Abstract
Although prospective memory (PM) is compromised in mild cognitive impairment (MCI), it is unclear which specific cognitive processes underlie these PM difficulties. We investigated older adults’ performance on a computerized event-based focal versus nonfocal PM task that made varying demands on the amount of attentional control required to support intention retrieval. Participants were nondemented individuals (mean age = 81.8 years; female = 66.1%) enrolled in a community-based longitudinal study, including those with amnestic MCI (aMCI), nonamnestic MCI (naMCI), subjective cognitive decline (SCD), and healthy controls (HC). Participants included in the primary analysis (n = 189) completed the PM task and recalled and/or recognized both focal and nonfocal PM cues presented in the task. Participants and their informants also completed a questionnaire assessing everyday PM failures. Relative to HC, those with aMCI and naMCI were significantly impaired in focal PM accuracy (p < .05). In a follow-up analysis that included 13 additional participants who successfully recalled and/or recognized at least one of the two PM cues, the naMCI group showed deficits in nonfocal PM accuracy (p < .05). There was a significant negative correlation between informant reports of PM difficulties and nonfocal PM accuracy (p < .01). PM failures in aMCI may be primarily related to impairment of spontaneous retrieval processes associated with the medial temporal lobe system, while PM failures in naMCI potentially indicate additional deficits in executive control functions and prefrontal systems. The observed focal versus nonfocal PM performance profiles in aMCI and naMCI may constitute specific behavioral markers of PM decline that result from compromise of separate neurocognitive systems.
Keywords: Memory, Prospective, Cues, Mild cognitive impairment, Subjective cognitive decline, Executive functions, Aging
INTRODUCTION
Prospective memory (PM), or memory for future actions, supports critical everyday tasks such as medication adherence (Park & Kidder, 1996). Previous research (Blanco-Campal, Coen, Lawlor, Walsh, & Burke, 2009; Costa et al., 2010; Karantzoulis, Troyer, & Rich, 2009; Schmitter-Edgecombe, Woo, & Greeley, 2009; Thompson, Henry, Rendell, Withall, & Brodaty, 2010; Troyer & Murphy, 2007) reports that PM is impaired in individuals with mild cognitive impairment (MCI) relative to cognitively healthy older adult controls, leading to the idea that PM dysfunction may be a marker of prodromal dementia. Recent meta-analytic results confirm the presence of large PM deficits in MCI and dementia relative to controls (van den Berg, Kant, & Postma, 2012), indicating that PM impairment is already well established by the MCI stage. Although the utility of PM-based measures has been confirmed in the neuropsychological evaluation of MCI (van den Berg et al., 2012) and PM markers may potentially yield diagnostic value for pre-MCI conditions, there is presently no consensus regarding what constitutes an optimal PM assessment.
PM is a multifaceted cognitive construct thought to rely on both retrospective memory and executive control functions. Retrospective memory supports the retrospective component of PM that is involved in encoding and retrieving the content of future intentions, while executive control functions mediate the prospective component of PM that assists in retrieving and initiating PM intentions at the proper moment (Einstein & McDaniel, 1990, 1996). The retrospective and prospective components of PM, respectively, are linked to the medial temporal lobe (MTL) and prefrontal systems (Burgess, Quayle, & Frith, 2001; West & Krompinger, 2005); these brain systems are associated with structural and functional brain changes in MCI and dementia (Bell-McGinty et al., 2005; Masdeu, Zubieta, & Arbizu, 2005; Scheltens, 2009). The complex nature of PM tasks (e.g., involving multiple cognitive systems), along with their reliance on neurocognitive systems that are highly vulnerable to age-related neuropathology, may account for the sensitivity of these tasks to preclinical dementia reported by some researchers (Blanco-Campal et al., 2009; Loewenstein et al., 2013). As PM mainly differs from retrospective memory in its additional dependence on internal control mechanisms (Craik, 1983, 1986), examining the prospective component as distinct from the retrospective component may provide insight on the unique diagnostic and predictive utility of PM-based tasks in distinguishing pathological and normal cognitive aging. The Multiprocess Theory of PM (McDaniel & Einstein, 2000; McDaniel, Guynn, Einstein, & Breneiser, 2004) posits that successful PM relies on both goal-directed processes, such as effortful strategic monitoring, and automatic processes, such as spontaneous retrieval, to support the prospective component of PM (i.e., to retrieve the PM intention when the PM cue is detected). Specifically, depending on the relation between the PM cue and the ongoing activity, either strategic monitoring will be required (i.e., when the PM cue is indistinct with regard to the ongoing activity) or spontaneous retrieval will be relied upon (i.e., when the PM cue is distinct with regard to the ongoing activity) to support PM.
In accordance with the Multiprocess Theory, a focal versus nonfocal test approach may be optimal for investigating the neurocognitive networks and brain systems that mediate the prospective component (Costa, Caltagirone, & Carlesimo, 2011; McDaniel, Shelton, Breneiser, Moynan, & Balota, 2011; Tam & Schmitter-Edgecombe, 2013). In a focal PM task, the ongoing activity directs focal attention to the PM cue and therefore places little executive control demand on the test-taker. For example, when the PM cue is a word embedded within an ongoing word-categorization task, both the cue and the words in the ongoing task are processed as single lexical units and the recruitment of high-level cognitive resources is not required for cue detection. Conversely, in a nonfocal PM task, the ongoing activity does not direct focal attention to the PM cue. Therefore, attentional processes are required for cue detection and the task poses a relatively higher level of executive control demand. For example, when the PM cue is a syllable embedded within a word-categorization task, the ongoing task does not support processing of syllables as it instead supports processing of words. In this case, strategic monitoring is necessary for cue detection to support retrieval of the PM intention. Focal PM accuracy is linked to spontaneous retrieval and the MTL system, while nonfocal PM accuracy is linked to strategic monitoring and other internal control mechanisms mediated by the prefrontal system (McDaniel et al., 2011; Reynolds, West, & Braver, 2009). Because a focal versus nonfocal PM paradigm allows the exploitation of either automatic or strategic processes based on the relationship of the PM cue to the ongoing task—if the retrospective component of PM (e.g., encoding, maintaining PM cue in memory) is intact, then this test approach permits examination of the neurocognitive systems that distinctly mediate the prospective component.
To our knowledge, only three studies in MCI and very mild dementia (Blanco-Campal et al., 2009; McDaniel et al., 2011; Tam and Schmitter-Edgecombe, 2013) use a focal versus nonfocal task approach to evaluate PM, and results differ. Blanco-Campal et al. (2009) used an event-based focal PM task in which the level of cognitive demand was manipulated by varying both the specificity of instructions and cue saliency. Half of the PM cues (words) were presented in plain text and the other half in italicized text (e.g., low/high saliency) within silly sentences (ongoing task), and participants were asked to say, “animal” (PM intention), whenever lion (PM cue) appeared in a sentence (focal condition) or whenever any animal (a total of 10 different animal names) appeared in a sentence (nonfocal condition). All conditions except the salient-specific condition showed greater power and sensitivity over traditional retrospective memory tests in differentiating amnestic mild cognitive impairment (aMCI) from healthy controls (HC). However, the non-salient non-specific condition (highest executive control demand) demonstrated the greatest discriminatory power. These findings emphasize the diagnostic value of PM-based assessments, particularly those that place a high demand on strategic attentional resources, and suggest the presence of early executive functioning difficulties in aMCI.
McDaniel et al. (2011) used a category-decision activity (ongoing task) in which either the target word (focal PM cue) or target syllable (nonfocal PM cue) was embedded as the exemplar in word-pair trials. Participants were to press a certain key when the target word or syllable appeared respectively in the focal or nonfocal condition (3 PM trials occurred in a total of 109 trials in each PM condition). Relative to controls, individuals with very mild Alzheimer’s disease (AD) (i.e., those with a clinical dementia rating of 0–0.5, which probably included some MCI individuals) were impaired on focal but not on nonfocal PM tasks, suggesting impairment of spontaneous retrieval processes but spared strategic monitoring processes in those with very mild AD. Although the authors reported that a possible floor effect for the nonfocal condition may have obscured detection of performance differences between the clinical and control groups, results from the within-subjects analyses of response latency and accuracy provided support that strategic monitoring was engaged in the nonfocal condition but not in the focal condition, as a latency cost of the PM tasks on ongoing activity performance (e.g., decreased speed) was only observed in the nonfocal condition. The different findings reported by Blanco-Campal et al. (2009) and McDaniel et al. (2011) may be due to differences in task features, with the former study possibly presenting higher PM and working memory demands because it required participants to respond to a greater number of PM cues.
