Abstract
Non-daily, or intermittent smokers (ITS) represent a growing pattern in adult smoking that needs to be explained by models of drug dependence. ITS regularly and voluntarily abstain from smoking, yet have difficulty quitting. We examine potential accounts of ITS’ smoking by exploring their experience of craving and withdrawal on the days they abstain. For three weeks, 146 ITS and 194 daily smokers used Ecological Momentary Assessment (EMA) to monitor craving, withdrawal, and smoking in real-time. ITS’ craving (p < .001) and arousal (p < .001) were significantly lower on the 34.4% of days when they abstained (compared to days they smoked), and they experienced no increases in withdrawal symptom. ITS who abstained for longer experienced lower craving, even on their first day of abstinence (p < .001). Within strata defined by longest duration of abstinence (1, 2-3, 4-6, ≥ 7 days), craving did not change over time, demonstrating no increase as resumption of smoking approached. Craving increased only at the moment smoking resumed. Further, duration of abstinence runs varied more within persons than across persons. These findings contradict the predictions of a model positing that craving recurs at fixed intervals. Findings are consistent with the hypothesis that ITS’ smoking is cued or primed by particular stimuli rather than by temporal cycles. These analyses demonstrate that ITS do not experience increased craving or withdrawal on days they do not smoke, and show neither signs of classical dependence nor regular cycles of craving and smoking.
Keywords: Smoking, dependence, ecological momentary assessment, non-daily smoking
Population surveys have uncovered an anomalous and growing pattern of smoking, indicating that approximately one third of adult smokers in the US do not smoke daily (Behavioral Risk Factor Surveillance System, Centers for Disease Control and Prevention, 2012; National Survey on Drug Use and Health, Substance Abuse and Mental Health Services Administration, 2013). Data from other countries also show that a substantial proportion of adult smokers in those countries are non-daily, or intermittent smokers (ITS; Korhonen, Broms, Levalahti, Koskenvuo, & Kaprio, 2009; Lindstrom & Ostergren, 2001; World Health Organization, 2007).
ITS’ smoking behavior is inconsistent with the smoking pattern expected of dependent smokers under the nicotine maintenance model of dependence, which rests on classical addiction theory (Benowitz, 2008; cf. DiFranza et al., 2011). Under this model, the typical developmental trajectory starts with a period of substantial exposure to nicotine, during which a user develops tolerance, escalates use, and experiences symptoms of withdrawal when abstaining, thus driving a need for regular dosing sufficient to maintain some minimal level of nicotine levels in the blood. ITS’ smoking behavior is inconsistent with nicotine maintenance, since they regularly abstain from smoking for days at a time. ITS abstain from smoking one of every three days, and often go for several days at a time without smoking (with the longest run of abstinence averaging 5 days over a 65-day reporting period; Shiffman, Tindle, et al., 2012). Yet, ITS have very poor quit rates, not much better than those of daily smokers (DS; Tindle & Shiffman, 2011), despite making repeated deliberate efforts to quit, which suggests substantial motivation to quit (ITS actually are more likely to make quit efforts than DS; Tindle & Shiffman, 2011).
Several explanations might be offered for ITS’ patterns of smoking. The simplest explanation is that they are simply not vulnerable to nicotine dependence, perhaps as a result of protective genetic factors. Indeed, even some daily smokers are not dependent (Goedeker & Tiffany, 2008; Donny & Dierker, 2007), and studies have demonstrated substantial genetic influence on nicotine dependence, even among those who take up smoking (Audrain-McGovern, et al, 2007; Bierut, et al., 2007; Chen, et al., 2012; Lerman & Berrettini, 2003). Thus, the process described by the theory may simply not apply to all individuals. And, indeed, most ITS score very low on psychometric measures of dependence (Shiffman, Ferguson, Dunbar, & Scholl, 2012; Shiffman, Tindle, et al., 2012). This leaves open the question of why ITS would have so much trouble quitting, and why they report experiencing craving, even intense craving (Shiffman et al., 2014b).
It could also be argued that ITS might be vulnerable to dependence, but their prior exposure to nicotine has not been adequate to instill dependence. This explanation is challenged by the fact that ITS in our recent sample take in at least as much nicotine from each cigarette as daily smokers (Shiffman, Dunbar, & Benowitz, 2014), and their smoking histories indicate that many have smoked for decades, having consumed, on average, more than 30,000 cigarettes (Shiffman, Tindle, et al., 2012). However, one could argue that some degree of continuous exposure might be necessary, and that intermittent exposure of the sort ITS now display might not be adequate. This renders interesting the behavior of “converted” ITS (“CITS”) who had a history of smoking daily for at least 6 months in comparison to “native” ITS (“NITS”) who have never smoked daily.
Another explanation is that ITS are dependent, and suffer craving and withdrawal, but simply tolerate the symptoms. As Fernando, Wellman, and DiFranza (2006, p. 340) put it, when not smoking, they may be “walking around in withdrawal.” Addiction theory does not posit that it is impossible for smokers to abstain, only that abstinence leads to craving and withdrawal symptoms that punish abstinence and prompt smoking. It is possible that, even on the days that they smoke, ITS – unlike DS – may not smoke enough to suppress withdrawal. To test this, we compare DS’ and ITS’ craving and withdrawal symptoms on smoking days. If ITS do not show elevated withdrawal symptoms, this would suggest they are not “walking around in withdrawal” on smoking days. However, craving and withdrawal may emerge on the days that they abstain completely, particularly as most nicotine is cleared overnight (Benowitz, 1992; Jarvik et al., 2000). Accordingly, we test whether ITS show signs of increased craving and withdrawal on the days when they abstain from smoking. Under classical dependence theory, failing to show nicotine maintenance and failing to develop craving and withdrawal when abstaining would firmly classify ITS as non-dependent, implying that some individuals are exempt from developing dependence even after years of nicotine intake.
However, a highly novel alternative model of nicotine dependence has recently been proposed that asserts that ITS are nicotine-dependent, but have just not progressed as far as daily smokers in the development of addiction. The Sensitization-Homeostasis Model (DiFranza & Wellman, 2005; DiFranza & Ursprung, 2008) posits that, from the very start of smoking, smokers become dependent, and experience regular, cyclical recurrences of craving that in turn drive smoking. This hypothesis – which we refer to as the “Craving Cycles Hypothesis” – posits that even modest exposures to nicotine (i.e., one cigarette) cause craving to recur at regular intervals, with the duration of the cycle being characteristic of the individual, based on the stage of dependence they currently occupy. Such cycles, or “latencies-to-craving” (Fernando et al., 2006; DiFranza & Ursprung, 2008; DiFranza et al., 2011) start out being long, but over time, become shorter, leading to more frequent smoking. By this account, ITS are dependent, in the same way as heavy smokers, but simply have long latencies-to-craving that allow them to go a day or more before they experience craving that motivates them to smoke. In support of this hypothesis are several studies in which light and intermittent smokers state, on retrospective global questionnaires or interviews, that after abstaining for a time, they begin to experience craving to smoke (DiFranza & Ursprung, 2008; DiFranza, Ursprung, & Carson, 2010; Fernando et al., 2006). This theory, with novel implications for our understanding of the development of nicotine dependence (and perhaps dependence in general), and for an account of non-daily smoking, deserves empirical attention. Proponents of the theory (Fernando et al., 2006) note the limitation of retrospective reports and call for testing of the model using prospective real-time measures. Here we report just such analyses, using Ecological Momentary Assessment (EMA; Shiffman, Stone, & Hufford, 2008) to assess ITS’ craving and withdrawal, as well as smoking, in real-time, as they traverse periods of smoking and abstinence.
The Craving Cycles Hypothesis produces novel and testable implications regarding the time course of craving. Because craving is recurring cyclically, it should begin to increase as the end of a period of abstinence (i.e., the recurrence of smoking) approaches. The model allows that smoking does not always follow immediately as craving reasserts itself – smokers may resist smoking or find it inconvenient – but posits that in such cases, craving will not abate, but rather intensify (DiFranza et al., 2010; DiFranza et al., 2011), thus suggesting that the upturn in craving should be even more readily observable. Accordingly, we assess time trends in craving during runs of abstinence, particularly assessing whether craving increases as the period of abstinence moves towards its conclusion.
The Craving Cycles Hypothesis also allows for some smoking that might not be driven by the cyclical recurrence of craving, positing that smokers might sometimes smoke “elective” cigarettes that are not driven by craving (DiFranza et al., 2011). To test whether this accounts for the resumption of smoking after a run of abstinence, we assessed the degree of craving reported at the time the first cigarette was smoked after days of abstinence. Reports of elevated craving leading up to smoking would imply that this was a “needed” cigarette based on cyclical craving, rather than an “elective” one based on other factors.
