Abstract
Background
US residents make 60 million international trips annually. Family practice providers need to be aware of travel-associated diseases affecting this growing mobile population.
Objective
To describe demographics, travel characteristics and clinical diagnoses of US residents who present ill after international travel.
Methods
Descriptive analysis of travel-associated morbidity and mortality among US travellers seeking care at 1 of the 22 US practices and clinics participating in the GeoSentinel Global Surveillance Network from January 2000 to December 2012.
Results
Of the 9624 ill US travellers included in the analysis, 3656 (38%) were tourist travellers, 2379 (25%) missionary/volunteer/research/aid workers (MVRA), 1580 (16%) travellers visiting friends and relatives (VFRs), 1394 (15%) business travellers and 593 (6%) student travellers. Median (interquartile range) travel duration was 20 days (10–60 days). Pre-travel advice was sought by 45%. Hospitalization was required by 7%. Compared with other groups of travellers, ill MVRA travellers returned from longer trips (median duration 61 days), while VFR travellers disproportionately required higher rates of inpatient care (24%) and less frequently had received pre-travel medical advice (20%). Illnesses of the gastrointestinal tract were the most common (58%), followed by systemic febrile illnesses (18%) and dermatologic disorders (17%). Three deaths were reported. Diagnoses varied according to the purpose of travel and region of exposure.
Conclusions
Returning ill US international travellers present with a broad spectrum of travel-associated diseases. Destination and reason for travel may help primary health care providers to generate an accurate differential diagnosis for the most common disorders and for those that may be life-threatening.
Keywords: Diagnosis, epidemiology, morbidity, prevention, surveillance, travel
Introduction
Among the 309 million US residents in 2010, 61.1 million international trips were taken by 29 million persons (1). International travellers are at risk for contracting infectious diseases, some of which are not endemic to the USA. Certain of these infections (e.g. malaria) may cause serious morbidity and mortality while others (e.g. Dengue) may also present a potential threat to the public health by extending its presence in USA (2–5). US health care providers, particularly those practicing in primary care settings, may encounter ill patients who have recently travelled. Familiarity with the more common travel-associated illnesses will assist in the development of appropriate differential diagnoses, and thus translate into best management, including consideration of infectious diseases consultation as needed.
Published reports of morbidity among US international travellers have been compiled, primarily in the context of single-centre studies or those that have focused on specific infectious diseases (6,7). We report the demographic characteristics, health care use and travel-associated morbidities of 9624 ill US travellers who visited 1 of 22 US GeoSentinel Surveillance Network clinics following international travel and highlight important considerations that can inform destination- and traveller-specific pre-travel counselling and the medical management of the ill-returned international traveller.
Methods
Data source
GeoSentinel Surveillance Network sites are specialized travel and tropical medicine clinics in 24 countries on 6 continents that systematically contribute clinical information on ill travellers (8,9). To be eligible for inclusion in the GeoSentinel database, patients must have crossed an international border within 10 years and be seeking medical care from a GeoSentinel clinician for a presumed travel-associated condition. Anonymous surveillance data are collected by the sites and entered into a structured query language database. Final diagnoses reported by physicians are assigned diagnosis codes chosen from a standardized list of >500 diagnoses. These diagnoses are grouped into 21 broad syndrome categories (10).
Inclusion criteria
We reviewed data entered into the GeoSentinel database from 1 January 2000 to 31 December 2012. Only ill travellers with either laboratory-confirmed or probable travel-associated diagnoses who were current US residents seen after return from international travel at 1 of the 22 US GeoSentinel Sites were included in the analysis. Travellers visiting friends and relatives (VFRs) were defined as immigrants, including their spouses and children, originally from a lower income country and now living in the United States, who travelled to the region of origin of self or family (11). Patients who had travelled for the purpose of immigration to USA, for medical tourism, or for military activities were excluded; the latter two categories were only recently available for coding in GeoSentinel and represented small numbers (n = 28).
Statistical analysis
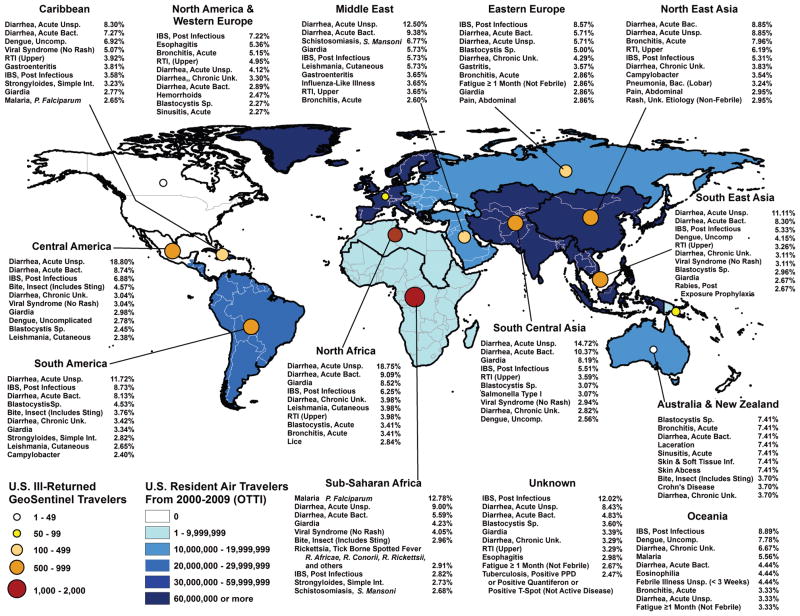
An analysis describing broad syndrome categories and specific diagnoses according to reason for travel [tourist, business, missionary/volunteer/research/aid work (MVRA), student and VFR], as well as world region of acquisition, was conducted using SAS 9.2 (Cary, NC). Figure 1 was generated by using ArcView GIS software (v. 10, ESRI, Redlands, CA).