Tam and Schmitter-Edgecombe (2013) found that, relative to controls, those with aMCI performed significantly worse on a nonfocal PM task in which the PM cue (background pattern) was embedded within a working memory task. There is evidence that PM and working memory systems compete for attentional resources in high (but not low) demand conditions (Pino, Poletti, & Caffarra, 2013). Therefore, similar to Blanco-Campal et al. (2009), this task may have also posed a greater demand on available attentional resources as compared with the test used by McDaniel et al. (2011). Results further revealed that although the nonfocal PM accuracy score in the control group correlated with neuropsychological tests of attention, working memory and executive functioning, the nonfocal accuracy score in the aMCI group was more strongly associated with tests of memory and language. The authors concluded that, although both retrospective and executive processes are required in the task, PM deficits observed in aMCI may be more attributable to impaired spontaneous retrieval processes and MTL structures than executive control dysfunction (Tam & Schmitter-Edgecombe, 2013).
Costa and colleagues (2011) suggest that inconsistent findings related to the nature of the cognitive difficulties underlying PM deficits in MCI may be due to the heterogeneity of PM tasks used across studies (e.g., time-based vs. event-based, PM vs. retrospective memory, laboratory-based vs. ecologically-orientated), variations in how MCI was classified, and, in some studies, the inclusion of different MCI subtypes within the same test group. For example, Thompson et al. (2010) combined individuals with aMCI and nonamnestic MCI (naMCI) into one group, even though aMCI is generally considered to be prodromal AD (Petersen, 2004) and naMCI, particularly of multiple domains, may convert to non-memory related dementias (Busse, Hensel, Guhne, Angermeyer, & Riedel-Heller, 2006; Petersen et al., 2001; see Petersen & Negash, 2008). Differential patterns of PM decline potentially associated with aMCI and naMCI remain to be clarified. Moreover, the fairly pronounced PM deficit in MCI raises questions about the possibility that subtle PM deficits may be present in pre-MCI conditions such as subjective cognitive decline (SCD).
The current study investigates the specific cognitive processes that underlie PM failures in the preclinical stages of dementia. Our study uses a focal versus nonfocal PM test that is already represented in the literature (Foster, McDaniel, Repovš, & Hershey, 2009; McDaniel et al., 2011), thereby reducing the attribution of potential group differences to differences in task features. The first hypothesis is that relative to healthy older adult controls (HC), those with aMCI (i.e., prodromal AD) will exhibit deficits only in focal PM accuracy, suggesting impairment of spontaneous retrieval processes and the MTL system (and spared strategic monitoring processes). We base this prediction on reported impairment of spontaneous retrieval processes (Blanco-Campal et al., 2009; Costa et al., 2010; Karantzoulis, Troyer, & Rich, 2009; Schmitter-Edgecombe et al., 2009; Thompson et al., 2010; Troyer & Murphy, 2007) and functional and structural aberrations of the MTL system in aMCI (Nickl-Jockschat et al., 2012). Although executive dysfunction has been reported in aMCI, in line with McDaniel et al. (2011), we do not expect to see a deficit in nonfocal PM in the aMCI group using this task, particularly as recent research conducted with a mixed group of single- and multiple-domain aMCI participants (Tam & Schmitter-Edgecombe, 2013) suggests lower PM performance (relative to HC) may be related to deficits in spontaneous processes and a compromised MTL system.
The second hypothesis is that, relative to HC, those with naMCI will show deficits in focal and nonfocal PM accuracy, suggesting dual impairment of spontaneous retrieval and strategic monitoring processes. This finding would suggest that both MTL and prefrontal structures are compromised in naMCI. This hypothesis stems from previous research such as Costa et al. (2010) who compared the retrospective and prospective components of PM using time- and event-based PM conditions. The time-based prospective component relied more on strategic monitoring (e.g., monitor the clock and execute the PM intention at the assigned times) and the event-based task relied more on spontaneous retrieval (e.g., execute PM intentions when bell rings). While both naMCI and aMCI groups performed significantly worse than HC, naMCI performed significantly worse than aMCI on the prospective component of the time-based task (highest executive demand condition). Therefore, it is reasonable to expect aMCI and naMCI to differ in nonfocal PM performance even if they both have deficits in spontaneous processes relative to HC.
The next hypothesis aims to determine whether subtle PM deficits are present in participants with subjective cognitive decline (SCD), despite otherwise normal performance on neuropsychological tests, who may represent a pre-MCI condition. Research supports the idea that SCD in otherwise cognitively healthy older adults may be linked to biomarkers consistent with AD pathology (Amariglio et al., 2012; Kryscio et al., 2014; Mosconi et al., 2008; Saykin et al., 2006; Scheef et al., 2012; Visser et al., 2009) and associated with the risk of future decline (Dufouil, Fuhrer, & Alperovitch, 2005; Jessen et al., 2010; Kryscio et al., 2014; Mitchell, Beaumont, Ferguson, Yadegarfar, & Stubbs, 2014; Reisberg, Shulman, Torossian, Leng, & Zhu, 2010; van Oijen, de Jong, Hoffman, Koudstaal, & Breteler, 2007). As PM deficits in MCI are reported to be comparable to those found in dementia (van den Berg et al., 2012), very early PM impairment may potentially be present in pre-MCI conditions. Relative to retrospective episodic memory tasks, PM tasks are more reliant on complex metacognitive activity and internal control mechanisms (Henry, MacLeod, Phillips, & Crawford, 2004; McDaniel & Einstein, 2011; Salthouse, Berish, & Siedlecki, 2004). Additionally, recent research found lower performance on a long-term, naturalistic PM task in SCD as compared to HC (Rabin et al., 2014). The engagement of multiple cognitive operations required by PM-based assessments may render them more sensitive, as compared to traditional retrospective memory tasks, to early or subtle cognitive deficits. Therefore, our third hypothesis is that participants with SCD will show significantly lower focal PM functioning than HC.
Because a decrease in processing speed is expected when cognitive resources are recruited to support controlled attentional processes (strategic monitoring) but not automatic processes (spontaneous retrieval), the fourth hypothesis is that the nonfocal (but not the focal) task will have a “latency cost” (Smith, 2003) or “slowing effect” (Einstein et al., 2005) on ongoing task performance and that this latency cost will be observed only in groups without strategic monitoring deficits. Specifically, spontaneous monitoring (revealed by cost) is expected to be intact in HC, thereby reducing the cognitive resources available to support the ongoing task when the nonfocal PM cue is present. This results in slower processing speeds on the ongoing task. As executive control problems are not a primary characteristic of the HC, SCD, and aMCI groups, we expect spontaneous monitoring to be intact for these groups. Thus, the mean ongoing task latency is predicted to be significantly longer in the nonfocal condition in comparison to the control condition for HC, SCD, and aMCI, while mean ongoing latencies in the focal and control conditions should not significantly differ. For the naMCI group, however, spontaneous monitoring deficits (e.g., due to executive dysfunction) should disrupt nonfocal PM accuracy. Therefore, we expect to see an absence of cost on ongoing task performance in naMCI, resulting in no significant differences in mean ongoing task latencies between the control and nonfocal conditions and the control and focal conditions.
Finally, we explore the possibility that laboratory-based PM performance will correspond to real-world PM functioning (Schmitter-Edgecombe et al., 2009; Twamley et al., 2008; Woods et al., 2008). The fifth hypothesis is that lower scores on the PM task will be associated with higher incidences of everyday PM difficulties on self and/or informant reports.