The Craving Cycles Hypothesis also makes a novel prediction about the distribution of periods, or “runs”, of abstinence. Specifically, it posits that the duration of runs of abstinence should be relatively stable within an individual, since each person is posited to exhibit a characteristic craving cycle duration for any given stage in their progression of dependence (DiFranza & Ursprung, 2008; DiFranza et al., 2010; DiFranza et al., 2011). We use data on multiple runs of abstinence reported by ITS to evaluate this hypothesis, testing the degree to which the length of abstinence runs varies within individuals.
Other accounts of ITS’ smoking behavior make different predictions about the temporal patterns of craving and of runs of abstinence. A stimulus control (Freeman & Lattal, 1992) account of ITS’ smoking would predict little temporal regularity. The stimulus control hypothesis of ITS’ smoking (Shiffman, Ferguson, et al., 2012) posits that ITS’ behavior is not driven by any fixed cycles of craving or periodic need for nicotine, but rather by exposure to specific stimuli and situations that prompt smoking (Shiffman & Paty, 2006). There is strong evidence for the triggering role of cues and for stimulus control in smoking among non-daily and non-dependent smokers. Data clearly show that ITS’ smoking is more closely associated than DS’ smoking with specific contexts, such as being with other smokers, consumption of alcohol, and engaging in social activity (Shiffman et al., 2014c), and similar dynamics apply to “chippers” – very light smokers, some of whom smoke daily (Shiffman et al., 1994). The role of conditioned cue responses and stimulus control is prominent in many theories of dependence (Marlatt & Gordon, 1985; Niaura et al., 1988; Robinson & Berridge, 1993), and has been demonstrated in both observational and experimental human studies and in experimental animal studies of nicotine and other drug use (DeGrandpre & Bickel, 1993; Kozlowski & Herman, 1984; Tiffany, 1990).
Under a stimulus control model of ITS smoking, craving during abstinence would not be expected to be cyclical, with no inherent time trend (though one might be introduced if inciting stimuli occurred at regular intervals, e.g., drinking in the evenings). Craving and smoking would recur when the appropriate cues and circumstances present themselves, without variation in craving as a function of abstinence duration. Even on the day that ITS resumed smoking, craving would not necessarily be expected to be rising or elevated, except around the time of actual smoking. Also, runs of abstinence would not be expected to be of any characteristic length for a given smoker; although some cues might recur periodically (e.g., drinking on weekends, or evenings), and perhaps even lead to anticipatory craving as the setting occasions approach, other smoking cues and occasions could present themselves at varying and perhaps unpredictable intervals.
Conditioned responses to immediate cues are not the only factor that could explain ITS’ patterns of craving and smoking. Priming effects may play a role. It is well-established that a dose of drug primes further drug-seeking (de Wit, 1996; Shaham, Shalev, Lu, de Wit & Stewart, 2003), which might be expressed as craving, as well as smoking. Thus, in the absence of smoking, craving would be low, but would rise once smoking took place, leading to higher craving on smoking days compared to abstinent days. Drug primed responses cannot explain the initial smoking episode that sets off this sequence (in experimental demonstrations, it is typically administered at random by an experimenter; Shaham et al., 2003), but priming effects could set in to drive continued and even escalating craving and smoking once the initial exposure was triggered by other factors, such as smoking cues. Moreover, other exogenous influences such as stressors can also act as primes for drug use (Erb, Shaham, & Stewart, 1996; Le et al., 1998; Shaham & Stewart, 1995).
Thus, EMA data on ITS’ runs of abstinence, and their experience during those runs, can inform theoretical accounts of their behavior and dependence. In this study, ITS carried electronic diaries for three weeks, recording each cigarette that they smoked. They were also “beeped” at random several times a day when they were not smoking (on both smoking and non-smoking days). Analyses contrasted ITS’ and DS’ craving and withdrawal on days when subjects were smoking, and also examined how ITS’ symptoms changed on days when they did not smoke. We also analyzed temporal trends in ITS’ craving across consecutive days of abstinence (“runs”), including analyses that examined trends in craving in the days leading up to resumption of smoking. Finally, we examined ITS’ craving on the day smoking resumed, and at the moment of resumption, and examined how patterns differed between CITS and NITS.
Methods
Participants
Participants were 340 Pittsburgh-area community volunteers recruited for this study via advertisement and promotion, with extra recruitment efforts focused on the African American community, because national surveys indicate that ITS are more likely to be racial/ethnic minorities (Tindle & Shiffman, 2012; Trinidad, Perez-Stable, Emery, Grana, & Messer, 2009). These were comprised of 146 ITS (83 CITS, 57 NITS, and six unknown) and 194 DS. To be eligible, volunteers had to be at least 21 years old, report smoking for at least three years and smoking at their current rate for at least three months, and not be planning to quit within the next month. ITS had to report smoking 4–27 days per month (no restrictions on number of cigarettes per day [CPD]), and DS had to report daily smoking of 5-30 cigarettes per day. To be classified as CITS, ITS had to report having previously smoked daily for at least six months. This sample largely overlaps with that reported in previous papers (Shiffman et al., 2014b, 2014c; Shiffman, Tindle, et al., 2012), but focuses on the subset of ITS (68.87%) who had EMA data that included at least one day of abstinence during monitoring.
The characteristics of the DS sample have been reported elsewhere (Shiffman et al., 2014b, 2014c). Among the ITS sample, the primary focus of the paper, average age was 33.16 (SD = 11.80) years old; 49.10% of the sample was female. The majority (87.10%) reported education beyond high school. As noted above, we oversampled African American smokers, who made up 22.60% of the sample, with 73.97% Caucasian, and 3.42% other ethnicities. We corrected for this deliberate oversampling by weighting the data to US national statistics (Kalton, 1983).
On average, ITS in this sample smoked on 53.11% of days (22.16%), smoking an average of 3.82 (2.82) cigarettes on those days. They had been smoking for 14.32 (SD = 11.15) years, consuming an estimated 31,233 cigarettes (Mdn = 10,420). Average duration of smoking trended toward being slightly longer (p = .06) among CITS (15.75 years [SD = 11.63]) compared to NITS (12.30 [SD = 10.10]), and CITS reported significantly more lifetime cigarettes (CITS: Mdn = 22,818 vs. NITS: Mdn = 4,252; p < .0001). The majority of ITS participants (61.08%) had Fagerström Test for Nicotine Dependence (FTND) scores of 0; the average FTND score was 0.83 (SD = 1.31). The mean total Nicotine Dependence Syndrome Scale (NDSS) score (in T-scores; see Shiffman, Ferguson, et al., 2012) was 27.61 (SD = 5.37) and the mean Wisconsin Inventory of Smoking Dependence Motives (WISDM) scores for Primary and Secondary Dependence Motives were 1.93 (SD = 0.89) and 2.57 (SD = 0.89), respectively. However, almost all ITS participants (89.13%; 93.63% among CITS) were considered dependent by the Hooked on Nicotine Checklist (HONC; Wellman et al., 2005).
Consistent with larger-scale epidemiological studies, ITS and DS differed on several demographic factors. ITS were younger (33.16 [SD = 11.80] vs 40.11 [SD = 11.68; p < .001), and reported more years of education (15.23 years [SD = 2.48] vs 13.69 [SD = 2.36], p < .001; 51.21% college graduates vs 19.95%, p < .001), and higher mean annual income (31.16 thousand [SD = 25.82] vs 25.06 thousand [SD = 22.46], p < .01; 52.77% over $25,000/year vs 37.53%, p < .01), but the income differences were accounted for by education. (Controlling for age and education did not change the results; consequently, we present the uncontrolled analyses.)
In the current sample, DS (55%) were slightly more likely than ITS (44%) to report smoking menthol cigarettes (p = 0.04; although see Shiffman, Tindle et al., 2012). As expected (Giovino et al., 2004), menthol smoking was more prevalent among AA smokers, almost all of whom (96%) reported smoking menthol cigarettes (vs 43% among Caucasian participants; p < .001). This was equally true among DS and ITS; there was no smoking status x race interaction (p = .69). Menthol smoking was unrelated to the length of the longest run of abstinence (see below), overall, or among Caucasian smokers, and whether longest run was analyzed by strata or as a continuous variable (p > .50).