Figure 1.
Top 10 diagnoses by world geographic regions visited among ill US travellers after return from international travel, GeoSentinel Surveillance Network, 2000–12.
Results
Demographic and travel characteristics
During 2000–12, 9624 ill-returned US travellers with 12 384 diagnoses were reported. Most travellers acquired their illness in subSaharan Africa (SSA) (25%), Central America (including Mexico) (18%) and South America (14%) (Table 1). Age and duration of travel differed according to reason for travel, and medical care was generally sought within 2 weeks (Table 1). Overall, 7% of travellers were treated as inpatients, while about one-quarter (24%) of VFR travellers required hospitalization. Pre-travel advice was obtained by almost half (45%) of all ill-returned travellers but only by 20% of VFR travellers.
Table 1.
Demographics of ill US residents after return from international travel presenting to US GeoSentinel Network Clinics, 2000–12a
| Reason for travel
| ||||||
|---|---|---|---|---|---|---|
| Characteristic | All Travellers
|
Business
|
MVRA
|
Student
|
Tourist
|
VFR
|
| n = 9624 | n = 1394 | n = 2379 | n = 593 | n = 3656 | n = 1580 | |
| Gender (%) | ||||||
| Male | 48.6 | 62.7 | 51.4 | 29.3 | 42.3 | 53.2 |
| Female | 51.5 | 37.4 | 48.6 | 70.7 | 57.7 | 46.8 |
| Country of birth (%) | ||||||
| USA | 77.9 | 78.6 | 91.5 | 91.4 | 86.2 | 32.3 |
| Non-USA | 22.1 | 21.5 | 8.5 | 8.6 | 13.8 | 67.7 |
| Age (years) | ||||||
| Median | 33 | 42 | 25 | 22 | 38 | 33 |
| Range | 0–94 | 4–88 | 1–92 | 12–61 | 0–94 | 0–91 |
| Age-groups (%) | ||||||
| ≤17 years | 6.8 | 0.9 | 2.8 | 4.4 | 4.1 | 25.1 |
| 18–64 years | 87.2 | 96.2 | 94.2 | 95.6 | 86.8 | 66.0 |
| ≥65 years | 6.1 | 3.0 | 3.0 | 0 | 9.1 | 8.9 |
| Travel duration (days) | ||||||
| Median | 20 | 14 | 61 | 42 | 13 | 30 |
| Interquartile range | 10–60 | 8–35 | 16–421 | 22–101.5 | 8–22 | 16–61 |
| Time from return to presentation (days) | ||||||
| Median | 13 | 16 | 17.5 | 11 | 11 | 10 |
| Interquartile range | 4–42 | 4–58 | 5–49 | 3–37 | 4–38 | 4–30 |
| Level of care (%) | ||||||
| Inpatient | 7.4 | 3.3 | 2.7 | 4.7 | 5.2 | 24.4 |
| Outpatient | 92.6 | 96.7 | 97.3 | 95.3 | 94.8 | 75.6 |
| Pre-travel medical advice (%) | ||||||
| Yes | 45.4 | 45.7 | 73.4 | 62.9 | 35.4 | 19.5 |
| No | 42.0 | 43.7 | 17.1 | 21.4 | 53.1 | 60.0 |
| Do not know | 12.6 | 10.6 | 9.5 | 15.7 | 11.5 | 20.5 |
| Region of exposure (%) | ||||||
| Central Americab | 17.5 | 9.0 | 14.3 | 15.7 | 27.2 | 8.4 |
| Caribbean | 10.0 | 7.0 | 7.3 | 2.0 | 9.8 | 19.8 |
| South America | 13.5 | 10.0 | 20.4 | 15.4 | 11.9 | 8.8 |
| South Central Asia | 9.0 | 13.6 | 5.6 | 12.3 | 7.3 | 13.2 |
| South East Asia | 7.8 | 8.2 | 7.4 | 5.3 | 8.7 | 7.0 |
| North East Asia | 3.9 | 8.6 | 2.3 | 6.2 | 3.5 | 3.0 |
| SubSaharan Africa | 25.4 | 26.6 | 34.0 | 31.8 | 14.8 | 32.4 |
| North Africa | 2.0 | 3.0 | 2.0 | 1.5 | 2.0 | 1.6 |
| Middle East | 2.2 | 3.6 | 0.7 | 3.3 | 2.6 | 2.2 |
| Oceania | 1.0 | 0.8 | 1.8 | 0.6 | 1.1 | 0.3 |
| Europe | 6.7 | 9.1 | 3.6 | 5.9 | 9.8 | 3.4 |
| Others | 0.8 | 0.6 | 0.6 | 0.2 | 1.4 | 0.1 |
MVRA, missionary, volunteers, research, aid work; VFR, visiting friends and relatives.
Missing values: age (84), sex (33), country of birth (1), pre-travel (0; note: combined missing values with ‘Don’t Know’), Hospitalization (19) and Travel reason (22). Data for region of exposure were missing (480) or not ascertainable (473).
Mexico was included.
Spectrum of disease
Illnesses of the gastrointestinal (GI) tract (58%), systemic febrile illnesses (18%) and dermatologic disorders (17%) were the leading diagnostic categories afflicting more than 90% of the travellers. Vaccine-preventable infections (VPI) were diagnosed in 2% of travellers. Three deaths were reported during the study period (Table 2).
Table 2.
