Abstract
Huntington’s disease (HD) is a prototypical dominantly inherited neurodegenerative disorder which is characterized by progressive cognitive deterioration, psychiatric disturbances and a movement disorder. The genetic cause of the illness is a CAG repeat expansion in the huntingtin gene, which leads to a polyglutamine expansion in the huntingtin protein. The exact mechanism by which mutant huntingtin causes HD is unknown, but it causes abnormalities in gene transcription, and both mitochondrial dysfunction and oxidative damage. Since the penetrance of HD is complete with CAG repeats greater than 39, patients can be diagnosed well before disease onset with genetic testing. Longitudinal studies of HD patients before disease onset have shown that subtle cognitive and motor deficits occur as much as 10 years before onset, as do reductions in glucose utilization and striatal atrophy. An increase in inflammation, as shown by elevated IL-6, occurs about 15 years before onset. Detection of these abnormalities may be useful in defining an optimal time for disease intervention to try to slow or halt the degenerative process. Although reducing gene expression with siRNA or shRNA is an attractive approach, other approaches targeting energy metabolism, inflammation and oxidative damage may be more easily and rapidly moved into the clinic. The recent PREQUEL study of coenzyme Q10 in presymptomatic gene carriers showed the feasibility of carrying out clinical trials to slow or halt the onset of HD. We review both the earliest detectable clinical and laboratory manifestations of HD, as well as potential neuroprotective therapies which could be utilized in presymptomatic HD.
Keywords: Presymptomatic Huntington’s disease, Coenzyme Q10, Bezafibrate, PGC-1alpha, SIRT1
Introduction
Huntington’s disease (HD) is a prototypical neurodegenerative disease which leads to progressive cognitive and physical deterioration.1 The illness is caused by a CAG repeat expansion which encodes polyglutamine. Progressive transcriptional dysregulation occurs in both the striatum and the cerebral cortex, leading to neuronal dysfunction and death. Another feature of the illness is mitochondrial dysfunction and an impairment of oxidative metabolism.2, 3 Since the disease is autosomal dominant with complete penetrance above a CAG repeat of 39, it can be reliably diagnosed in its presymptomatic phase. This raises the possibility of carrying out clinical trials in presymptomatic gene positive individuals, in order to delay or halt the neurodegenerative process. The feasibility of this was recently shown in the PREQUEL phase 2 study of coenzyme Q. It is therefore important to be able to detect the illness in its earliest phase before clinical diagnosis. We review both the earliest clinical, imaging and biochemical changes which occur in presymptomatic gene positive patients, as well as a number of promising therapies which could be utilized for treatment, with a particular emphasis on halting the mitochondrial dysfunction and oxidative damage.
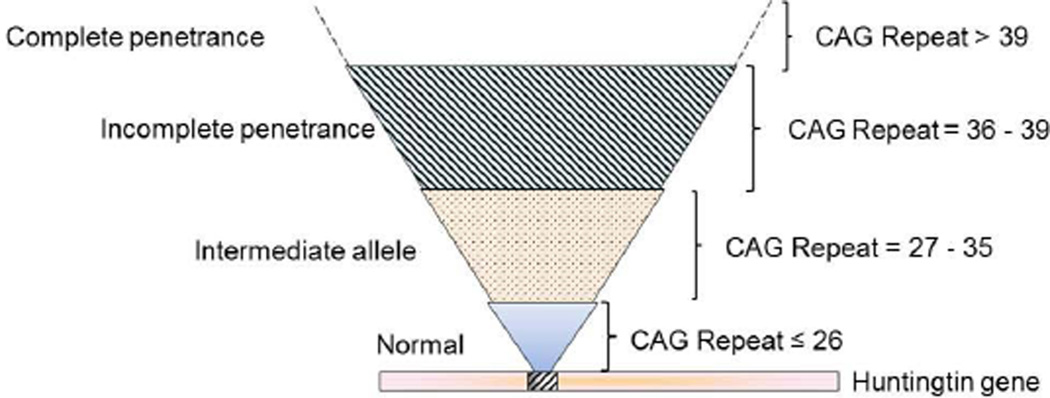
In Western countries, it is estimated that about five to seven people per 100,000 are affected by HD. The number of CAG repeats in the HTT gene is the main predictor of the age of disease-onset. Patients with HD have CAG repeat lengths above 36, with variable penetrance of repeat lengths 36–39 and complete penetrance with 40 or more repeats; longer repeat lengths (>60) have been associated with juvenile-onset HD.1 Having up to 26 CAG copies is considered normal, whereas CAG expansions in the 27–35 range are referred to as intermediate alleles (IA) (Figure 1).4 This range confers meiotic instability and can expand into the HD range when passed to subsequent generations, especially when inherited from the father.5 Individuals at risk of inheriting the expanded CAG nucleotide can be identified before clinical onset by predictive genetic testing.6 Although the length of CAG repeats can be used for predicting age of onset, the CAG repeat length seems to contribute less to the rate of progression, and understanding the determinants of rate of progression, could help in finding targets for therapeutic intervention.7
Figure 1.
CAG repeat size and penetrance of HD
HD patients develop progressive motor impairment, gradual cognitive decline, and psychological disturbances, and the patient eventually becomes completely dependent on others for daily functioning. Metabolic abnormalities such as wasting and altered energy expenditure are increasingly recognized as clinical hallmarks of the disease. Pathologically, there is a preferential and progressive loss of the medium spiny neurons (MSNs) in the striatum, as well as cortical pyramidal neurons, and degeneration of other brain regions later in the disease. There is progressive disability, and death usually occurs 15 to 20 years after onset. Individuals with HD frequently die due to secondary complications such as choking, infection, or heart failure. There are no currently available treatments to delay disease onset or retard its progression, and the focus of medical care is limited to symptom management and maximizing function. Transcriptional dysregulation, protein aggregation, mitochondrial dysfunction, and enhanced oxidative stress have been implicated in the disease pathogenesis. A key feature of HD patients is pronounced weight loss, despite sustained caloric intake and deficits in energy expenditure have been linked with mitochondrial dysfunction in HD.2, 8–10
Despite great progress since the identification of the gene mutation, the critical pathway by which the HD gene mutation leads to neuronal dysfunction and death has not yet been established. The function of normal huntingtin protein has also not been fully elucidated, but it is known to be associated with synaptic vesicles and microtubules, and it plays a critical role in nerve cell function, transcriptional regulation, mRNA processing, and as an essential scaffold protein regulating axonal transport of vesicles including brain-derived neurotrophic factor (BDNF).11–16 The huntingtin protein plays a role in activating the glycolytic enzyme GAPDH, to supply energy from glycolysis for fast axonal transport of vesicles.17 Both gain-of-function (for mutant huntingtin) and loss-of-function (for normal huntingtin) may contribute to HD pathogenesis.
