Abstract
We aimed to identify brain functional correlates of working memory performance in aging, in hopes of facilitating understanding of mechanisms that promote better versus worse working memory in late-life. Among 64 healthy adults, aged 23 to 78, we examined the relationship between age, working memory performance, and brain functional response during task performance. We focused on the association between working memory load-modulated functional response and individual differences in performance and whether these function-performance relationships differed with age. As expected, older age was associated with poorer working memory performance. Older age was also associated with reduced load-modulated activation including in bilateral prefrontal and parietal regions and left caudate as well as reduced deactivation including in the medial prefrontal cortex. Contrary to findings of hyperactivation in aging, we found no evidence of increased activation with older age. Positive associations identified between brain response and performance did not differ with age. Our findings suggest that the neural mechanisms underlying better versus worse working memory performance are age-invariant across adulthood, and argue against a pattern of functional reorganization in aging. Results are discussed within the broader literature, in which significant heterogeneity in findings between studies has been common.
Keywords: Aging, Working memory, Cognition, Functional neuroimaging, fMRI, Individual differences
INTRODUCTION
Working memory declines, on average, in aging (Park et al., 2002). Yet, decline may not be inevitable, motivating the study of neural mechanisms underlying individual differences in working memory performance in aging. The aging brain may show functional reorganization, recruiting different regions than the young to perform tasks. Although it remains unclear whether such changes are beneficial for performance (i.e., compensatory), older adults have shown patterns of increased bilateral activation during task performance, known as hemispheric asymmetry reduction in older adults (HAROLD) (Cabeza, 2002), and greater frontal and reduced occipital activation, known as posterior–anterior shift in aging (PASA) (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). Additionally, older adults appear deficient in “turning off” the default-mode network during task performance (Grady, Springer, Hongwanishkul, McIntosh, & Winocur, 2006).
Studies of working memory have yielded complex results regarding the effect of age on brain function. Some have supported HAROLD (Reuter-Lorenz et al., 2000; Sala-Llonch et al., 2012), with age-related increases in bilateral frontal activation. Within the dorsolateral prefrontal cortex, some studies found hyperactivation in aging (reviewed in Rajah and D’Esposito, 2005), but others found reduced (e.g., Rypma, Prabhakaran, Desmond, & Gabrieli, 2001) or equivalent activation in older versus younger adults (Rypma, Berger, Genova, Rebbechi, & D’Esposito, 2005). The effect of age on activation differs even between subregions of the prefrontal cortex (Rajah & D’Esposito, 2005) and by working memory load (Cappell, Gmeindl, & Reuter-Lorenz, 2010; Nagel et al., 2011; Prakash, Heo, Voss, Patterson, & Kramer, 2012). Older adults may show hyperactivation at low loads, hypoactivation at higher loads, or a lack of loadmodulated enhancement in activation in frontal and parietal regions. Less extensively studied, age-related alterations in working memory-related deactivation include reduced default-mode network deactivation (Prakash et al., 2012; Sala-Llonch et al., 2012; Sambataro et al., 2010).
Examining functional correlates of performance and whether function-performance relationships differ with age is needed to understand whether unique neural mechanisms support better versus worse cognition in late-life. Few studies of working memory have assessed individual differences in aging, with no consensus on the nature of function-performance relationships or whether associations differ with age. Some found the same “greater activation, better performance” relationship in younger and older adults (Nagel et al., 2011), others found this association in younger but not older adults (Prakash et al., 2012) or only in older adults with greater activation being detrimental in younger adults (Rypma, Eldreth, & Rebbechi, 2007). Evidence suggests a “greater deactivation, better performance” relationship in younger and older adults (Sambataro et al., 2010), this holding in older but not younger adults (Prakash et al., 2012), or even that high-performing older adults activate default-mode regions (Sala-Llonch et al., 2012). Notably, only one of these studies (Nagel et al., 2011) statistically tested for an interaction between age and function in predicting performance.