Diagnosis groups, selected subgroup and aetiologic diagnoses and proportion of hospitalization for 9624 ill-returning US international travellers
| Diagnosisa | N (%) of US traveller | (%) hospitalized |
|---|---|---|
| Acute diarrhoeab | 2897 (30.1) | 2.9 |
| Unspecified aetiology | 1081 (37.3) | 1.7 |
| Bacterial aetiology | 897 (31.0) | 2.5 |
| Parasitic aetiology | 650 (22.4) | 3.2 |
| Chronic diarrhoeac | 1008 (10.5) | 0.5 |
| Post-infectious IBS | 591 (58.6) | 0.2 |
| Nondiarrhoeal gastrointestinal disorderd | 1709 (17.8) | 4.0 |
| Strongyloidiais, simple intestinal | 206 (12.1) | 5.3 |
| Schistosomiasise | 96 (5.6) | 6.5 |
| Systemic febrile illness, allf | 1748 (18.2) | 23.8 |
| Malariag | 479 (27.4) | 48.6 |
| Viral syndrome | 324 (18.5) | 4.0 |
| Dengueh | 209 (12.0) | 18.3 |
| Mononucleosis syndromei | 152 (8.7) | 2.1 |
| Enteric feverj | 106 (6.1) | 34.9 |
| Rickettsial infectionsk | 82 (4.7) | 6.1 |
| Dermatologic disorder, alll | 1594 (16.6) | 4.0 |
| Arthropod bites | 319 (20.0) | 1.3 |
| Bacterial skin infections | 150 (9.4) | 10.7 |
| Fungal skin infections | 107 (6.7) | 1.0 |
| Cutaneous leishmaniasism | 98 (6.1) | 3.1 |
| Respiratory disorder, alln | 1042 (10.8) | 6.8 |
| Hyperactive airway diseaseo | 271 (26.0) | 5.0 |
| Upper respiratory tract infection | 286 (27.4) | 1.1 |
| Nonspecific | 578 (6.0) | 4.7 |
| Chronic disease | 250 (2.6) | 11.4 |
| Neurologic disorder | 204 (2.1) | 14.0 |
| Genitourinary/STDp | 245 (2.5) | 11.4 |
| Psychologic | 229 (2.4) | 3.2 |
| Injury | 226 (2.3) | 11.2 |
| Tissue parasitesq | 149 (1.5) | 9.0 |
| Oral/dental disorder | 139 (1.4) | 3.7 |
| Vaccine-preventable diseaser | 178 (1.8) | 28.1 |
IBS, irritable bowel syndrome, STD, sexually transmitted diseases.
Three deaths were reported during the study period, the associated diagnoses were acute respiratory infection and sepsis. No associated diagnosis was reported for the third case.
Specific diagnoses included infections with Giardia spp. (n = 359), Campylobacter spp. (n = 136), Entamoeba histolytica (n = 89), Salmonella spp. (n = 40), Shigella spp. (n = 16), Cryptosporidium spp. (n = 42) and Dientamoeba fragilis (n = 37) and Clostridium difficile-associated disease (n = 54).
Includes travellers with pre-existing (n = 32) and new onset (n = 13) inflammatory bowel disease.
Includes travellers with acute hepatitis [n = 69; hepatitis A virus (n = 26), hepatitis E virus (n =7), hepatitis B virus (n = 5), hepatitis C virus (n = 1), unspecified (n = 30)], echinococcosis (n = 9) and intestinal ascaris (n = 45).
Most identified species were Schistosoma mansoni (n = 92) and S. japonicum (n = 4).
Rare specific diagnosis were histoplasmosis (n = 21), acute brucellosis (n = 19), leptospirosis (n = 18), extrapulmonary infection with Mycobacterium tuberculosis (n = 13), relapsing fever (n = 2), Chikungunya fever (n = 11), acute HIV infection (n = 7), Q fever (n = 5), coccidiomycosis (n = 3) and African trypanosomiasis (n = 3).
Includes cases of severe and complicated malaria (n = 26). Malaria was caused by infections with Plasmodium falciparum (n = 328), P. vivax (n = 61), P. ovale (n = 14) and P. malariae (n = 8).
Dengue cases were reported as uncomplicated (n = 205) and complicated (i.e. Dengue hemorrhagic fever and Dengue shock syndrome, according to the WHO criteria (n = 4).
Includes infections with Epstein–Barr virus (n = 79), cytomegalovirus (n = 30) and Toxoplasma gondii (n = 18).
Enteric fever was caused by infections with S. Typhi (n = 58), S. Paratyphi (n = 16) and unspecified species (n = 32).
Rickettsial infections were mostly due to tick-borne-spotted fever-associated species (n = 68), or to flea-borne Rickettsia typhi (n = 4).
Less common specific diagnoses were animal bites (n = 96), scabies (n = 51), myiasis (n = 47), cutaneous larva migrans (n = 45), leprosy (n = 5) and tungiasis (n = 15).
Most cases were acquired in Costa Rica (n = 22) and Peru (n = 12).
Other less common specific diagnoses were acute sinusitis (n = 100), bacterial (lobar) pneumonia (n = 91), and pulmonary infection with Mycobacterium tuberculosis (n = 25).
Hyperactive airway disease represents the diagnoses of asthma, acute and chronic bronchitis and bronchospasm.
Includes infections with S. haematobium (n = 49).
Tissue parasites represent mostly filarial infections (n = 90), and infections with schistosomiasis, human species not further specified (n = 37).
The most common diagnoses included enteric fever [n = 106, S. Typhi (n = 58), S. Paratyphi (n = 16), unspecified (n = 32), acute viral hepatitis (n = 31), hepatitis A virus (n = 26), hepatitis B virus (n = 5) and influenza [n = 29, influenza A virus (n = 21), influenza 2009 H1N1 virus (n = 6), influenza B virus (n = 2)].
