Abstract
Objective
To determine whether prenatal diagnosis lowers the risk of preoperative brain injury by assessing differences in the incidence of preoperative brain injury across centers.
Study design
From 2 prospective cohorts of newborns with complex congenital heart disease studied by preoperative cerebral magnetic resonance imaging, one cohort from the University Medical Center Utrecht (UMCU) and a combined cohort from the University of California San Francisco (UCSF) and University of British Columbia (UBC), patients with aortic arch obstruction were selected and their imaging and clinical course reviewed.
Results
Birth characteristics were comparable between UMCU (n = 33) and UCSF/UBC (n = 54). Patients had a hypoplastic aortic arch with either coarctation/interruption or hypoplastic left heart syndrome. In subjects with prenatal diagnosis, there was a significant difference in the prevalence of white matter injury (WMI) between centers (11 of 22 [50%] at UMCU vs 4 of 30 [13%] at UCSF/UBC; P < .01). Prenatal diagnosis was protective for WMI at UCSF/UBC (13% prenatal diagnoses vs 50% postnatal diagnoses; P < .01), but not at UMCU (50% vs 46%, respectively; P > .99). Differences in clinical practice between prenatally diagnosed subjects at UMCU vs UCSF/UBC included older age at surgery, less time spent in the intensive care unit, greater use of diuretics, less use of total parenteral nutrition (P < .01), and a greater incidence of infections (P = .01). In patients diagnosed postnatally, the prevalence of WMI was similar in the 2 centers (46%at UMCU vs 50% at UCSF/UBC; P > .99). Stroke prevalence was similar in the 2 centers regardless of prenatal diagnosis (prenatal diagnosis: 4.5% at Utrecht vs 6.7% at UCSF/UBC, P = .75; postnatal diagnosis: 9.1% vs 13%, respectively, P > .99).
Conclusion
Prenatal diagnosis can be protective for WMI, but this protection may be dependent on specific clinical management practices that differ across centers.
Neonates with complex congenital heart disease are at high risk for cerebral injury. Newborns with aortic arch obstruction, particularly those with single-ventricle physiology, have some of the highest rates of injury.1 At school age, approximately one-third of these children manifest problems, varying from motor problems to difficulties in executive function.2,3 Preoperative cranial magnetic resonance imaging (MRI) studies have revealed evidence of injury in 28%–43% of these patients, with this percentage increasing to 34%–72% after surgery.4–7 The majority of the lesions detected on MRI are white matter injury (WMI), and a small proportion are strokes.
Brain injuries occur as the cumulative result of both the genetic background of the patient and the altered circulation during fetal, preoperative, intraoperative, and postoperative periods.8 Numerous different risk factors are involved, including clinical management practices, such as balloon atrial septostomy and timing of surgery.9,10 A surprising degree of clinical practice pattern variability exists across major pediatric congenital heart surgery programs. A recent single-ventricle reconstruction trial testing the effects of different Norwood shunt types among North American centers found significant variation in rates of common clinical practices, including prenatal diagnosis (55%–85%), preoperative intubation (29%–91%), and enteral feeding (1%–100%).11
Prenatal diagnosis particularly affects clinical practice, allowing for planned delivery and perinatal management in a tertiary care center. Changes in clinical care afforded by prenatal diagnosis have been postulated to influence both surgical and neurodevelopmental outcomes.12,13 Although prenatal diagnosis did not appear to be protective for preoperative brain injury in a large group of neonates with a wide range of cardiac diagnoses,14 this does not exclude the possibility of a beneficial effect in specific defects, such as aortic arch obstructions. In newborns with transposition of the great arteries (TGA), prenatal diagnosis was not associated with improved early neurodevelopmental outcomes.15 At school age, however, although IQ, language, and memory was normal in children with prenatally diagnosed TGA and those with postnatally diagnosed TGA, the latter had a higher prevalence of neurocognitive deficits and worse executive function.16
For newborns with aortic arch obstruction, prenatal diagnosis allows for early initiation of prostaglandin E2 therapy to maintain ductal patency and results in improved preoperative clinical status.17 The effect on brain injury remains unknown. We focused on rates of preoperative brain injury in neonates with aortic arch obstruction as related to the presence of prenatal diagnosis and clinical practice differences across centers.
Methods
Our analysis used data from 2 prospective cohorts at 3 centers performing MRI scans before and after neonatal cardiac surgery: University Medical Center Utrecht (Utrecht, The Netherlands [UMCU]), and a longstanding collaboration of the University of California San Francisco (UCSF) and the University of British Columbia (UBC; Vancouver, Canada).1,7,18 For our analysis, UCSF and UBC were considered a single center owing to the smaller sample size of patients with arch obstruction at UBC (n = 10) and initial comparisons showing similar perioperative management at these 2 centers (Table I; available at www.jpeds.com). Informed consent was obtained from all participating parents and from the institutional Medical Ethical Boards.
Table I.