Research involving presymptomatic testing and identification of a biomarker for detection of disease onset and progression, and for monitoring treatment effects has garnered special attention in recent years. HD studies typically rely on the United Huntington’s Disease Rating Scale (UHDRS) for outcome measures, which includes a battery of tests that evaluate motor skills, cognition, behavior, activities of daily living and independence. This assessment tool however may be insensitive in detecting the earliest stages of the illness. Researchers over the past several years have emphasized the need to reliably identify subtle changes that can be measured in the presymptomatic stages of the disease. We below briefly discuss aspects of presymptomatic testing, and therapies being developed for neuroprotection.
Presymptomatic testing
The first genetic marker for HD was identified in 1983, and after a decade’s effort, scientists finally isolated the HD gene in 1993. This discovery made genetic testing possible, which involves screening of a small sample of blood to detect the presence or absence of the HD mutation in leukocytes. Presymptomatic testing is available for at risk individuals who have a family history of HD, but as yet no symptoms themselves. The testing involves a neurological examination, pretest genetic and psychologic counseling, and follow up. The purpose of the neurological examination is to determine whether or not the person requesting testing is showing any clinical symptoms of HD. Pretest counseling informs the individual about the manifestations of HD, the level of risk, and about the testing procedure (including the test's limitations, the accuracy of the test, and possible outcomes). Despite the availability of genetic testing, most patients prefer not to have it, which is understandable since no proven neuroprotective treatment is presently available. Only about 5% to 20% of people at risk for the disease request testing when approached by registries or testing centers.18, 19
Identification of the Earliest Subtle Physiological Changes during Prodromal period
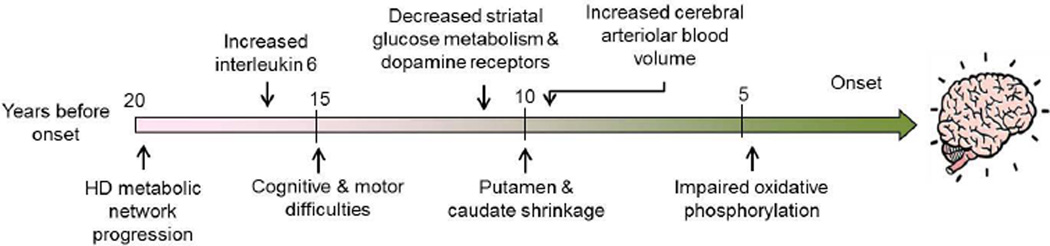
It is important to be able to identify early biomarkers for HD since they may identify an optimal time for therapeutic interventions. It may be necessary to initiate neuroprotective therapies during the prodromal period in the very earliest phases of HD, before clinical onset. Starting approximately fifteen years before the onset of motor symptoms subtle brain changes may occur as well as some subtle cognitive difficulties and difficulties with motor function (Figure 2).20–22 It is known that regional brain atrophy, such as reductions of putaminal volume and caudate shrinkage occur 10 years or more before the onset of clinically-diagnosable HD.20–27 Blood markers such as IL-6 levels are increased as many as 16 years before clinical diagnosis,28 and it correlates with disease progression.29 The chemokines eotaxin-3, MIP-1β and eotaxin also show linear increases in plasma with disease progression.30 Plasma 24S-hydroxycholesterol is reduced in HD plasma, but does not worsen with disease progression.31 The H2A histone family member Y (H2AFY) was shown to be a potential HD blood biomarker which is associated with disease activity, and showed a drug response to a histone deacetylase (HDAC) inhibitor.32
Figure 2.
Physiological Changes during Prodromal period
Using time-resolved Förster resonance energy transfer (TR-FRET) immunoassay, Weiss et al. (2012) found that mHtt is present in the peripheral immune system, and that mean mHtt levels in monocytes, T cells, and B cells are significantly increased in premanifest mutation carriers as compared to controls.33 The levels are also significantly different in premanifest gene carriers as compared to those with clinical onset. The monocyte and T cell mHtt levels were significantly associated with disease burden scores and caudate atrophy rates in patients with manifest HD, indicating that the mHtt levels track HD progression. Furthermore, there are a number of studies which have looked at both cerebral glucose metabolism and cerebral blood flow in the prodromal period of HD.23, 34–36 Both, the striatal glucose metabolism as well as dopamine D2 receptor binding, are reduced in presymptomatic HD gene carriers.34, 36, 37
Recently, Tang et al. (2013) used a computational approach to identify a functional brain network associated with the progression of preclinical HD.38 They studied 2 longitudinal cohorts of premanifest HD mutation carriers using [18F]-fluorodeoxyglucose PET to measure cerebral metabolic activity, and [11C]-raclopride PET and structural MRI, to measure concurrent declines in caudate/putamen D2 neuroreceptor binding and tissue volume. They reported that amongst the various imaging markers analyzed, metabolic network activity was the most sensitive to disease progression, as demonstrated by its rapid rate of progression and high expression at the time of phenoconversion. Modeling suggested that metabolic network progression begins 19–20 years before phenoconversion, anteceding the basal ganglia volume-loss pattern by 3 years, and continuing at a constant rate in the remaining preclinical years, and in the period following phenoconversion.38
Hua et al. (2013) examined arteriolar cerebral blood volume (CBVa) using an inflo-based vascular-space-occupancy (iVASO) technique, which is a noninvasive magnetic resonance imaging method at ultrahigh field (7T). They investigated alterations in CBVa in 7 prodromal HD subjects with an estimated years-to-onset of 8.3 + 3.8 years. The subjects showed some subtle motor abnormalities but no cerebral or striatal atrophy. Interestingly the CBVa was significantly greater in prodromal HD patients in the frontal, and parietal and temporal cortex, with relative differences from controls of 30–50%. Significant correlations were observed between gray matter CBVa in the frontal cortex and the CAG Age Product (CAP) score and estimated years-to-onset.39 In transgenic mouse models of HD, the total CBV in cerebral cortex and striatum was also increased.40–43
These findings show that both blood flow and metabolic abnormalities occur presymptomatically. The increase in CBV is unexpected since there are reductions in glucose metabolism in both the striatum and the cerebral cortex in presymptomatic HD subjects.34, 36, 37 There, however, may be alterations in both CBV and glucose metabolism which occur over time. There are reports of increased glucose metabolism in some areas of cerebral cortex in HD patients, and it is possible that there is an initial increase in glucose metabolism preceding the reductions in glucose uptake.44 We carried out studies of glucose utilization in several HD transgenic mouse models including N171-82Q and HdhQ111 knockin transgenic mice. We observed there was an initial increase in glucose utilization in both the cerebral cortex and striatum, followed by a reduced glucose utilization which occurred over time (Browne and Beal, unpublished findings). This may be reflective of impaired oxidative phosphorylation, which is initially compensated by an increase in glucose uptake and glycolysis, but then is followed by neuronal impairment and reduced glucose metabolism as the disease progresses.