We investigated how individual differences in load-modulated activation and deactivation relate to working memory performance in aging. We hypothesized that older age would be associated with increased prefrontal activation, and that older adults who showed greater prefrontal activation would perform relatively better, while greater prefrontal activation would be detrimental for performance in younger adults. We predicted older adults would show reduced deactivation in default-mode regions (e.g., medial prefrontal cortex and posterior cingulate), and that there would be a stronger positive relationship between deactivation and performance with older age. Our study extends the literature by investigating individual differences across the adult lifespan and statistically testing whether function-performance relationships differ by age to infer whether there are unique neural mechanisms underlying better versus worse performance in late-life.
METHODS
Participants
Participants were 64 healthy adults (ages 23–78; 63% female; 80% Caucasian; education: mean = 15.5 years, SD = 2.3) from three prior studies in which individuals completed functional magnetic resonance imaging (fMRI) during an N-back working memory task. Exclusion criteria common across studies included contraindications for MRI, left-handedness, loss of consciousness >15 min, or self-reported history of an Axis I disorder.
Procedures
Demographics and health status were obtained via interview or questionnaires. Neuroimaging data were collected at the UCSD Keck Center for Functional Magnetic Resonance Imaging. All but six participants completed a verbal intelligence (VIQ) measure [the American National Adult Reading Test (Grober, Sliwinski, & Korey, 1991) or North American Adult Reading Test (Blair & Spreen, 1989)]. All participants provided written informed consent to the study in which they enrolled. The original studies, and this secondary analysis of combined data, were approved by the UCSD Institutional Review Board and/or the VA San Diego Healthcare System’s R&D Committee as appropriate.
Working Memory Task
The N-back task is a block-design fMRI paradigm. Single letters appeared on the screen for 1 s, followed by an asterisk for 1.5 s. During “0-back,” participants were to press a button when the letter “X” appeared. During 1-, 2-, and 3-back conditions, participants were to press a button when the current letter matched that presented one, two, or three letters before. Each block contained 11 letters (three targets). Responses were recorded throughout the 2.5-s trial interval. Two of the original studies used a version with four blocks of all conditions. One study used a version with six blocks of 0-back, five blocks each of 1- and 2-back, but no blocks of 3-back. The N-back task was otherwise identical across studies; the same stimuli (i.e., letters of identical font, size, and color) were presented on the same background, following the same presentation rate, using the same button box device for response. We aimed to capture working memory performance on the most challenging condition all participants completed, thus we examined d’ on the 2-back condition, a signal detection metric that incorporates “hits” and false positives (higher d’ = better performance), and well as mean 2-back reaction time (RT).
Neuroimaging Procedures
Images were collected on one of two 3-Tesla General Electric magnetic resonance scanners with an 8-channel head coil. A high-resolution T1-weighted anatomical image was collected, and blood-oxygen-level-dependent (BOLD) signal during the N-back task was measured using gradient echo echoplanar imaging. Functional scan parameters varied between studies (see Appendix 1), and one study used two variations of scan parameters. To account for these differences, a variable reflecting the study each individual participated in was included as a covariate (detailed below).
Data Analysis
Neuroimaging data analysis
AFNI software (Cox, 1996) was used to process the fMRI data. Field map corrections were applied, if available (see Appendix 1). For all functional data, a 6mm FWHM spatial filter was applied and individual’s functional data were aligned to their anatomical scan and transformed into Talairach space. We visually examined each participant’s data for motion and censored out time-points with visually obvious motion. To be included in the present study, participants were required to have visually obvious motion in no more than one-third of time-points. For examination of functional response, we used a general linear model with a baseline and linear trend plus regressors for trial type (0-, 1-, 2-, or 3-back)/(0-, 1-, or 2-back) and parameters to account for residual motion. We calculated brain response during the 1- and 2-back conditions compared to 0-back as baseline (i.e., 1>0 and 2>0). We created a map of the fit coefficient for the contrast between the 2-back and 1-back conditions (i.e., 2>1). We aimed to examine the load-modulated brain response specific to the most challenging condition (i.e., 2-back), thus the 2>1 fit coefficient maps serve as the focus of our analyses.