Characteristics of the UCSF and UBC cohorts
| UCSF (n = 44) | UBC (n = 10) | P value | |
|---|---|---|---|
| Preoperative injury, n (%) | |||
| Any WMI | 14 (31) | 2 (20) | .71 |
| Mild | 7 (16) | 2 (20) | .66 |
| Moderate or severe | 7 (16) | 0 | .33 |
| Stroke | 2 (4.4) | 2 (10) | .15 |
| Birth characteristics | |||
| Gestational age, wk, median (IQR) | 39.0 (38.0–39.9) | 39.0 (38.0–40.0) | .92 |
| Birth weight, g, median (IQR) | 3227 (2982–3516) | 3590 (2758–4355) | .35 |
| Male sex, n (%) | 28 (62) | 8 (80) | .47 |
| Genetic syndrome, n (%) | 5 (11) | 0 (0) | .57 |
| Prenatal diagnosis, n (%) | 25 (56) | 6 (60) | >.99 |
| Diagnosis of HLHS, n (%) | 25 (57) | 4 (40) | .49 |
| Perinatal course | |||
| Cesarean delivery, n (%) | 11 (25) | 5 (50) | .14 |
| 5-min Apgar score, median (IQR) | 9 (8–9) | 9 (9–9) | .11 |
| Neonatal course, median (IQR) | |||
| Age at presentation, d | 0 (0–0) | 0 (0–0) | .44 |
| Age at MRI, d | 5 (3–6) | 2 (1–8) | .10 |
| Age at surgery, d | 7 (6–11) | 6 (2–15) | .14 |
| Time in ICU, % | 100 (69–100) | 100 (34–100) | .71 |
| Non-ICU days, n | 0 (0–2) | 0 (0–6) | .92 |
| Hemodynamics and management | |||
| LCOS, n/N (%) | 16/36 (44) | 2/9 (22) | .28 |
| CPR, n (%) | 2 (4.4) | 0 | >.99 |
| Mechanical ventilation, n (%) | 30 (68) | 3 (30) | .04 |
| Ventilator strategy, n (%)4 | 4 (8.9) | 0 | >.99 |
| Sedatives, n/N (%) | 34/43 (77) | 4/9 (44) | .10 |
| Prostaglandin, n (%) | 45 (100) | 8 (80) | .03 |
| Inotropes, n/N (%) | 15/43 (34) | 1 (10) | .25 |
| Diuretics, n/N (%) | 12/43 (27) | 2/9 (22) | >.99 |
| Total parenteral nutrition, n/N (%) | 30/37 (81) | 2/9 (22) | <.01 |
| Enteral feeding, n/N (%) | 13/41 (32) | 6 (60) | .15 |
| Lowest hemoglobin, median (IQR) | 12.7 (11.5–13.4) | 13.7 (12.6–15.4) | .05 |
| Inflammation, n (%) | |||
| Infection | 2 (4.4) | 1 (10) | .47 |
| Antibiotics | 24 (53) | 3 (30) | .30 |
For this study, the preoperative scans of all enrolled neonates with aortic arch obstruction were used. The 3 centers had comparable MRI protocols, resulting in a similar sensitivity for identifying abnormalities.
At UMCU, MRI was performed with a 1.5-T scanner (Philips Medical Systems, Best, The Netherlands). MRI included 2-mm-thick sagittal T1-, transverse T2-, and inversion recovery-weighted sequences. An echo-planar imaging technique was used for diffusion-weighted imaging (DWI) (repetition time [TR], 3800–5200 msec; echo time [TE], 89 msec), with a 180 × 180-mm field of view, 4-mm-thick sections, a 0-mm section gap, and b factors of 0 and 1000 s/mm2 (1.5 T). At UCSF, a 1.5-T Signa Echo-Speed System (GE Medical Systems, Waukesha, Wisconsin) was used. Imaging included T1-weighted sagittal spin-echo images (TR, 600 msec; TE, 8 msec; field of view, 20 cm; slice thickness, 3 mm; section gap, 1 mm), dual-echo T2-weighted spin-echo images (TR, 3000 msec; TE, 60 msec; field of view, 8.3–13.5 cm; slice thickness, 4 mm; section gap, 2 mm), coronal volumetric 3-dimensional gradient echo images with radiofrequency spoiling images (TR, 36 msec; TE, 3.5 msec; field of view, 22 cm; slice thickness, 1 mm, section gap, 0), and average diffusivity map echo-planar acquisition (TR, 8000 msec; TE, 150 msec; field of view, 36 3 27 cm; slice thickness, 5 mm, section gap, 0). At UBC, MRI studies were performed with a Siemens 1.5-T Avanto system (Siemens, Erlangen, Germany) using VB 13A software, and included 3-dimensional coronal volumetric T1-weighted images (TR, 36 msec; TE, 9.2 msec; field of view, 200 mm; slice thickness, 1 mm; section gap, 0) and axial fast spin-echo T2-weighted images (TR, 4610 msec; TE, 107 msec; field of view, 160 mm; slice thickness, 4 mm; section gap, 0.2 mm). Average diffusivity maps were generated from diffusion tensor imaging acquired with a multirepetition, single-shot echo planar sequence with 12 gradient directions (TR, 4900 msec; TE, 104 msec; field of view, 160 mm; slice thickness, 3 mm, section gap, 0); b,¼0, 600, and 700 s/mm2; and an in-plane resolution of 1.3 mm.