There is also evidence that there are reduced high-energy phosphates in response to an exercise stimulus in muscle in both presymptomatic and symptomatic HD subjects.45 One prior study examined cerebral blood flow and oxygen extraction as well as glucose utilization, and showed that there was an increase in the relative glucose utilization in HD patients, consistent with an increase in glycolysis.37 This observation however could reflect the reduced numbers of mitochondria in striatal spiny neurons that we documented in both HD postmortem brain tissue and transgenic mice.46, 47 Reduced numbers of mitochondria may require increased glucose uptake and metabolism as a compensation to maintain cellular energy levels and synaptic activity. We previously reported increased lactate in cerebral cortex of HD patients that occurred presymptomatically.48
The increased arteriolar CBV observed in the prodromal HD subjects may also reflect an increase in blood flow and glucose delivery in order to compensate for a defect in oxidative phosphorylation, with an increase in glycolysis.39 It would be, therefore, of great interest to determine the time course of the alterations in CBV which occur in the prodromal HD subjects, and whether the CBVa eventually returns to baseline, and then becomes reduced with disease progression.
PREDICT-HD and TRACK-HD
Presymptomatic patients with HD show reduced levels of striatal dopamine receptors, and abnormal metabolites in nuclear magnetic resonance spectroscopy several years before the presence of the clinical findings.49–53 Currently, two large studies: the PREDICT-HD and the TRACK-HD studies are utilizing multicenter observations to identify a panel of biomarkers that could be used as efficacy end points in future trials.21, 22, 54, 55 In PREDICT-HD, researchers are following hundreds of US and Australian prodromal participants with detailed imaging, cognitive, blood, and other measures. Motor abnormalities, and anatomical and behavioral changes preceding clinical onset have been reported in this study.56–58 Paulsen et al. (2013) recently reported the analysis of data collected over a span of 10 years from more than 1000 PREDICT-HD participants with prodromal HD.54 They identified a number of cognitive tasks sensitive to longitudinal decline, and a progression gradient was observed, with participants who were less affected showing a smaller decline as compared to those nearing disease diagnosis. They ranked the effect sizes of the various cognitive tests and similar to previous findings the largest effect size was found for the Symbol Digital Modality Test (SDMT; a task requiring coordination of visual scanning, working memory, fine motor speed, and concentration) suggesting that this task is highly sensitive and consistent for tracking decline in prodromal HD.54
TRACK-HD is a study following a smaller number of individuals in Europe and Canada, with similar measures as PREDICT-HD, but the study includes early-stage patients and more frequent visits, with additional quantitative motor and oculomotor measures. The identification and validation of biomarkers in these longitudinal studies, will not only provide potential endpoints for use in a disease modifying clinical trial, but also will help in guiding the nature and timing of therapeutic interventions. The TRACK-HD studies reported various potential outcome measures with strong effect sizes in dictating potential utility in preHD clinical trials.55 Longitudinal changes over 36 months in imaging, quantitative motor, and cognitive measures were evident in individuals who were fewer than 10.8 years from predicted symptom onset (designated as preHD-B group). By contrast, despite striatal and white-matter loss, there was little evidence of significant functional decline in premanifest individuals who were more than 10.8 years from disease onset (designated preHD-A group).55 The only abnormality in preHD-A subjects was a larger interval between taps during finger tapping task than in controls. The preHD-B group also showed the extended tap interval, and additionally brain atrophy, and a decline greater than controls on the SDMT. Others previously reported significant cognitive declines over 2 years, and in agreement with Tabrizi et al. (2013), the rate of decline increased with estimated proximity to diagnosis.59 Overall these studies show that finger tapping speed and interval, and cognitive dysfunction detected by the SDMT test accompany striatal atrophy in subjects less than 10 years from diagnosis, and they worsen approaching the disease onset.
The Prospective Huntington At Risk Observational Study (PHAROS)
The Prospective Huntington At Risk Observational Study (PHAROS) enrolled adults at risk for HD. They were assessed approximately every 9 months with the UHDRS by investigators unaware of participants’ gene status. UHDRS scores were compared according to the Huntingtin gene CAG repeat number: expanded >36, intermediate 27–35, and nonexpanded controls <26. As mentioned previously in this review, CAG expansions ranging from 27–35 CAG repeats are considered intermediate (IA) and nonpathologic i.e. they have no direct phenotypic consequences, with recently reported exceptions.60, 61 However, as an unstable or mutable normal allele, this intermediate allele (IA) has the small risk of expanding into the disease range upon germline transmission especially with paternal transmission. Therefore, IA carriers, though unaffected themselves, may pass an expanded version of the HD gene to their offspring to manifest disease in the next generation.5 Killoran et al. (2013) recently described the clinical phenotype conferred by the intermediate-length huntingtin allele CAG repeat expansion in a population-based study.62 The authors compared the clinical characteristics of individuals with IA (27–35 CAG repeats) to those with alleles below 27 CAG repeats. Fifty of 983 participants (5.1%) in PHAROS had IA, and as a group, they did not differ from the controls on UHDRS motor, cognitive and functional assessments. However, the IA participants exhibited significantly worse behavioral changes (suicidal thoughts and apathy) than the controls, and on these measures more closely resemble those with CAG expansions above 35. The authors concluded that this behavioral phenotype may represent a prodromal stage of HD, with the potential for subsequent clinical manifestations, or it may be a part of a distinct phenotype due to pathologic changes independent of the CAG expansion length.62 These findings are in agreement with those of Ha et al. (2012),63 who found subtle, but relevant, disease manifestations in patients with intermediate CAG repeats. This study has potential implications for genetic counseling both for individuals at risk for HD, and perhaps even for the 5%–6% of the general population that carries an HD IA.64, 65
Feasibility of Clinical Trials in Presymptomatic HD Gene Carriers
It is possible that presymptomatic therapy in HD gene carriers aiming to slow the onset and progression of the illness may be more effective than treatment in symptomatic patients in whom there is already substantial neuronal death of 50% or more in the basal ganglia. A prerequisite for such an approach is that the therapeutic intervention is known to be safe without substantial side effects. One such study was recently completed using coenzyme Q10 (CoQ10), which has an excellent safety record. This study of 90 premanifest HD patients is called PREQUEL (Study in PRE-manifest Huntington’s disease of coenzyme Q10 (UbiquinonE) Leading to preventive trials) and it used 600, 1200 and 2400 mg of CoQ10 daily. This was well tolerated in these patients, and there was an attempt to demonstrate the biological activity of CoQ10 through changes in plasma 8-OHdG, a marker of oxidative stress. This was unsuccessful since no reductions in 8-OHdG were observed. This study however was the first multi-center interventional trial in participants with pre-manifest HD, showing that such trials can be designed and carried out successfully.