Procedures
During EMA monitoring, participants completed five study visits, at which they retrospectively reported cigarette consumption on each day since the previous session using the timeline follow-back (TLFB; Sobell, Sobell, & Maisto, 1979) procedure. The average look-back period for the TLFB data – the length of time between study visits during the EMA period – was 6.18 days (SD = 1.88). Participants also completed an extensive baseline questionnaire that included various measures to assess nicotine dependence (see Shiffman, Ferguson et al., 2012). Smoking history was assessed to identify ITS who had previously smoked daily for at least six months (CITS; n = 83) and those who had never done so (NITS, n = 57); this measure was not obtained from six ITS, who were excluded from CITS/NITS analyses.
EMA Procedures
Participants monitored their real-time smoking behavior over a period of approximately three weeks using electronic diaries (22.5 [SD = 4.12] days of monitoring). EMA procedures are described in detail in Shiffman et al. (2014c), and were similar to those used in other studies (Shiffman et al., 2002; Shiffman et al., 2006). Briefly, participants engaged in event-based monitoring of smoking behavior, in which they were instructed to report every cigarette as they initiated smoking during the monitoring period. To avoid excess burden, especially among DS, the electronic diaries randomly selected a subset of these occasions for assessment. Participants could also report at end of day any smoking they had not reported in real time. In addition to cigarette-event based assessments, the ED also prompted individuals at random times to complete brief assessments approximately 3-4 times each day when not smoking. On days that subjects smoked, these non-smoking assessments were constrained to fall at least 15 minutes after a smoking event.
Both smoking and non-smoking assessments covered multiple domains (see Shiffman et al., 2014c, 2014c for a complete description of assessment content). Of importance to this current study, during each assessment participants responded to a number of questions pertaining to current internal psychological state, including cigarette craving, mood, and other items relating to symptoms of nicotine withdrawal. Craving and restlessness were assessed on a 0-100 scale (ranging from NO!! to YES!!). Participants also rated overall mood and energy level, along with 14 mood-related adjectives on the same scale, specifically: able to focus; active; angry/frustrated; bored; calm/relaxed; difficulty concentrating; enthusiastic; happy; irritable; miserable; nervous/tense; quiet/sleepy; restless; and sad. Two more general items assessed bi-polar scales tapping affective tone (negative to positive) and arousal (low to high). These 16 items were summarized using factor analysis into four derived scales (as T scores: M = 50, SD = 10): Negative Affect, Positive Affect, Arousal, and Attention Disturbance (Shiffman et al., 2014c). Past studies have shown such assessments to be sensitive to withdrawal (Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006; Shiffman et al., 2006; Shiffman et al., 1995), with the exception of Arousal, which, despite a tendency to decrease during abstinence, has not demonstrated consistent response to abstinence, and is not considered part of the nicotine withdrawal syndrome (American Psychiatric Association [APA], 2013; Hughes, 2007; Hughes et al., 1990; Shiffman et al., 2006).
Data reduction & analytic plan
Using both EMA and TLFB data, we identified days on which participants were abstinent. Individual monitoring days were considered abstinence days only if both EMA and TLFB data both indicated no smoking. Days on which EMA data were available only for part of the day (e.g., the first and last days of monitoring) were excluded because unobserved smoking could have occurred. We then identified “runs” of days in which abstinence continued over consecutive days, and identified the longest run of abstinence for each participant.
In one set of analyses, smoking days were compared to abstinent days (expressed as an indicator variable) on mean craving and five withdrawal measures (restlessness, negative affect, positive affect, arousal, attention disturbance) in six separate models using Generalized Estimation Equations (GEE [Zeger, Liang, & Albert, 1988], specifying an AR(1) autoregressive covariance structure); we report the predicted means from these models along with model-based empirical standard errors. We extended these models to test for differences in mean craving and withdrawal measures by smokers’ history of daily smoking (CITS vs. NITS), by including the interaction between CITS/NITS status and abstinent day stratum. In order to determine whether ITS experienced any withdrawal at all, we also compared the levels of craving and withdrawal reported on smoking days to levels experienced by the comparison sample of DS. Separate GEE models (as above, specifying AR(1) covariance structure) assessed smoker type (ITS vs. DS) as a predictor of mean withdrawal measures (restlessness, negative affect, positive affect, arousal, attention disturbance; separate analyses) reported in non-smoking events on smoking days.
A further analysis examined trends over consecutive days of abstinence. We analyzed this in two ways. First, we began with the first day of abstinence and examined trends as time moved forward to detect changes in mean daily craving as abstinence progressed. Sequential day of abstinence within a “run” (i.e., first abstinent day=1; second abstinent day=2 etc.; modeled as a continuous variable) was used to predict craving (treated as a continuous variable; range: 0-100). Second, we began with the end of an abstinence run and analyzed craving going backwards in time. In these models, the number of days until the end of a run (i.e., last day of run=-1; second to last day of run=-2 etc.) was used to predict mean craving on each day; they examined the prediction from the Craving Cycles Hypothesis that craving would increase as one approached the resumption of smoking.
In both the forward and backward analyses, we first examined these trends in craving over time in the sample as a whole. Subsequent analyses stratified the sample by the length of participants’ longest run of abstinence (i.e., separate analysis for each group: 1 day [n = 39], 2-3 days [n = 56], 4-6 days [n = 32], and ≥ 7 days [n = 19]), to distinguish individual differences (in both duration of abstinence and in craving) from trends over time. Participants could contribute multiple runs of abstinence, varying in duration, to the dataset, but the participant-level stratification was based on the duration of each participant's longest run of abstinence. A subject-level variable indicating stratum was included in these models. In a separate analysis, we also tested for an interaction between time (days in abstinence) and stratum, to examine whether the patterns of craving over time were consistent across strata. Finally, we assessed for differences in mean craving and withdrawal measures over time across individuals’ history of daily smoking (CITS vs. NITS) by including the interaction between sequential abstinence day (and days to resumption) and participants’ CITS /NITS status within longest run strata.
Population average modeling (GEE) was used to address the primary questions regarding changes in mean daily craving and withdrawal across days (Hubbard et al., 2010). Additionally, we used hierarchical linear modeling, which partitions between- and within-subject variance (unconditional model, no covariates; SAS Proc Mixed; Singer, 1998), to compute the percent of variance in run-length that was attributable to subjects (between-person variation) versus runs-within-subjects. This analysis was limited to the 90 participants who had at least three runs in the dataset (average = 4.51 runs; SD = 1.65).
Finally, to more closely examine craving as resumption of smoking approached, we used GEE to analyze craving on the day smoking actually resumed, comparing craving reported in non-smoking assessments in the hours preceding resumption to that on the preceding day (the last day of the abstinence run), with the contrast indicated by a dummy variable (0/1). We also compared the level of craving reported at the time the first cigarette was actually smoked to the craving reported at randomly-scheduled assessments earlier on the resumption day (with the contrast indicated by a dummy variable 0/1). This analysis was limited to runs where cigarettes were recorded in real time on the resumption day (rather than only at end of day, for example), and included an indicator variable for the stratum of the participant's longest run as a covariate. We also tested for differences according to smokers’ history of daily smoking (CITS vs. NITS) in a follow-up model, by adding an indicator variable for CITS vs. NITS and its interaction with resumption day status. All analyses were conducted using SAS (version 9.3).
Results
Patterns of abstinence
During 3,104 eligible participant-days of EMA observation, we observed 1,059 participant-days of abstinence, accounting for about one-third of each participants’ days, on average (34.41%; SD = 24.49%, Mdn = 26.20%). Participants reported a total of 484 individual runs of abstinence, with an average of 3.32 runs per participant (SD = 2.00, Mdn = 3 lasting an average of 2.17 days (SD = 2.24, Mdn = 1).
Within-person variance in duration of abstinence
There was considerable variability in the duration of abstinence runs, both between- and within-subjects. Among participants with at least three individual runs, average run duration was 2.13 days, the within-subject standard deviation (across runs) averaged 1.30 days (SD = 1.19, Mdn = 0.97) and the average within-subject range was 2.72 days (SD = 2.42, Mdn = 2). To quantify the stability of run-length within persons, we computed the percent of variance in run length attributable to individuals, i.e., the proportion of between-person variance to total variance. Only 12.13% of the variance in run length was attributable to between-subject differences; that is, run length varied more than 8 times more within a given smoker than it did across smokers. This pattern held for both CITS (16.60% attributable to subjects) and NITS (6.90%).