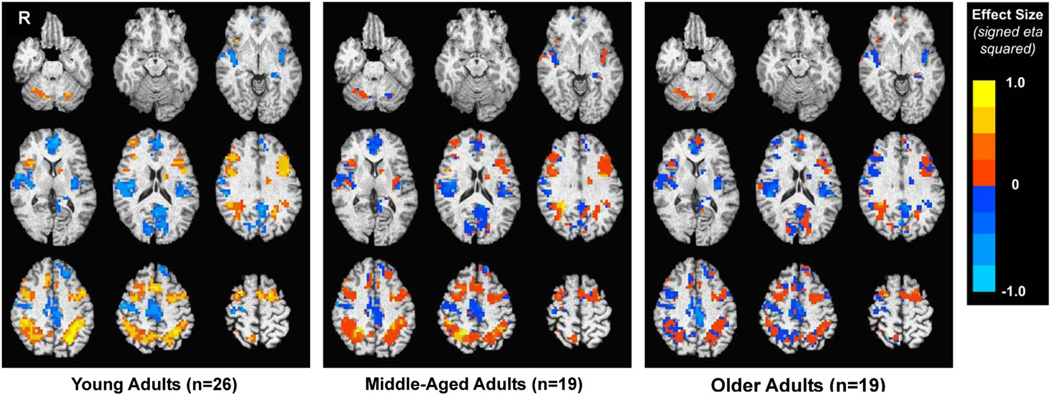
Regions-of-interest (ROIs) were created based on areas that were task-relevant in any age group, using the 2> 1 fit coefficient maps. With AFNI program 3dttest + +, we conducted one-sample t tests separately for young (23- to 39-year-olds), middle-aged (40- to 59-year-olds), and older adults (≥60-year-olds) to reveal clusters significantly activated (i.e., positive fit coefficient) or deactivated (i.e., negative fit coefficient) in each group (p <.01; cluster size = 22 voxels determined with AlphaSim given 4-mm3 voxels and 6-mm FWHM blur), controlling for the effect of study. Then, we created a mask combining these clusters, resulting in 15 ROIs (see Figure 1 and Table 1) that were activated or deactivated in one or more age group. We created our ROIs in this manner to facilitate our ability to detect regions that might be differentially activated or deactivated in different ages, given the possibility that older adults might recruit different regions than younger individuals.
Fig. 1.
ROIs and the unthresholded one-sample t test results (2> 1 Contrast) shown only within the ROIs, separately by age group. ROIs were created by combining clusters that were task-activated or deactivated (2> 1) in any age group, controlling for study.
Table 1.