Therapeutic Approaches
A number of approaches to treat HD have been identified, and are being readied for the clinic. These include direct targeting of the HD gene and its protein product mutant huntingtin with RNAi, antisense RNA oligonucleotides and antibodies. Use of siRNA decreases mutant HTT expression and ameliorates the phenotype in mouse models of HD.66–69 Promising results have also been shown with antisense oligonucleotides infused directly into the lateral ventricles of mouse models of HD.70, 71 These approaches have shown promise in transgenic mice, but there are obstacles which will need to be overcome to ensure success in the clinic, such as delivery to the appropriate sites, and overcoming immunologic defenses.
Coenzyme Q10 (CoQ10) is critically involved in the electron transport chain and is a scavenger of free radicals.72 We showed that CoQ10 significantly blocks 3-nitropropionic acid (3-NP) induced striatal lesion volumes, and it reduces lipid and DNA oxidation. In addition, it exerts neuroprotective effects in improving motor performance and extending the survival of the R6/2 transgenic mouse model of HD.73 In patients with manifest HD, 600 mg daily of CoQ10 and remacemide (CARE-HD) showed a trend towards slowing HD progression with CoQ10 treatment.74 Subsequently, another study (Pre2CARE) of CoQ10 in manifest HD and healthy controls demonstrated a relative plateau in plasma CoQ10 levels above 2400 mg daily.75 As a consequence a second phase 3 clinical trial examining the efficacy of CoQ10 at a dose of 2400 mg daily (2CARE) is presently being carried out by the Huntington Study Group. This trial is enrolling 608 patients randomized to CoQ10 or placebo followed for 5 years, with the primary outcome of change in total functional capacity.
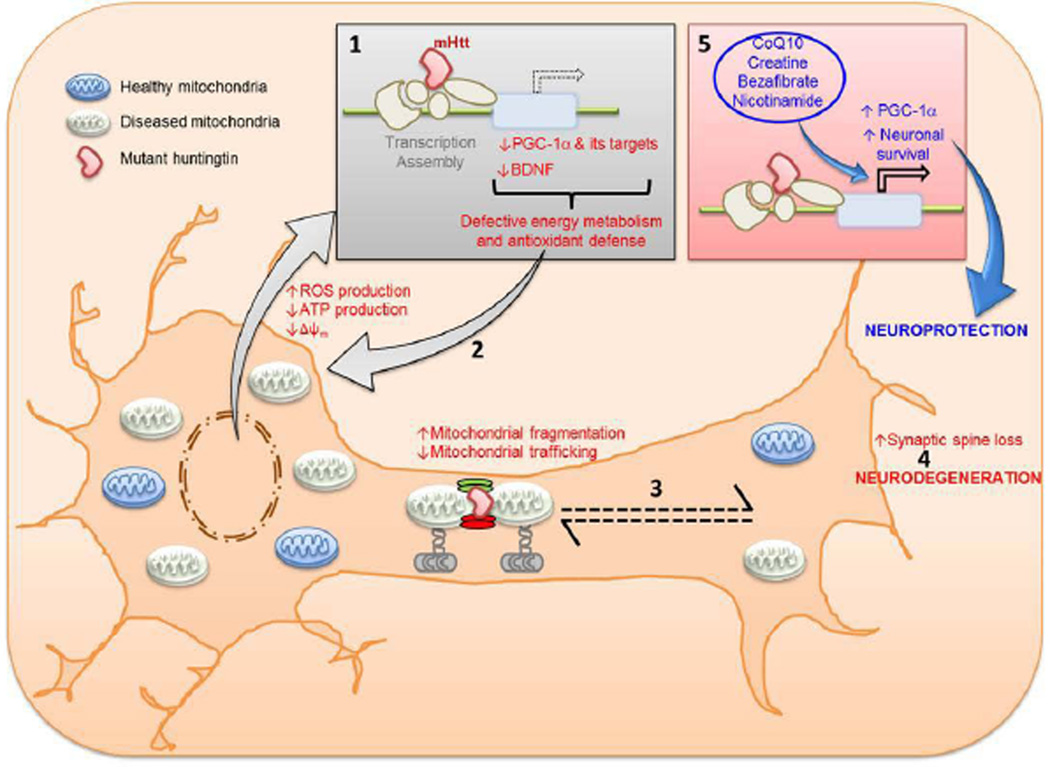
As noted, there is a strong body of evidence implicating impaired metabolism in the pathogenesis of HD which is at least in part caused by a deficiency of the transcriptional co-regulator PGC-1α.10, 76–80 It has been shown that haplotypes in the gene coding for PGC-1α influence age of onset in HD, as do polymorphisms in its downstream transcription factors NRF-1 and Tfam 81–83. This raises the prospect of therapies aimed at correcting this deficiency. There are a number of pharmacologic approaches to increasing PGC-1α including activation of PPAR nuclear receptors (Figure 3). Thiazolidinediones including pioglitazone activate PPARs, and increase PGC-1α, which exerts neuroprotective effects in both cellular and transgenic mouse models of HD.84, 85 We showed that administration of the pan-PPAR agonist bezafibrate was efficacious in improving rotarod performance and survival, as well as striatal atrophy, and atrophy of the striatal medium spiny neurons.47 It also ameliorated the depletion of type I muscle fibers and lipid vacuolation of brown adipose tissue. The numbers of mitochondria were depleted in both muscle fibers and striatal neurons of the R6/2 HD transgenic mice. Bezafibrate treatment produced a significant increase in numbers of mitochondria in both muscle fibers and in the striatal spiny neurons in the R6/2 HD transgenic mice, returning them to numbers similar to those in littermate control mice. There was also a reduction in immunostaining for malondialdehyde (MDA), and MDA levels by HPLC were reduced in the striatum of R6/2 mice after bezafibrate treatment.47
Figure 3.