METHODS
Participants and Procedures
Participants were originally 208 nondemented older adults recruited from the Einstein Aging Study (EAS), a community-based longitudinal study of cognitive aging in older adults aged 70 and above residing in the Bronx, NY. Participants were recruited through systematic sampling from Medicare or voter registration lists (Lipton et al., 2003; Katz et al., 2012). EAS exclusion criteria include severe audiovisual impairment and medical conditions that prevent successful completion of neuropsychological assessment, non-English speaking, and being homebound. We did not approach EAS participants with dementia and clinically significant depression. After study initiation, we excluded 6 participants who failed to recognize any PM cues and 13 participants who recognized only one of the PM cues (either focal or nonfocal) on a retrospective memory quiz, suggesting possible difficulties with the retrospective component of PM. This left 189 participants eligible for our primary analysis. All participants provided written informed consent in accordance with procedures approved by the Institutional Committee for the Protection of Human Subjects. Participants were assessed during two sessions: first, on their annual EAS visit (see Katz et al., 2012 for details); second, approximately two weeks later when they completed experimental and standardized measures of PM. Transportation to and from the EAS was provided, along with lunch and $25 for participation.
Mean age of the participants was 81.83 (SD = 5.19) years, mean years of education was 14.62 (SD = 3.14), 66.1% of participants were female, and 62.4% identified as White. We took a psychometric approach to classifying study participants (Rabin, Chi, et al., 2014; Rabin, Wang, Katz, & Lipton, 2014). First, we established robust norms for 13 neuropsychological tests for 411 EAS participants who were dementia free for 3 years (and did not include participants in the current study); next, a principal component analysis yielded three underlying cognitive factors: global/verbal, executive/processing speed, and memory (see Appendix A). For participants in the current study, cognitive domain scores were calculated as the average of the Z scores of the neuropsychological tests within each cognitive factor using means and SDs from the robust sample, stratified by age group (70–79 and 80 and above).
MCI was classified in 33 participants whose cognitive domain scores were considerably lower (>1 SD) than the mean of the robust sample on one or more cognitive factors and who endorsed at least one cognitive complaint on EAS self-report measures. MCI was further subdivided into aMCI in 15 participants whose cognitive factor Z scores were below 1 SD on the memory or memory plus executive/processing speed and/or global domains and into naMCI for 18 participants whose cognitive factor Z scores were below 1 SD on the executive and/or global domains.
SCD was classified in 58 cognitively intact participants (i.e., cognitive factor Z scores for all three domains did not fall considerably lower (>1 SD) than the mean of the robust sample) who exceeded an optimal cut point for self and/or informant complaints. We used cognitive complaints items from previous research (Rabin et al., 2012) to derive scores that were the proportion of positive responses. Subsequently, we derived an optimal cut point from a receiver operating characteristic (ROC) analysis, stratified by young-old (age 70–79) and old-old (age 80 and above) groups, which used the robust sample and was based on the cross-sectional association between the self or informant complaint and MCI (Rabin, Wang, et al., 2014).
HC was classified in 98 cognitively intact participants whose cognitive factor Z scores for all three domains did not fall considerably lower (>1 SD) than the mean of the robust sample and who did not exceed the optimal cut point for self and/or informant complaints.
MEASURES
Primary Outcome Measure: Laboratory-based PM task
We used a computerized PM task used by Foster et al. (2009), based on the paradigm introduced by Einstein and colleagues (2005; see Figure 1), comprised of control, focal, and nonfocal conditions administered in randomized order across participants. The control condition consisted of a word-categorization activity that also served as the ongoing task in the PM conditions, in which participants made a categorization decision about an on-screen word pair. Instructions were to press Y (for yes) or N (for no) on the keyboard to indicate whether the lower-case word on the left belonged to the capitalized category (capitalized word) on the right. We presented an equal number of yes and no trials. Ongoing task latency was recorded in milliseconds (ms) for each of the 160 word-pair trials, and the mean was calculated for each participant.
Fig. 1.
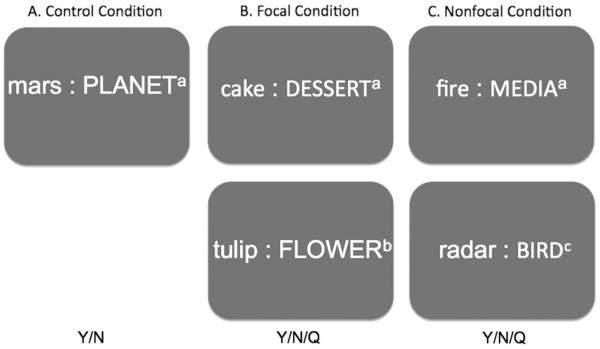
Visual display of the laboratory-based prospective memory task. A: The control condition contained a word-categorization task on which participants responded by pressing Y or N (yes or no) with 160 word-pair trials presented in random order. B: The focal condition contained a word-categorization task that additionally presented the focal PM cue, “tulip”, four times as the exemplar (e.g., appearing once on every 40th trial amongst a total of 165 word-pair trials that were presented in randomized order) and participants were required to respond by pressing Y or N (e.g., categorization), or Q when “tulip” appeared (e.g., PM intention). C: The nonfocal condition contained a word-categorization task that additionally presented the nonfocal PM cue, “rad”, within the words “radiator”, “radical”, “radio”, and “radar”, four times as the exemplar (e.g., appearing once on every 40th trial amongst a total of 165 word-pair trials that were presented in randomized order) and participants were required to respond by pressing Y or N (e.g., categorization), or Q when “tulip” appeared (e.g., PM intention). a: word-categorization trial containing no PM cue. b: word-categorization trial containing a focal PM cue. c: word-categorization trial containing a nonfocal PM cue.
In the focal and nonfocal PM conditions, participants were instructed to additionally press the “Q” key immediately after the word “tulip” (focal condition) or the syllable “rad” (nonfocal condition) appeared on-screen. “Tulip” was the focal cue and “rad” was the nonfocal cue (presented as “radiator”, “radical”, “radio”, and “radar” in random order). The PM intention was to press the “Q” key. The PM cues were always presented on the left side of the screen, as the exemplar in the word pair, and appeared four times in both conditions of 165 word-pair trials. As the ongoing task was a word-categorization activity, it granted focal attention to words and not to syllables; therefore, spontaneous retrieval should support intention retrieval in the focal condition, while strategic monitoring should support intention retrieval in the nonfocal condition.
Participants received one point if they correctly executed the PM intention (pressed “Q”) on a trial that contained a PM cue or on the following trial. Participants completed the word-categorization task (answered Y/N) before executing the PM intention. Total focal and nonfocal PM accuracy scores were calculated as the sum of correct responses in each condition and ranged from 0 to 4. Ongoing task latency was recorded in milliseconds (ms) for each of the 165 word-pair trials, although we excluded the PM trials (and the four trials that followed) when calculating the mean for each participant to allow participants to return to normal performance after seeing a PM cue. At the end of the entire session, participants were tested on their recall and recognition (only if they failed recall) of both PM cues.
Self- and Informant Report Measures
The Comprehensive Assessment of Prospective Memory, Section B (CAPMB) is a 39-item questionnaire that assesses how problematic everyday PM failures are to an individual (Chau, Lee, Fleming, Roche, & Shum, 2007). The CAPMB includes self- and informant-rated versions. Each item describes a PM failure and participants indicate “how much of a problem” each listed memory failures is (scale 1 to 5) with “1” representing “no problem at all” and “5” representing “a very serious problem.” A “not applicable” (N/A) option is also available. We used the CAPMB total score, the average rating of all items answered, excluding N/A responses.
The short form of the Geriatric Depression Scale (GDS; Sheikh & Yesavage, 1986) is a self-reported measure of depressive symptoms, using a yes/no rating scale. Scores range from 0 to 15, and scores of 5 or higher suggest clinical depression (Almeida & Almeida, 1999; Marc, Raue, & Bruce, 2008).
Statistical Analyses
We calculated the descriptive statistics of mean and standard deviation for continuous variables and frequency and percentage for the categorical variables. We used analysis of variance (ANOVA) to compare the continuous variables and the Pearson chi-square test to compare the categorical variables. As the GDS had a skewed distribution, scores were logarithmically transformed to allow for parametric analysis. In the between-group comparisons for the HC, SCD, and MCI groups, we used ANOVA to test for differences on measures of PM accuracy and ongoing task latency. We also performed analysis of covariance (ANCOVA) comparisons, adjusting for significantly different variables from the demographic and clinical characteristics comparisons. Planned contrasts (independent t-tests) using adjusted means were performed to test Hypotheses 1, 2, and 3. We calculated effect sizes using partial eta square for the ANOVA and ANCOVA comparisons and eta square for the planned contrasts. Within-subjects analyses of PM performance, comparing focal and nonfocal PM accuracy scores, and of latency costs, comparing ongoing task latencies in the control versus the focal and nonfocal conditions, were conducted using repeated measures ANOVA and were followed by post hoc analyses using paired t-tests. Due to the small cell size in certain groups, we performed the Fisher’s exact test to analyze group differences with regard to the percentage of participants who failed the retrospective test. Pearson correlation analysis was performed for different measures of prospective memory performance. All p-values were two-tailed with an alpha level of .05. We used SPSS Version 22 for all analyses.
