Abstract
Objectives
Adherence to the American Association for the Study of Liver Disease (AASLD) guidelines for the management of chronic hepatitis B (CHB) has not been systematically assessed. We sought to comprehensively evaluate adherence to five key areas of these guidelines. We also evaluated physician and patient factors underlying nonadherence, and predictors of nonadherence such as physician type, patient demographic factors, and phase of CHB infection.
Methods
Nine hundred and sixty-two adult patients were retrospectively identified. Each patient chart was reviewed in detail. The primary outcome was adherence to five areas of the AASLD guidelines: (i) timely alanine aminotransferase (ALT)/hepatitis B virus DNA level checks needed to monitor inactive carrier and immune-tolerant phases; (ii) liver biopsy to guide decisions on initiating treatment; (iii) treatment initiation when indicated; (iv) hepatocellular carcinoma (HCC) screening; (v) testing for hepatitis A virus (HAV) immunity, HIV, and hepatitis C virus (HCV) co-infections.
Results
Sixty percent did not undergo clinically indicated liver biopsies, largely owing to physician nonadherence. Eighty-nine percent of these missed biopsies were needed to further assess possible e-antigen-negative CHB. A high treatment initiation rate was found for the treatment eligible, but 121 patients had unclear treatment eligibility as they warranted, but did not undergo, liver biopsy. Forty-five percent did not have timely HCC screening, although gastroenterology physicians had the highest odds of adherence, and 29% did not have timely CHB lab assessment; patients seen by gastroenterologists had twice the odds compared with primary care physicians of undergoing timely lab monitoring. Thirty-five, 24, and 54% were not tested for HAV, HCV, and HIV co-infections.
Conclusions
Our findings show remarkably poor adherence to AASLD guidelines, particularly in the areas of liver biopsy, timely HCC and ALT monitoring, and testing for co-infection. These findings call for greater efforts to meet physician knowledge gaps, incorporation of decision support tools, and improved communication among providers.
Introduction
In the United States, there are an estimated 1.4–2 million people chronically infected with hepatitis B (1), defined as hepatitis B surface antigen positivity for more than 6 months (2,3). Management of chronic hepatitis B (CHB) is complex, because not all phases in its natural history require treatment, liver enzymes can be normal despite significant viral damage to the liver, and hepatocellular carcinoma (HCC) can develop in the absence of cirrhosis or liver enzyme abnormalities. Hepatitis B spontaneously fluctuates between different phases in its natural history, and therefore close monitoring is essential even when treatment is not needed in a particular phase. Failure to capture patients who evolve into phases needing treatment significantly increases the risk of complications, including cirrhosis and HCC (1,4). Treatment can prevent 15–25% of premature deaths from cirrhosis or HCC (1).
In the current movement toward improved quality of care in the United States, clinical practice guidelines have an essential role in guiding and standardizing CHB management, thus providing a set of processes useful for assessing the quality of health-care delivery. The American Association for the Study of Liver Diseases (AASLD) has published guidelines to assist health-care providers in the management and treatment of CHB, including setting standards for timely alanine aminotransferase (ALT) and viral load monitoring for inactive carriers and immune-tolerant patients (i.e., phases that do not require treatment), liver biopsy to guide treatment decisions, criteria for treatment initiation, screening for HCC, and testing for hepatitis A, hepatitis C, and HIV co-infections (2). A previous study has shown that less than one-third of CHB patients receive appropriate laboratory screening (5). Another study of gastroenterologists' HCC screening practices for CHB patients showed that only 60% performed at least annual HCC screening (6). Undertreatment of eligible patients has also been found, with 28% found not to receive the required treatment that could prevent cirrhosis and HCC (7).
Although these studies have addressed adherence to individual areas of CHB management, no study has comprehensively evaluated adherence to the multiple essential areas encompassed by the AASLD guidelines; indeed, no previous studies have addressed adherence to liver biopsy guidelines or testing for co-infection. Furthermore, we sought to address the etiology of nonadherence, specifically physician vs. patient factors. Finally, we sought to understand the predictors of nonadherence such as physician type, patient demographic factors, and phase of CHB infection.
This study sought to evaluate adherence to AASLD guidelines (latest update, 2009) for the management of CHB in five key areas: monitoring of liver enzymes and viral load, liver biopsy, treatment of eligible patients, HCC screening, and testing for exposure to HAV along with HCV and HIV co-infections.
Methods
Study population
We retrospectively identified 1,127 adult patients with CHB (defined as positive hepatitis B surface antigen or detectable hepatitis B virus (HBV) DNA for at least 6 months) seen at our medical center and satellite community health centers and clinics during the period from 1 January 2005 to 30 September 2012 identified through the Research Patient Data Registry (8) at Partners Healthcare. This database is composed of records from over 4.5 million patients from 1988 to the present and contains both inpatient and outpatient encounters, laboratory test results, imaging, and other clinical data. We performed a text search using the term “chronic hepatitis B.” Only those patients who had documented the presence of hepatitis B surface antigen or HBV DNA for at least 6 months were included in the study.