Transcriptional interference of mutant huntingtin (mHtt) with PGC-1α produces defective energy metabolism and antioxidant defense (1) resulting in increased reactive oxygen species production, which in turn damage more mitochondria (2). Mutant huntingtin also interferes with mitochondrial fission-fusion process tipping the balance towards increased fission and interferes with vesicular transport (3). The net result of these impairments is low ATP at nerve terminals which culminates in neuronal death (4). Pharmacologic treatments with CoQ10, creatine, bezafibrate and nicotinamide increase PGC-1α and protect against neuronal death (5).
A number of other approaches to modulating PGC-1α and ameliorating mitochondrial dysfunction have great promise. Activation of SIRT1 results in deacetylation of PGC-1α which increases its activity. Genetically increasing SIRT1 is neuroprotective in transgenic mouse models of HD, while a deficiency exacerbates the phenotype and reduces survival.86, 87 Another approach to activating sirtuins is to increase NAD+ levels by administration of nicotinamide precursors, such as nicotinamide riboside.88 This has the advantage of activating both SIRT1 and SIRT3, leading to increased PGC-1α, as well as induction of antioxidant enzymes, and SIRT3 mediated increases in SOD2 and mitochondrial reduced glutathione. Nicotinamide by itself upregulated the gene expression levels of brain-derived neurotrophic factor (BDNF) and PGC-1α, and improved the motor phenotype in the R6/1 transgenic mouse model of HD.89
Another consequence of PGC-1α deficiency is reduced expression of antioxidant enzymes and increased oxidative damage, which occurs in both HD transgenic mice and in postmortem brain tissue and body fluids of HD patients. There are single-nucleotide polymorphisms in the OGG1 and XPC genes which are implicated in repair of oxidative damage to DNA, with age of onset in HD 90. A deficiency of OGG1 prevents age-dependent CAG repeat expansions suggesting that expansion occurs during removal of oxidized bases 91. Another source of oxidative damage is aberrant increases in NADPH oxidase activity.92 The importance of oxidative stress in HD pathogenesis was recently confirmed by the finding that increased expression of glutathione peroxidase is neuroprotective in animal models of HD.93 A number of mitochondria-targeted antioxidants are being developed. Administration of creatine exerts antioxidant and neuroprotective effects in transgenic mouse models of HD, and it is now in a phase III clinical trial in HD (CREST). A particularly interesting approach is using the antioxidant TEMPOL linked to gramicidin (XJB-5-131), which localizes it to the inner mitochondrial membrane.94 Administration of XJB-5-131 to a transgenic mouse model of HD resulted in improvement in mitochondrial function, reduced generation of ROS, reduced loss of striatal neurons, and amelioration of behavioral deficits.95 Several other compounds have been developed which can specifically target mitochondria. These include compounds such as mitoQ, a form of coenzyme Q linked to triphosphonium ions, which results in selective accumulation within mitochondria. There are also novel peptide antioxidants termed SS31 and SS20 which bind to the inner mitochondrial membrane, and are neuroprotective in transgenic mouse models of amyotrophic lateral sclerosis (ALS) as well as in neurotoxin models.73, 96
Another approach to ameliorating oxidative damage is to activate the Nrf2/ARE transcriptional pathway, which leads to increased expression of hemeoxygenase 1, NADPH-oxidoreductase, antioxidant enzymes, heat shock proteins, and enzymes which synthesize glutathione. We showed that treatment with CDDO-methylamide, a triterpenoid, produced significant rescue against striatal lesions caused by the neurotoxin 3-NP. CDDO- methylamide reduced markers of oxidative damage such as malondialdehyde, F(2)-Isoprostanes, 8-hydroxy-2-deoxyguanosine, 3-nitrotyrosine, and it improved glutathione homeostasis.97 We also showed that administration of the triterpenoids CDDO-ethylamide and CDDO-trifluoroethylamide reduced oxidative stress, improved motor impairment, reduced striatal atrophy and increased survival in a transgenic mouse model of HD.98 Another activator of the Nrf2/ARE pathway is dimethylfumarate, which showed efficacy in phase III clinical trials in multiple sclerosis, and was recently approved for clinical use. It also produced neuroprotective effects in two different transgenic mouse models of HD.99
Conclusions
Findings from longitudinal studies of presymptomatic HD patients show that subtle, but reproducible deficits occur on fine motor tasks such as finger tapping, and on neuropsychologic tests. These test abnormalities, as well as reductions of putaminal volume, occur 10 years or more before disease onset.24–26 The study of loss of D2 dopamine receptors, reductions in glucose uptake, CBVa, high energy phosphates, and metabolic network activity may culminate in improved markers of disease progression. It therefore may be possible to detect both clinical alterations in motor and cognitive functions, which are reflected by striatal atrophy, as well as increases in IL-6 and mHtt in leukocytes, which occur as many as 16 years before clinical diagnosis, to identify the optimal time to initiate neuroprotective therapies. A number of new approaches are available which may lead to effective neuroprotective agents to slow or halt the progression of HD. There is substantial evidence that metabolic alterations associated with a deficiency of PGC-1α directly contribute to the mitochondrial dysfunction and oxidative damage which play an important role in HD pathogenesis. Eventually the ability to detect and monitor disease progression may allow us to treat patients before disease onset, and to intervene with neuroprotective treatments, in order to slow or prevent disease progression.
Acknowledgments
The research for this study was supported by NIH grant [P01AG14930].