Association between Functional Response in each ROI with Age and Working Memory Performance
| 2back > 1back ROIs |
# Voxels | Center of mass (xyz) |
Mean fit coefficient (Beta) |
Association between Age and Function (Standardized Regression Coefficient) |
Association between 2back > 1back Function and Performance (Standardized Regression Coefficient) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 > 0 | 2 > 0 | 2 > 1 | 1 > 0 | 2 > 0 | 2 > 1 | 2-back d’ | 2-back RT | ||||||
| Task-activated ROIs | |||||||||||||
| L prefrontal (IFG, MFG, SFG) | 427 | 25.8 | −8.5 | 41.5 | .05 | .17 | .12 | −.01 | −.42** | −.39** | .28 | −.24 | |
| R parietal (IPL, SPL) | 399 | −31.4 | 54.2 | 41.5 | .04 | .18 | .14 | .12 | −.31* | −.42** | .34* | −.23 | |
| L parietal (IPL, SPL) | 284 | 31.5 | 53.7 | 40.7 | .02 | .15 | .14 | .06 | −.37** | −.41** | .31* | −.22 | |
| R prefrontal (IFG, MFG) | 205 | −34.1 | −5.7 | 43.8 | .05 | .16 | .11 | .10 | −.28* | −.33* | .35* | −.25 | |
| R prefrontal (MFG) | 42 | −39.5 | −28.0 | 28.4 | .08 | .20 | .12 | −.14 | −.49** | −.36* | .40** † | −.26 | |
| R cerebellum | 35 | −25.7 | 62.9 | −22.6 | −.01 | .16 | .17 | .03 | .11 | .09 | −.06 | −.10 | |
| R prefrontal (MFG) | 32 | −35.4 | −48.0 | 18.0 | .10 | .23 | .13 | .11 | −.24 | −.40** | .21 | −.11 | |
| L cerebellum | 29 | 26.7 | 64.2 | −24.6 | .03 | .13 | .11 | .08 | .02 | −.03 | .07 | −.15 | |
| R insula & IFG | 25 | −35.6 | −18.8 | 7.4 | .07 | .16 | .09 | .04 | −.28* | −.34* | .18 | −.10 | |
| L caudate | 24 | 15.2 | 1.8 | 19.0 | −.02 | .03 | .05 | .09 | −.28* | −.38** | .22 | −.17 | |
| Task-deactivated ROIs | |||||||||||||
| B cingulate, posterior cingulate gyrus, & cuneus | 423 | 1.3 | 48.1 | 31.2 | −.13 | −.22 | −.09 | .19 | .37* | .34* | −.23 | .04 | |
| R insula & precentral gyrus | 420 | −44.5 | 15.8 | 25.1 | −.04 | −.14 | −.10 | .31* | .39** | .28 | −.11 | .01 | |
| B medial frontal gyrus | 211 | 5.8 | −45.4 | 22.3 | −.16 | −.28 | −.12 | .23 | .45** | .44** | −.33* | .09 | |
| L insula | 149 | 39 | 18.6 | 11.8 | −.03 | −.12 | −.09 | .38** | .31* | .07 | −.16 | .02 | |
| L hippocampus & parahippocampal gyrus | 33 | 22.6 | 36.5 | −0.7 | −.07 | −.12 | −.05 | .12 | .33* | .32* | −.27 | .04 | |
Note: Standardized regression coefficients for age and performance associations are adjusted for study.
p <.05.
Significant after Bonferroni correction.
Scatterplot of association shown in Supplementary Figure 1.
R = right, L = left, B = bilateral, IPL = inferior parietal lobule, IFG = inferior frontal gyrus, MFG = middle frontal gyrus, SFG = superior frontal gyrus.
Statistical Analysis
We evaluated whether participant characteristics/performance differed by study (by means of analysis of variance or χ2 tests) or were associated with age (by means of t tests or correlations) to understand potential confounds. Using regression models controlling for study, we tested whether our sample showed the expected age-associated poorer working memory d’ and increased RT. We then conducted regression models to investigate associations between functional response (i.e., fit coefficient for 1> 0, 2 >0, and 2> 1) within our 15 ROIs and (1) age, (2) d’, and (3) RT, respectively (controlling for study). To protect against multiple comparisons, we applied a Bonferroni correction to each set of these analyses (Bonferroni correction for bivariate associations: p = .05/15 = .0033). To investigate whether the relationship between load-modulated functional response (i.e., 2> 1) and performance differed by age, we conducted a series of regression models with either d’ or RT as the outcome and the following predictors: (1) study, (2) age, (3) 2 >1 ROI functional response, and (4) age × ROI response, applying the same Bonferroni correction.
RESULTS
Supplementary Tables 1 and 2 detail participant characteristics/ performance by study and age. Although older age was associated with higher VIQ (r = .28; p = .03), VIQ was unassociated with N-back performance (d’: r = .07; p = .61; RT: r = .04; p = .76), thus we did not include VIQ as a covariate.