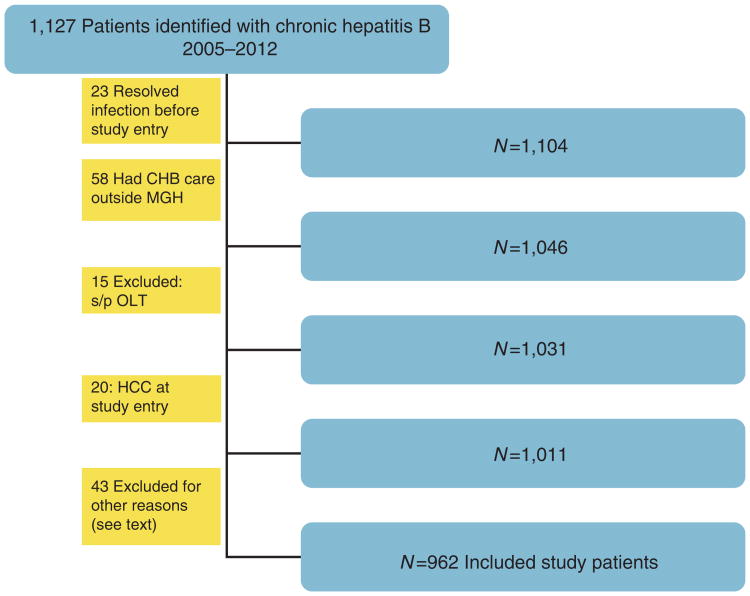
One hundred and sixty-five patients were excluded from the study (Figure 1). Exclusion criteria included resolved infection (refers to hepatitis B surface antigen seroclearance in CHB patients or following acute hepatitis B) before study entry, receipt of HBV management outside our hospital system, liver transplantation before study entry, diagnosis of HCC at study entry, age < 18 years, deceased during study period, stopped follow-up owing to insurance or change of residence, or patients in hospice care. Six patients with reactivation of hepatitis B due to immune-suppressive therapy and two patients with HDV co-infection were also excluded. Although hepatitis D co-infection is an important issue, given the very small number of co-infected patients in this cohort, we did not feel that reasonable conclusions could be drawn about adherence to guidelines.
Figure 1.
Flow chart of study patient inclusion and exclusion.
Nine hundred and sixty-two CHB patients were included in the study. Five patients had clear transitions through multiple phases in the natural history of hepatitis B during the study period. Therefore, all analyses were performed, with each phase as a separate encounter for a total of 972 encounters in 962 patients.
Data collection
Each patient chart including labs, imaging, liver biopsies, and clinic notes were analyzed in detail by two independent reviewers to ensure accuracy. In the event of discordant conclusions, a third reviewer (a senior hepatologist) adjudicated. Pertinent information was recorded into a patient database.
Outcomes of interest
The primary outcome was adherence to AASLD guidelines for CHB management in five key areas:
Timely ALT/HBV DNA checks needed to monitor inactive carrier (annual) and immune-tolerant phases (bi-annual). These two phases do not require treatment but need close monitoring. Adherence was defined as lab checks (ALT/HBV DNA) at least every 12 months for inactive carriers, or every 6 months for immune-tolerant patients. To assess adherence to ALT and HBV DNA monitoring, the time since the previous blood test was counted for adherence. Adherence was defined as lab checks (ALT/HBV DNA) performed at least every 12 months for inactive carriers, or every 6 months for immune-tolerant patients; a 3-month grace period (i.e., every 15 months for inactive carriers or 9 months for immune-tolerant patients) was permitted and counted as adherent.
Liver biopsy when indicated according to AASLD guide-lines in order to guide decisions on treatment initiation (see Appendix A1 : Summary of AASLD guidelines according to chronic hepatitis B phases).
Treatment initiation with antiviral therapy when indicated by AASLD guidelines (see Appendix A1).
HCC screening with liver imaging and/or alpha-fetoprotein (AFP) at least every 12 months. Nonadherence was counted as HCC screening with liver imaging or AFP less frequently than every 12 months during the study period; a 3-month grace period (i.e., every 15 months) was permitted and counted as adherent.
Assessment for HAV immunity and for the presence of HIV and HCV co-infection.
For each of the areas above, we also conducted detailed review of clinic notes, labs, and imaging studies to assess patient vs. physician factors in nonadherence. Patient factors were defined as missed appointments, loss to follow-up, and refusal of the proposed intervention. Physician factors were defined as failure to order the recommended lab, biopsy, imaging study, or medication as indicated by guidelines.
Using multivariable logistic regression models, we examined the following three variables as predictors of the five areas in the primary outcome:
Physician types: there were four physician types primarily responsible for managing CHB in our cohort: primary care physicians (PCPs), gastroenterologists (including hepatologists), infectious disease specialists, and “other”, which refers to a mix of obstetrics, oncology, and emergency department physicians. Although these specialties do not typically manage hepatitis B, the physicians in this category were caring for patients who were not under the care of gastroenterology (GI), infectious disease, or PCPs. If patients visited both PCPs and specialists, the responsibility for management was attributed to primary care if the specialist note stated that the patient could be followed by primary care and did not require return for a specialist appointment. For example, many inactive carriers saw a specialist once but were sent back with recommendations for routine monitoring (e.g., labs and HCC screening) to be implemented by primary care. If patients had more than one visit to see a specialist for CHB, then this was considered specialist management rather than primary care.
Patient demographic factors: dichomotized variables of age ≥45 years, gender, White vs. non-White, and English vs. non-English language as a primary language of communication. The age cutoff of 45 years was chosen as an average age because the AASLD HCC screening guidelines state that men from endemic areas > 40 years and women from endemic area > 50 years should be screened.
Phase of CHB infection: see summary table below of five phases (See Appendix A1).