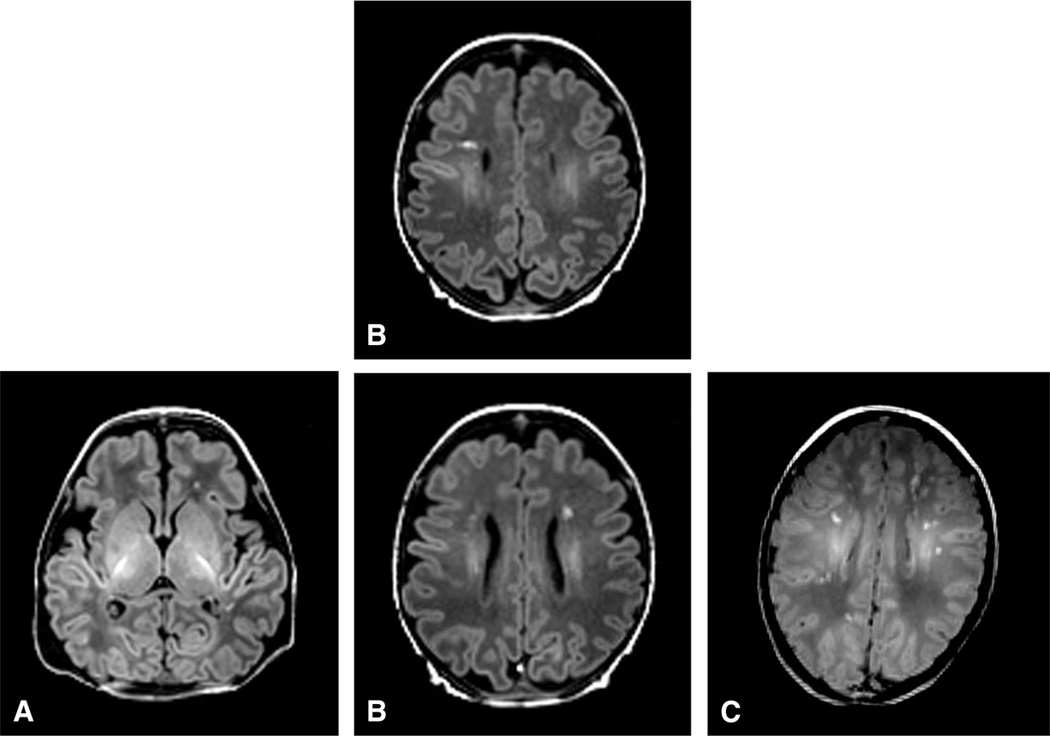
All scans were assessed for evidence of stroke and WMI by a single reviewer (K.P.), using conventional T1-weighted imaging, T2-weighted imaging, and DWI. WMI was scored as defined previously19 and as depicted in the Figure (available at www.jpeds.com). Mild WMI was defined as no more than 3 lesions each no larger than 2 mm; moderate, as 3 or more lesions or areas larger than 2 mm; and severe, as involvement of approximately >5% of the hemisphere.
Figure.
Classification of WMI severity. A, Mild WMI was defined as no more than 3 lesions each no larger than 2 mm. B, Moderate WMI was defined as 3 or more lesions or areas larger than 2 mm. C, Severe WMI was defined as involvement of approximately >5% of the hemisphere.
Clinical data were collected by retrospective chart review. Only data for the time period before preoperative MRI were analyzed, and thus events that may have occurred between the preoperative MRI and surgery were not included.
All daily physician progress notes and transfer notes were used, as were all available laboratory data. Low cardiac output syndrome (LCOS) was defined as either the use of cardiopulmonary resuscitation (CPR) or the presence of at least 3 of any of the following variables: clinical signs of LCOS, such as tachycardia, cool extremities, poor pulses, or oliguria; laboratory data showing an increased base deficit >3 mEq/L or a lactate >3 mmol/L; or an intervention such as administration of inotropes, high-dose prostaglandin, or HCO3−.20 LCOS was only scored for patients with sufficient clinical records to establish the diagnosis. Infections were assessed as defined previously.18 All clinical and imaging data were analysed separately by category of prenatal diagnosis.
Binary variables were compared between groups using the Fisher exact test. Continuous variables were compared using the Mann-Whitney U test. Given the sample size, univariate analyses were performed. All analyses were performed using SPSS version 15 (SPSS, Chicago, Illinois).
Results
Eighty-eight cases with aortic arch obstruction were available for the study, including 33 from UMCU and 54 from UCSF/UBC (44 from UCSF and 10 from UBC). Of the 52 patients in the prenatally diagnosed group, 25 (48%) had hypoplastic left heart syndrome (HLHS), and the remainder had other aortic arch obstructions necessitating univentricular or biventricular repair. Of the 35 patients in the postnatally diagnosed group, 12 (34%) had HLHS (P = .27, prenatal vs postnatal groups for diagnosis of HLHS). Specific cardiac diagnoses are listed in Table II (available at www.jpeds.com).
Table II.
Cardiac diagnoses and procedures performed at Utrecht and UCSF/UBC
| Utrecht, n | UCSF/UBC, n | ||||
|---|---|---|---|---|---|
| Diagnosis | Surgery | Prenatal (n = 22) |
Postnatal (n = 11) |
Prenatal (n = 30) |
Prenatal (n = 30) |
| HLHS | Norwood procedure | 7 | 1 | 18 | 11 |
| Other diagnoses | |||||
| HRHS (TGA with TA atresia or stenosis) | Norwood procedure | 2 | 0 | 3 | 1 |
| DILV, TGA, CoA | Norwood procedure | 1 | 0 | 0 | 0 |
| DILV with CoA or hypoplastic aortic arch | Norwood procedure | 1 | 0 | 1 | 0 |
| Complete AVSD, LVOTO, hypoplastic MV, atrial isomerism | Norwood procedure | 0 | 0 | 1 | 0 |
| HLHC21 | Biventricular repair | 3 | 4 | 0 | 0 |
| CoA (with or without VSD and/or ASD) | Aortic arch reconstruction, ASD and/or VSD closure | 1 | 3 | 2 | 5 |
| IAA (with or without VSD and/or ASD) | Aortic arch reconstruction, ASD and/or VSD closure | 0 | 1 | 1 | 2 |
| Taussig-Bing anomaly with CoA or IAA | Arterial switch, aortic arch reconstruction, VSD closure | 4 | 0 | 3 | 1 |
| TGA with IAA or CoA, VSD, and/or ASD | Arterial switch, aortic arch reconstruction, VSD and/or ASD closure | 2 | 0 | 1 | 0 |
| DORV, CoA, VSD | VSD tunnel repair, aortic arch reconstruction | 0 | 1 | 0 | 1 |
| Complete AVSD, LVOTO, hypoplastic aortic arch, situs inversus | AVSD repair, LVOTO resection, aortic arch reconstruction | 1 | 0 | 0 | 0 |
| IAA type B with hypoplastic aortic arch, severe LVOTO, VSD | Ross-Konno, aortic arch reconstruction, VSD closure | 0 | 1 | 0 | 0 |
| DORV, AVSD, CoA, MV atresia | Hybrid procedure: PA banding, aortic arch and PDA stenting | 0 | 0 | 0 | 1 |
| IAA, large A-P window | Patch plasty aortic arch, repair A-P window | 0 | 0 | 0 | 1 |
| Truncus arteriosus, IAA, VSD | Aortic arch reconstruction, RV-PA conduit, VSD closure | 0 | 0 | 0 | 1 |
| Total | 15 | 10 | 12 | 13 | |
A-P, aortopulmonary; ASD, atrial septal defect; AVSD, atrioventricular septal defect; CoA, coarctation of the aortic arch; DILV, double-inlet left ventricle; DORV, double-outlet right ventricle; HLHC, hypoplastic left heart complex21; HRHS, hypoplastic right heart syndrome; IAA, interrupted aortic arch; LVOTO, left ventricular outflow tract obstruction; MV, mitral valve; PA, pulmonary artery; PDA, patent ductus arteriosus; RV, right ventricle; TA, tricuspid atresia; VSD, ventricular septal defect.