RESULTS
Participant Characteristics
Table 1 shows the demographic and clinical characteristic comparisons for the HC, SCD, and MCI groups. Race/ethnicity significantly differed, with naMCI having the greatest percentage of non-white participants. Education significantly differed, with SCD having the greatest number of mean years of education. There were no significant differences for the comparisons with age, sex, and GDS.
Table 1.
Demographic and clinical characteristics of the healthy control, subjective cognitive decline, amnestic mild cognitive impairment, and non-amnestic mild cognitive impairment groups (n = 189)
| Variable | HC M (SD) or # (%) (n = 98) |
SCD M (SD) or # (%) (n = 58) |
aMCI M (SD) or # (%) (n = 15) |
naMCIM(SD) or # (%) (n = 18) |
p |
|---|---|---|---|---|---|
| Age (years) | 81.4 (5.3) | 82.5 (4.6) | 83.1 (6.6) | 81.2 (5.1) | .433 |
| Sex (women) | 62 (63.3) | 39 (67.2) | 9 (60.0) | 15 (83.3) | .388 |
| Race/Ethnicity (non-white) | 37 (37.8) | 15 (25.9) | 6 (40.0) | 13 (72.2) | .005 |
| Education (years) | 14.8 (3.1) | 15.2 (3.1) | 14.6 (3.3) | 12.2 (2.1) | .004 |
| GDS | 1.2 (1.5) | 2.4 (3.0) | 2.3 (2.3) | 1.2 (1.5) | .198 |
Note. M = mean; SD = standard deviation; HC = healthy control; SCD = subjective cognitive decline; aMCI = amnestic mild cognitive impairment; naMCI = non-amnestic mild cognitive impairment; GDS = Geriatric Depression Scale; GDS data were logarithmic transformed and data shown are original values for ease of interpretation. For continuous variables: p values are based on univariate analysis of variance. For categorical variables: p values are based on the Pearson chi-square test. Similar patterns for significance between the groups occurred when we included all participants who showed successful recall and/or recognition of at least one of the PM cues (n = 202).
Retrospective Memory Assessment
Of the original 208 participants, 189 (90.7%) successfully recalled and/or recognized both focal and nonfocal cues. There was no overall significant difference (p = .726) for the groups. Of the original participants, 13 (6.3%) successfully recalled and/or recognized only one PM cue. Among this subset, there was an overall significant difference (p < .001) for the groups with the percentages greatest for naMCI as compared to HC, SCD, and aMCI [3.9% (n = 4) in the HC group, 3.3% (n = 2) in the SCD group, 6.3% (n = 1) in the aMCI group, and 37.7% (n = 6) in the naMCI group]. Additionally, of the original participants, 6 (2.9%) failed to recognize any PM cues.
Performance on the Computerized Prospective Memory Task
Prospective Memory Accuracy Score
Table 2 shows the analyses for the PM accuracy comparisons for the participant groups. There was an overall significance for focal PM accuracy between the groups in ANOVA that was maintained in ANCOVA, where race/ethnicity and education were included as covariates. Planned contrasts showed that HC had a significantly greater mean than aMCI, t(109) = 2.61 p = .010, η2 = .04, and naMCI, t(110) = 2.56, p = .011, η2 = .04 but not SCD (p = .807). For nonfocal PM accuracy, there was a trend toward overall significance between the groups in ANOVA (p = .06) and no overall significance between groups in ANCOVA. Planned contrasts showed that HC had a greater mean than naMCI, only with a trend toward significance, t(110) = 1.87, p = .06, η2 = .02, but not SCD (p = .359) or aMCI (p = .500).
Table 2.
Analyses for group differences between the healthy control, subjective cognitive decline, amnestic mild cognitive impairment, and non-amnestic mild cognitive impairment groups on measurements of prospective memory (n = 189)
| Variable | HC M (SD) (n = 98) |
SCD M (SD) (n = 58) |
aMCI M (SD) (n = 15) |
naMCI M (SD) (n = 18) |
ANOVA F-value (p) |
ANCOVA F-value (p) |
Effect size |
|---|---|---|---|---|---|---|---|
| Prospective Memory Accuracy | |||||||
| Focal PM Accuracy | 3.42 (1.21) | 3.50 (.90) | 2.69 (1.50) | 2.63 (1.63) | 4.28 (.006) | 4.04 (.008) | .07 |
| Nonfocal PM Accuracy | 2.67 (1.41) | 2.83 (1.21) | 2.25 (1.40) | 1.58 (1.42) | 2.53 (.059) | 1.09 (.36) | .04 |
Note. M = mean; SD = standard deviation; HC = healthy control; SCD = subjective cognitive decline; aMCI = amnestic mild cognitive impairment; naMCI = non-amnestic mild cognitive impairment; ANOVA = analysis of variance; ANCOVA = analysis of covariance. Sample size slightly varies due to omission of scores by certain participants. ANCOVA was used to compare group differences on all variables, adjusting for race/ethnicity and education. All effect sizes are partial eta square.
In a follow-up analysis, we included all participants who showed successful recall and/or recognition of at least one of the PM cues (n = 202) as an approach for increasing sample size. We present the results in Table 3. ANOVA showed an overall significance for focal PM accuracy that was maintained in ANCOVA where race/ethnicity and education were included as covariates. Planned contrasts showed that HC had a significantly greater mean than aMCI t(114) = 2.35, p = .020, η2 = .03 and naMCI t(114) = 3.64, p = .001, η2 = .07, but not SCD (p = .990). ANOVA showed an overall significance for nonfocal PM accuracy, F(3,193) = 2.86, p = 0.040, η2 = .05 that was maintained in ANCOVA. Planned contrasts showed that HC had a significantly greater mean than naMCI t(114) = 3.03, p = .003, η2 = .05, but not SCD (p = .688) or aMCI (p = .258).
Table 3.
Follow-up analyses for group differences between the healthy control, subjective cognitive decline, amnestic mild cognitive impairment, and non-amnestic mild cognitive impairment groups on measurements of prospective memory (n = 202)
| Variable | HC M (SD) (n = 102) |
SCD M (SD) (n = 60) |
aMCI M (SD) (n = 16) |
naMCI M (SD) (n = 24) |
ANOVA F-value (p) |
ANCOVA F-value (p) |
Effect size |
|---|---|---|---|---|---|---|---|
| Prospective Memory Accuracy | |||||||
| Focal PM Accuracy | 3.42 (1.20) | 3.45 (1.00) | 2.69 (1.50) | 2.36 (1.60) | 6.21 (<.001) | 5.43 (.001) | .09 |
| Nonfocal PM Accuracy | 2.65 (1.39) | 2.81 (1.21) | 2.40 (1.35) | 1.79 (1.65) | 5.24 (.002) | 2.87 (.038) | .08 |
Note. M = mean; SD = standard deviation; HC = healthy control; SCD = subjective cognitive decline; aMCI = amnestic mild cognitive impairment; naMCI = non-amnestic mild cognitive impairment; ANOVA = analysis of variance; ANCOVA = analysis of covariance. Sample size slightly varies due to omission of scores by certain participants. ANCOVA was used to compare group differences on all variables, adjusting for race/ethnicity and education. All effect sizes are partial eta square.
Ongoing Task Latency
Table 4 compares within-subjects differences in ongoing task latency for three levels of the computerized PM test separately for each participant group. Repeated measures ANOVA showed overall significance for ongoing task latency for HC, SCD, and aMCI but not for the naMCI group. Post hoc paired t-test analyses were performed for focal versus control and also nonfocal versus control. For HC, nonfocal had a significantly greater task latency than control (p < .001) but no significant task latency difference for focal versus control (p = .792). For SCD, nonfocal had a significantly greater task latency than control (p < .001) but no significant task latency difference for focal versus control (p = .534). For aMCI, nonfocal had a significantly greater task latency than control (p = .002) but no significant task latency difference for focal versus control (p = .755).