Statistical analysis
We calculated median and interquartile range for continuous variables, frequencies, and proportions (%) for categorical variables. We applied the χ 2-test or Fisher's exact test whenever appropriate to analyze associations between categorical variables. Normally, distributed continuous variables were evaluated by the Student's t -test, and nonparametric continuous variables were evaluated by the Wilcoxon rank sum test. Multivariable logistic regressions, which used variables chosen to adjust for confounding, were performed to assess relationships between physician type, CHB infection phase, and patient demographics associated with each of the five areas of guideline recommendations. The percentage of patients with missing data was small, ranging from 0 to 8%. The analyses were based on complete cases (subjects with no missing data). Significance is defined as a P value less than or equal to 0.01, which reflects the Bonferroni correction to adjust for five outcomes. SAS version 9.2 (SAS Institute, Cary, NC) was used for statistical analyses. This study was approved by the Partners Human Research Committee.
Results
Patient demographic characteristics for the 962 study patients are shown in Table 1. Median age was 45 (interquartile range: 37, 56) years, 43% (415) were female, 75% (732) were non-White, and 20% (198) did not list English as their primary language. The largest proportion of patients, 41% (395), was inactive carriers; the smallest proportion was immune tolerant, 1% (13). Primary responsibility for the management of CHB was provided by gastroenterologists in 65% (630) and PCP in 26% (254). The duration of follow-up differed for each patient. The purpose of the study was to assess the application of guidelines for CHB management during any duration of follow-up. The mean follow-up duration was 3.1 years (s.d., 1.1 years). Any patient who was diagnosed with CHB between 2005 and 2012 was followed up for the duration of their care during this time period.
Table 1. Demographic and clinical characteristics of the study population: total n =972.
| Patient demographics | |||
|---|---|---|---|
| Percent | Number | ||
| Age (median years) | 45 (37, 56) | ||
| Male | 57 | 556 | |
| Female | 43 | 415 | |
| White | 21 | 204 | |
| Non-White | 75 | 732 | |
| English primary language | 74 | 719 | |
| Non-English primary language | 20 | 198 | |
| Phases of hepatitis B infection | |||
| Inactive carrier | 41 | 395 | |
| Immune tolerant | 1 | 13 | |
| Immune active (eAg +) | 14 | 138 | |
| e-Antigen-negative chronic hepatitis B | 16 | 159 | |
| Possible eAg-negative hepatitis (liver biopsy needed) | 15 | 146 | |
| Already on therapy | 11 | 108 | |
| Unclear phase (missing eAg) | 1 | 13 | |
| Type of physician managing hepatitis B | |||
| Gastroenterology/hepatology | 65 | 630 | |
| Primary care | 26 | 254 | |
| Infectious disease | 5 | 49 | |
| Other physician types | 3.9 | 39 |
Timely CHB Lab (ALT/HBV DNA) assessment
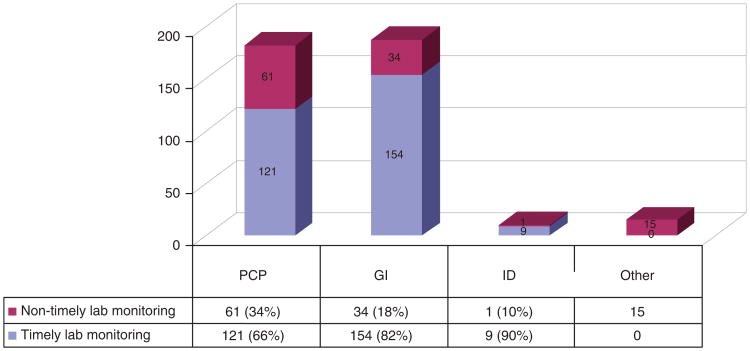
Among 395 inactive carriers, 113 (29%) did not undergo timely, defined as at least annual, CHB lab assessment. Figure 2 shows adherence to this guideline by physician type. Of the 13 immune-tolerant patients in this study who also warranted annual lab monitoring, only 1 did not undergo timely lab checks.
Figure 2.
Adherence to timely alanine aminotransferase (ALT)/hepatitis B virus (HBV) DNA monitoring for inactive carriers by physician type (n = 395).
In multivariable analysis after controlling for patient demographic variables, patients seen by gastroenterologists had more than two times greater odds of undergoing at least annual hepatitis B lab monitoring compared with those seen by PCPs (odds ratio: 2.3, 95% confidence interval: 1.4–3.8, P= 0.001, Supplementary Table S1 online).
Patient demographic variables of age (dichotomized at > or < 45 years), gender, race (white vs. non-White), and language (English vs. non-English) did not predict physician nonadherence to timely ALT assessment.
Liver biopsy nonadherence
A total of 203 (21%) of 972 patients merited liver biopsies as recommended by AASLD guidelines in order to clarify their infection phase and treatment eligibility. Forty percent (n=82) of these biopsy-eligible patients underwent appropriate liver biopsy, but 60% (n=121) did not undergo liver biopsy despite the clinical indication.