Footnotes
Authors declare no conflict of interest
References
- 1.Vonsattel JP, DiFiglia M. Huntington disease. J Neuropathol Exp Neurol. 1998;57(5):369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Johri A, Beal MF. Mitochondrial dysfunction in neurodegenerative diseases. The Journal of pharmacology and experimental therapeutics. 2012;342(3):619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johri A, Beal MF. Antioxidants in Huntington's disease. Biochimica et biophysica acta. 2012;1822(5):664–674. doi: 10.1016/j.bbadis.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semaka A, Creighton S, Warby S, Hayden MR. Predictive testing for Huntington disease: interpretation and significance of intermediate alleles. Clinical genetics. 2006;70(4):283–294. doi: 10.1111/j.1399-0004.2006.00668.x. [DOI] [PubMed] [Google Scholar]
- 5.Maat-Kievit A, Losekoot M, Van Den Boer-Van Den Berg H, et al. New problems in testing for Huntington's disease: the issue of intermediate and reduced penetrance alleles. J Med Genet. 2001;38(4):E12. doi: 10.1136/jmg.38.4.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wexler NS, Lorimer J, Porter J, et al. Venezuelan kindreds reveal that genetic and environmental factors modulate Huntington's disease age of onset. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(10):3498–3503. doi: 10.1073/pnas.0308679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenblatt A, Liang KY, Zhou H, et al. The association of CAG repeat length with clinical progression in Huntington disease. Neurology. 2006;66(7):1016–1020. doi: 10.1212/01.wnl.0000204230.16619.d9. [DOI] [PubMed] [Google Scholar]
- 8.Browne SE, Beal MF. The energetics of Huntington's disease. Neurochem Res. 2004;29(3):531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- 9.Mochel F, Haller RG. Energy deficit in Huntington disease: why it matters. The Journal of clinical investigation. 2011;121(2):493–499. doi: 10.1172/JCI45691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johri A, Chandra A, Flint Beal M. PGC-1alpha, mitochondrial dysfunction, and Huntington's disease. Free radical biology & medicine. 2013;62:37–46. doi: 10.1016/j.freeradbiomed.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiFiglia M, Sapp E, Chase K, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14(5):1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 12.Velier J, Kim M, Schwarz C, et al. Wild-type and mutant huntingtins function in vesicle trafficking in the secretory and endocytic pathways. Experimental neurology. 1998;152(1):34–40. doi: 10.1006/exnr.1998.6832. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier LR, Charrin BC, Borrell-Pages M, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118(1):127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Colin E, Zala D, Liot G, et al. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27(15):2124–2134. doi: 10.1038/emboj.2008.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caviston JP, Holzbaur EL. Huntingtin as an essential integrator of intracellular vesicular trafficking. Trends Cell Biol. 2009;19(4):147–155. doi: 10.1016/j.tcb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godin JD, Colombo K, Molina-Calavita M, et al. Huntingtin is required for mitotic spindle orientation and mammalian neurogenesis. Neuron. 2010;67(3):392–406. doi: 10.1016/j.neuron.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Zala D, Hinckelmann MV, Yu H, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152(3):479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 18.Semaka A, Hayden M. Evidence-Based Genetic Counselling Implications for Huntington Disease Intermediate Allele Predictive Test Results. Clinical genetics. 2013 doi: 10.1111/cge.12324. [DOI] [PubMed] [Google Scholar]
- 19.Tibben A. Predictive testing for Huntington's disease. Brain research bulletin. 2007;72(2–3):165–171. doi: 10.1016/j.brainresbull.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Paulsen JS, Nopoulos PC, Aylward E, et al. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain research bulletin. 2010;82(3–4):201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet neurology. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- 22.Tabrizi SJ, Reilmann R, Roos RA, et al. Potential endpoints for clinical trials in premanifest and early Huntington's disease in the TRACK-HD study: analysis of 24 month observational data. Lancet neurology. 2012;11(1):42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- 23.Harris GJ, Codori AM, Lewis RF, Schmidt E, Bedi A, Brandt J. Reduced basal ganglia blood flow and volume in pre-symptomatic, gene-tested persons at-risk for Huntington's disease. Brain : a journal of neurology. 1999;122(Pt 9):1667–1678. doi: 10.1093/brain/122.9.1667. [DOI] [PubMed] [Google Scholar]
- 24.Aylward EH, Li Q, Stine OC, et al. Longitudinal change in basal ganglia volume in patients with Huntington's disease. Neurology. 1997;48(2):394–399. doi: 10.1212/wnl.48.2.394. [DOI] [PubMed] [Google Scholar]
- 25.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology. 2004;63(1):66–72. doi: 10.1212/01.wnl.0000132965.14653.d1. [DOI] [PubMed] [Google Scholar]
- 26.Aylward EH, Nopoulos PC, Ross CA, et al. Longitudinal change in regional brain volumes in prodromal Huntington disease. Journal of neurology, neurosurgery, and psychiatry. 2011;82(4):405–410. doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgiou-Karistianis N, Scahill R, Tabrizi SJ, Squitieri F, Aylward E. Structural MRI in Huntington's disease and recommendations for its potential use in clinical trials. Neuroscience and biobehavioral reviews. 2013;37(3):480–490. doi: 10.1016/j.neubiorev.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Bjorkqvist M, Wild EJ, Thiele J, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington's disease. The Journal of experimental medicine. 2008;205(8):1869–1877. doi: 10.1084/jem.20080178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalrymple A, Wild EJ, Joubert R, et al. Proteomic profiling of plasma in Huntington's disease reveals neuroinflammatory activation and biomarker candidates. Journal of proteome research. 2007;6(7):2833–2840. doi: 10.1021/pr0700753. [DOI] [PubMed] [Google Scholar]
- 30.Wild E, Magnusson A, Lahiri N, et al. Abnormal peripheral chemokine profile in Huntington's disease. PLoS currents. 2011;3:RRN1231. doi: 10.1371/currents.RRN1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leoni V, Mariotti C, Tabrizi SJ, et al. Plasma 24S-hydroxycholesterol and caudate MRI in pre-manifest and early Huntington's disease. Brain : a journal of neurology. 2008;131(Pt 11):2851–2859. doi: 10.1093/brain/awn212. [DOI] [PubMed] [Google Scholar]