Table 4.
Analyses for within-subjects differences in the healthy control, subjective cognitive decline, amnestic mild cognitive impairment, and non-amnestic mild cognitive impairment groups on measurements of ongoing task latency (n =189)
| Control M (SD) ms | Focal M (SD) ms | Nonfocal M (SD) ms | ANOVA F-value (p) | MSE | Effect size | |
|---|---|---|---|---|---|---|
| HC (n = 98) | 1,652.73 (352.54) | 1,647.60 (353.27) | 1,907.55 (464.99) | 56.81 (<.001) | 49,081.46 | .38 |
| SCD (n = 58) | 1,748.59 (447.88) | 1,734.76 (431.31) | 1,945.62 (589.30) | 17.94 (<.001) | 65,097.23 | .24 |
| aMCI (n = 15) | 1,730.23 (353.92) | 1,742.27 (343.34) | 2,087.50 (556.42) | 11.55 (.001) | 59,418.61 | .45 |
| naMCI (n = 18) | 2,167.46 (486.93) | 2,069.61 (474.63) | 2,035.14 (529.88) | 2.29 (.119) | 32,981.89 | .13 |
Note. M = mean; SD = standard deviation; HC = healthy control; SCD = subjective cognitive decline; aMCI = amnestic mild cognitive impairment; naMCI = non-amnestic mild cognitive impairment; ANOVA = analysis of variance; ms = milliseconds; MSE = means square error. Sample size slightly varies due to omission of scores by certain participants. All effect sizes are partial eta square.
PM Score Correlations with Self- and Informant-Report of PM Function
Table 5 shows Pearson correlation analyses for laboratory PM performance and self- and informant-reported concerns about everyday prospective memory failures on the CAPMB. There was a statistically significant small positive correlation between the nonfocal and focal PM accuracy scores. There was a statistically significant large small negative correlation between the nonfocal PM accuracy score and the informant-reported CAMPB score, but no significant correlation with the self-reported CAMPB score. The focal PM accuracy score did not significantly correlate with the self-reported or informant-reported CAMPB scores.
Table 5.
Pearson correlations for laboratory prospective memory performance and self- and informant-reported concern of everyday PM (n = 189)
| Variable | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Nonfocal Condition-Prospective Memory score | 1.00 | |||
| 2. Focal Condition-Prospective Memory score | 0.183** | 1.00 | ||
| 3. CAMPB, Self (PM) | −0.111 | 0.027 | 1.00 | |
| 4. CAMPB, Informant (PM) | −0.221** | −0.072 | 0.124 | 1.00 |
Note. CAMPB = Comprehensive Assessment of Prospective Memory, Section B; Self = self-reported; Informant = informant-reported; PM = prospective memory total score.
p < .01.
DISCUSSION
In a sample of community-dwelling nondemented older adults, relative to healthy controls, those with aMCI and naMCI performed significantly worse on the focal condition of a computerized PM test. In a follow-up analysis with a slightly larger sample, which included 13 individuals who showed only partial recall and/or recognition on the retrospective memory quiz, results additionally showed that those with naMCI also performed significantly worse on the nonfocal condition. Participants with subjective cognitive decline (SCD), despite normal performance on standard neuropsychological tests, exhibited PM accuracy consistent with intact performance and did not differ from the HC group on either condition. Comparisons of mean ongoing task latencies for the three conditions of the PM task showed a latency cost of the nonfocal condition but not of the focal condition on ongoing task latency for those with HC, SCD, and aMCI, but not for naMCI. Lastly, greater nonfocal PM accuracy scores measured using the laboratory-based test significantly correlated with greater informant-reported difficulty with everyday PM tasks.
Nonfocal and focal PM accuracy scores are respectively linked to strategic monitoring and spontaneous retrieval processes (McDaniel et al., 2011; Reynolds et al., 2009). Results from both our main and follow-up analyses showed impairment of focal but not of nonfocal PM in aMCI, which supports the first hypothesis, suggesting that aMCI disrupts processes that support spontaneous retrieval of intended actions and thereby interferes with focal PM accuracy. The pattern of focal versus nonfocal PM performance above is consistent with McDaniel et al. (2011), who showed a preferential deficit in focal PM in very early AD. Furthermore, our results were obtained in the absence of a floor effect, possibly because we included four instead of three PM trials in each condition, which may have increased sensitivity. Additionally, our findings are consistent with Tam and Schmitter-Edgecombe’s (2013) correlational analyses, which suggested that the PM impairment in aMCI is more strongly associated with compromised spontaneous retrieval processes and MTL integrity. Our cost analysis (see below) revealed slower performance on the ongoing task in the nonfocal versus the control condition but comparable latencies between the focal and control conditions, suggesting that attentional resources were deployed to support strategic monitoring for the PM cues in the nonfocal condition but not in the focal condition. These findings provide evidence that PM failures in aMCI are related to impairment of spontaneous retrieval processes and the MTL system but not to problems with strategic monitoring and prefrontal systems, which is consistent with reports of key structural decline of the MTL and hippocampal structures in aMCI (Mielke et al., 2012; Nickl-Jockschat et al., 2012; Nobili et al., 2009). Overall, our findings suggest that preferential deficits in focal PM accuracy may represent a signature marker of PM decline in aMCI that is comparable to what is seen in early AD (McDaniel et al., 2011).
Results from our main analysis revealed that relative to HC, the naMCI group showed significantly lower focal PM performance, suggesting deficits in spontaneous retrieval and the MTL system. There was no overall significant relationship between groups on nonfocal PM accuracy with either ANOVA or ANCOVA. Planned contrasts showed only a trend toward significance when nonfocal PM accuracy was compared between the HC and naMCI groups. These results partially confirm the second hypothesis, in which we expected focal and nonfocal PM impairment in this group. The focal PM deficits observed in naMCI indicate that despite normal performance on retrospective memory tests individuals with naMCI are impaired in spontaneous retrieval processes when performing PM tasks; this lends further support to previous research (Costa et al., 2010, 2011; Schmitter-Edgecombe et al., 2009; Thompson et al., 2010) that shows declarative memory ability cannot completely account for PM performance. On face value, the fact that naMCI did not exhibit significantly lower nonfocal PM performance relative to HC appears to suggest that strategic monitoring processes and prefrontal systems are spared in this group. This conclusion would be inconsistent with Costa and colleagues (2010), in which a dysexecutive MCI group relative to HC showed deficits in both high and low cognitive demand conditions of a PM task (e.g., requiring the higher and lower levels of attentional resources, respectively).
Furthermore, the above interpretation conflicts with the results of our cost analysis (see below), which indicated slower ongoing task latencies on the nonfocal versus the control condition and comparable latencies between the focal and control conditions for all groups except naMCI. This suggests that for the HC, SCD, and aMCI groups, for which we did not expect primary executive control deficits, attentional resources were recruited to support strategic monitoring in the nonfocal condition, resulting in slower reaction times on the ongoing task, but not in the focal condition. Conversely, the naMCI group showed comparable ongoing task latencies in the nonfocal versus control condition and in the focal versus control condition, suggesting that attention-demanding processes were not recruited to support strategic monitoring on the nonfocal (or the focal) task. This presents evidence that strategic monitoring was disrupted in the naMCI group, resulting in an absence of latency cost of the nonfocal PM task on ongoing task latency. As participants were classified into the naMCI group based on low performance on executive functioning/processing speed and global/verbal tasks, one can infer that disruption of strategic monitoring processes was associated with deficits in these neuropsychological domains (see Methods and Appendix A for specific tasks).