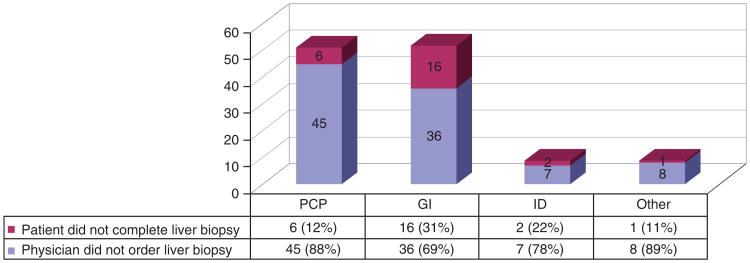
Figure 3 shows that 79% (n = 96) of the 121 missed liver biopsies were due to physician nonadherence and 21% (n = 25) were due to patient factors such as refusal or loss to follow-up. Furthermore, 89% (108 of 121) patients who missed liver biopsies warranted biopsy to further assess possible e-antigen (e-Ag)-negative hepatitis (core promoter/precore mutation), as defined by persistently elevated viral load > 2,000 IU ml−1 with normal or only minimally elevated ALTs. Supplementary Figure S1 online shows that 78% (84 of 108) of these missed biopsies to evaluate possible e-Ag-negative hepatitis were due to physician nonadherence. If moderate inflammation or severe fibrosis had been found on liver biopsy, AASLD guidelines would have recommended treatment initiation. No significant differences in nonadherence were found among different physician types, nor among different patient demographic groups. In all groups, physician nonadherence was higher than patient nonadherence.
Figure 3.
Physician vs. patient nonadherence for missed liver biopsies (n = 121).
Adherence to guidelines for treatment initiation
Supplementary Table S2 online depicts the characteristics of 31% (297 of 972) of patients who were treatment eligible during the study period, excluding those who were already on treatment at the start of the study period. Only three patients who were treatment eligible did not receive treatment. All three were in the e-antigen-positive immune-active phase; two were not treated because of patient refusal, and one because the physician did not offer treatment.
One hundred and twenty-one patients had unclear treatment eligibility since they warranted, but did not undergo, liver biopsy to clarify their disease activity.
HCC screening
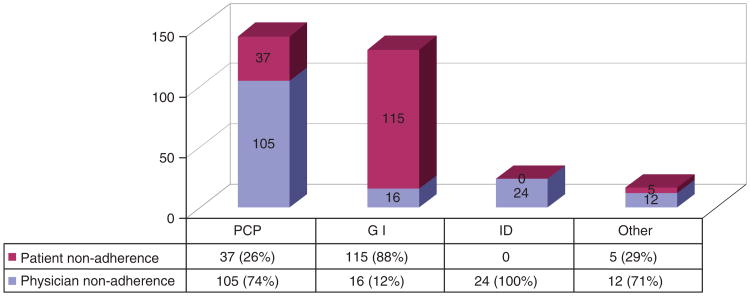
Six hundred and ninety-six patients warranted annual HCC screening by guidelines, but 45% of this group (314) did not undergo timely screening, defined as liver imaging or AFP less frequently than every 12 months during the study period (a 3-month grace period was permitted). Three hundred and fourteen patients fell into this group, of which 71 never had HCC screening during the study period. For the 243 patients who had nontimely HCC screening (but at least one HCC screening), the mean interval of screening was 3.9 years (s.d., 1.4 years).
Figure 4 shows that for non-GI physicians, physician failure to order timely HCC screening was the most common cause of missed HCC screening.
Figure 4.
Nonadherence to hepatocellular carcinoma (HCC) screening by physician type (n = 314).
In the multivariable regression analysis (Table 2a) after controlling for patient demographic factors, GI physicians had significantly greater odds of conducting timely HCC screening compared with all other physician types.
Table 2a. Multivariable analysis of physician type associated with timely HCC screening, controlling for patient demographic factors.
| Physician type | Odds ratio | 95% CI | P value |
|---|---|---|---|
| GI vs. PCP | 6.67 | 4.5–9.7 | < 0.0001 |
| GI vs. ID | 3.69 | 1.8–7.6 | 0.0005 |
| GI vs. other | 5.57 | 2.5–12.6 | < 0.0001 |
| ID vs. other | 1.56 | 0.5–4.3 | 0.48 |
| ID vs. PCP | 1.83 | 0.8–3.8 | 0.13 |
| Other vs. PCP | 1.25 | 0.5–2.7 | 0.67 |
CI, confidence interval; GI, gastroenterology; ID, infectious disease; PCP, primary care physician.
Supplementary Table S3 online shows the multivariable analysis of the relationship between patient demographic factors and HCC screening, controlling for physician type. Patients aged 45 years or more had seven times greater odds than younger patients who were HCC screening eligible to have timely screening (odds ratio: 7.46, 95% confidence interval: 5–11, P<0.0001).
Table 2b shows the multivariable analysis of the relationship between CHB infection phase and missed HCC screening, controlling for patient demographic and physician factors. Inactive carriers had significantly greater odds compared with immune-active patients and eAg-negative hepatitis patients of missing HCC screening; patients with possible e-Ag-negative hepatitis had significantly greater odds compared with definitive immune-active patients and e-Ag-negative hepatitis patients of missing HCC screening.
Table 2b. Multivariable analysis of CHB phase associated with non-timely HCC screening.
| CHB phase | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Inactive carriers vs. immune active patients | 2.26 | 1.4–3.8 | 0.002 |
| Inactive carriers vs. eAg-negative hepatitis | 3.28 | 2.0–5.4 | < 0.0001 |
| Inactive carriers vs. possible eAg-negative hepatitis | 1.01 | 0.6–1.6 | 0.96 |
| Possible eAg-negative hepatitis vs. immune active patients | 2.24 | 1.2–4.1 | 0.01 |
| Possible eAg-negative hepatitis vs. e-Ag-negative hepatitis | 3.25 | 1.8–5.9 | < 0.0001 |
| Immune active vs. eAg-negative hepatitis | 1.45 | 0.8–2.7 | 0.25 |
CHB, chronic hepatitis B; CI, confidence interval; HCC, hepatocellular carcinoma.