Craving and withdrawal on abstinent days versus smoking days
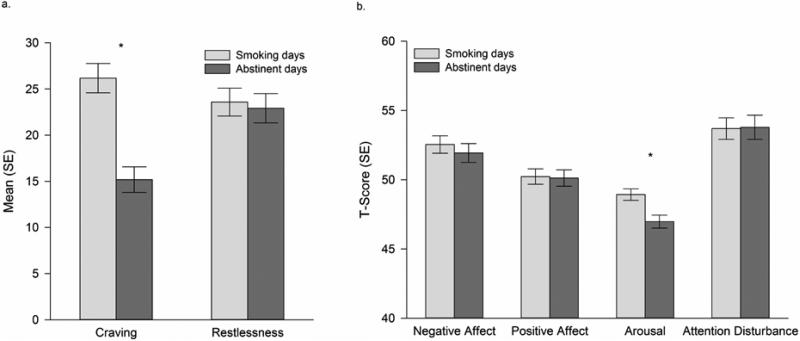
To assess the effects of abstinence on craving and withdrawal, we compared symptoms on smoking days with those on abstinent days, using symptom reports that had been collected at non-smoking times (i.e., excluding smoking occasions). The results are shown in Figure 1. Craving intensity was significantly lower (absolute difference = 10.99, on the 0-100 scale, p < .001) on abstinent days than on smoking days (Figure 1, Panel A). There was no statistically significant difference between smoking and abstinent days for affective withdrawal, restlessness, or attention disturbance (Figure 1, Panel B; absolute differences < 0.67, T-score). Arousal scores were significantly lower on abstinent days than on smoking days (absolute difference = 1.96, , T-score, p < .001). There were no differences between NITS and CITS in comparisons of abstinent and smoking days: both groups showed the same patterns evident in the group as a whole (data not shown).
Figure 1.
Symptoms reported on smoking versus abstinent days. All symptom reports were obtained at non-smoking occasions. Panel a shows raw means for craving and restlessness, which are highly-responsive to abstinence (Shiffman, Ferguson, et al., 2006). Panel b shows standardized factor scores, expressed as T-scores (M = 50, SD = 10) for dimensions relevant to nicotine withdrawal. * p < .001.
We also compared ITS’ craving and withdrawal severity on smoking days to equivalent data from a comparison sample of DS. On smoking days, ITS, on average, reported experiencing significantly lower levels of craving than DS (27.49 vs. 59.17, 0-100 scale; p < .001). The two groups did not differ in their self-reported levels of affective withdrawal, restlessness or arousal (all p-values > .13), and ITS actually reported marginally higher levels of attention disturbance on smoking days than did DS (ITS: 53.78 vs. DS: 51.67, T-scored; p = .049).
Trends over days as abstinence progressed
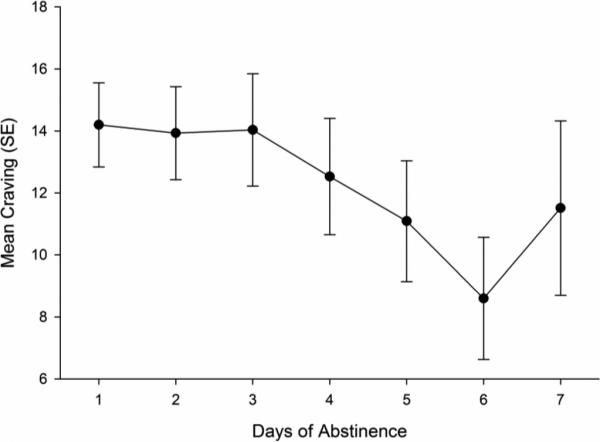
As shown in Figure 2, using data from all persons and all days, craving seemed to decrease as abstinence grew longer, with a significant linear trend over days (p < .006), and no significant quadratic trend (p > .05). While the pattern seen in Figure 2 could be due to genuine within-subjects temporal trends, it could also be due to the fact that different participants – who may differ in dependence – contributed differentially to different parts of the temporal trends, as illustrated in Table 1. For example, the estimate for Day 3 consisted of data from 77 participants (52.74% of the sample) who were observed abstaining for at least three days in a row at least once during monitoring. However, the estimate for Day 7 was based on only those 19 participants (13.04% of the sample) who were observed abstaining for at least seven days. Since participants able to abstain longer may differ from those unable to achieve longer abstinence (we have shown that they are less dependent, for example; Shiffman, Ferguson, et al., 2012), this would confound between-subject differences in dependence with within-subject temporal trends.
Figure 2.
Self-reported mean craving by day of abstinence (n = 146).
Table 1.
Sample size across consecutive days of abstinence (n = 146).
| Duration of Abstinence | Number of participants contributing any data at this duration | Number of subject-days of data at this duration | Number of observations at this duration |
|---|---|---|---|
| 1 | 146 | 484 | 1710 |
| 2 | 107 | 210 | 774 |
| 3 | 77 | 120 | 460 |
| 4 | 51 | 73 | 246 |
| 5 | 37 | 47 | 170 |
| 6 | 31 | 35 | 133 |
| 7 | 19 | 20 | 79 |
| 8-10 | 35æ | 13† | 47† |
| 11-15 | 15æ | 4† | 15† |
| 16-23(max) | 3æ | 2† | 5† |
Notes:
Participant count reflects the number of participants contributing data to any of the durations of abstinence within the grouping
Entries are the average number of subject-days / observations in available for analysis in each day in the run of abstinence.
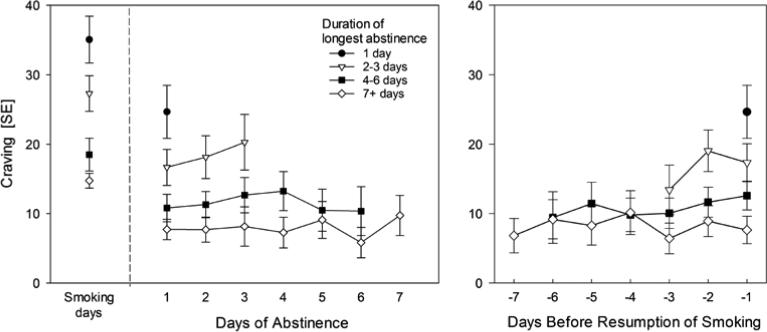
Accordingly, we stratified the sample of participants based on their longest run of abstinence (1 day, 2-3 days, 4-6 days, ≥ 7 days), and then analyzed and plotted the temporal trends within these participant strata, using data from all days and all runs, including those that were shorter than the participant's longest run. To ensure adequate sample size of days (see Table 1), we truncated the length of runs at seven days. Figure 3 (Panel A) reveals how these analyses distinguished between- and within-subject variation. To analyze the influence of between-subject differences in craving, we compared craving reported on the very first day of abstinence in each run (thus holding time constant and including data from all participants) across groups of participants that differed in maximum run-length (grouped by strata, as shown in Figure 3). Participants who abstained longer showed lower craving even on the very first day of their runs (Means: 1 day: 24.66, 2-3 days: 16.66, 4-6 days: 10.81, ≥ 7 days: 7.72; p < .001); the strata also differed in terms of their dependence, with dependence generally decreasing as the longest run of abstinence increased, but most of the differences distinguishing only the group that abstained for one day from those who abstained seven days or more (Table 2). The apparent decrease in craving seen in Figure 2 is due to the progressive decrease over time in the proportion of the participants who reported longer runs of abstinence (Table 1); that is, it is due to between-subject differences, not within-subject changes over time.
Figure 3.
Both panels stratify participants (not runs themselves, or days) by the longest run of abstinence evident in the EMA data. Panel A shows craving intensity over days of abstinence, running forward from the first day of a run of abstinence, as well as showing the average craving intensity reported by each stratum during their smoking days. Panel B shows craving intensity over days as the resumption of smoking approaches (i.e., smoking occurred on Day 0).
Table 2.