Several factors may have prevented our ability to detect a nonfocal PM deficit in naMCI in our main analysis. Our computerized task, which presented only two PM cues and four PM trials per condition, resulted in a restricted range of performance. Future studies should consider increasing the complexity and number of PM trials on this task to increase discriminative power. We also had a limited sample size. In light of this, we repeated the above analyses using a slightly larger sample that included the 13 participants whom were excluded from the main analysis because they demonstrated only partial recognition/recall (e.g., only one of the two PM cues) on the retrospective memory quiz. These 13 individuals were not originally included in the main analysis to ensure that differences in focal and nonfocal PM accuracy were due to spontaneous retrieval and strategic monitoring processes that mediate the prospective component of PM, and not due to problems with retrospective memory (e.g., poor encoding). However, of these 13 participants, 7 responded at least once on the PM condition that contained the cue not recognized on the quiz, and 5 of these 7 individuals received a perfect PM accuracy score for the PM condition in question, indicating intact memory for that cue during testing. Because we queried participants at the end of the entire session rather than immediately after each experimental block, it is possible that some participants mistakenly believed we were only asking about the most recently completed condition (e.g., responding with only one PM cue). Taken together, we believed there was support for intact retrospective memory in these individuals and included them in our follow-up analysis. Results showed an overall significant association of group on nonfocal PM accuracy with both ANOVA and ANCOVA. Planned contrasts further showed impairment of both focal and nonfocal PM accuracy in naMCI, relative to HC, which is in-line with Costa and colleagues (2010). Considering the two analytical approaches above, our findings provide some evidence for dual focal and nonfocal PM deficits in naMCI, as proposed by our second hypothesis, as well as preliminary support for behavioral markers that may distinguish PM decline in those with naMCI.
We did not find support for the third hypothesis, which predicted a role for our PM task in detecting subtle cognitive decline not readily detected by traditional episodic memory tasks in individuals with SCD. The SCD group did not demonstrate significantly lower scores than HC on focal PM accuracy or nonfocal PM accuracy, suggesting spared spontaneous retrieval and strategic monitoring processes. Recent research from our lab suggests that using long-term and more naturalistic PM tasks might be a better way to tap into the subtle cognitive problems experienced by those with SCD (Rabin, Chi et al., 2014). Therefore, it is possible that the task used here was not optimal for distinguishing HC from SCD.
The fourth hypothesis predicted that, in concordance with the Multiprocess Theory of PM (McDaniel & Einstein, 2000; McDaniel et al., 2004), mean ongoing task latency in the nonfocal condition would be significantly longer relative to the control condition (e.g., showing a latency cost when strategic monitoring was required), while mean latencies would not differ between the focal and control conditions (e.g., showing no latency cost when spontaneous retrieval was relied upon). We hypothesized that this pattern would be observed in HC, SCD, and aMCI, for which strategic monitoring was expected to be intact, and not in the naMCI group, for which strategic monitoring deficits were expected to interfere with nonfocal PM. Our results show this pattern and reinforce the idea that focal and nonfocal PM accuracy scores are respectively related to spontaneous processes and effortful, strategic monitoring, as reported in Foster et al. (2009) and McDaniel et al. (2011). Furthermore, these results support the presence of nonfocal PM deficits in naMCI.
Consistent with the fifth hypothesis, informant-report of PM difficulties negatively correlated with nonfocal PM accuracy but no relationship was found with focal PM accuracy. This suggests that everyday PM failures detected by informants are associated with deficits in executive control functions related to self-monitoring rather than impairment of spontaneous processes. There is increasing support that informant reports of cognition may improve the prediction of AD conversion over objective cognitive assessment (Rabin et al., 2012), and may better predict conversion to dementia than self-reports (Gifford et al., 2014). Similar to Foster et al. (2009), we did not find a correlation between PM scores and self-reported PM failures. Poor associations between self-report instruments and objective tests of prospective remembering are common (Burgess et al., 2006; Chaytor & Schmitter-Edgecombe, 2003). Overall, our correlational results underscore the importance of informant report of everyday cognitive problems in pre-dementia conditions, suggesting certain PM paradigms may tap real-world decline observed by knowledgeable others.
In conclusion, the study results suggest impairment of focal PM accuracy in aMCI and impairment of focal PM and potentially of nonfocal PM accuracy in naMCI. These deficits may constitute specific behavioral markers of PM decline. In accordance with the Multiprocess Theory of PM (McDaniel & Einstein, 2000; McDaniel et al., 2004), our results are consistent with the notion that aMCI produces a deficient reflective-associative retrieval system but not an impaired attentional-executive system, disrupting hippocampal but not prefrontal functioning, while naMCI compromises both of these systems, producing respective functional problems. Findings may inform compensatory strategies for improving PM in aMCI and naMCI. For example, using external prompts and detailed instructions simultaneously (Andrade et al., 2005; Martin-Saez, Deakins, Winson, Watson, & Wilson, 2011; Simoni et al., 2009; Wilson, Emslie, Quirk, & Evans, 2001) may be beneficial, compensating for both executive functioning and memory deficits. Nonfocal PM accuracy is negatively correlated to informant-reported concern of everyday PM failures. Overall, our findings emphasize the importance of including items related to executive dysfunction on informant report questionnaires, as well as the importance of informant involvement during the diagnostic process.
ACKNOWLEDGMENTS
The authors thank Dr. Erin Foster for generously sharing the PM measure used in this study. We are appreciative of Milushka Elbulok-Charcape, Valdiva Da Silva, John Flynn, Tangeria Adams, Erica Meltzer, Dr. David Owen, Charlotte Magnotta, Wendy Ramratan, Dr. Molly Zimmerman, Dr. Richard Lipton, and Mindy Katz for their contributions. This project was supported by funding from the National Institute on Aging (NIA) and National Institute of General Medical Sciences (SC2AG039235), NIA (AG03949), National Science Foundation (NSF Award #1156870), Czap Foundation, and The Leonard and Sylvia Marx Foundation.
APPENDIX A
Neuropsychological Tests Utilized in Psychometric Classification of Participants
| Neuropsychological tests | Factor loadings |
|---|---|
| Global/Verbal Factor | |
| Boston Naming Test, Total Correct Without Semantic Cue |
0.433 |
| WAIS-R Information, Raw Score | 0.738 |
| WAIS-R Similarities, Raw Score | 0.495 |
| WAIS-R Vocabulary, Raw Score | 0.771 |
| Letter Fluency, Total Words F-A-S Across Three 1-Minute Trials |
0.375 |
| WAIS-R Digit Span, Raw Score | 0.738 |
| Executive/Processing Speed Factor | |
| WAIS-R Block Design, Raw Score | 0.599 |
| WAIS-R Digit Symbol, Raw Score | 0.779 |
| Trail Making Test – Part A (time), Seconds to task completion |
−0.684 |
| Trail Making Test – Part B (time), Score after 300 seconds |
−0.658 |
| Memory Factor | |
| FCSRT, Total Free Recall Across Three Test Trials | 0.421 |
| WMS-R, Logical Memory I Subtest (LM I) Raw Score Across Three 1-Minute Trials |
0.256 |
| Category Fluency, Total Words (animals, vegetables, and fruits) Across Three 1-Minute Trial |
0.920 |
For the principal component analysis, orthogonal varimax rotation was used with Kaiser-Meyer-Olkin Measure of Sampling Adequacy = .89 and significant Bartlett's Test of Sphericity (p < .001). The 13 neuropsychological tests were: (1) verbal episodic memory/word learning—free recall from the Free and Cued Selective Reminding Test (FCSRT; Grober & Buschke, 1987); (2) verbal episodic memory/story recall—Logical Memory I subtest of the Wechsler Memory Scale-Revised (WMS-R; Wechsler, 1987); (3) verbal fluency/word generation according to an initial letter—Letter Fluency (Spreen & Strauss, 1998); (4) verbal fluency/naming exemplars from a category—Category Fluency (Rosen, 1980); (5) confrontation naming—short form of the Boston Naming Test (BNT, Kaplan, Goodglass, & Weintraub, 1983); (6–7) visuomotor tracking, divided attention, and cognitive flexibility—Trail Making Test Parts A and B (Reitan 1958); and select subtests of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997), including (8) visuospatial organization—Block Design, (9) psychomotor processing speed—Digit Symbol-Coding, (10) auditory attention and working memory—Digit Span, (1) general fund of knowledge—Information, (12) vocabulary level—Vocabulary, and (13) verbal abstraction of categories—Similarities. The three factors identified by exploratory factor analysis were as follows: (1) global/verbal (Boston Naming, Information, Similarities, Vocabulary, Letter Fluency, Digit Span), (2) executive/processing speed (Block Design, Digit Symbol-Coding, and Trail Making Test Parts A & B), and memory (FCSRT, Category Fluency, Logical Memory). For clinical reasons (i.e., the fact that Logical Memory I assesses narrative memory under a free recall condition), we included Logical Memory I as part of the memory factor even though it loaded more strongly (0.381) on the global/verbal factor.