Illnesses of the GI tract
Overall, 30% and 11% of travellers were diagnosed with acute and chronic diarrhoea, respectively (Table 2). Among those with acute diarrhoea, the most commonly detected infectious pathogens were Giardia spp. (12.4%), Campylobacter spp. (4.7%), Entamoeba histolytica (3.1%) and Salmonella spp. (1.4%), while those with chronic diarrhoea were predominantly diagnosed with post-infectious irritable bowel syndrome (PI-IBS) (58.6%). The leading nondiarrhoeal infections among travellers with a (GI) diagnosis were intestinal strongyloidiasis (12.1%) and schistosomiasis (5.6%).
Systemic febrile illnesses
Malaria, diagnosed in 27.4% of travellers with fever, was the most common identified aetiologic diagnosis followed by dengue (12.0%) and mononucleosis syndrome (8.7%) (Table 2). Enteric fever [including infections with Salmonella enterica serotype Typhi (S. Typhi), Salmonella enterica serotype Paratyphi (S. paratyphi), and unspecified species] and rickettsial infections, mostly due to tick-borne-spotted fever-associated species, were diagnosed in 6.1% and 4.7% of travellers with fever, respectively.
Dermatologic disorders
Most patients with skin problems were diagnosed with arthropod bites (20%), followed by bacterial infections (9%), fungal infections (7%) and cutaneous leishmaniasis (6%) (Table 2).
Travel-associated diagnoses according to reason for travel and region of exposure
Among most travellers regardless of the world region of exposure acute and chronic diarrhoea accounted for 40%–60% of the reported diagnoses (Figure 2). Acute unspecified diarrhoea and acute bacterial diarrhoea were among the top three diagnoses among US residents returning ill from all regions except North America/Western Europe, Australia/New Zealand and Oceania. Giardia spp.-related acute diarrhoea was a top 10 diagnosis in travellers returning ill from North Africa, Middle East, South Central Asia, SSA and Central America (Figure 1).
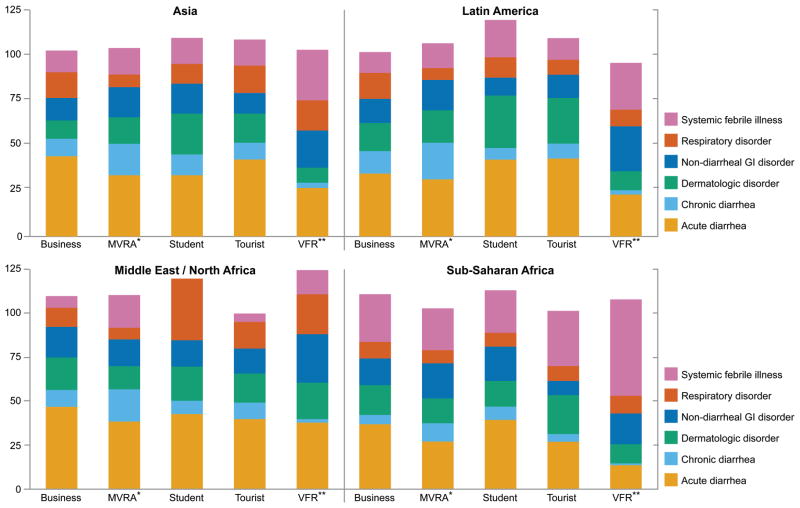
Figure 2.
Broad diagnostic categories according to purpose of travel and region of exposure in US residents after return from international travel, US GeoSentinel Clinics, 2000–12. Patients can have ≥1 diagnosis in different categories. Latin America includes Mexico, and countries in the Caribbean, Central and South America. MVRA, missionary, volunteer, research, aid work; VFR, visitng friends and relatives.
Nondiarrhoeal GI disorders accounted for a large proportion of diagnoses among VFR travellers returning from Latin America, North Africa/Middle East and Asia (Figure 2). Simple intestinal strongyloidiasis was noted disproportionately among VFR travellers and MVRA travellers with exposure in Latin America and SSA (Table 3), while Schistosoma mansoni was primarily diagnosed in travellers who returned from the Middle East/North Africa and SSA (Table 3). In fact, infection with S. mansoni was one of the leading diagnoses among those returning ill from the Middle East, with most diagnoses among travellers returned from Yemen, Sudan and Egypt (Figure 1).
Table 3.