Baseline nicotine dependence by longest run strata (n = 146).
| Longest Run of Abstinence Observed During Monitoring | ||||
|---|---|---|---|---|
| Dependence Measure | 1 Day (n = 39) | 2-3 Days (n = 56) | 4-6 Days (n = 32) | ≥ 7 Days◆ (n = 19) |
| Cigarettes/day (on smoking days) | 5.31 (2.78)** | 3.87 (3.16) | 3.99 (3.27) | 2.77 (1.98) |
| HONC (range 0 – 10) | 5.22 (2.31)* | 4.03 (2.86) | 3.79 (2.89) | 3.54 (2.80) |
| HONC=0 | 3.24% | 12.87% | 9.98% | 15.21% |
| FTND (range 0 – 10) | 1.38 (1.44) | 0.76 (1.23) | 0.52 (1.11) | 0.79 (1.36) |
| FTND=0 | 40.42% | 60.06% | 74.93% | 68.96% |
| NDSS (z score) | −1.99 (0.56)** | −2.22 (0.56) | −2.37 (0.43) | −2.45 (0.39) |
| WISDM | ||||
| Primary (range 1 – 7) | 2.46 (0.89)*** | 1.95 (0.95)* | 1.66 (0.57) | 1.45 (0.49) |
| Secondary (range 1 – 7) | 2.86 (0.88)** | 2.64 (0.86)* | 2.47 (0.82) | 2.15 (0.83) |
Notes: Numbers represent Mean (SD), except where percentages are indicated. All analyses weighted by race. HONC = Hooked on Nicotine Checklist. FTND = Fagerström Test of Nicotine Dependence. NDSS = Nicotine Dependence Syndrome Scale. WISDM = Wisconsin Inventory of Smoking Dependence Motives (separate scores for the Primary and Secondary Dependence Motives). Differences between the strata were tested with ANOVA, comparing each group to the group whose longest abstinence was ≥ 7 Days).
p < .05
p < .01
p < .001.
Reference group for contrasts.
To assess changes over time, we analyzed the temporal trends within these participant strata, and found no within-subjects temporal trends over time in any stratum (all p values > .32; absolute differences across strata scores < 3.93). Within strata, we also examined whether the temporal trends differed between CITS and NITS (that is, a time X group [CITS/NITS] interaction). There was a difference in the trends only among participants in the stratum with the longest runs (at least seven days; p < .05): within this stratum, NITS showed a significant decline in craving over days (p < .05), whereas CITS’ craving did not show any reliable trend (p > .15).
Trends over days as resumption approached
We also analyzed the same data while construing time as running backwards from the day of smoking resumption (i.e., with days classified as being one day before resumption, two days before resumption, etc.; Figure 3, Panel B). This would capture rising craving as smoking resumption approached (as posited by the Craving Cycles Hypothesis). As in the earlier analysis, participants who abstained longer showed lower craving even on the day immediately preceding the end of a run of abstinence (Means: 1 day: 24.66, 2-3 days: 17.32, 4-6 days: 12.58, ≥ 7 days: 7.64; p < .001). This analysis also showed no temporal trend – within strata, there were neither linear (all p values > .16; absolute differences across strata < 5.64, 0-100 scale) nor quadratic (all p values > .21) trends indicating changes in craving leading up to the day smoking was resumed. Again, CITS and NITS differed (time X group interaction) only within the stratum of subjects who reported at least seven days of abstinence (p < .002). Separate analyses for CITS and NITS (within this stratum) showed opposite trends: over days, craving was decreasing among CITS (p < .025) but increasing among NITS (p < .023).
Craving on the day of resumption and time of resumption
A final set of analyses examined the course of craving as the run of abstinence ended and smoking resumed. We compared craving reported on the last day of abstinence with craving on the day smoking resumed, but during the period before the first cigarette was actually smoked (by at least one hour). The analyses further examined the craving reported at the time the first cigarette was actually smoked. On the day of resumption, prior to smoking, craving was as low (18.54 [SE = 2.17]) as that on the last day of abstinence (15.35 [SE = 2.03], p > .10), but then rose substantially and significantly at the moment of smoking resumption (55.88 [SE = 2.54], p < .001). In other words, craving increased at the moment of smoking, but not before. This pattern was similar for both for NITS and CITS, except that craving before smoking was lower (p < .001) for CITS (14.63 [SE = 2.83]) compared to NITS (24.17 [SE = 3.37]) and the rise in craving at the moment of smoking resumption was greater for CITS than NITS (to 59.03 [SE = 3.14] vs. 52.92 [SE = 4.28]; difference in change from pre-smoking craving, p < .01).
Discussion
This study of smoking and abstinent days among ITS confirms that they do not show signs of dependence expected under the classic nicotine-maintenance model of dependence. Having observed that ITS abstain from smoking for days at a time, we tested whether they ‘paid the price’ for abstinence in craving and withdrawal. The results were clear: when ITS abstained, there were no increases in the core affective symptoms of withdrawal, and craving actually was lower than on days when they had smoked, not higher. There was also no evidence that they suffer a withdrawal syndrome during periods of abstinence on the days that they do smoke. On the days they were smoking, when assessed during periods of abstinence, their craving was also lower than that of DS smoking ad libitum, and their affective distress was no different. Thus, ITS seem not to have developed typical dependence, despite substantial nicotine exposure (Shiffman et al. 2014a), perhaps due to protection by genetic or other factors (Bierut, et al., 2007; Chen et al., 2012; Lerman & Berrettini, 2003). Genetic differences between ITS and dependent DS should be examined as one way of gaining insight into genetic vulnerability to nicotine dependence.
If ITS are not dependent, and are not motivated to smoke by a need to limit the negative experience of withdrawal (that is, they are not “trough-avoiders,” to use Russell's (1971) terminology), they may instead be motivated to smoke in order to experience the rewarding acute effects of nicotine (Russell's “peak-seekers”). If ITS experience little craving or withdrawal during periods of abstinence, it could suggest that their smoking is driven not by fear of the negative consequences of abstinence, but instead by a desire to experience the positive rewards of smoking.
The absence of dependence, and the putative role of peak-seeing does not explain why ITS’ craving would actually decrease, rather than stay the same, on days they abstained. One explanation is that the act of smoking itself may incite – or prime – craving. While smoking has been shown to immediately relieve craving (Jarvik et al., 2000; Perkins, Karelitz, Conklin, Sayette & Giedgowd, 2010; Rose, Behm, Westman, Bates, & Salley, 2003), smoking (and drug use in general) can also prime further use (de Wit, 1996; Shaham, Adamson, Grocki, & Corrigall; Shaham et al., 2003), and, likely, craving. As this account already assumes an initial smoking episode, it is incomplete, however.
Further explanation may rest on the effect of exposure to stimuli that may prompt smoking or that have come to acquire stimulus control over smoking through conditioning. We have shown that, across individuals, ITS’ smoking is tied to particular situations and settings (Shiffman et al., 2014c), such as when others are smoking and when ITS are drinking. Beyond these linkages to particular stimuli across smokers, analyses have demonstrated even stronger linkages when idiographic or idiosyncratic stimulus links are considered, taking into account that different individuals may respond to different stimuli. These analyses show that ITS' (Shiffman, Dunbar, & Ferguson, 2015; Shiffman & Paty, 2006) smoking is under very tight stimulus control indeed; for example, analysis showed that one could predict whether ITS were smoking (versus being in a randomly-selected non-smoking situation) from EMA measures of their situational context with 95% accuracy. Considering that the EMA assessment would inevitably be incomplete (e.g., not distinguishing between one friend and another who may have different histories and different associations for the smoker) this is remarkably tight stimulus control. This suggests that ITS may experience the impulse to smoke – expressed as craving – only, or at least primarily, when they encounter particular stimuli, and this may help explain the variation in the length of their runs of abstinence and the drops in craving during those periods.
The explanation of ITS smoking patterns based on stimulus control does not rest solely on data about smoking patterns in this sample of ITS. The role of smoking cues and stimulus control has also been amply documented among very light smokers (“Chippers”; Shiffman & Paty, 2006), and even among DS, whose smoking is also associated with alcohol or coffee consumption, the presence of other smokers, and other cues (Shapiro et al., 2002; Shiffman et al., 2002; Shiffman et al., 2014c; Thrul, Buhler, & Ferguson, 2014). The role of such cues is demonstrably even stronger in relation to relapse when smokers are abstinent (Ferguson & Shiffman, 2009; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996); we have suggested that the power of stimulus control over daily and dependent smokers is partially masked by ongoing smoking, and re-emerges in abstinence, particularly after initial withdrawal symptoms start to subside (Shiffman et al., 2015). Laboratory experiments using cue reactivity methods (Carter & Tiffany, 1999) have also documented the role of cueing stimuli in eliciting craving and smoking. Nor is the role of cues and stimulus control limited to smoking: similar relationships have been documented for other drug use, both in the field (Epstein et al., 2009; Hopper et al., 2006; Hussong, Hicks, Levy, & Curran, 2001) and in the lab (see Carter & Tiffany, 1999). In animal studies, where experimental control is possible, studies have demonstrated that pairing cues with drug availability creates very strong stimulus control, and, particularly in the case of nicotine, cues are capable of sustaining self-administration behavior (Caggiula et al., 2001) and of triggering reinstatement after extinction (Liu, Caggiula, Palmatier, Donny, & Sved, 2008), which has been seen as a model for relapse (Shaham & Miczek, 2003). Thus, a robust body of literature supports the importance of stimulus control as an important influence on drug self-administration in general and on smoking in particular.