Older age was associated with worse and slower N-back performance (d’: β = −.42; p = .001; RT: β = .38; p = .002). As shown in Table 1, older age was associated with reduced 2>1 activation in task-activated ROIs, particularly in bilateral prefrontal and parietal regions and left caudate (where age associations survived Bonferroni correction). Older age was also associated with reduced 2>1 deactivation in task-deactivated ROIs, particularly within bilateral medial frontal gyrus. ROIs with age-associated reductions in activation/ deactivation showed performance associations with d’ but not RT, with a positive association between right prefrontal activation and d’ surviving Bonferroni correction (see scatterplot in Supplementary Figure 1). Regression models testing whether associations between ROI functional response and performance differed with age revealed no significant age × ROI interactions (all interaction terms p>.05). (All β-values reported here and in Table 1 are standardized regression coefficients).
DISCUSSION
We predicted older adults would show greater prefrontal activation during a working memory task than younger adults, in line with previous studies showing hyperactivation in aging. Instead, older age was associated with decreased load-modulated activation, particularly in bilateral prefrontal and parietal regions and left caudate. A possible explanation for this finding is that older adults in our study did not achieve comparable overall performance levels to younger individuals. Working memory performance can be impacted by age-related reductions in working memory capacity and older adults’ increased susceptibility to interference (Gazzaley, Sheridan, Cooney, & D’Esposito, 2007). Thus, older adults may have recruited fewer functional resources in correspondence with these deteriorations. Although not universally observed, other studies of working memory have found age-related decreases in load-modulated prefrontal and/or parietal activation (Cappell et al., 2010; Nagel et al., 2011; Prakash et al., 2012; Rypma et al., 2001). Some of these (e.g., Cappell et al., 2010) did find age-related hyperactivation at low loads (when older adults performed as well as younger adults), suggesting the aging brain has a limited ability to functionally compensate. However, our results diverge from these as well, as we found no age-related hyperactivation even at the lowest load. Although reasons for such heterogeneous findings remain unclear, a recent study suggests that age differences in functional patterns may depend on the specific working memory component process being examined. Namely, Macpherson et al. (2014) found age-related frontal hyperactivation during working memory encoding, but frontal hypoactivation in older age during retrieval. The latter pattern mirrors our results, raising the possibility that our study may have best captured functional correlates of retrieval rather than encoding. We are unable to tease apart these components in our study, but the potential for differential age effects by working memory component warrants additional research.
In addition to task activation, we examined task-related deactivation, motivated by the growing evidence of the importance of default-mode network function. As hypothesized, older age was associated with reduced load-modulated deactivation during working memory performance, most strongly within the medial prefrontal cortex, the frontal hub of the default-mode network. Our findings support the notion that older adults are less capable of suppressing default-mode function while engaging in a cognitive task.
Our direct statistical testing for interactions revealed no evidence that the relationship between working memory performance and brain function differs with age. Our findings of a lack of hyperactivation in aging and a lack of unique function-performance relationships suggest there are not functional changes that come online in aging in response to a declining working memory system. Instead, we found that greater right prefrontal activation was the strongest predictor of better versus worse performance (d’), regardless of age. We found no functional associations with RT; it may be that the functional correlates of performance speed are not well-captured by our a priori focus on regions involved in load-modulated brain response.
Our conclusions are limited by our cross-sectional design and combination of similar samples that differed in some respects, and the effects of which may not have been entirely removed by treating “study” as a covariate. We cannot rule out the possibly that our results were influenced by altered hemodynamic response in aging, as we did not measure resting cerebral blood flow nor did we find regions of hyperactivation with aging. However, using the contrast between BOLD signal response to 2-back versus 1-back as we did makes it more likely our findings reflect age differences in task-related response rather than neurovascular differences (D’Esposito, Deouell, & Gazzaley, 2003).
