1–35. [Google Scholar]
- 8.Swensson A, Holmquist CE, Lundgren KD. Injury to the respiratory tract by isocyanates used in making lacquers. Br. J. Ind. Med. 1955;12(1):50–53. doi: 10.1136/oem.12.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher AA. Contact Dermatitis. Philadelphia, Pa.: 1967. Polyurethanes (“isocyanate resins”) pp. 134–135. [Google Scholar]
- 10.Upjohn Company. Technical Bulletin No. 105. Kalamazoo, Mich.: Upjohn Company; 1970. Urethanes: engineering, medical control and toxicologic considerations. [Google Scholar]
- 11.Lofgren DJ, Walley TL, Peters PM, Weis ML. MDI exposure for spray-on truck bed lining. Appl. Occup. Environ. Hyg. 2003;18(10):772–779. doi: 10.1080/10473220301441. [DOI] [PubMed] [Google Scholar]
- 12.Hettick JM, Siegel PD. Determination of the toluene diisocyanate binding sites on human serum albumin by tandem mass spectrometry. Anal. Biochem. 2011;414(2):232–238. doi: 10.1016/j.ab.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Wisnewski AV, Hettick JM, Siegel PD. Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin. Chem. Res. Toxicol. 2011;24(10):1686–1693. doi: 10.1021/tx2002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hettick JM, Siegel PD. Comparative analysis of aromatic diisocyanate conjugation to human albumin utilizing multiplexed tandem mass spectrometry. International J. Mass Spectrometry. 2012;309:168–175. [Google Scholar]
- 15.Lemus R, Lukinskeine L, Bier ME, Wisnewski AV, Redlich CA, Karol MH. Development of immunoassays for biomonitoring of hexamethylene diisocyanate exposure. Environ. Health Perspect. 2001;109(11):1103–1108. doi: 10.1289/ehp.011091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruwona TB, Johnson VJ, Schmechel D, Simoyi RH, Beezhold D, Siegel PD. Monoclonal antibodies against toluene diisocyanate haptenated proteins from vapor-exposed mice. Hybridoma (Larchmt.) 2010;29(3):221–229. doi: 10.1089/hyb.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruwona TB, Johnson VJ, Hettick JM, et al. Production, characterization and utility of a panel of monoclonal antibodies for the detection of toluene diisocyanate haptenated proteins. J. Immunol. Methods. 2011;373(1–2):127–135. doi: 10.1016/j.jim.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Wisnewski AV, Liu J. Molecular determinants of humoral immune specificity for the occupational allergen, methylene diphenyl diisocyanate. Mol. Immunol. 2013;54(2):233–237. doi: 10.1016/j.molimm.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kent UM. Purification of antibodies using ammonium sulfate fractionation or gel filtration. Methods Mol. Biol. 1999;115:11–18. doi: 10.1385/1-59259-213-9:11. [DOI] [PubMed] [Google Scholar]
- 20.Green BJ, Cummings KJ, Rittenour WR, et al. Occupational sensitization to soy allergens in workers at a processing facility. Clin. Exp. Allergy. 2011;41(7):1022–1030. doi: 10.1111/j.1365-2222.2011.03756.x. [DOI] [PubMed] [Google Scholar]
- 21.Stone KL, Williams KR. Enzymatic digestion of protein in solution and in SDS polyacrylamide gels. In: Walker JM, editor. The Protein Protocols Handbook. Totowa, N.J: Humana Press; 2002. pp. 511–521. [Google Scholar]
- 22.Chakraborty AB, Berger SJ, Gebler JC. Use of an integrated MS-multiplexed MS/MS data acquisition strategy for high-coverage peptide mapping studies. Rapid Comm. Mass Spectrom. 2007;21(5):730–744. doi: 10.1002/rcm.2888. [DOI] [PubMed] [Google Scholar]
- 23.Marks R, Bosma MJ. Truncated mu (mu’) chains in murine IgM. Evidence that mu’ chains lack variable regions. J. Exp. Med. 1985;162(6):1862–1877. doi: 10.1084/jem.162.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gledhill A, Wake A, Hext P, Leibold E, Shiotsuka R. Absorption, distribution, metabolism and excretion of an inhalation dose of [14C] 4,4′-methylenediphenyl diisocyanate in the male rat. Xenobioticaaa. 2005;35(3):273–292. doi: 10.1080/00498250500057591. [DOI] [PubMed] [Google Scholar]
- 25.Budnik LT, Nowak D, Merget R, Lemiere C, Baur X. Elimination kinetics of diisocyanates after specific inhalative challenges in humans: Mass spectrometry analysis as a basis for biomonitoring strategies. J. Occup. Med. Toxicol. 2011;6(1):9. doi: 10.1186/1745-6673-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy AL, Brown WE. Biochemical and historadioautographic characterisation of the distribution of radioactivity following exposure to 14C-MDI aerosol. International Isocyanate Institute. 1999:159–175. (Report #11321) [Google Scholar]