Of course, other processes may also be operative in determining when ITS smoke or crave smoking. Stimulus control and priming processes may act synergistically: conditioned cues or discriminative stimuli for smoking may trigger initial smoking, which in turn may prime further smoking, perhaps leading to bouts of concentrated smoking.
Also, the fact that the duration of runs of abstinence was related to psychometric measures of dependence (see Shiffman Ferguson et al., 2012) and correlated with craving even on the first non-smoking day suggests that some modicum of dependence also plays a role, even in these non-daily smokers. These findings are consistent with both the classical model of dependence and with the Sensitization-Homeostasis Model (DiFranza & Wellman, 2005). They are harder to explain on the basis of priming or stimulus control, since craving should rise in response to situational cues or priming stimuli and not from individual differences in internal drives. This suggests that ITS’ smoking is not wholly explained by peak-seeking, but by some mix of situational peak-seeking and dependence motives. This suggests that the ITS may be vary substantially in the motives that drive and maintain their smoking, with some showing signs of developing dependence, and some being more cue-driven.
Although ITS as a group were demonstrably not dependent in the sense of suffering craving and withdrawal when abstinent, the influence of stimulus control, via conditioned cues and priming stimuli, may itself exert a degree of control over ITS’ smoking behavior that amounts to a kind of dependence of a different type. Consistent with this, in analyses of ITS’ profiles on the WISDM (Shiffman, Dunbar, Scholl, & Tindle, 2012), we found that ITS had relatively higher scores on what Piasecki, Piper, & Baker (2010) have called “secondary” dependence motives – those not associated with traditional dependence processes such as craving and withdrawal-relief. Indeed, strikingly, ITS’ two highest scores were on scales assessing smoking in response to cues, including social cues. This stimulus-control-based dependence may help explain why ITS’ smoking behavior is so persistent and resistant to change despite the absence of classical dependence.
Arousal was also lower on non-smoking days. Arousal is not consistently affected by nicotine withdrawal (APA, 2013; Hughes, 2007; Hughes et al., 1990; Shiffman et al., 2006), but nicotine is known to affect arousal (Parrott, 1998), and it has been proposed that smokers might smoke in order to manage arousal (Frith, 1971). This might explain why ITS are more likely to smoke under high-arousal conditions (Shiffman et al., 2014c). Accordingly, the lower arousal observed on non-smoking days may reflect the absence of situations that raise arousal and trigger smoking, rather than withdrawal, or the absence of nicotine effects, per se. We examined trends in craving over consecutive days of abstinence. After accounting for between-subject variations in the maximum duration of abstinence, craving did not increase as abstinence progressed. That ITS can go for days without experiencing craving is consistent both with a peak-seeking model of ITS and with the Craving Cycles Hypothesis, which posits that individuals exhibit a need to smoke at characteristic intervals, and that these intervals can be quite long at early stages of dependence. However, the latter model implies that one should see craving rise as each smoker's interval comes to an end. We did not observe increased craving as a run of abstinence ended (Figure 3, Panel B); a finding that is at odds with this theory's predictions. The Craving Cycles Hypothesis allows for some cigarettes to be smoked even when they are not needed – i.e., with no craving – but that does not seem to be the case here, as we saw craving rise again at the time ITS actually resumed smoking. This pattern – no trend in craving approaching the smoking day, but an acute rise when smoking resumes – is more consistent with stimulus-control or priming models of smoking than with a model positing a characteristic interval between cigarettes. To be clear, these data do not explicitly demonstrate the role of stimulus control in ITS’ smoking – that is evident in other analyses showing that ITS’ smoking is more strongly related to a variety of stimuli (Shiffman et al., 2014c) – but they are compatible with it.
We did see some limited differences in patterns in craving over time among CITS and NITS (as indicated by group-by-time interactions), but only among those who had abstained for at least seven days during EMA monitoring. NITS who had abstained for seven days or more showed decreasing craving over the first seven days of abstinence. Examining trends on craving as resumption of smoking approached, we found that CITS’ craving was decreasing, while NITS’ craving was increasing. These patterns do not seem consistent with the Craving Cycles Hypothesis; one would have expected CITS, who are more dependent (Shiffman, Ferguson, et al., 2012) to be especially likely to show increasing craving. In any case, these differences between CITS and NITS who abstained for seven days or more should be interpreted cautiously, as they are based on a small number of select subjects (eight CITS and 11 NITS) and observations.
The Craving Cycles Hypothesis also posits that, at any one time in a smoker's development of dependence, each individual smoker has a particular interval at which he or she needs to smoke (DiFranza et al., 2011), implying that periods of abstinence should be roughly consistent in duration within individuals. We did not observe this. Rather, participants’ periods of abstinence ranged widely; indeed, by far most of the variation (> 85%) in duration of abstinence was within persons, not between persons. Even what little temporal consistency of smoking was observed could be due to exogenous factors, such as regular recurrence of certain cues, such as socializing and drinking on weekends, rather than to the endogenous cycles posited by the Craving Cycles Model.
The Craving Cycles Model proposes to explain some of the variation in the timing of smoking via two processes (DiFranza & Ursprung, 2008; DiFranza et al., 2010; DiFranza et al., 2011). First, smokers might decide not to smoke even if their craving has risen, though this is said to lead to intensification of craving (DiFranza et al., 2010; DiFranza et al., 2011). However, we showed that craving did not rise, either in the days leading up to resumption, or even on the day of resumption itself (prior to resumption), until the actual moment of resumption. The theory also allows that smokers might smoke ‘electively’ even if they have no craving (DiFranza et al., 2011). This would imply that the cigarettes that terminated runs of abstinence would be marked by little or no craving, but that is not what we observed: participants reported considerable craving when they smoked that first cigarette after a run of abstinence. Thus, the data contradict the posited stability of intervals of craving and smoking.
It is important to emphasize that the ITS studied here were experienced smokers whose behavior would be expected to conform to models of dependence. They had been smoking for over a decade and reported having consumed over 40,000 cigarettes, on average. Further, although they were not dependent according to most measures and theories, almost all (89%) were considered dependent according the HONC, using criteria associated with the DiFranza et al's model, which also posits that dependence can begin with the first cigarette (Scragg, Wellman, Laugesen, & DiFranza, 2008). Thus, these smokers’ behavior would have been expected to conform to the model.
Although ITS do not seem to fit the template for dependence in either the classical withdrawal-avoidance model nor the Sensitization-Homeostasis Model, they nevertheless show what is perhaps the most important characteristic or product of dependence – an inability to quit. ITS make frequent quit attempts (indeed, more frequently than daily smokers), and their failure rate is only slightly lower than that of daily smokers (Tindle & Shiffman, 2011). Put another way, their pattern of smoking is sufficient to keep them smoking for years (14.32 years, on average, in this sample) despite multiple quit attempts (4.35 attempts, on average, in this sample). It is not fully clear how to explain this persistence. Some ITS do seem to demonstrate some degree of dependence, with slightly elevated scores on traditional psychometric measures of dependence, and the data presented show that these individuals demonstrate shorter periods of abstinence (see also Shiffman, Ferguson, et al., 2012) and more intense craving when they abstain for even one day. Yet, this does not seem adequate to explain the overwhelming rates of failure at quitting among ITS as a whole. Some of the explanation may lie in the strong linkages between their smoking and environmental stimuli (Shiffman et al., 2014c) that may elicit or prime smoking. Exposure to proximal cues plays a very prominent role in triggering lapses that lead to relapse even among heavy and dependent smokers (Ferguson & Shiffman, 2010; Shiffman et al., 1996). They may play an even more prominent role in making quitting difficult for ITS.
A potential limitation to our study is that it is based on self-reported data. Smokers in this study self-reported smoking status; as such, it is possible that the classification of some subjects as non-daily smokers was incorrect. Days that we have categorized as non-smoking / abstinence days may actually have been days when participants were simply non-compliant with reporting. However, to minimize the likelihood of this, we cross-checked smoking data to reconcile realtime EMA cigarette counts with TLFB reported at study visits. Our observations were typically based on three weeks of smoking behavior, and we observed a limited number of runs of abstinence, though there is little reason to think that this affected the estimate of within-person stability.