- 32.Hu Y, Chopra V, Chopra R, et al. Transcriptional modulator H2A histone family, member Y (H2AFY) marks Huntington disease activity in man and mouse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(41):17141–17146. doi: 10.1073/pnas.1104409108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiss A, Trager U, Wild EJ, et al. Mutant huntingtin fragmentation in immune cells tracks Huntington's disease progression. The Journal of clinical investigation. 2012;122(10):3731–3736. doi: 10.1172/JCI64565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonini A, Leenders KL, Spiegel R, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain : a journal of neurology. 1996;119(Pt 6):2085–2095. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 35.Esmaeilzadeh M, Ciarmiello A, Squitieri F. Seeking brain biomarkers for preventive therapy in Huntington disease. CNS neuroscience & therapeutics. 2011;17(5):368–386. doi: 10.1111/j.1755-5949.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin H, Kim MH, Lee SJ, et al. Decreased Metabolism in the Cerebral Cortex in Early-Stage Huntington's Disease: A Possible Biomarker of Disease Progression? Journal of clinical neurology. 2013;9(1):21–25. doi: 10.3988/jcn.2013.9.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powers WJ, Videen TO, Markham J, et al. Selective defect of in vivo glycolysis in early Huntington's disease striatum. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(8):2945–2949. doi: 10.1073/pnas.0609833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang CC, Feigin A, Ma Y, et al. Metabolic network as a progression biomarker of premanifest Huntington's disease. The Journal of clinical investigation. 2013;123(9):4076–4088. doi: 10.1172/JCI69411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua J, Unschuld PG, Margolis RL, van Zijl PC, Ross CA. Elevated arteriolar cerebral blood volume in prodromal Huntington's disease. Movement disorders : official journal of the Movement Disorder Society. 2013 doi: 10.1002/mds.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vis JC, Nicholson LF, Faull RL, Evans WH, Severs NJ, Green CR. Connexin expression in Huntington's diseased human brain. Cell biology international. 1998;22(11–12):837–847. doi: 10.1006/cbir.1998.0388. [DOI] [PubMed] [Google Scholar]
- 41.Franciosi S, Ryu JK, Shim Y, et al. Age-dependent neurovascular abnormalities and altered microglial morphology in the YAC128 mouse model of Huntington disease. Neurobiology of disease. 2012;45(1):438–449. doi: 10.1016/j.nbd.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Lin CHC, Lin M. Magnetic resonance microscopic angiography visualization of abnormal microvasculature in a transgenic mouse model of Huntington’s disease. Proceedings of the ISMRM 18th Scientific Meeting and Exhibition; May 1–7; Stockholm, Sweden. 2010. [Google Scholar]
- 43.Cepeda-Prado E, Popp S, Khan U, et al. R6/2 Huntington's disease mice develop early and progressive abnormal brain metabolism and seizures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(19):6456–6467. doi: 10.1523/JNEUROSCI.0388-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feigin A, Tang C, Ma Y, et al. Thalamic metabolism and symptom onset in preclinical Huntington's disease. Brain : a journal of neurology. 2007;130(Pt 11):2858–2867. doi: 10.1093/brain/awm217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lodi R, Schapira AH, Manners D, et al. Abnormal in vivo skeletal muscle energy metabolism in Huntington's disease and dentatorubropallidoluysian atrophy. Annals of neurology. 2000;48(1):72–76. [PubMed] [Google Scholar]
- 46.Kim J, Moody JP, Edgerly CK, et al. Mitochondrial loss, dysfunction and altered dynamics in Huntington's disease. Human molecular genetics. 2010;19(20):3919–3935. doi: 10.1093/hmg/ddq306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johri A, Calingasan NY, Hennessey TM, et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington's disease. Human molecular genetics. 2012;21(5):1124–1137. doi: 10.1093/hmg/ddr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkins BG, Rosas HD, Chen YC, et al. 1H NMR spectroscopy studies of Huntington's disease: correlations with CAG repeat numbers. Neurology. 1998;50(5):1357–1365. doi: 10.1212/wnl.50.5.1357. [DOI] [PubMed] [Google Scholar]
- 49.Paulsen JS, Zhao H, Stout JC, et al. Clinical markers of early disease in persons near onset of Huntington's disease. Neurology. 2001;57(4):658–662. doi: 10.1212/wnl.57.4.658. [DOI] [PubMed] [Google Scholar]
- 50.Paulsen JS, Zimbelman JL, Hinton SC, et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington's Disease. AJNR American journal of neuroradiology. 2004;25(10):1715–1721. [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen JS, Hayden M, Stout JC, et al. Preparing for preventive clinical trials: the Predict-HD study. Archives of neurology. 2006;63(6):883–890. doi: 10.1001/archneur.63.6.883. [DOI] [PubMed] [Google Scholar]
- 52.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the Predict-HD study. Journal of neurology, neurosurgery, and psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wild EJ, Tabrizi SJ. Predict-HD and the future of therapeutic trials. Lancet neurology. 2006;5(9):724–725. doi: 10.1016/S1474-4422(06)70531-2. [DOI] [PubMed] [Google Scholar]
- 54.Paulsen JS, Smith MM, Long JD the PHDi, coordinators of the Huntington Study G. Cognitive decline in prodromal Huntington Disease: implications for clinical trials. Journal of neurology, neurosurgery, and psychiatry. 2013 doi: 10.1136/jnnp-2013-305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabrizi SJ, Scahill RI, Owen G, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet neurology. 2013;12(7):637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 56.Beglinger LJ, Paulsen JS, Watson DB, et al. Obsessive and compulsive symptoms in prediagnosed Huntington's disease. The Journal of clinical psychiatry. 2008;69(11):1758–1765. doi: 10.4088/jcp.v69n1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Biglan KM, Ross CA, Langbehn DR, et al. Motor abnormalities in premanifest persons with Huntington's disease: the PREDICT-HD study. Movement disorders : official journal of the Movement Disorder Society. 2009;24(12):1763–1772. doi: 10.1002/mds.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kloppel S, Chu C, Tan GC, et al. Automatic detection of preclinical neurodegeneration: presymptomatic Huntington disease. Neurology. 2009;72(5):426–431. doi: 10.1212/01.wnl.0000341768.28646.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowe KC, Paulsen JS, Langbehn DR, et al. Self-paced timing detects and tracks change in prodromal Huntington disease. Neuropsychology. 2010;24(4):435–442. doi: 10.1037/a0018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Potter NT, Spector EB, Prior TW. Technical standards and guidelines for Huntington disease testing. Genetics in medicine : official journal of the American College of Medical Genetics. 2004;6(1):61–65. doi: 10.1097/01.gim.0000106165.74751.15. [DOI] [PubMed] [Google Scholar]
- 61.Squitieri F, Jankovic J. Huntington's disease: how intermediate are intermediate repeat lengths? Movement disorders : official journal of the Movement Disorder Society. 2012;27(14):1714–1717. doi: 10.1002/mds.25172. [DOI] [PubMed] [Google Scholar]
- 62.Killoran A, Biglan KM, Jankovic J, et al. Characterization of the Huntington intermediate CAG repeat expansion phenotype in PHAROS. Neurology. 2013;80(22):2022–2027. doi: 10.1212/WNL.0b013e318294b304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha AD, Beck CA, Jankovic J. Intermediate CAG Repeats in Huntington's Disease: Analysis of COHORT. Tremor and other hyperkinetic movements. 2012;2 doi: 10.7916/D8FF3R2P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feigin A. Redefining the genetic risk for Huntington disease. Neurology. 2013;80(22):2004–2005. doi: 10.1212/WNL.0b013e318294b49b. [DOI] [PubMed] [Google Scholar]