Assessment for HAV immunity and HBV, HIV co-infection
Supplementary Figures S2A–C online demonstrate adherence to co-infection testing guidelines by physician type. A total of 35% (345) patients were not tested for hepatitis A immunity, 24% (n = 230) were not tested for hepatitis C, and 54% (n = 529) were not tested for HIV co-infection.
In multivariable analysis controlling for patient demographic factors (Table 3), PCPs, GI, and infectious disease physicians all had higher odds of testing for HAV immunity compared with other (i.e., obstetrics, oncology, and so on) physician types. Infectious disease physicians had significantly higher odds of testing for HCV co-infection than PCPs or GI physicians. Infectious disease physicians also had the highest odds of testing for HIV co-infection compared with the other three physician types.
Table 3. Multivariable analysis of physician type associated with testing for co-infections, controlling for patient demographic factors.
| Physician type | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Testing for HAV | |||
| GI vs. other | 3.40 | 1.6–7.1 | 0.003 |
| PCP vs. other | 3.27 | 1.5–7.0 | 0.003 |
| ID vs. other | 6.45 | 2.3–18.2 | 0.003 |
| GI vs. PCP | 1.04 | 0.8–1.4 | 0.56 |
| ID vs. PCP | 1.97 | 0.9–4.4 | 0.78 |
| ID vs. GI | 1.90 | 0.9–4.1 | 0.23 |
| Testing for HCV | |||
| ID vs. PCP | 3.70 | 1.1–12.8 | < 0.0001 |
| ID vs. GI | 4.40 | 1.3–14.4, | < 0.0001 |
| ID vs. other | 3.66 | 0.9–15.4 | 0.77 |
| Other vs. PCP | 1.02 | 0.4–2.4 | 0.55 |
| Other vs. GI | 1.19 | 0.5–2.7 | 0.96 |
| PCP vs. GI | 1.16 | 0.8–1.7 | 0.67 |
| Testing for HIV | |||
| ID vs. PCP | 16.39 | 4.9–55.0 | < 0.0001 |
| ID vs. GI | 18.92 | 5.8–62.5 | < 0.0001 |
| ID vs. other | 10.51 | 2.6–41.7 | < 0.0001 |
| Other vs. PCP | 1.56 | 0.7–3.3 | 0.84 |
| Other vs. GI | 1.80 | 0.9–3.7 | 0.26 |
| PCP vs. GI | 1.16 | 0.8–1.6 | 0.35 |
CI, confidence interval; GI, gastroenterology; HAV, hepatitis A virus; HCV, hepatitis C virus; PCP, primary care physician.
Supplementary Figures S3A–C online show adherence to co-infection testing by patient demographic factors of age, gender, race, and language.
In the multivariable model (Supplementary Table S4 online) controlling for physician type, patients younger than 45 years had two times higher odds of being tested for HIV than older patients (odds ratio: 2.05, 95% confidence interval: 1.5–2.8, P < 0.0001). No significant associations were found for HAV or HCV testing.
Discussion
The results of this study demonstrate poor adherence to five principal areas of CHB management delineated by AASLD guidelines. No previous studies have so comprehensively examined adherence to hepatitis B management guidelines, particularly assessment of patient and physician barriers to adherence, in a large cohort of nearly 1,000 patients at a large academic medical center. We believe that the findings in our study are generalizable to other urban tertiary medical centers in the United States. Massachusetts General Hospital is located in an urban center with 12 community satellite clinics, and therefore it has a diverse population mix in terms of ethnicity and socioeconomic background.
This is the first study to examine the rate of adherence to AASLD liver biopsy guidelines in CHB management. Our study found a remarkably high rate of missed liver biopsies (i.e., guideline-recommended biopsies that were not performed), most of which were due to physician nonadherence across all physician types, including gastroenterologists. There are multiple barriers to liver biopsy in clinical practice, and many clinicians have understandable justifications for not ordering liver biopsy. For example, physicians may be hesitant to recommend liver biopsy owing to its risk for complications such as intra-abdominal bleeding. Patients may also decline biopsy for the same reasons. As new, noninvasive tests for liver fibrosis become validated in CHB, we anticipate that the rates of assessment for liver fibrosis using these noninvasive tests will grow. Our study finding underscores the difficulty in following through on idealized guidelines. This finding may also highlight the knowledge gap about liver biopsy indications that should be the focus of continuing medical education for physicians. Nearly 90% of these missed biopsies could have elucidated cases of possible e-Ag-negative hepatitis (core promoter/precore mutation), as defined by persistently elevated viral load > 2,000 IU ml −1 but with normal or only mildly elevated ALTs. In HBeAg-negative patients, an HBV DNA > 20,000 IU ml−1 was chosen as a cutoff for initiating therapy based on non-histology-based natural history studies that revealed a high rate of cirrhosis development (9), but later histology-based studies showed that HBV DNA > 2,000 IU ml−1 is associated with high rates of fibrosis (10,11). Several studies have found that HBV-infected patients with even normal (19 Ul−1 for women, 30 Ul−1 for men) and/or mildly elevated ALT values may have significant histologic disease and can be at an increased risk of mortality from liver disease, especially those above the age of 40 years (12). A recent study by Sanai et al. (13) performed on 366 patients with liver biopsies to assess the accuracy of guideline-defined thresholds in identifying patients with > F2 fibrosis showed an unacceptably high miss rate of one-half of all cases of significant fibrosis. A meta-analysis of studies of liver histology in HBeAg-negative patients with normal or minimally elevated ALT and HBV DNA >2,000 IU ml−1 showed that 8% had moderate or severe fibrosis (14).