In summary, this study documented that ITS regularly and voluntarily undergo periods of abstinence without experiencing craving or withdrawal. Indeed, craving was actually considerably lower on abstinent days than on smoking days. There were also no consistent trends towards increasing symptoms over multi-day runs of abstinence, even among CITS, who had previously smoked daily. Among ITS who had multiple runs of abstinence, there was little consistency in the length of these runs within individuals. These findings are at odds with the Craving Cycles Hypothesis, but are consistent with a stimulus control model that asserts that ITS are peak-seekers who smoke for the acute effects of nicotine at particular times in response to particular contexts.
Lay summary.
It had been hypothesized that even non-daily intermittent smokers (ITS) need to smoke at regular intervals characteristic of the particular smoker. This study contradicts that hypothesis by showing that craving is lower on days ITS abstain, and does not begin to rise even as renewed smoking approaches. Also, the duration of runs of abstinence varied more within ITS individuals than across individuals.
Acknowledgments
This work was supported by grant R01-DA020742 (Shiffman) from the National Institutes of Health, National Institute on Drug Abuse. Additional support for authors was provided by Cancer Council Tasmania (Ferguson), and by grant 1R01DA034629-01A1 (Tindle). The authors are grateful to Sarah Scholl for overseeing conduct of the study, to Neha Mehta for preparation of data displays, and to Alexandra Cardy for editorial assistance.
References
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders. Fifth ed. Washington, DC: 2013. [Google Scholar]
- Audrain-McGovern J, Al Koudsi N, Rodriguez D, Wileyto EP, Shields PG, Tyndale RF. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119(1):e264–274. doi: 10.1542/peds.2006-1583. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Cigarette smoking and nicotine addiction. The Medical clinics of North America. 1992;76(2):415–437. doi: 10.1016/s0025-7125(16)30360-1. [DOI] [PubMed] [Google Scholar]
- Benowitz N. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clinical Pharmacology and Therapeutics. 2008;83(4):531–541. doi: 10.1038/clpt.2008.3. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacology Biochemistry and Behavior. 2001;70(4):515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. doi: 10.1046/j.1360-0443.1999.9433273.x. [PubMed] [Google Scholar]
- Centers for Disease Conrol and Prevention . Behavioral Risk Factor Surveillance System Survey Data. U.S. Department of Health and Human Services; 2012. [2013 Nov 22]. from http://apps.nccd.cdc.gov/brfss/ [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of current cigarette smoking among adults and changes in prevalence of current and some day smoking — United States, 1996 – 2001. Morbidity and Mortality Weekly Report. 2003;52:303–307. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Cigarette smoking among adults -- United States, 2007. Morbidity and Mortality Weekly Report. 2008;57(45):1221–1226. [PubMed] [Google Scholar]
- Chen LS, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, Bierut LJ. Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine & Tobacco Research. 2012;14(4):425–433. doi: 10.1093/ntr/ntr231. doi: 10.1093/ntr/ntr231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrandpre RJ, Bickel WK. Stimulus control and drug dependence. The Psychological Record. 1993;43(4):651–666. [Google Scholar]
- de Wit H. Priming effects with drugs and other reinforcers. Experimental and Clinical Psychopharmacology. 1996;4(1):5–10. [Google Scholar]
- DiFranza JR, Ursprung WW. The latency to the onset of nicotine withdrawal: a test of the sensitization-homeostasis theory. Addict Behav. 2008;33(9):1148–1153. doi: 10.1016/j.addbeh.2008.04.011. doi: 10.1016/j.addbeh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Ursprung WW, Carson A. New insights into the compulsion to use tobacco from an adolescent case-series. Journal of Adolscence. 2010;33(1):209–214. doi: 10.1016/j.adolescence.2009.03.009. doi: 10.1016/j.adolescence.2009.03.009. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ. A sensitization-homeostasis model of nicotine craving, withdrawal, and tolerance: integrating the clinical and basic science literature. Nicotine Tob Res. 2005;7(1):9–26. doi: 10.1080/14622200412331328538. doi: 10.1080/14622200412331328538. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Wellman RJ, Mermelstein R, Pbert L, Klein JD, Sargent JD, Winickoff JP. The natural history and diagnosis of nicotine addiction. Current Reviews in Pediatrics. 2011;72(2):88–96. [Google Scholar]
- Donny EC, Dierker LC. The absence of DSM-IV nicotine dependence in moderate-to-heavy daily smokers. Drug and Alcohol Dependence. 2007;89(1):93–96. doi: 10.1016/j.drugalcdep.2006.11.019. doi: 10.1016/j.drugalcdep.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Archives of General Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128(4):408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36(3):235–243. doi: 10.1016/j.jsat.2008.06.005. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. Effect of high-dose nicotine patch on the characteristics of lapse episodes. Health Psychology. 2010;29:358–366. doi: 10.1037/a0019367. doi: 10.1037/a0019367. [DOI] [PubMed] [Google Scholar]
- Fernando WWSA, Wellman RJ, DiFranza JR. The relationship between level of cigarette consumption and latency to the onset of retrospectively reported withdrawal symptoms. Psychopharmacology. 2006;188(3):335–342. doi: 10.1007/s00213-006-0497-x. doi: DOI 10.1007/s00213-006-0497-x. [DOI] [PubMed] [Google Scholar]
- Freeman TJ, Lattal KA. Stimulus control of behavioral history. Journal of the Experimental Analysis of Behavior. 1992;57(1):5–15. doi: 10.1901/jeab.1992.57-5. doi: 10.1901/jeab.1992.57-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD. Smoking behaviour and its relation to the smoker's immediate experience. British Journal of Social and Clinical Psychology. 1971;10:73–78. doi: 10.1111/j.2044-8260.1971.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Sidney S, Gfroerer JC, O'Malley PM, Allen JA, Richter PA, Cummings KM. Epidemiology of menthol cigarette use. Nicotine and Tobacco Research. 2004;6(Suppl 1):S67–81. doi: 10.1080/14622203710001649696. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Goedeker KC, Tiffany ST. On the nature of nicotine addiction: A taxometric analysis. Journal of Abnormal Psychology. 2008;117:896–909. doi: 10.1037/a0013296. doi: 10.1037/a0013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Su Z, Looby AR, Ryan ET, Penetar DM, Palmer CM, Lukas SE. Incidence and patterns of polydrug use and craving for ecstasy in regular ecstasy users: An ecological momentary assessment study. Drug and Alcohol Dependence. 2006;85(3):221–235. doi: 10.1016/j.drugalcdep.2006.04.012. doi: 10.1016/j.drugalcdep.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Hubbard AE, Ahern J, Fleischer NL, Van der Laan M, Lippman SA, Jewell N, Satariano WA. To GEE or not to GEE: comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology. 2010;21(4):467–474. doi: 10.1097/EDE.0b013e3181caeb90. doi: 10.1097/EDE.0b013e3181caeb90. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9(3):315–327. doi: 10.1080/14622200701188919. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami DK, Kozlowski LT, Annis HM, Cappell HD, Vingilis ER. Effects of abstinence from tobacco: A critical review Research advances in alcohol and drug problems. Plenum Publishing; New York: 1990. pp. 317–398. [Google Scholar]
- Hussong AM, Hicks RE, Levy SA, Curran PJ. Specifying the relations between affect and heavy alcohol use among young adults. Journal of Abnormal Psychology. 2001;110(3):449–461. doi: 10.1037//0021-843x.110.3.449. doi: 10.1037//0021-843X.110.3.449. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000;66(3):553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kalton G. SAGE University Paper series on Quantitative Applications in the Social Sciences. SAGE Publications, Inc; Beverly Hills and London: 1983. Introduction to Survey Sampling. series no 07-035. [Google Scholar]
- Korhonen T, Broms U, Levalahti E, Koskenvuo M, Kaprio J. Characteristic and health consequences of intermittent smoking: Long-term follow-up among Finnish adult twins. Nicotine and Tobacco Research. 2009;11(2):148–155. doi: 10.1093/ntr/ntn023. doi: 10.1093/ntr/ntn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Herman CP. The interaction of psychosocial and biological determinants of tobacco use: More on the Boundary Model. Journal of Applied Social Psychology. 1984;14(3):244–256. doi: 10:-1111/j.15559-1816.1984.tb02234x. [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135(2):169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Lerman C, Berrettini W. Elucidating the role of genetic factors in smoking behavior and nicotine dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2003;118B(1):48–54. doi: 10.1002/ajmg.b.10003. doi: 10.1002/ajmg.b.10003. [DOI] [PubMed] [Google Scholar]