Table III shows the prevalence of WMI and stroke in both centers by prenatal diagnosis. In the UCSF/UBC cohort, 4 of 30 (13%) prenatally diagnosed patients had WMI, compared with 12 of 24 (50%) of the postnatally diagnosed patients, representing a risk reduction of 37% (P < .01). In the UMCU cohort, the prevalence of preoperative WMI did not differ significantly between the prenatally diagnosed and postnatally diagnosed groups (11 of 22 [50%] vs 5 of 11 [46%]; P > .99). There was a significant difference in the prevalence of injury between the Utrecht and UCSF/UBC cohorts in patients diagnosed prenatally (50% vs 13%; P < .01). In both centers, mild WMI was most common (7 of 11 patients at Utrecht vs 3 of 4 patients at UCSF/UBC), and there were no cases of severe WMI. At UMCU, 6 of the 11 cases with WMI had lower apparent diffusion coefficient (ADC) values on DWI, suggesting recent injury. At UCSF/UBC, none of the 4 patients with WMI had areas of restricted diffusion.
Table III.
Preoperative injury
| Diagnosis | UMCU | UCSF/UBC | P value |
|---|---|---|---|
| Prenatal diagnosis | n = 22 | n = 30 | |
| Any WMI | 11 (50) | 4 (13) | <.01 |
| Mild | 7 (32) | 3 (10) | .05 |
| Moderate | 4 (18) | 1 (3.3) | .07 |
| Stroke | 1 (4.5) | 2 (6.7) | .75 |
| Postnatal diagnosis | n = 11 | n = 24 | |
| Any WMI | 5 (46) | 12 (50) | >.99 |
| Mild | 0 (0) | 6 (25) | .15 |
| Moderate-severe | 5 (46) | 6 (25) | .26 |
| Stroke | 1 (9.1) | 3 (13) | >.99 |
Values stated as number of patients (% of center).
The status of prenatal diagnosis did not affect the occurrence of stroke at either center (UMCU: 1 of 22 [4.5%] prenatally diagnosed vs 1 of 10 (10%) postnatally diagnosed, P > .99; UCSF/UBC: 2 of 30 [6.7%] prenatally diagnosed vs 3 of 21 [13%] postnatally diagnosed, P = .64). The incidence of stroke in prenatally diagnosed patients did not differ between centers (1 of 22 [4.5%] at UMCU vs 2 of 30 [6.7%] at UCSF/UBC; P = .75).
To explore the risk factors accounting for the difference in prevalence of brain injury across centers, we examined clinical management practices by status of prenatal diagnosis. Differences in perinatal data and in postnatal management are outlined in Table IV. There were no between-group differences in birth characteristics, although there was a trend toward more cases of HLHS at UCSF/UBC (32% at UMCU vs 60% at UCSF/UBC; P = .06). Both 1-minute and 5-minute Apgar scores were significantly higher at Utrecht (median 1-minute score, 9 at UMCU vs 8 at UCSF/UBC, P = .02; median 5-minute score, 9 at UMCU vs 9 UCSF/UBC, P = .02).
Table IV.
Clinical characteristics and management practices by center
| Prenatal diagnosis | Postnatal diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | UMCU (n = 22) |
UCSF/UBC (n = 30) |
P (UMCU vs UCSF/UBC) |
UMCU (n = 11) |
UCSF/UBC (n = 24) |
P (UMCU vs UCSF/UBC) |
| Birth characteristics | ||||||
| Gestational age, wk, median (IQR) | 39.0 (38.3–40.0) | 38.7 (38.0–39.3) | .28 | 39.1 (38.6–40.1) | 39.3 (38.5–40.3) | .89 |
| Birth weight, g, median (IQR) | 3320 (3030–3660) | 3245 (2971–3688) | .99 | 3400 (3240–3516) | 3278 (3043–3528) | .39 |
| Male sex, n (%) | 19 (86) | 21 (70) | .20 | 8 (73) | 14 (58) | .48 |
| Genetic syndrome, n (%) | 2 (9.5) | 2 (6.7) | .71 | 1 (9.1) | 3 (13) | >.99 |
| Diagnosis of HLHS, n (%)* | 7 (32) | 18 (60) | .06 | 1 (9.1) | 11 (46) | .06 |
| Year, median (IQR) | 2010 (2009–2011) | 2007 (2005–2010) | <.01 | 2010 (2009–2011) | 2006 (2005–2009) | <.01 |
| Perinatal course | ||||||
| Cesarean delivery, n/N (%) | 3/22 (13.6) | 9 (30) | .20 | 0 (0) | 7 (29) | .07 |
| 5-min Apgar score, median (IQR) | 9 (9–10) | 9 (8–9) | .02 | 10 (9–10) | 9 (8–9) | .03 |
| Neonatal course, median (IQR) | ||||||
| Age at presentation, d | 0 (0–0) | 0 (0–0) | >.99 | 3 (1–9) | 0 (0–4) | .06 |
| Age at MRI, d | 7 (6–9) | 3 (2–5) | <.01 | 11 (7–13) | 6 (5–10) | .03 |
| Age at surgery, d | 9 (7–13) | 6 (4–8) | <.01 | 12 (10–15) | 9 (7–12) | .07 |
| Time in ICU, %, median (IQR) | 25 (16–70) | 100 (100–100) | <.01 | 29 (18–46) | 60 (31–86) | .03 |
| Non-ICU days, n, median (IQR) | 3 (2–6) | 0 (0–0) | <.01 | 8 (3–9) | 2 (1–4) | .04 |