Proportions of selected aetiologic diagnoses according to purpose of travel and region of exposure, GeoSentinel Surveillance Network, 2000–12
| Reason for travel (col %)
|
Region of exposurea (col %)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Business n = 1394 | MVRA n = 2379 | Student n = 593 | Tourist n = 3656 | VFR n = 1580 | Latin Americab n = 3547 | Asia n = 1795 | Sub-Saharan Africa n = 2199 | Middle East/North Africa n = 368 | Otherc n = 742 | |
| Acute diarrhoea due to Giardia spp., n = 359 | 68 (4.9) | 87 (3.7) | 22 (3.7) | 126 (3.4) | 55 (3.5) | 108 (3.0) | 87 (4.8) | 93 (4.2) | 26 (7.1) | 12 (1.6) |
| Post-infectious irritable bowel syndrome, n = 591 | 87 (6.2) | 233 (9.8) | 33 (5.6) | 217 (5.9) | 18 (1.1) | 237 (6.7) | 97 (5.4) | 62 (2.8) | 22 (6.0) | 56 (7.5) |
| Strongyloidiasis (simple intestinal), n = 206 | 11 (0.8) | 58 (2.4) | 11 (1.9) | 42 (1.1) | 84 (5.3) | 86 (2.4) | 30 (1.7) | 60 (2.7) | 3 (0.8) | 5 (0.7) |
| Schistosomiasis (S. mansoni), n = 92 | 7 (0.5) | 46 (1.9) | 7 (1.2) | 14 (0.4) | 18 (1.1) | 5 (0.1) | 1 (<0.1) | 59 (2.7) | 16 (4.3) | 2 (0.3) |
| Malaria (P. falciparum), n = 328 | 28 (2.0) | 44 (1.8) | 14 (2.4) | 34 (0.9) | 208 (13.2) | 26 (0.7) | 7 (0.4) | 281 (12.8) | 5 (1.4) | 1 (0.1) |
| Dengue fever, n = 209 | 23 (1.6) | 53 (2.2) | 11 (1.9) | 72 (2.0) | 50 (3.2) | 123 (3.5) | 49 (2.7) | 23 (1.0) | 1 (0.3) | 7 (0.9) |
| Enteric fever, n = 106 | 9 (0.6) | 21 (0.9) | 2 (0.3) | 32 (0.9) | 42 (2.7) | 30 (0.8) | 53 (3.0) | 17 (0.8) | 0 (0.0) | 2 (0.3) |
| Rickettsiosis, n = 82 | 6 (0.4) | 14 (0.6) | 2 (0.3) | 57 (1.6) | 3 (0.2) | 4 (0.1) | 5 (0.3) | 68 (3.1) | 2 (0.5) | 2 (0.3) |
| Cutaneous leishmaniais, n = 98 | 9 (0.6) | 22 (0.9) | 13 (2.2) | 44 (1.2) | 10 (0.6) | 67 (1.9) | 3 (0.2) | 5 (0.2) | 18 (4.9) | 2 (0.3) |
MVRA, missionary, volunteer, research, aid work, VFR, visiting friends and relatives.
Missing or not ascertainable exposure region: Giardia spp. 33 (9%); IBS 117 (20%); Strongyloidiasis 22 (11%); Schistosomiasis 9 (10%); P. falciparum malaria 8 (2%); Dengue 6 (3%); Enteric 4 (4%); Rickettsiosis 1 (1%) and Leishmaniasis 3 (3%).
Includes Mexico and countries in the Caribbean, Central and South America.
‘Other’ region includes North America, Australia/New Zealand, Western/Eastern Europe and Oceania.
Systemic febrile illness was disproportionately encountered among ill VFR travellers returning from Asia and SSA (Figure 2). The relative morbidity due to malaria [infection with Plasmodium falciparum (Pf)] and enteric fever was greatest among VFR travellers, while rickettsial infections disproportionately affected tourist travellers (Table 3). Malaria due to Pf and rickettsiosis were observed predominantly after exposure in SSA (Table 3; Figure 1). Dengue was disproportionately observed among travellers returning ill from Latin America and Asia, while enteric fever was predominantly diagnosed in ill travellers returning from Asia (Table 3). Especially among travellers with exposure to the Caribbean, South East Asia, and Oceania dengue was noted as a leading diagnosis (Figure 1).
Dermatologic disorders were disproportionally diagnosed in ill student travellers with exposure in Latin America and Asia (Figure 2). Cutaneous leishmaniasis was diagnosed with greater frequency in those returning ill from Latin America and Middle East/North Africa (Table 3; Figure 1).
Discussion
We report an extended spectrum of disease among nearly 10 000 ill-returned US travellers during 2000–12 that may reflect a high degree of global mobility of the US public (1). The acquisition and aetiology of travel-associated diseases varied with destination and purpose of travel. Since most travellers do not visit specialized clinics for advice prior to travel nor for illness following travel, it is important for primary care providers to be familiar with the epidemiology of commonly acquired and life-threatening travel-associated conditions. This knowledge could lead to better preparation of departing international travellers, and result in faster and more accurate diagnosis and medical management of ill-returned travellers, thereby reducing complications and even mortality (4,12).
Acute diarrhoea was previously estimated to be the leading diagnosis among travellers, affecting 9.5–15.9 million US travellers annually (13). Similarly, we found that diarrhoea was the most common condition reported among US travellers returning ill from all regions of the world. In clinical practice, the aetiology of acute diarrhoea is usually unknown. Patients may be managed by either self-treatment during or after travel, or by empiric antibiotic treatment following travel from their provider without pursuing routine stool cultures/studies and thus precluding the detection of the more common bacterial aetiologies (e.g. enterotoxogenic/enterohaemorrhagic Escherichia coli, Salmonella spp, Shigella spp, Campylobacter spp (3,14,15)). The frequent use of empiric antibiotics among international travellers with diarrhoea may have favoured the identification of Giardia spp. in this analysis.
Post-infectious irritable bowel syndrome (PI-IBS) has become increasingly recognized as a major cause of travel-associated morbidity (16). In our analysis, MVRA travellers frequently presented with PI-IBS. However, the relevance of PI-IBS as one of the top 10 diagnoses for most regions must be viewed carefully in the context of an estimated background IBS prevalence of 10–15% in the US population (17).
Strongyloidiasis was frequently diagnosed in VFR and MVRA travellers. Diagnosis was made by either examination of the stool for ova and parasites or by serology. An extended sojourn in endemic regions with environmental exposure to water or soil favours infection (12). Among VFR travellers born in the tropics, the diagnosis of strongyloidiasis cannot necessarily be attributed to the most recent trip, since without treatment it can be considered a lifelong infection acquired prior to emigration to USA. Nevertheless, it is still important to establish this diagnosis in at-risk persons to prevent complications of strongyloidiasis (e.g. if they are later given immunosuppressive medication or undergo an organ transplantation (12)).