The treatment initiation rate was excellent in our study, which is consistent with another study showing a treatment rate of 72% for treatment-eligible patients (7) as defined by the AASLD guidelines. However, this high treatment rate is misleading because 121 patients needed further evaluation with liver biopsy to determine treatment eligibility; they represent potential missed opportunity for treatment, which could have prevented morbidity and mortality from cirrhosis and HCC. A retrospective analysis by Tong et al. (4) found that 30–53% of patients who later developed HCC or died from cirrhosis were not found to be eligible for treatment on the basis of AASLD guidelines for immune-active e-Ag-positive and -negative patients without liver biopsy. This potential under-treatment of patients is supported by a recent study estimating that only 10–15% of potentially eligible individuals receive treatment (1). A cost-effectiveness study showed that if the number of patients eligible for antiviral therapy could be increased from 4 to 15% through effective screening programs and appropriate evaluation for treatment, the cost of medical intervention could save up to $ 82,000 USD per quality-adjusted life year (15). Another study of an uninsured immigrant population in California showed a 16% treatment rate, but this was a mostly uninsured group of patients (16).
No previous study has assessed adherence to testing for hepatitis A immunity, hepatitis C, or HIV co-infections. One-third of the patients were not tested for hepatitis A immunity, which could represent missed opportunities to provide vaccine protection, which could lead to serious clinical consequences. For example, one study found that 11 of 20 patients with acute hepatitis A and CHB developed fulminant or submassive liver necrosis, whereas 100 patients with acute hepatitis A and no underlying chronic liver disease recovered fully (17). Compared with infectious disease physicians, gastroenterologists were often remiss in their adherence to testing for HCV and HIV co-infection.
Adherence to other guideline recommendations was also sub-optimal. Almost one-third of inactive carriers in our study did not have monitoring of ALT at least every 12 months, although gastroenterologists were more likely to perform timely monitoring than PCPs. This finding is consistent with a large retrospective study assessing adherence to AASLD CHB laboratory monitoring guidelines, which found that ∼ 50% of patients failed to undergo timely ALT assessment (5).
Almost half of the patients in the cohort who required HCC screening did not undergo imaging or AFP at least annually; nonadherence was largely due to physician failure to order HCC screening rather than patient nonadherence, except in the case of GI specialists who had the highest odds of conducting timely HCC screening. Our findings are consistent with another study of gastroenterologist screening practices for CHB patients, which showed that only 60% performed at least annual HCC screening (6). A survey study of 459 physicians (18) found that physicians with a higher knowledge of HBV management and the use of HCC screening as a quality measure increased the odds of HCC screening. Another study surveying 109 physicians about self-reported HCC screening found that one-third of providers surveyed were not familiar with AASLD guidelines endorsing surveillance (19). Clearly, targeted physician education is needed to address these knowledge gaps to improve HCC screening rates. In our study, inactive carriers had significantly greater odds compared with immune-active eAg-positive patients and eAg-negative hepatitis patients of missing HCC screening. One possible explanation is that the latter two groups have more alarming ALT and viral load levels compared with inactive carriers, which captures physician attention to liver disease and HCC screening. Ours is the first study to examine the relationship between HBV infection phase and HCC screening.
Our study highlights important physician nonadherence to guidelines, especially in the areas of liver biopsy and HCC screening. With the increased nationwide implementation of electronic medical records, decision support with the use of automatic reminders for HCC screening and flags for consideration of liver biopsy when viral load is > 2,000 IU ml−1 may greatly improve the quality of care. In this regard, a recent study by Mayorga et al. demonstrated a significant improvement in guideline adherence for the management of variceal bleed in cirrhotics through the use of an electronic order set (20). Through the use of an electronic order set for decision support, the rate of antibiotic administration and time to antibiotic/octreotide administration were both significantly improved.
Our chart reviews of clinic notes highlighted several types of patient barriers to guideline adherence, including reluctance to take long-term medication owing to fear of side effects, costs associated with treatment, fear of stigmatization, and patient loss to follow-up, findings that are supported by multiple other studies (21–23). A qualitative study conducted in Singapore on barriers to managing chronic inactive carriers with hepatitis B (24) found poor patient compliance with follow-up owing to the asymptomatic nature of the illness and poor understanding of the disease. In this context, long-term benefits were intangible, whereas short-term costs of clinic visits and physical discomfort (such as lab draws) were more concrete deterrents to regular follow-up. Zhang et al. (7) also found that patient loss to follow-up and patient refusal of medication were main explanations for nontreatment of those who were treatment eligible. Other studies have cited cultural and language barriers, lack of access to medical care owing to lack of insurance, and difficulty navigating the US health-care system as important patient barriers (22,25).
Several additional notable deficiencies emerged during the detailed review of clinic notes. Clear ownership of the management of inactive carriers emerged as an important theme. For example, inactive carriers were of en seen by the gastroenterologist once, and were then sent back to the PCP for monitoring with labs and imaging. However, PCPs still deferred monitoring to the specialist, and thus ultimately no physician was monitoring the patient. Not all gastroenterologists are consistent in this practice; some will manage inactive carriers with annual visits and some will refer back to the PCP after the initial consult, especially if the patient does not reside locally. Perhaps, one solution is that PCPs should always assess viral load, ALT, AFP, and abdominal ultrasonography during routine visits if records do not disclose recent studies in the system. We also found that PCP clinic notes would sometimes indicate referral to a GI specialist, but without actual subsequent clinic visits. Further chart investigation revealed missing referral orders, or that patients would miss specialist appointment because there was significant delay between referral and actual appointment date. The solutions to these system issues may be unique to each institution, but identification of these barriers is the first step to addressing them.