- Lindstrom M, Ostergren P. Intermittent and daily smokers: Two different socioeconomic patterns, and diverging influence of social participation. Tobacco Control. 2001;10:258–266. doi: 10.1136/tc.10.3.258. doi: 10.1136/tc.10.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Sved AF. Cue-induced reinstatement of nicotine-seeking behavior in rats: effect of bupropion, persistence over repeated tests, and its dependence on training dose. Psychopharmacology (Berl) 2008;196(3):365–375. doi: 10.1007/s00213-007-0967-9. doi: 10.1007/s00213-007-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press; New York, N.Y.: 1985. [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, Abrams DB. Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology. 1988;97(2):133–152. doi: 10.1037//0021-843x.97.2.133. [DOI] [PubMed] [Google Scholar]
- Parrott A. Nesbitt's Paradox resolved? Stress and arousal modulation during cigarette smoking. Addiction. 1998;93:27–39. doi: 10.1046/j.1360-0443.1998.931274.x. doi: 10.1046/j.1360-0443.1998.931274.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz J, Conklin CA, Sayette M, Giedgowd G. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychiatry. 2010;67:707–714. doi: 10.1016/j.biopsych.2009.12.017. doi: 10.1016/j.biopsych.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Piper ME, Baker T. Refining the tobacco dependence phenotype using the Wisconsin Inventory of Smoking Dependence Motives: II. Evidence from a laboratory self-administration assay. Journal of Abnormal Psychology. 2010;119(3):513–523. doi: 10.1037/a0020235. doi: 10.1037/a0020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–29. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Bates JE, Salley A. Pharmacologic and sensorimotor components of satiation in cigarette smoking. Pharmacology Biochemistry and Behavior. 2003;76(2):243–250. doi: 10.1016/j.pbb.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Russell MAH. Cigarette smoking: The natural history of a dependence disorder. British Journal of Medical Psychology. 1971;44:1–16. doi: 10.1111/j.2044-8341.1971.tb02141.x. doi: 10.1111/j.2044-8341.1971.tb02141.x. [DOI] [PubMed] [Google Scholar]
- Scragg R, Wellman RJ, Laugesen M, DiFranza JR. Diminished autonomy over tobacco can appear after the first cigarette. Addictive Behaviors. 2008;33(5):689–698. doi: 10.1016/j.addbeh.2007.12.002. doi: 10.1016/j.addbeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Adamson LK, Grocki S, Corrigall WA. Reinstatement and spontaneous recovery of nicotine seeking in rats. Psychopharmacology (Berl) 1997;130(4):396–403. doi: 10.1007/s002130050256. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Miczek KA. Reinstatement - toward a model of relapse. Psychopharmacology. 2003;168(1-2):1–2. doi: DOI 10.1007/s00213-003-1469-z. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168(1-2):3–20. doi: 10.1007/s00213-002-1224-x. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119(3):334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shapiro D, Jamner LD, Davtdov DM, James W. Situations and moods associated with smoking in everyday life. Psychology of Addictive Behavior. 2002;16(4):342–345. doi: 10.1037//0893-164x.16.4.342. doi: 10.1037/0893-164X.16.4.342. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Smoking cessation attempts and use of smoking cessation treatments in the United States. American Journal of Prevention Medicine. 2008;34:102–111. doi: 10.1016/j.amepre.2007.09.033. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Benowitz NL. A comparison of nicotine biomarkers and smoking patterns in daily and non-daily smokers. Cancer Epidemiology, Biomarkers, & Prevention. 2014a;23(7):1264–1272. doi: 10.1158/1055-9965.EPI-13-1014. doi: 10.1158/1055-9965.epi-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Ferguson SG. Stimulus control in intermittent and daily smokers. Psychology of Addictive Behaviors. 2015 doi: 10.1037/adb0000052. doi: 10.1037/adb0000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Craving in intermittent and daily smokers during ad libitum smoking. Nictotine and Tobacco Research. 2014b doi: 10.1093/ntr/ntu023. doi: 10.1093/ntr/ntu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, Ferguson SG. Smoking patterns and stimulus control in intermittent and daily smokers. PLoS ONE. 2014c;9(3):e89911. doi: 10.1371/journal.pone.0089911. doi: 10.1371/journal.pone.0089911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA. Smoking motives of daily and non-daily smokers: A profile analysis. Drug and Alcohol Dependence. 2012;126:362–368. doi: 10.1016/j.drugalcdep.2012.05.037. doi: 10.1016/j.drugalcdep.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Dunbar MS, Scholl SM. Tobacco dependence among intermittent smokers. Nicotine & Tobacco Research. 2012;14(11):1372–1381. doi: 10.1093/ntr/nts097. doi: 10.1093/ntr/nts097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis M, Shadel W. Reduction of abstinence-induced withdrawal and craving using high dose nicotine replacement therapy. Psychopharmacolgy. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. doi: 10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, Gnys M. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. Journal of Abnormal Psychology. 2002;111(4):531–545. doi: 10.1037//0021-843x.111.4.531. doi: 10.1037/0021-843X.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards T. Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology. 1996;64:993–1002. doi: 10.1037//0022-006x.64.5.993. doi: 10.1037/0022-006X.64.5.993. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Patten CA, Gwaltney CJ, Paty JA, Gnys M, Kassel JD, Balabanis M. Natural history of nicotine withdrawal. Addiction. 2006;101:1822–1832. doi: 10.1111/j.1360-0443.2006.01635.x. doi: 10.1111/j.1360-0443.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. Journal of Abnormal Psychology. 2006;115(3):509–523. doi: 10.1037/0021-843X.115.3.509. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Elash C. Nicotine withdrawal in chippers and regular smokers: Subjective and cognitive effects. Health Psychology. 1995;14(4):301–309. doi: 10.1037//0278-6133.14.4.301. doi: 10.1037//0278-6133.14.4.301. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JD, Hickcox M. First lapses to smoking: Within-subjects analyses of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. doi: 10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. doi: 10.1037/1064-1297.2.2.126. [Google Scholar]
- Shiffman S, Scharf DM, Shadel WG, Gwaltney CJ, Dang Q, Paton SM, Clark DB. Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology. 2006;74(2):276–285. doi: 10.1037/0022-006X.74.2.276. doi: 10.1037/0022-006X.74.2.276. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Experimental and Clinical Psychopharmacology. 2012;20(4):264–277. doi: 10.1037/a0027546. doi: 10.1037/a0027546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. doi: 10.2307/1165280. [Google Scholar]
- Sobell MB, Sobell LC, Maisto SA. Time-Line Follow-Back assessment method [TLFB]. In: Lettieri DJ, Nelson JE, Sayers MA, editors. Alcoholism Treatment Assessment Research Instruments. (NIAAA Ttreatment Handbook Series, Vol 2) National Institute on Alcoholism and Alcohol Abuse; Rockville, MD: 1979. pp. 167–188. [Google Scholar]
- Substance Abuse and Mental Health Services Administration [2013 Nov 22];Results from the 2012 National Survey on Drug Use and Health: National Findings. 2013 from http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/DetTabs/NSDUH-DetTabsSect6peTabs1to54-2012.htm#Tab6.5B. [PubMed]
- Thrul J, Buhler A, Ferguson SG. Situational and mood factors associated with smoking in young adult light and heavy smokers. Drug and Alcohol Review. 2014;33(4):420–427. doi: 10.1111/dar.12164. doi: 10.1111/dar.12164. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug use behavior: Role of automatic and non-automatic processes. Psychological Review. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tindle HA, Shiffman S. Smoking cessation behavior among intermittent smokers versus daily smokers. American Journal of Public Health. 2011;101(7):e1–3. doi: 10.2105/AJPH.2011.300186. doi: 10.2105/AJPH.2011.300186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Perez-Stable EJ, Emery SL, White MM, Grana RA, Messer KS. Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine & Tobacco Research. 2009;11:203–210. doi: 10.1093/ntr/ntn018. doi:10.1093/ntr/ntn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman RJ, DiFranza JR, Savageau JA, Godiwala S, Friedman K, Hazelton J. Measuring adults' loss of autonomy over nicotine use: The Hooked on Nicotine Checklist. Nicotine and Tobacco Research. 2005;7:157–161. doi: 10.1080/14622200412331328394. doi: 10.1080/14622200412331328394. [DOI] [PubMed] [Google Scholar]
- World Health Organization [Jan 4 2008];WHO global infobase on-line. 2007 from World Health Organization www.who.iint/infobase/report.aspx.
- Zeger SL, Liang K, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]