| Hemodynamics and management | ||||||
| LCOS, n (%)† | 4 (22) | 4 (17) | .71 | 9 (90) | 14 (67) | .22 |
| CPR, n (%) | 0 (0) | 0 (0) | >.99 | 3 (27) | 2 (8.3) | .14 |
| Balloon atrioseptostomy, n (%) | 0 (0) | 1 (3.3) | >.99 | 0 (0) | 1 (2.9) | >.99 |
| Mechanical ventilation, n (%) | 6 (27) | 13 (43) | .26 | 8 (73) | 20 (83) | .65 |
| Ventilator strategy, n (%)‡ | 0 (0) | 4 (13) | .13 | 0 (0) | 0 (0) | >.99 |
| Sedatives, n (%)† | 7 (31.8) | 16 (57) | .09 | 8 (73) | 21 (88) | .35 |
| Prostaglandin, n (%) | 19 (86) | 29 (97) | .17 | 10 (91) | 23 (96) | .54 |
| Inotropes, n (%) | 4 (18) | 2 (6.9) | .38 | 9 (82) | 14 (58) | .26 |
| Diuretics, n (%)† | 13 (59) | 5 (17) | <.01 | 6 (54) | 9 (39) | .48 |
| Total parenteral nutrition, n (%)† | 2 (9.1) | 16 (64) | <.01 | 1 (9.1) | 16 (80.0) | <.01 |
| Enteral feeding, n (%)† | 22 (100) | 3 (10) | <.01 | 11 (100) | 16 (73) | .08 |
| Lowest hemoglobin, median (IQR) | 14.6 (13.0–17.6) | 13.3 (12.5–14.6) | .13 | 13.0 (11.6–15.3) | 12.1 (11.3–13.3) | .06 |
| Inflammation, n (%) | ||||||
| Infection | 5 (23) | 0 (0) | .01 | 1 (9.1) | 3 (13) | >.99 |
| Antibiotics | 7 (32) | 4 (13) | .11 | 4 (36) | 22 (92) | <.01 |
Specific diagnoses are listed in Table II.
Data were available for a limited number of patients: LCOS, n = 74; sedatives, n = 84; diuretics, n = 86; total parenteral nutrition, n = 79; enteral feeding, n = 85. Percentages shown are only of patients with sufficient data.
Special ventilator strategies included ventilation with hypoxic gas and permissive hypercapnia.
Intensive care unit (ICU) admittance policy differed in the 2 groups. At UMCU, patients diagnosed prenatally with a cardiac condition were admitted to the pediatric ICU immediately after birth for stabilization and, once stable (usually the next day), transferred to the pediatric cardiology ward (with less intensive monitoring). Readmittance to the ICU before surgery occurred only in the event of hemodynamic instability. In contrast, at UCSF/UBC, all patients were cared for in the pediatric cardiac ICU or neonatal ICU until undergoing surgery. These management differences are reflected in the percentage of preoperative time spent on the ICU (median, 25% at UMCU vs 100% at UCSF/UBC; P < .01). In addition, the number of non-ICU days differed between groups (median, 3 days at UMCU vs 0 days at UCSF/UBC; P < .01).
The timing of surgery was later at UMCU than at UCSF (median, 9 days vs 6 days; P < .01). Because MRI is usually planned shortly before surgery at UMCU, the day of MRI also was later than at UCSF (median, 7 days vs 3 days; P < .01). The prevalence of CPR and LCOS did not differ between the centers (with data available for 18 of 22 UMCU patients [81%] and for 25 of 30 UCSF/UBC patients [83%]). Despite the greater number of ICU admissions at UCSF/UBC, the number of patients requiring mechanical ventilation and inotropic medications were similar at the 2 centers. However, more patients at UMCU received diuretics (59% at UMCU vs 17% at UCSF/UBC; P < .01). In contrast, more patients at UCSF/UBC received total parenteral nutrition (9.1% at UMCU vs 64% at UCSF/UBC; P < .01). Enteral feeding was more common at UMCU (100% vs 10%; P < .01). Finally, infections before surgery were more common at UMCU (23% vs none at UCSF; P = .01). Infections included 3 culture-confirmed bloodstream infections (2 with Staphylococcus aureus and 1 with a coagulase-negative Staphylococcus) and 2 pneumonias (documented on chest radiographs), all of which were treated with antibiotics for at least 5 days.
Of note, when UBC patients were excluded from the analyses, thus comparing UMCU only with UCSF, all of the aforementioned differences remained significant.
There was no significant between-group difference in the incidence of WMI (46% vs 50%; P > .99) (Table III). All of the UMCU cases had moderate WMI, and all had decreased ADC values. At UCSF/UBC, severity was evenly distributed over mild and moderate to severe (1 case of severe WMI), and 5 of 12 cases (42%) had decreased ADC values. Stroke prevalence was similar at the 2 centers (1 of 11 [9.1%] at UMCU vs 3 of 24 [13%] at UCSF/UBC; P > .99).