There are several limitations to the current study. The analysis of patient and physician factors for nonadherence to guidelines was performed retrospectively, which sometimes limited details about why a patient was lost to follow-up or declined treatment. For example, information on alcohol and drug abuse was often not found in the clinic notes, and this could be an under-reported patient barrier to guideline adherence. A prospective study would provide more detailed information on patient adherence barriers.
Conclusion
In this large cohort of CHB patients attending clinics affiliated with a large academic medical center, adherence to AASLD guide-lines for CHB management is poor, particularly with respect to liver biopsy to assess for possible e-Ag-negative chronic hepatitis, timely HCC and ALT monitoring, and testing for HAV immunity and HCV, HIV co-infection. Further efforts to improve education of providers regarding the tenets of optimal HBV management, incorporation of decision support tools in the age of electronic medical records, and improved communication between providers regarding the status of shared patients are essential for improvement of overall outcomes for CHB.
Supplementary Material
Study Highlights.
What is Current Knowledge
There is poor adherence to American Association for the Study of Liver Disease (AASLD) guidelines in three areas of chronic hepatitis B (CHB) management: monitoring alanine aminotransferase (ALT) in inactive carriers, hepatocellular carcinoma (HCC) screening, and treatment.
What is New Here
-
First study to assess adherence to AASLD liver biopsy guidelines in CHB management.
Sixty percent of patients did not undergo clinically indicated liver biopsies, largely owing to physician nonadherence. Eighty-nine percent of these missed biopsies were warranted to further assess possible e-antigen (e-Ag)-negative CHB.
A high treatment initiation rate was found for the treatment eligible, but 121 patients had unclear treatment eligibility since they warranted, but did not undergo, liver biopsy. A total of 45% did not undergo timely HCC screening, although gastroenterology (GI) physicians had the highest odds of adherence.
Twenty-nine percent of inactive carriers did not undergo timely CHB lab assessment.
Patients seen by gastroenterologists were twice as likely compared with primary care physicians (PCPs) to undergo timely lab monitoring.
First study to assess adherence to co-infection testing.
Thirty-five, 24, and 54% were not tested for hepatitis A, hepatitis C, and HIV co-infections.
Acknowledgments
Financial support: Financial assistance was provided by Bristol Myers Squibb Virology Fellowship; and NIH DK007191 (Ying Wu, Kara B. Johnson) DK078772 (Raymond T. Chung).
Appendix A1
Table A1. Summary of AASLD guidelines according to chronic hepatitis B phases.
| Immune tolerant | Immune active (e-antigen positive) | Inactive carrier | e-Antigen-negative chronic hepatitis: (core promoter/precore mutation) | Possible e-antigen-negative hepatitisa | |
|---|---|---|---|---|---|
| HBeAg status | + | + | - | - | - |
| HBV DNA | ≥20,000 IU ml−1 | ≥20,000 IU ml−1 | HBV DNA < 2,000 IU ml−1 | HBV DNA > 20,000 IU ml−1 | > 2,000 IU ml−1 |
| ALT | Normal (≤ 19 Ul−1 for women, ≤ 30 Ul−1 for men) | ≥2×ULN (38 Ul−1 for women, 60 Ul−1 for men) | Normal (≥ 19 Ul−1 for women, ≥ 30 Ul−1 for men) | ≥2×ULN (38 UI−1 for women, 60 Ul−1 for men) | ALT < 2×ULN (38 Ul−1 for women, 60 Ul−1 for men) |
| AASLD recommendation | No treatment; check ALT q6 mo. If ALT becomes elevated, check HBV DNA and ALT q3 mo. Liver biopsy if persistent elevations in ALT or viral load or patient's age > 40 (distinguish immune tolerant vs. immune active). nitiate treatment if biopsy shows moderate/severe inflammation or significant fibrosis. | Treat until 6 months after HBeAg + seroconversion. Liver biopsy before tx is optional. | No treatment; check ALT q12 mo. If ALT becomes elevated, check HBV DNA and ALT q3 mo. Liver biopsy if persistent elevations in ALT or viral load (distinguish inactive carrier and e-antigen-negative chronic hepatitis B). Initiate treatment if biopsy shows moderate/severe inflammation or significant fibrosis | Perform liver biopsy if persistent elevations in ALT or HBV DNA: distinguish between inactive carrier and e-antigen-negative CHB. Initiate treatment if biopsy shows moderate/severe inflammation or significant fibrosis | |
| AASLD HCC Screening Guidelines for all phases | |||||
The following types of patients should be screened with ultrasound at least every 12 months. Alpha-fetoprotein can be used if ultrasound is not available.
| |||||
AASLD, American Association for the Study of Liver Disease; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCC, hepatitis C virus.
Phase defined by authors.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662.
Footnotes
Supplementary Material is linked to the online version of the paper at http://www.nature.com/ajg
Guarantor of the article: Ying Wu, MD, MSc.
Specific author contributions: Study conception, study design, data collection, data interpretation, manuscript preparation, and revision: Ying Wu; data collection, data interpretation, manuscript preparation, and revision: Kara B. Johnson; data collection, data interpretation, and manuscript preparation: Giorgio Roccaro; data analysis: Hui Zheng; data collection and data interpretation: Joanna Lopez; data collection and data interpretation: Nneka Ufere, Omar Kattanstudy design, manuscript preparation, and manuscript revision: Raymond T. Chung.