Schistosoma mansoni is another important pathogen attributed to environmental exposure. Endemic areas include Africa, tropical South America and parts of the Middle East and Asia. The greatest proportion was observed among travellers returning ill from North Africa/Middle East and SSA. Exposure (swimming, bathing or wading) to freshwater lakes, streams and rivers is the primary risk factor, and outbreaks of acute schistosomiasis among travel groups have been described by GeoSentinel sites (18–21). Affected travellers commonly present 2–8 weeks after exposure with a clinical syndrome that may include fever, cough, malaise, headache, hepatosplenomegaly and eosinophilia, although asymptomatic infection can occur (22). Some clinicians recommend screening by schistosomiasis serology (at least 3 months following return) for travellers who return from affected areas with a history of freshwater exposure (23).
Malaria was the most common aetiologic diagnosis among febrile travellers and frequently required hospitalization. Unspecified febrile illnesses and viral syndromes are likely under-represented in this analysis, because such patients may not seek medical attention at all or may not visit specialized travel/tropical medicine clinics. Malaria is particularly important to recognize due to the risk of serious morbidity and mortality associated with misdiagnosis and delays in the initiation of treatment. With headache, nausea, vomiting and low-grade fever, the clinical presentation in travellers is often nonspecific and may mimic other diseases, including influenza, septicaemia, gastroenteritis, urinary tract infection and viral syndromes (24). Although in some countries the number of imported malaria cases may have decreased, the most recent US surveillance data from 2011 reported a 14% increase above 2010 (25). By far, VFR travellers with exposure in SSA represent the leading group of travellers at risk for malaria. Although our analysis could not directly assess use of malaria prophylaxis, CDC data from 2009 demonstrated that VFR travellers had the lowest proportion using prophylaxis compared with other traveller groups (26). When patients are seen with a fever following travel to a malaria endemic area (www.cdc.gov/malaria/map/), malaria smears should be performed (and a rapid malaria test done, if available) and read by an experienced microscopist. This may involve an urgent referral to a larger community or university hospital emergency room or to an infectious disease or tropical medicine clinician.
As in other studies, rickettsial infections, primarily African tick-bite fever, were diagnosed almost exclusively in tourist travellers after exposure in SSA (27). Risk factors reported include male gender, travel during the late summer months of southern Africa (March–May), and game hunting (28). Transmission is by tick bite, often during safaris, and patients may present with fever and an inoculation eschar. These patients may also be quite ill and require hospitalization and treatment with doxycycline.
Dengue is an important travel-associated viral infection, capable of causing significant morbidity. Furthermore, dengue has the potential to be introduced in the United States because the vector mosquitoes are in abundance here (5,29). Most dengue cases in this study were diagnosed in ill travellers returning from Latin America and Asia. Over the last decade, dengue activity has significantly increased in dengue-endemic regions in Latin America that are major destinations for US travellers (30). Therefore most dengue cases in USA have previously been reported among travellers returning from the Caribbean, Mexico and Central America (6). Travellers returning with dengue typically present within 14 days of their last possible exposure with an illness characterized by fever, headache, rash, myalgia/arthralgia and vomiting/nausea. Dengue haemorrhagic fever/dengue shock syndrome is only rarely seen in travellers, yet hemorrhagic manifestations have been frequently reported (31). Diagnosis still relies mostly on serologic testing and treatment is supportive. Educating all travellers to endemic regions about mosquito bite avoidance measures and the importance of prompt medical evaluation of fever are key. An effective and safe dengue vaccine will be a welcome tool to protect international travellers in the future (32).
Vaccine-preventable infections (VPI) were rarely diagnosed; most were due to infections with Salmonella enterica serotype Typhi (S. Typhi) and hepatitis A virus. The true burden of VPI may be higher, since most travellers with respiratory infections due to either the common cold or influenza do not seek care at a specialized travel clinic. These diagnoses would be seen more frequently in the primary care setting. It is helpful to know that influenza occurs year-round in the tropics so that even if travellers return from the tropics ill with respiratory infection during the summer, it is reasonable to test for influenza. Enteric fever disproportionally affected VFR travellers and travellers with exposure in Asia. A recent review of US typhoid fever surveillance data from 1999 to 2006 also observed that most cases were noted among travellers to the Indian subcontinent (67%) and in VFR travellers (66% (33)). Further, S. Typhi isolates acquired in such countries were 8 and 20 times more likely to be multidrug- and nalidixic acid-resistant, respectively (33). Hence, a typhoid fever vaccine for US travellers to typhoid-fever endemic areas represents an important intervention to help reduce the burden of typhoid fever and the spread of resistant strains of S. Typhi in USA (33). However, given that typhoid vaccine is only 70% effective, meticulous food and water hygiene practices remain important as preventive measures. Diagnosis is most frequently made by blood cultures, though stool and urine cultures should be obtained as well. Bone marrow cultures are the most sensitive, but are rarely done. Because many strains are now resistant to quinolone antibiotics, a parenteral third generation cephalosporin should be used prior to obtaining sensitivity testing.
Leishmaniasis has been increasingly reported in travellers, corresponding to its recent emergence in previously nonendemic countries (34). While visceral and mucocutaneous leishmaniasis were not at all and only rarely (n = 1) reported during the study period, respectively, cutaneous leishmaniasis was identified as one of the most common dermatologic diagnoses among ill-returning US travellers. Latin America is considered the principal region for travellers to acquire cutaneous leishmaniasis, yet destinations around the Mediterranean, including southern Europe, North Africa and Middle East, as shown in this analysis, are other important areas of acquisition (34). Small macular skin lesions at the site of sandfly bites that evolve over weeks into pruritic, erythematous papules or nodules are highly suspicious and returning travellers with such a lesion may benefit from expert evaluation, including biopsy.