- 65.Hogarth P. Huntington disease: How many repeats does it take? Neurology. 2013;80(22):e241–e243. doi: 10.1212/WNL.0b013e3182984b31. [DOI] [PubMed] [Google Scholar]
- 66.DiFiglia M, Sena-Esteves M, Chase K, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boudreau RL, McBride JL, Martins I, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington's disease mice. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(6):1053–1063. doi: 10.1038/mt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kordasiewicz HB, Stanek LM, Wancewicz EV, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanek LMYW, Angus S, Sardi PS, Hayden MR, Hung GH, Bennett CF, Cheng SH, Shihabuddin LS. Antisense oligonucleotide-mediated correction of transcriptional dysregulation is correlated with behavioral benefits in the YAC128 mouse model of Huntington's disease. J Huntingtons Dis. 2013;2:217–228. doi: 10.3233/JHD-130057. [DOI] [PubMed] [Google Scholar]
- 70.Pfister EL, Zamore PD. Huntington's disease: silencing a brutal killer. Experimental neurology. 2009;220(2):226–229. doi: 10.1016/j.expneurol.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godinho BM, Ogier JR, Darcy R, O'Driscoll CM, Cryan JF. Self-assembling modified beta-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: focus on Huntington's disease. Molecular pharmaceutics. 2013;10(2):640–649. doi: 10.1021/mp3003946. [DOI] [PubMed] [Google Scholar]
- 72.Beal MF, Shults CW. Effects of Coenzyme Q10 in Huntington's disease and early Parkinson's disease. BioFactors. 2003;18(1–4):153–161. doi: 10.1002/biof.5520180218. [DOI] [PubMed] [Google Scholar]
- 73.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxidants & redox signaling. 2009;11(9):2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huntington Study G. A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 2001;57(3):397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]
- 75.Huntington Study Group Pre CI, Hyson HC, Kieburtz K, et al. Safety and tolerability of high-dosage coenzyme Q10 in Huntington's disease and healthy subjects. Movement disorders : official journal of the Movement Disorder Society. 2010;25(12):1924–1928. doi: 10.1002/mds.22408. [DOI] [PubMed] [Google Scholar]
- 76.Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington's disease transgenic mice implicate PGC-1alpha in Huntington's disease neurodegeneration. Cell metabolism. 2006;4(5):349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 78.Chaturvedi RK, Adhihetty P, Shukla S, et al. Impaired PGC-1alpha function in muscle in Huntington's disease. Human molecular genetics. 2009;18(16):3048–3065. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington's disease following chronic energy deprivation. Human molecular genetics. 2010;19(16):3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johri A, Starkov AA, Chandra A, et al. Truncated peroxisome proliferator-activated receptor-gamma coactivator 1alpha splice variant is severely altered in Huntington's disease. Neuro-degenerative diseases. 2011;8(6):496–503. doi: 10.1159/000327910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weydt P, Soyal SM, Gellera C, et al. The gene coding for PGC-1alpha modifies age at onset in Huntington's Disease. Mol Neurodegener. 2009;4:3. doi: 10.1186/1750-1326-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taherzadeh-Fard E, Saft C, Andrich J, Wieczorek S, Arning L. PGC-1alpha as modifier of onset age in Huntington disease. Mol Neurodegener. 2009;4:10. doi: 10.1186/1750-1326-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Soyal SM, Felder TK, Auer S, et al. A greatly extended PPARGC1A genomic locus encodes several new brain-specific isoforms and influences Huntington disease age of onset. Human molecular genetics. 2012;21(15):3461–3473. doi: 10.1093/hmg/dds177. [DOI] [PubMed] [Google Scholar]
- 84.Chiang MC, Chern Y, Huang RN. PPARgamma rescue of the mitochondrial dysfunction in Huntington's disease. Neurobiology of disease. 2012;45(1):322–328. doi: 10.1016/j.nbd.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 85.Jin YN, Hwang WY, Jo C, Johnson GV. Metabolic state determines sensitivity to cellular stress in Huntington disease: normalization by activation of PPARgamma. PloS one. 2012;7(1):e30406. doi: 10.1371/journal.pone.0030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nature medicine. 2012;18(1):153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jeong H, Cohen DE, Cui L, et al. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nature medicine. 2012;18(1):159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang T, Chan NY, Sauve AA. Syntheses of nicotinamide riboside and derivatives: effective agents for increasing nicotinamide adenine dinucleotide concentrations in mammalian cells. Journal of medicinal chemistry. 2007;50(26):6458–6461. doi: 10.1021/jm701001c. [DOI] [PubMed] [Google Scholar]
- 89.Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington's disease. Neurobiology of disease. 2011;41(1):43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berger F, Vaslin L, Belin L, et al. The impact of single-nucleotide polymorphisms (SNPs) in OGG1 and XPC on the age at onset of Huntington disease. Mutation research. 2013;755(2):115–119. doi: 10.1016/j.mrgentox.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 91.Ayala-Pena S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington's disease pathogenesis. Free radical biology & medicine. 2013;62:102–110. doi: 10.1016/j.freeradbiomed.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valencia A, Sapp E, Kimm JS, et al. Elevated NADPH oxidase activity contributes to oxidative stress and cell death in Huntington's disease. Human molecular genetics. 2013;22(6):1112–1131. doi: 10.1093/hmg/dds516. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Mason RP, Casu M, Butler N, et al. Glutathione peroxidase activity is neuroprotective in models of Huntington's disease. Nature genetics. 2013 doi: 10.1038/ng.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang J, Kurnikov I, Belikova NA, et al. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. The Journal of pharmacology and experimental therapeutics. 2007;320(3):1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 95.Xun Z, Rivera-Sanchez S, Ayala-Pena S, et al. Targeting of XJB-5-131 to mitochondria suppresses oxidative DNA damage and motor decline in a mouse model of Huntington's disease. Cell reports. 2012;2(5):1137–1142. doi: 10.1016/j.celrep.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petri S, Kiaei M, Damiano M, et al. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. Journal of neurochemistry. 2006;98(4):1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 97.Yang L, Calingasan NY, Thomas B, et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PloS one. 2009;4(6):e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stack C, Ho D, Wille E, et al. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free radical biology & medicine. 2010;49(2):147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellrichmann G, Petrasch-Parwez E, Lee DH, et al. Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington's disease. PloS one. 2011;6(1):e16172. doi: 10.1371/journal.pone.0016172. [DOI] [PMC free article] [PubMed] [Google Scholar]