Footnotes
The authors do not have any conflicts of interest to disclose.
REFERENCES
- Almeida OP, Almeida SA. Short versions of the geriatric depression scale: A study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. International Journal of Geriatric Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Andrade AS, McGruder HF, Wu AW, Celano SA, Skolasky RL, Selnes OA, McArthur JC. A programmable prompting device improves adherence to highly active antiretroviral therapy in HIV-infected subjects with memory impairment. Clinical Infectious Diseases. 2005;41(6):875–882. doi: 10.1086/432877. doi:10.1086/432877. [DOI] [PubMed] [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. doi:10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-McGinty S, Lopez OL, Cidis Meltzer C, Scanlon J, Whyte EM, DeKosky ST, Becker JT. Differential cortical atrophy in subgroups of mild cognitive impairment. Archives of Neurology. 2005;62(9):1393–1397. doi: 10.1001/archneur.62.9.1393. doi:10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- Blanco-Campal A, Coen RF, Lawlor BA, Walsh JB, Burke TE. Detection of prospective memory deficits in mild cognitive impairment of suspected Alzheimer's disease etiology using a novel event-based prospective memory task. Journal of the International Neuropsychological Society. 2009;15(1):154–159. doi: 10.1017/S1355617708090127. doi:10.1017/S1355617708090127. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates LM, Dawson DR, Channon S. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Journal of the International Neuropsychological Society. 2006;12(2):194–209. doi: 10.1017/S1355617706060310. doi:10.1017/S1355617706060310. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Quayle A, Frith CD. Brain regions involved in prospective memory as determined by positron emission tomography. Neuropsychologia. 2001;39(6):545–555. doi: 10.1016/s0028-3932(00)00149-4. doi:10.1016/S0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. doi:10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- Chau LT, Lee JB, Fleming J, Roche N, Shum D. Reliability and normative data for the Comprehensive Assessment of Prospective Memory (CAPM) Neuropsychological Rehabilitation. 2007;17(6):707–722. doi: 10.1080/09602010600923926. doi:10.1080/09602010600923926. [DOI] [PubMed] [Google Scholar]
- Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychology Review. 2003;13(4):181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. doi:10.1023/B:NERV.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- Costa A, Caltagirone C, Carlesimo GA. Prospective memory impairment in mild cognitive impairment: An analytical review. Neuropsychology Review. 2011;21(4):390–404. doi: 10.1007/s11065-011-9172-z. doi:10.1007/s11065-011-9172-z. [DOI] [PubMed] [Google Scholar]
- Costa A, Perri R, Serra L, Barban F, Gatto I, Zabberoni S, Carlesimo GA. Prospective memory functioning in mild cognitive impairment. Neuropsychology. 2010;24(3):327–404. doi: 10.1037/a0018015. doi:10.1037/a0018015. [DOI] [PubMed] [Google Scholar]
- Craik FIM. On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society, Series B, Biological Sciences. 1983;302:341–359. [Google Scholar]
- Craik FIM. A functional account of age differences in memory. In: Klix F, Hagendorf H, editors. Human memory and cognitive capabilities: Mechanisms and performances. Elsevier; Amsterdam: 1986. pp. 409–422. [Google Scholar]
- Dufouil C, Fuhrer R, Alperovitch A. Subjective cognitive complaints and cognitive decline: Consequence or predictor? The epidemiology of vascular aging study. Journal of the American Geriatics Society. 2005;53(4):616–621. doi: 10.1111/j.1532-5415.2005.53209.x. doi:10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Normal aging and prospective memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1990;16(4):717–726. doi: 10.1037//0278-7393.16.4.717. doi:10.1037/0278-7393.16.4.717. [DOI] [PubMed] [Google Scholar]
- Einstein GO, McDaniel MA. Retrieval processes in prospective memory: Theoretical approaches and some new empirical findings. In: Bradimonte M, Einstein GO, McDaniel MA, editors. Prospective memory: Theory and applications. Erlbaum; Mahwah: 1996. pp. 115–141. [Google Scholar]
- Einstein GO, McDaniel MA, Thomas R, Mayfield S, Shank H, Morrisette N, Breneiser J. Multiple processes in prospective memory retrieval: Factors determining monitoring versus spontaneous retrieval. Journal of Experimental Psychology: General. 2005;134(3):327–342. doi: 10.1037/0096-3445.134.3.327. doi:10.1037/0096-3445.134.3.327. [DOI] [PubMed] [Google Scholar]
- Foster ER, McDaniel MA, Repovš G, Hershey T. Prospective memory in Parkinson disease across laboratory and self-reported everyday performance. Neuropsychology. 2009;23(3):347–358. doi: 10.1037/a0014692. doi:10.1037/a0014692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J, Kowall N, Jefferson AL. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimer’s & Dementia. 2014;10:319–327. doi: 10.1016/j.jalz.2013.02.007. doi:10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Buschke H. Genuine memory deficits in dementia. Developmental Neuropsychology. 1987;3(1):13–36. doi:10.1080/87565648709540361. [Google Scholar]
- Henry JD, MacLeod MS, Phillips LH, Crawford JR. A meta-analytic review of prospective memory and aging. Psychology and Aging. 2004;19(1):27–39. doi: 10.1037/0882-7974.19.1.27. doi:10.1037/0882-7974.19.1.27. [DOI] [PubMed] [Google Scholar]
- Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kolsch H, Bickel H. Prediction of dementia by subjective memory impairment: Effects of severity and temporal association with cognitive impairment. Archives of General Psychiatry. 2010;67(4):414–422. doi: 10.1001/archgenpsychiatry.2010.30. doi:10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. 1983. [Google Scholar]
- Karantzoulis S, Troyer AK, Rich JB. Prospective memory in amnestic mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;15(3):407–415. doi: 10.1017/S1355617709090596. [DOI] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Derby CA. Age and sex specific prevalence and incidence of mild cognitive impairment, dementia and Alzheimer’s dementia in blacks and whites: A report from the Einstein Aging Study. Alzheimer Disease & Associated Disorders. 2012;26(4):335–343. doi: 10.1097/WAD.0b013e31823dbcfc. doi:10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryscio RJ, Abner EL, Cooper GE, Fardo DW, Jicha GA, Nelson PT, Schmitt FA. Self-reported memory complaints Implications from a longitudinal cohort with autopsies. Neurology. 2014;7:1359–1365. doi: 10.1212/WNL.0000000000000856. doi:10.1212/WNL.0000000000000856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton R, Katz MJ, Kuslansky G, Sliwinski MJ, Stewart W, Verghese J, Buschke H. Screening for dementia by telephone using the memory impairment screen. Journal of the American Geriatric Society. 2003;51(10):1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. doi:10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein D, Curiel R, Crocco E, Czaja S, Levin B, Wahlestedt C, Wright C. Prospective memory deficits in English-and Spanish-speaking patients with mild cognitive impairment (MCI) and PreMCI. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2013;9(4):P323–P323. [Google Scholar]
- Marc LG, Raue PJ, Bruce ML. Screening performance of the Geriatric Depression Scale (GDS-15) in a diverse elderly home care population. The American Journal of Geriatric Psychiatry. 2008;16(11):914–921. doi: 10.1097/JGP.0b013e318186bd67. doi:10.1097/JGP.0b013e318186bd67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Saez M, Deakins J, Winson R, Watson P, Wilson BA. A 10-year follow up of a paging service for people with memory and planning problems within a healthcare system: How do recent users differ from the original users? Neuropsychological Rehabilitation. 2011;21(6):769–783. doi: 10.1080/09602011.2011.614378. doi:10.1080/09602011.2011.614378. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Zubieta JL, Arbizu J. Neuroimaging as a marker of the onset and progression of Alzheimer’s disease. Journal of the Neurological Sciences. 2005;236(1–2):55–64. doi: 10.1016/j.jns.2005.05.001. doi:10.1016/j.jns.2005.05.001. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Einstein GO. Strategic and automatic processes in prospective memory retrieval: A multiprocess framework. Applied Cognitive Psychology. 2000;14(7):S127–S144. doi:10.1002/acp.775. [Google Scholar]