In neonates diagnosed with congenital heart disease postnatally (n = 35), again birth characteristics were similar at the 2 centers, but with a trend toward a higher prevalence of HLHS at UCSF/UBC (9.1% at UMCU vs 46% at UCSF/UBC; P = .06) (Table IV). A trend towards a higher rate of cesarean delivery at UCSF/UBC was also noted (none in UMCU vs 29% at UCSF/UBC; P = .07). Five-minute Apgar score was higher at UMCU (median, 10 at UMCU vs 9 at UCSF/UBC; P = .03).
Patients often presented at a level 2 center and were then transferred to a level 3 center (UMCU or UCSF/UBC). There was a trend toward a later day of initial presentation (at any hospital, usually the level 2 hospital) in the UMCU group (median, day 3 at UMCU vs day 0 [day of birth] at UCSF/UBC; P = .06).
As in the group of patients diagnosed prenatally, MRI scans were performed at a later age at UMCU, and there was a trend toward a later day of surgery at Utrecht (median age at MRI, 11 days at UMCU vs 6 days at UCSF/UBC, P = .03; median day of surgery, 12 days at UMCU vs 9 days at UCSF/UBC, P = .07). In addition, less time was spent on the ICU at UMCU (29% of the time in UMCU vs 60% at UCSF/UBC; P = .03; and 8 non-ICU days at UMCU vs 2 at UCSF/UBC; P = .04).
Combining the patients of both centers, we assessed for preoperative factors associated with the presence of WMI. Results are presented in Table V. In the overall cohort, the protective effect of prenatal diagnosis was of borderline significance (P = .07).
Table V.
Risk factors for WMI
| Prenatal diagnosis | Postnatal diagnosis | |||||
|---|---|---|---|---|---|---|
| Variables | No WMI (n = 37) | WMI (n = 15) |
P (no WMI vs WMI) |
No WMI (n = 18) | WMI (n = 17) |
P (no WMI vs WMI) |
| Birth characteristics | ||||||
| Gestational age, wk, median (IQR) | 39 (38–39) | 39 (38–40) | .55 | 39 (39–40) | 40 (39–41) | .14 |
| Birth weight, g, median (IQR) | 3290 (3010–3695) | 3237 (2739–3655) | .44 | 3234 (2941–3375) | 3412 (3140–3569) | .10 |
| Male sex, n (%) | 28 (76) | 12 (80) | >.99 | 11 (61) | 11 (65) | >.99 |
| Genetic syndrome, n (%) | 4 (11) | 0 (0) | .57 | 4 (22) | 0 (0) | .10 |
| Diagnosis of HLHS, n (%) | 19 (51) | 6 (40) | .55 | 4 (22) | 8 (47) | .16 |
| Year, median (IQR) | 2008 (2006–2010) | 2009 (2005–2010) | .24 | 2009 (2004–2010) | 2007 (2006–2010) | .94 |
| Perinatal course | ||||||
| Cesarean delivery, n (%) | 10 (27) | 2 (13) | .47 | 4 (22) | 3 (18) | >.99 |
| 5-min Apgar score, median (IQR) | 9 (9–9) | 9 (8–9) | .55 | 9 (8–9) | 9 (9–9) | .80 |
| Neonatal course, median (IQR) | ||||||
| Age at presentation, d | 0 (0–0) | 0 (0–0) | >.99 | 1 (0–8) | 2 (0–5) | .81 |
| Age at MRI scan, d | 4 (2–6) | 6 (4–8) | <.01 | 8 (5–12) | 7 (5–12) | .53 |
| Age at surgery, d | 7 (5–8) | 9 (6–12) | .07 | 11 (7–14) | 11 (8–15) | .84 |
| Time in ICU, %, median (IQR) | 100 (64–100) | 67 (25–100) | .16 | 31 (19–85) | 60 (32–76) | .13 |
| Non-ICU days, median (IQR) | 0 (0–3) | 2 (0–6) | .09 | 6 (1–10) | 3 (1–7) | .31 |
| Hemodynamics and management | ||||||
| LCOS, n (%)* | 5 (15) | 3 (33) | .22 | 8 (53) | 15 (94) | .02 |
| CPR, n (%) | 0 (0) | 0 (0) | >.99 | 0 (0) | 5 (31) | .02 |
| Balloon atrioseptostomy, n (%) | 1 (2.7) | 0 (0) | >.99 | 0 (0) | 1 (5.9) | .49 |
| Mechanical ventilation, n (%) | 11 (30) | 8 (53) | .13 | 11 (61) | 17 (100) | .01 |
| Ventilator strategy, n (%)† | 3 (8.1) | 1 (6.7) | >.99 | 0 | 0 | |
| Sedatives, n (%)* | 15 (43) | 8 (53) | .55 | 12 (67) | 17 (100) | .02 |
| Prostaglandin, n (%) | 35 (95) | 13 (87) | .57 | 16 (89) | 17 (100) | .49 |
| Inotropes, n (%) | 3 (8.3) | 3 (20) | .34 | 9 (50) | 14 (82) | .08 |
| Diuretics, n (%)* | 10 (28) | 8 (53) | .11 | 6 (35) | 9 (53) | .49 |
| Total parenteral nutrition, n (%)* | 13 (39) | 5 (36) | >.99 | 7 (47) | 10 (63) | .48 |
| Enteral feeding, n (%)* | 14 (39) | 11 (73) | .03 | 13 (77) | 14 (88) | .66 |
| Lowest hemoglobin, median (IQR) | 13.9 (13.0–16.2) | 13.0 (12.3–17.1) | .46 | 12.2 (11.4–14.3) | 12.2 (11.3–13.3) | .63 |
| Inflammation, n (%) | ||||||
| Infection | 1 (2.7) | 4 (27) | .02 | 3 (17) | 1 (5.9) | .60 |
| Antibiotics | 5 (14) | 6 (40) | .06 | 13 (72) | 13 (77) | >.99 |
Data were available for a limited number of patients: LCOS, n = 74; sedatives, n = 84; diuretics, n = 86; total parenteral nutrition, n = 79; and enteral feeding, n = 85. Percentages shown are only for patients with sufficient data.