Aside from travel destination, purpose of travel is an important determinant of travel-associated morbidity. As shown previously (35–37), US-VFR travellers in this analysis were found to rarely avail themselves of pre-travel preventive services, while they were disproportionally affected by conditions that require hospitalization. Our findings once more underscore that travel health, and specifically malaria-relevant prevention messages, need to be clearly targeted toward VFR populations (26). Innovative models are needed to engage this group of travellers and to mitigate barriers to access of preventive pre-travel health services (38).
MVRA and student travellers embarked on travel of above-average duration and were more likely to have received pre-travel care compared with the other travellers. The trips of such travellers are commonly pre-arranged and organized by aid, religious or educational institutions that frequently require pre-travel care (39). Despite this, US student travellers in our analysis still faced health problems that were most likely related to longer and more intimate environmental exposures. According to a recent survey, student travellers’ health problems included infections (70%), followed by psychological distress (10%) and injuries (8% (40)). Likewise, other studies of long-term travellers, including humanitarian aid workers, identified vector-borne and contact-transmitted diseases, as well as psychological problems, as common (41).
GeoSentinel data do not allow a complete epidemiologic analysis of all ill-returning US travellers. In particular, GeoSentinel has limited capture of patients with mild and self-limited diseases, those presenting to nonspecialized primary and emergency care settings, and those with more severe travel-associated diseases requiring hospitalization. Furthermore, data on well travellers are not captured, making it impossible to calculate true incidence rates or risk. GeoSentinel data primarily permit analysis of morbidity among travellers in the context of location of exposure and reason for travel. Data for region of exposure were missing or not ascertainable for 973 (10%) of reported diagnoses, which may have limited our analysis of region-specific morbidity. However, only in a minority of travellers with certain aetiologic diagnoses was no exposure region recorded (Table 3). Despite these limitations, the travellers included in this analysis represent a sentinel sample of US travellers, allowing these data to provide insight into the complex epidemiology of travel-associated morbidity.
In summary, US clinicians in family practice as well as in other primary care areas are increasingly likely to encounter ill travellers, and continuing medical education curricula need to keep pace with the increasing mobility of the population. Inquiry about recent travel needs to be considered as an important routine question in medical history-taking. Information on purpose of travel and destination represents important determinants guiding clinicians for optimal post-travel care and may inform public health planners in developing preventive pre-travel care strategies.
Acknowledgments
We thank all contributors to the GeoSentinel Surveillance Network, and Kevin Liske for preparing the map. The findings and conclusions in this report are the findings and conclusions of the authors and do not necessarily represent the views of the US Centres for Disease Control and Prevention.
Funding: US Centres for Disease Control and Prevention (5U50CK000189), International Society of Travel Medicine.
Footnotes
Conflict of interest: Dr. Connor reported receiving royalties from Elsevier for ‘Travel Medicine, 3rd Edition’. Dr. Marano reported that she is an employee of GlaxoSmithKline Vaccines and that she is a GlaxoSmithKline stock option owner. No other author reported conflict of interest.
Ethical approval: GeoSentinel’s data collection protocol was reviewed and classified by the Centres for Disease Control and Prevention as public health surveillance and not as human subjects research requiring submission to institutional review boards.
References
- 1.US Department of Commerce, ITA, Office of Travel and Tourism Industries. [accessed August 12, 2014];United States Resident Travel Abroad. 2010 Released July 2012. http://tinet.ita.doc.gov.
- 2.LaRocque RC, Rao SR, Lee J, Ansdell V, et al. Global TravEpiNet Consortium. Global TravEpiNet: a national consortium of clinics providing care to international travelers–analysis of demographic characteristics, travel destinations, and pretravel healthcare of high-risk US international travelers, 2009–2011. Clin Infect Dis. 2012;54:455–62. doi: 10.1093/cid/cir839. [DOI] [PubMed] [Google Scholar]
- 3.Hill DR, Ericsson CD, Pearson RD, et al. Infectious Diseases Society of America. The practice of travel medicine: guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1499–539. doi: 10.1086/508782. [DOI] [PubMed] [Google Scholar]
- 4.Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among U.S. travelers, 1963–2001. Ann Intern Med. 2004;141:547–55. doi: 10.7326/0003-4819-141-7-200410050-00012. [DOI] [PubMed] [Google Scholar]
- 5.Morens DM, Fauci AS. Dengue and hemorrhagic fever: a potential threat to public health in the United States. JAMA. 2008;299:214–6. doi: 10.1001/jama.2007.31-a. [DOI] [PubMed] [Google Scholar]
- 6.Mohammed HP, Ramos MM, Rivera A, et al. Travel-associated dengue infections in the United States, 1996 to 2005. J Travel Med. 2010;17:8–14. doi: 10.1111/j.1708-8305.2009.00374.x. [DOI] [PubMed] [Google Scholar]