- 27.Schutze D, Sepai O, Lewalter J, Miksche L, Henschler D, Sabbioni G. Biomonitoring of workers exposed to 4,4′-methylenedianiline or 4,4′-methylenediphenyl diisocyanate. Carcinogenesis. 1995;16(3):573–582. doi: 10.1093/carcin/16.3.573. [DOI] [PubMed] [Google Scholar]
- 28.Sepai O, Henschler D, Sabbioni G. Albumin adducts, hemoglobin adducts and urinary metabolites in workers exposed to 4,4′-methylenediphenyl diisocyanate. Carcinogenesis. 1995;16(10):2583–2587. doi: 10.1093/carcin/16.10.2583. [DOI] [PubMed] [Google Scholar]
- 29.Johannesson G, Sennbro CJ, Willix P, Lindh CH, Jonsson BA. Identification and characterisation of adducts between serum albumin and 4,4′-methylenediphenyl diisocyanate (MDI) in human plasma. Arch. Toxicol. 2004;78(7):378–383. doi: 10.1007/s00204-004-0555-2. [DOI] [PubMed] [Google Scholar]
- 30.Wisnewski AV, Srivastava R, Herick C, et al. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2000;162(6):2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- 31.Jin R, Day BW, Karol MH. Toluene diisocyanate protein adducts in the bronchoalveolar lavage of guinea pigs exposed to vapors of the chemical. Chem. Res. Toxicol. 1993;6(6):906–912. doi: 10.1021/tx00036a023. [DOI] [PubMed] [Google Scholar]
- 32.Sparer J, Stowe MH, Bello D, Liu Y, Gore RJ, Youngs F, et al. Isocyanate exposures in autobody shop work: The SPRAY study. J. Occup. Environ. Hyg. 2004;1(9):570–581. doi: 10.1080/15459620490485909. [DOI] [PubMed] [Google Scholar]
- 33.Woskie SR, Sparer J, Gore RJ, et al. Determinants of isocyanate exposures in auto body repair and refinishing shops. Ann. Occup. Hyg. 2004;48(5):393–403. doi: 10.1093/annhyg/meh021. [DOI] [PubMed] [Google Scholar]
- 34.Sennbro CJ, Lindh CH, Mattsson C, Jonsson BA, Tinnerberg H. Biological monitoring of exposure to 1,5-naphthalene diisocyanate and 4,4′-methylenediphenyl diisocyanate. Int. Arch. Occup. Environ. Health. 2006;79(8):647–653. doi: 10.1007/s00420-006-0096-5. [DOI] [PubMed] [Google Scholar]
- 35.Karol MH, Alarie Y. Antigens which detect IgE antibodies in workers sensitive to toluene diisocyanate. Clin. Allergy. 1980;10(1):101–109. doi: 10.1111/j.1365-2222.1980.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein DI, Ott MG, Woolhiser M, Lummus Z, Graham C. Evaluation of antibody binding to diisocyanate protein conjugates in a general population. Ann. Allergy Asthma Immunol. 2006;97(3):357–364. doi: 10.1016/S1081-1206(10)60801-0. [DOI] [PubMed] [Google Scholar]
- 37.Hofstetter W, Heusser CH, Blaser K. Non-specific binding of mouse IgM antibodies to lipid antigens. J. Neuroimmunol. 1985;7(4):207–214. doi: 10.1016/s0165-5728(84)80020-x. [DOI] [PubMed] [Google Scholar]
- 38.Glee PM, Pincus SH, McNamer DK, Smith MJ, Burritt JB, Cutler JE. Peptide ligands that bind IgM antibodies and block interaction with antigen. J. Immunol. 1999;163(2):826–833. [PubMed] [Google Scholar]
- 39.Karol MH, Jin R, Lantz RC. Immunohistochemical detection of toluene diisocyanate (TDI) adducts in pulmonary tissue of guinea pigs following inhalation exposure. Inhalation Toxicol. 1997;9(2):63–84. [Google Scholar]
- 40.Lange RW, Lantz RC, Stolz DB, et al. Toluene diisocyanate colocalizes with tubulin on cilia of differentiated human airway epithelial cells. Toxicol. Sci. 1999;50(1):64–71. doi: 10.1093/toxsci/50.1.64. [DOI] [PubMed] [Google Scholar]
- 41.Mhike M, Chipinda I, Hettick JM, et al. Characterization of methylene diphenyl diisocyanate-haptenated human serum albumin and hemoglobin. Anal Biochem. 1999;440(2):159–175. doi: 10.1016/j.ab.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luna LG, Green BJ, Zhang F, Seigel PD, Bartels MJ. Enrichment and measurement of a signature MDI human serum albumin peptide adduct. Poster presented at Isocyanates and Health: Past, Present and Future; Potomac, Md.. April 3–4, 2013. [Google Scholar]
- 43.Sabbioni G, Dongari N, Kumar A. Determination of a new biomarker in subjects exposed to 4,4′-methylenediphenyl diisocyanate. Biomarkers. 2010;15(6):508–515. doi: 10.3109/1354750X.2010.490880. [DOI] [PubMed] [Google Scholar]