Conflict of Interest: Potential competing interests: None.
References
- 1.Cohen C, Holmberg SD, McMahon BJ, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18:377–83. doi: 10.1111/j.1365-2893.2010.01401.x. [DOI] [PubMed] [Google Scholar]
- 2.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. The National Academies Press; Washington, DC: 2010. [PubMed] [Google Scholar]
- 4.Tong MJ, Hsu L, Chang PW, et al. Evaluation of current treatment recommendations for chronic hepatitis B: a 2011 update. J Gastroenterol Hepatol. 2011;26:829–35. doi: 10.1111/j.1440-1746.2011.06623.x. [DOI] [PubMed] [Google Scholar]
- 5.Juday T, Tang H, Harris M, et al. Adherence to chronic hepatitis B treatment guideline recommendations for laboratory monitoring of patients who are not receiving antiviral treatment. J Gen Intern Med. 2011;26:239–44. doi: 10.1007/s11606-010-1549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepato-cellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54:2712–21. doi: 10.1007/s10620-009-1015-x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Ristau JT, Trinh HN, et al. Undertreatment of Asian chronic hepatitis B patients on the basis of standard guidelines: a community-based study. Dig Dis Sci. 2012;57:1373–83. doi: 10.1007/s10620-012-2137-0. [DOI] [PubMed] [Google Scholar]
- 8.Nalichowski R, Keogh D, Chueh HC, et al. Calculating the benefits of a research patient data repository. AMIA Annu Symp Proc. 2006;1044 [PMC free article] [PubMed] [Google Scholar]
- 9.Iloeje UH, Yang HI, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Gastroenterology. 2006;130:678–86. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Sanai FM, Helmy A, Bzeizi KI, et al. Discriminant value of serum HBV DNA levels as predictors of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2011;18:e217–25. doi: 10.1111/j.1365-2893.2011.01437.x. [DOI] [PubMed] [Google Scholar]
- 11.Papatheodoridis GV, Manesis EK, Manolakopoulos S, et al. Is there a meaningful serum hepatitis B virus DNA cutoff level for therapeutic decisions in hepatitis B e antigen-negative chronic hepatitis B virus infection? Hepatology. 2008;48:1451–9. doi: 10.1002/hep.22518. [DOI] [PubMed] [Google Scholar]
- 12.Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376–84. doi: 10.1053/j.gastro.2008.02.075. [DOI] [PubMed] [Google Scholar]
- 13.Sanai FM, Babatin MA, Bzeizi KI, et al. Accuracy of international guidelines for identifying significant fibrosis in hepatitis B e antigen-negative patients with chronic hepatitis. Clin Gastroenterol Hepatol. 2013;11:1493–1499. e2. doi: 10.1016/j.cgh.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Papatheodoridis GV, Manolakopoulos S, Liaw YF, et al. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196–202. doi: 10.1016/j.jhep.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuijzen IK, Toy M, Hahné SJ, et al. Screening and early treatment of migrants for chronic hepatitis B virus infection is cost-effective. Gastroenterology. 2010;138:522–30. doi: 10.1053/j.gastro.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 16.Jung CW, Tan J, Tan N, et al. Evidence for the insufficient evaluation and undertreatment of chronic hepatitis B infection in a predominantly low-income and immigrant population. J Gastroenterol Hepatol. 2010;25:369–75. doi: 10.1111/j.1440-1746.2009.06023.x. [DOI] [PubMed] [Google Scholar]
- 17.Pramoolsinsap C, Poovorawan Y, Hirsch P, et al. Acute, hepatitis-A super-infection in HBV carriers, or chronic liver disease related to HBV or HCV. Ann Trop Med Parasitol. 1999;93:745–51. doi: 10.1080/00034989958005. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen TT, Gildengorin G, Truong A, et al. Factors influencing physicians' screening behavior for liver cancer among high-risk patients. J Gen Intern Med. 2007;22:523–6. doi: 10.1007/s11606-007-0128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56:1516–23. doi: 10.1007/s10620-010-1439-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayorga CA, Rockey DC. Clinical utility of a standardized electronic order set for the management of acute upper gastrointestinal hemorrhage in patients with cirrhosis. Clin Gastroenterol Hepatol. 2013;11:1342–8. doi: 10.1016/j.cgh.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Malespin M, Wong S, Siqueira F, et al. Barriers to treatment of hepatitis B in an urban Chinatown community. J Clin Gastroenterol. 2012;46:e66–70. doi: 10.1097/MCG.0b013e31824e159c. [DOI] [PubMed] [Google Scholar]
- 22.Wiegand J, van Bömmel F, Berg T. Management of chronic hepatitis B: status and challenges beyond treatment guidelines. Semin Liver Dis. 2010;30:361–77. doi: 10.1055/s-0030-1267537. [DOI] [PubMed] [Google Scholar]
- 23.Hu KQ. Hepatitis B virus (HBV) infection in Asian and Pacific Islander Americans (APIAs): how can we do better for this special population? Am J Gastroenterol. 2008;103:1824–33. doi: 10.1111/j.1572-0241.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 24.Tan NC, Cheah SL. What barriers do primary care physicians face in the management of patients with chronic hepatitis B infection in primary care? Singapore Med J. 2005;46:333–9. [PubMed] [Google Scholar]
- 25.Hwang JP, Roundtree AK, Engebretson JC, et al. Medical care of hepatitis B among Asian American populations: perspectives from three provider groups. J Gen Intern Med. 2010;25:220–7. doi: 10.1007/s11606-009-1204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.