- McDaniel MA, Guynn MJ, Einstein GO, Breneiser J. Cue-focused and reflexive-associative processes in prospective memory retrieval. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30(3):605–614. doi: 10.1037/0278-7393.30.3.605. doi:10.1037/0278-7393.30.3.605. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Shelton JT, Breneiser JE, Moynan S, Balota DA. Focal and nonfocal prospective memory performance in very mild dementia: A signature decline. Neuropsychology. 2011;25(3):387–396. doi: 10.1037/a0021682. doi:10.1037/a0021682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Okonkwo OC, Oishi K, Mori S, Tighe S, Miller MI, Lyketsos CG. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimer's & Dementia. 2012;8(2):105–113. doi: 10.1016/j.jalz.2011.05.2416. doi:10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: Meta-analysis. Acta Psychiatrica Scandinavica. 2014 doi: 10.1111/acps.12336. doi:10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, de Leon MJ. Hypometabolism and altered cerebrospinal fluid markers in normal apolipo-protein E E4 carriers with subjective memory complaints. Biological Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. doi:10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Kleiman A, Schulz JB, Schneider F, Laird AR, Fox PT, Reetz K. Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: A meta-analysis. Brain Structure and Function. 2012;217(1):115–125. doi: 10.1007/s00429-011-0333-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobili F, De Carli F, Frisoni GB, Portet F, Verhey F, Rodriguez G, Visser PJ. SPECT predictors of cognitive decline and Alzheimer's disease in mild cognitive impairment. Journal of Alzheimer's Disease. 2009;17(4):761–772. doi: 10.3233/JAD-2009-1091. doi:10.3233/JAD-2009-1091. [DOI] [PubMed] [Google Scholar]
- Park DC, Kidder DP. Prospective memory and medication adherence. In: Brandimont M, Einstein G, McDaniel M, editors. Prospective memory: Theory and applications. Lawrence Erlbaum Associates Publishers; Mahwah, NJ: 1996. pp. 369–390. [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. doi:10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Winblad B. Current concepts in mild cognitive impairment. Archives of Neurology. 2001;58(12):1985. doi: 10.1001/archneur.58.12.1985. doi:10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Negash S. Mild cognitive impairment: An overview. CNS Spectrums. 2008;13(1):45–53. doi: 10.1017/s1092852900016151. [DOI] [PubMed] [Google Scholar]
- Pino O, Poletti F, Caffarra P. Cognitive demand and reminders effect on time-based prospective memory in Amnesic Mild Cognitive Impairment (aMCI) and in healthy elderly. Open Journal of Medical Psychology. 2013;2:35–46. doi:10.4236/ojmp.2013.21007. [Google Scholar]
- Rabin LA, Chi SY, Wang C, Fogel J, Kann SJ, Aronov A. Prospective memory on a novel clinical task in older adults with mild cognitive impairment and subjective cognitive decline. Neuropsychological Rehabilitation. 2014;24:868–893. doi: 10.1080/09602011.2014.915855. doi:10.1080/09602011.2014.915855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Wang C, Katz MJ, Derby CA, Buschke H, Lipton RB. Predicting Alzheimer’s disease: Neuropsychological tests, self-reports, and informant reports of cognitive difficulties. Journal of the American Geriatrics Society. 2012;60(6):1128–1134. doi: 10.1111/j.1532-5415.2012.03956.x. doi:10.1111/j.1532-5415.2012.03956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Wang C, Katz MJ, Lipton RB. A psychometric approach to the classification of subjective cognitive decline and mild cognitive impairment. Presented at the Annual Meeting of the International Neuropsychological Society; Seattle, Washington. Feb, 2014. [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer's & Dementia. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. doi:10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. doi:10.2466/pms.1958.8.3.271. [Google Scholar]
- Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cerebral Cortex. 2009;19(5):1208–1221. doi: 10.1093/cercor/bhn164. doi:10.1093/cercor/bhn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen W. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2(2):135–146. doi:10.1080/01688638008403788. [Google Scholar]
- Salthouse TA, Berish DE, Siedlecki KL. Construct validity and age sensitivity of prospective memory. Memory & Cognition. 2004;32(7):1133–1148. doi: 10.3758/bf03196887. doi:10.3758/BF03196887. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. doi:10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. doi:10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- Scheltens P. Imaging in Alzheimer’s disease. Dialogues in Clinical Neuroscience. 2009;11(2):191–199. doi: 10.31887/DCNS.2009.11.2/pscheltens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing multiple memory deficits and their relation to everyday functioning in individuals with mild cognitive impairment. Neuropsychology. 2009;23(2):168–177. doi: 10.1037/a0014186. doi:10.1037/a0014186. [DOI] [PubMed] [Google Scholar]
- Sheikh VI, Yesavage VA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. Haworth Press; New York: 1986. pp. 165–174. [Google Scholar]
- Simoni JM, Huh D, Frick PA, Pearson CR, Andrasik MP, Dunbar PJ, Hooton TM. Peer support and pager messaging to promote antiretroviral modifying therapy in Seattle: A randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes. 2009;52(4):465–473. doi: 10.1097/qai.0b013e3181b9300c. doi:10.1097/QAI.0b013e3181b9300c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE. The cost of remembering to remember in event-based prospective memory: Investigating the capacity demands of delayed intention performance. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2003;29(3):347–361. doi: 10.1037/0278-7393.29.3.347. doi:10.1037/0278-7393.29.3.347. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. Oxford University Press; New York: 1998. [Google Scholar]
- Tam JW, Schmitter-Edgecombe M. Event-based prospective memory and everyday forgetting in healthy older adults and individuals with mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2013;35(3):279–290. doi: 10.1080/13803395.2013.770823. doi:10.1080/13803395.2013.770823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C, Henry JD, Rendell PG, Withall, Brodaty H. Prospective memory function in mild cognitive impairment and early dementia. Journal of the International Neuropsychological Society. 2010;16(2):318–325. doi: 10.1017/S1355617709991354. doi:10.1017/S1355617709991354. [DOI] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ. Memory for intentions in amnestic mild cognitive impairment: Time-and event-based prospective memory. Journal of the International Neuropsychological Society. 2007;13(2):365–369. doi: 10.1017/S1355617707070452. doi:10.1017/S1355617707070452. [DOI] [PubMed] [Google Scholar]
- Twamley EW, Woods SP, Zurhellen CH, Vertinski M, Narvaez JM, Mausbach BT, Jeste DV. Neuropsychological substrates and everyday functioning implications of prospective memory impairment in schizophrenia. Schizophrenia Research. 2008;106(1):42–49. doi: 10.1016/j.schres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg E, Kant N, Postma A. Remember to buy milk on the way home! A meta-analytic review of prospective memory in mild cognitive impairment and dementia. Journal of the International Neuropsychological Society. 2012;18(4):706–716. doi: 10.1017/S1355617712000331. doi:10.1017/S1355617712000331. [DOI] [PubMed] [Google Scholar]
- van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimer’s & Dementia. 2007;3(2):92–97. doi: 10.1016/j.jalz.2007.01.011. doi:10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund L-O, Freund-Levi Y, Blennow K. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: A prospective cohort study. Lancet Neurology. 2009;8(7):619–627. doi: 10.1016/S1474-4422(09)70139-5. doi:10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. The Psychological Corporation; San Antonio: 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- West R, Krompinger J. Neural correlates of prospective and retrospective memory. Neuropsychologia. 2005;43(3):418–433. doi: 10.1016/j.neuropsychologia.2004.06.012. doi:10.1016/j.neuropsychologia.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Emslie HC, Quirk K, Evans JJ. Reducing everyday memory and planning problems by means of a paging system: A randomised control crossover study. Journal of Neurology, Neurosurgery, & Psychiatry. 2001;70(4):477–482. doi: 10.1136/jnnp.70.4.477. doi:10.1136/jnnp.70.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Iudicello JE, Moran LM, Carey CL, Dawson MS, Grant I. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology. 2008;22(1):110. doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