Special ventilator strategies included ventilation with hypoxic gas and permissive hypercapnia.
In prenatally diagnosed patients, increased age at MRI and surgery showed a significant association with more WMI (patients without WMI had an MRI at a median age of 4 days and underwent surgery at 7 days, vs 6 days and 9 days, respectively, in the group with WMI; P < .01 and P = .07, respectively). Enteral feeding was associated with occurrence of WMI (39% in the no WMI group vs 73% in the WMI group; P = .03). Infection also was associated with increased risk (2.7% in the no WMI group vs 27% in the WMI group; P = .02).
For postnatally diagnosed patients, significant risk factors for WMI were CPR, LCOS, and the need for mechanical ventilation and sedatives (P = .02, P = .02, P = .01, and P = .02, respectively).
Discussion
The fact that the 2 cohorts had very similar birth characteristics but still a very different prevalence of WMI makes this a highly suitable group for studying the potential of various postnatal care strategies to reduce the burden of brain injury. In the time leading up to the surgical procedure, the most apparent difference between the 2 centers was the difference in timing of surgery (and, consequently, of preoperative MRI). The question arises as to whether the white matter lesions were more established in the UMCU patients because they underwent MRI later. This is not likely, given that both centers used sensitive MRI techniques, such as DWI, which will show injury, such as white matter lesions, as early as hours after the insult (although the slice thickness of DWI is greater than that of conventional T1 and T2 sequences; 4 mm vs 2 mm). Furthermore, in the UMCU population, the abundance of DWI-positive lesions suggests that at least part of the injury has a postnatal onset, considering that lesions remain DWI-positive for up to 7–8 days, and that the median day of MRI was 7 days in the prenatally diagnosed UMCU patients and 11 days in the postnatally diagnosed UMCU patients.
Taking the foregoing findings into account, we hypothesize that the increasing abundance of WMI while awaiting surgery may be related in part to the ongoing suboptimal hemodynamic state. In newborns with TGA, lower oxygen saturation and longer time to surgery also have been associated with higher rates of WMI.4,9 Moreover, in a recent study of infants with various cardiac diagnoses, the subgroup with preoperative WMI showed a trend toward higher lactate values.14 In patients with single-ventricle physiology, such as HLHS, there is a delicate balance between pulmonary and systemic perfusion, and in the first days of life, as pulmonary vascular resistance falls and pulmonary blood flow increases, this may be at the expense of the systemic and cerebral circulation. Increasing pulmonary blood flow carries a risk of cerebral hypoperfusion. Patients underwent surgery at a median of 9 days at UMCU, compared with 6 days at UCSF/UBC. The longer interval until surgery may put these neonates at greater risk for systemic and cerebral hypoperfusion, especially when they are not continuously admitted to the ICU and closely monitored. A recent study of risk factors for preoperative injury in neonates with HLHS by Goff et al21 reported a relatively low prevalence of WMI of 19%, which may be attributed to both the early age at surgery (mean, 3.6 days) and the high percentage of cases diagnosed prenatally (86%). This low frequency of WMI in the setting of prenatal diagnosis is consistent with the prevalence observed at UCSF/UBC. The only other risk factor identified in this study was brain immaturity, a finding also observed in the UCSF/UBC cohort.22
Other management differences between the 2 centers that may have important effects on cerebral injury must be taken into account as well. The first of these is the higher incidence of preoperative infections before surgery in UMCU. The association between infection and WMI has been observed before in both neonates undergoing cardiac surgery and preterm neonates.18,23 Furthermore, the use of diuretics was much more common in the UMCU group. Although commonly administered to treat tachypnea and pulmonary edema, diuretics carry a risk of reducing preload and further compromising systemic perfusion, which may result in a higher prevalence of WMI. Finally, provision of total parenteral nutrition, instead of enteral nutrition, was more common at UCSF/UBC. Giving the increasing evidence indicating that proper nutrition is essential for brain protection in neonates, total parenteral nutrition may deliver more trophic factors to the brain, helping minimize the risk of WMI. Alternatively, enteral feeding may lead to increased abdominal perfusion, at the expense of cerebral blood flow in ductal-dependent circulation.
In patients diagnosed after birth, the prevalence of WMI was approximately the same (50%) in the 2 centers. The risk factors for WMI in this group were all markers of the state in which these patients presented with their cardiac lesion: LCOS and cardiac arrest, with a subsequent need for mechanical ventilation and sedation. These abnormal circulatory states presumably result in a primary brain injury at the time of presentation. No other center-specific management differences apparently affected the overall risk of injury; however, there was a trend toward milder forms of WMI at UCSF/UBC in this group, perhaps reflecting the same preoperative management differences between the centers as in the prenatally diagnosed group (ie, time spent on the ICU and total parenteral nutrition). Another explanation may be the later age of identification of a cardiac condition in the UMCU patients (3 days vs day of birth at UCSF/UBC), which may reflect the more common practice of home births in The Netherlands.
Although there was a large discrepancy in WMI burden between centers, this did not apply to stroke incidence, which was similar in the 2 centers, suggesting less influence of preoperative management on this phenomenon. This may be related to the fact that thromboembolic processes are the most important cause of stroke. Balloon atrial septostomy has been identified as a risk factor for stroke in newborns with TGA. In neonates with aortic arch obstruction, balloon atrial septostomy is much less common.10
The present study has some important limitations. Although the number of cases analyzed is quite substantial for this very specific cardiac patient group, the separate assessment of prenatally diagnosed and postnatally diagnosed patients precluded multivariate testing of risk factors for WMI. Furthermore, although we made every effort to collect extensive data to explore possible important clinical factors, we cannot exclude the possibility that other important information might have been overlooked. In particular, given the differences in care location (eg, ICU vs ward), important physiological data (eg, blood pressure) and laboratory data (eg, lactate, blood gases) were not collected uniformly at each center, making meaningful analysis of these data impossible. In the future, a prospective study in which hemodynamic data are continuously recorded during the entire preoperative course will better identify possible risk factors for cerebral injury, especially WMI. Only then can we effectively intervene, aiming for the lowest possible burden of injury before these neonates undergo their necessary cardiac surgery.