The literature on brain functional correlates of working memory in aging is characterized by highly variable findings. Although our study cannot explain this heterogeneity, several factors may be important to consider in future research. It is currently difficult to compare findings between studies, given the diversity in methods used. In addition to differences in the component processes of working memory investigated (e.g., encoding, maintenance, retrieval), studies vary in the types of tasks used (e.g., N-back tasks, recognition-based tasks, spatial tasks) and the working memory loads examined. This variability likely gives rise to different patterns of functional response and varying associations between function with age and performance. Diverse analytical approaches also induce variability, such as block design versus event-related studies, differences in fMRI preprocessing, ROI versus whole-brain analyses, and whether or not statistical analyses directly test for interaction effects. Future research in this field would benefit from standardization of methods and study replication, so that we might be able to draw clearer conclusions about the neural mechanisms that promote better versus worse working memory ability in aging.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by R01 MH083968 awarded to Dr. Eyler, R01 AG024506 awarded to Dr. Drummond, and the NIMH T-32 Fellowship in Geriatric Mental Health (T32 MH019934) awarded to Dilip V. Jeste, M.D. Infrastructure support was provided to one of the studies by the VA San Diego Center for Excellence in Stress and Mental Health. Writing of this manuscript was supported by Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the San Francisco Veterans Affairs Medical Center, and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC).
APPENDIX 1
Functional Scan Parameters by Study
| Study | n | Scanner | Functional scan parameters |
|---|---|---|---|
| 1 | 9 | 1 | 32 slices, 195 reps, slice thickness = 4 mm, TR = 2500 ms, TE = 32, flip angle = 90°, echo = 30 ms. Field maps collected and applied to correct for image distortion.** |
| 2 | 29 | 2 | 32 slices, 195 reps, slice thickness = 4 mm, TR = 2500 ms, TE = 32, flip angle = 90°, echo = 30 ms. Field maps collected and applied. |
| 3a | 15 | 2 | 32 slices, 195 reps, slice thickness = 4 mm, TR = 2500 ms, TE = 30, flip angle = 90°, echo = 30 ms. Field maps not collected. |
| 3b | 11 | 2* | 32 slices, 195 reps, slice thickness = 4 mm, TR = 2500 ms, TE = 30, flip angle = 90°, echo = 30 ms. Field maps were not collected. |
Except for one participant on scanner 1.
Field map corrections not available for two individuals due to technical error.
Footnotes
The authors have no conflicts of interest to disclose.
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1355617714000824
REFERENCES
- Blair JR, Spreen O. Predicting premorbid IQ: A revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3(2):129–136. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46(4):462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4(11):863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21(5):532. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neuroscience. 2006;18(2):227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M, Korey SR. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Macpherson HN, White DJ, Ellis KA, Stough C, Camfield D, Silberstein R, Pipingas A. Age-related changes to the neural correlates of working memory which emerge after midlife. Frontiers in Aging Neuroscience. 2014;6:70. doi: 10.3389/fnagi.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li S, Nyberg L, Backman L, Lindenberger U, Heekeren HR. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. Journal of Cognitive Neuroscience. 2011;23(8):2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17(2):299–320. [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: Implications for cognitive performance. Behavioural Brain Research. 2012;230(1):192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of Cognitive Neuroscience. 2000;12(1):174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D’Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41:582–594. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: A multiple component analysis. Cortex. 2007;43:65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychology and Aging. 2001;16(3):371–384. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R, Arenaza-Urquijo EM, Valls-Pedret C, Vidal-Piñeiro D, Bargalló N, Junqué C, Bartrés-Faz D. Dynamic functional reorganizations and relationship with working memory performance in healthy aging. Frontiers in Human Neuroscience. 2012;6:152. doi: 10.3389/fnhum.2012.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambataro F, Murty VP, Callicott JH, Tan HY, Das S, Weinberger DR, Mattay VS. Age-related alterations in default mode network: Impact on working memory performance. Neurobiology of Aging. 2010;31(5):839–852. doi: 10.1016/j.neurobiolaging.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.