In conclusion, our data suggest that optimizing the care of neonates with aortic arch obstruction before they undergo surgery may provide an opportunity to fully realize the potential of prenatal cardiac diagnosis to improve the brain health of these vulnerable neonates.
Acknowledgments
Supported by the Canadian Institutes of Health Research (MOP93780), the National Institutes of Health (RO1 NS40117, R01NS063876, P50 NS35902 and PO1 NS082330), National Center for Research Resources (5-M01-RR-01271), the March of Dimes Foundation (5-FY05-1231, #6-FY2009-303), the American Heart Association (0365018Y), and the Larry L. Hillblom Foundation (2002/3E). S.A. was funded by a travel grant from the European Society for Paediatric Research. S.M. is the Bloorview Children’s Hospital Chair in Paediatric Neuroscience (from September 2012), and received support as a Tier 2 Canada Research Chair in Neonatal Neuroscience and a Michael Smith Foundation for Health Research Scholar award (to July 2012).
We thank Niels Blanken (Dept. of Radiology, Utrecht) for his expertise, as well as Mark Chalmers (UBC) and Veronica DiSantiago (UCSF) for help with retrieval of patient data.
Glossary
- ADC
Apparent diffusion coefficient
- CPR
Cardiopulmonary resuscitation
- DWI
Diffusion-weighted imaging
- HLHS
Hypoplastic left heart syndrome
- ICU
Intensive care unit
- LCOS
Low cardiac output syndrome
- MRI
Magnetic resonance imaging
- TE
Echo time
- TGA
Transposition of the great arteries
- TR
Repetition time
- UBC
University of British Columbia
- UCSF
University of California San Francisco
- WMI
White matter injury
Footnotes
The authors declare no conflicts of interest.
References
- 1.Block AJ, McQuillen PS, Chau V, Glass H, Poskitt KJ, Barkovich AJ, et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:550–557. doi: 10.1016/j.jtcvs.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148:72–77. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 4.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127:692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ., III Survival after reconstructive surgery for hypoplastic left heart syndrome: a 15-year experience from a single institution. Circulation. 2000;102(19) Suppl 3:III136–III141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 6.Dent CL, Spaeth JP, Jones BV, Schwartz SM, Glauser TA, Hallinan B, et al. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. doi: 10.1016/j.jtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 8.Tabbutt S, Gaynor JW, Newburger JW. Neurodevelopmental outcomes after congenital heart surgery and strategies for improvement. Curr Opin Cardiol. 2012;27:82–91. doi: 10.1097/HCO.0b013e328350197b. [DOI] [PubMed] [Google Scholar]
- 9.Petit CJ, Rome JJ, Wernovsky G, Mason SE, Shera DM, Nicolson SC, et al. Preoperative brain injury in transposition of the great arteries is associated with oxygenation and time to surgery, not balloon atrial septostomy. Circulation. 2009;119:709–716. doi: 10.1161/CIRCULATIONAHA.107.760819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113:280–285. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 11.Pasquali SK, Ohye RG, Lu M, Kaltman J, Caldarone CA, Pizarro C, et al. Variation in perioperative care across centers for infants undergoing the norwood procedure. J Thorac Cardiovasc Surg. 2012;144:915–921. doi: 10.1016/j.jtcvs.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Frommelt MA. Does fetal diagnosis make a difference? Clin Perinatol. 2005;32:877–890. viii. doi: 10.1016/j.clp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Yates RS. The influence of prenatal diagnosis on postnatal outcome in patients with structural congenital heart disease. Prenat Diagn. 2004;24:1143–1149. doi: 10.1002/pd.1072. [DOI] [PubMed] [Google Scholar]
- 14.Beca J, Gunn JK, Coleman L, Hope A, Reed PW, Hunt RW, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett JM, Wypij D, Bellinger DC, Rappaport LA, Heffner LJ, Jonas RA, et al. Effect of prenatal diagnosis on outcomes in D-transposition of the great arteries. Pediatrics. 2004;113:e335–e340. doi: 10.1542/peds.113.4.e335. [DOI] [PubMed] [Google Scholar]
- 16.Calderon J, Angeard N, Moutier S, Plumet MH, Jambaque I, Bonnet D. Impact of prenatal diagnosis on neurocognitive outcomes in children with transposition of the great arteries. J Pediatr. 2012;161:94–98. e1. doi: 10.1016/j.jpeds.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Sivarajan V, Penny DJ, Filan P, Brizard C, Shekerdemian LS. Impact of antenatal diagnosis of hypoplastic left heart syndrome on the clinical presentation and surgical outcomes: the Australian experience. J Paediatr Child Health. 2009;45:112–117. doi: 10.1111/j.1440-1754.2008.01438.x. [DOI] [PubMed] [Google Scholar]
- 18.Glass HC, Bowman C, Chau V, Moosa A, Hersh AL, Campbell A, et al. Infection and white matter injury in infants with congenital cardiac disease. Cardiol Young. 2011;21:562–571. doi: 10.1017/S1047951111000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 21.Goff DA, Shera DM, Tang S, Lavin NA, Durning SM, Nicolson SC, et al. Risk factors for preoperative periventricular leukomalacia in term neonates with hypoplastic left heart syndrome are patient-related. J Thorac Cardiovasc Surg. 2014;147:1312–1318. doi: 10.1016/j.jtcvs.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimitropoulos A, McQuillen PS, Sethi V, Moosa A, Chau V, Xu D, et al. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]