- 7.Nilles EJ, Arguin PM. Imported malaria: an update. Am J Emerg Med. 2012;30:972–80. doi: 10.1016/j.ajem.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Freedman DO, Kozarsky PE, Weld LH, Cetron MS. GeoSentinel: the global emerging infections sentinel network of the International Society of Travel Medicine. J Travel Med. 1999;6:94–8. doi: 10.1111/j.1708-8305.1999.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 9.CDC. Surveillance for Travel-Related Disease – GeoSentinel Surveillance System, United States, 1997–2011. MMWR Morbid Mortal Wkly Rep. 2013;62:1–23. [PubMed] [Google Scholar]
- 10.Freedman DO, Weld LH, Kozarsky PE, Fisk T, et al. GeoSentinel Surveillance Network. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354:119–30. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 11.Keystone JS. Immigrants returning home to visit friends and relatives (VFRs) In: Brunette GW, Kozarsky PE, Magill AJ, Shlim DR, Whatley AD, editors. CDC Health Information for International Travel 2012. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 12.Boulware DR, Stauffer WM, Hendel-Paterson BR, et al. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med. 2007;120:545.e1–8. doi: 10.1016/j.amjmed.2006.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paredes-Paredes M, Flores-Figueroa J, Dupont HL. Advances in the treatment of travelers’ diarrhea. Curr Gastroenterol Rep. 2011;13:402–7. doi: 10.1007/s11894-011-0208-6. [DOI] [PubMed] [Google Scholar]
- 14.DuPont HL, Khan FM. Travelers’ diarrhea: epidemiology, microbiology, prevention, and therapy. J Travel Med. 1994;1:84–93. doi: 10.1111/j.1708-8305.1994.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 15.Greenwood Z, Black J, Weld L, et al. GeoSentinel Surveillance Network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221–8. doi: 10.1111/j.1708-8305.2008.00203.x. [DOI] [PubMed] [Google Scholar]
- 16.Pitzurra R, Fried M, Rogler G, et al. Irritable bowel syndrome among a cohort of European travelers to resource-limited destinations. J Travel Med. 2011;18:250–6. doi: 10.1111/j.1708-8305.2011.00529.x. [DOI] [PubMed] [Google Scholar]
- 17.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691–9. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 18.Leshem E, Maor Y, Meltzer E, Assous M, Schwartz E. Acute schistosomiasis outbreak: clinical features and economic impact. Clin Infect Dis. 2008;47:1499–506. doi: 10.1086/593191. [DOI] [PubMed] [Google Scholar]
- 19.Nicolls DJ, Weld LH, Schwartz E, et al. GeoSentinel Surveillance Network. Characteristics of schistosomiasis in travelers reported to the GeoSentinel Surveillance Network 1997–2008. Am J Trop Med Hyg. 2008;79:729–34. [PubMed] [Google Scholar]
- 20.Morgan OW, Brunette G, Kapella BK, et al. Schistosomiasis among recreational users of Upper Nile River, Uganda, 2007. Emerg Infect Dis. 2010;16:866–8. doi: 10.3201/eid1605.091740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartz E, Kozarsky P, Wilson M, et al. Schistosome infection among river rafters on Omo River, Ethiopia. J Travel Med. 2005;12:3–8. doi: 10.2310/7060.2005.00002. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz E. Schistosomiasis. In: Schwartz E, editor. Tropical Diseases in Travelers. Oxford, UK: Wiley-Blackwell; 2009. pp. 229–42. [Google Scholar]
- 23.Meltzer E, Artom G, Marva E, et al. Schistosomiasis among travelers: new aspects of an old disease. Emerg Infect Dis. 2006;12:1696–700. doi: 10.3201/eid1211.060340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadim B, Beherens RH. Malaria: an update for physicians. In: Moellering RC, Zumla A, Keiser J, editors. Tropical Diseases, Infectious Disease Clinic of North America. Vol. 26. 2012. pp. 243–59. [DOI] [PubMed] [Google Scholar]
- 25.CDC. Malaria Surveillance – United States, 2011. MMWR Morbid Mortal Wkly Rep. 2013;62:1–17. [PubMed] [Google Scholar]
- 26.CDC. Malaria Surveillance – United States, 2009. MMWR Morbid Mortal Wkly Rep. 2011;60:1–15. [PubMed] [Google Scholar]
- 27.Walker DH. Rickettsial diseases in travelers. Travel Med Infect Dis. 2003;1:35–40. doi: 10.1016/S1477-8939(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 28.Jensenius M, Fournier PE, Vene S, et al. Norwegian African Tick Bite Fever Study Group. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin Infect Dis. 2003;36:1411–7. doi: 10.1086/375083. [DOI] [PubMed] [Google Scholar]
- 29.CDC. Locally acquired dengue---Key West, Florida, 2009–2010. MMWR Morbid Mortal Wkly Rep. 2010;59:577–81. [PubMed] [Google Scholar]
- 30.San Martín JL, Brathwaite O, Zambrano B, et al. The epidemiology of dengue in the americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–35. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wichmann O, Gascon J, Schunk M, et al. European Network on Surveillance of Imported Infectious Diseases. Severe dengue virus infection in travelers: risk factors and laboratory indicators. J Infect Dis. 2007;195:1089–96. doi: 10.1086/512680. [DOI] [PubMed] [Google Scholar]
- 32.Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ. Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep. 2010;12:157–64. doi: 10.1007/s11908-010-0102-7. [DOI] [PubMed] [Google Scholar]
- 33.Lynch MF, Blanton EM, Bulens S, et al. Typhoid fever in the United States, 1999–2006. JAMA. 2009;302:859–65. doi: 10.1001/jama.2009.1229. [DOI] [PubMed] [Google Scholar]
- 34.Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032–9. doi: 10.1016/j.ijid.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Leder K, Tong S, Weld L, et al. GeoSentinel Surveillance Network. Illness in travelers visiting friends and relatives: a review of the GeoSentinel Surveillance Network. Clin Infect Dis. 2006;43:1185–93. doi: 10.1086/507893. [DOI] [PubMed] [Google Scholar]
- 36.Bacaner N, Stauffer B, Boulware DR, Walker PF, Keystone JS. Travel medicine considerations for North American immigrants visiting friends and relatives. JAMA. 2004;291:2856–64. doi: 10.1001/jama.291.23.2856. [DOI] [PubMed] [Google Scholar]
- 37.Angell SY, Cetron MS. Health disparities among travelers visiting friends and relatives abroad. Ann Intern Med. 2005;142:67–72. doi: 10.7326/0003-4819-142-1-200501040-00013. [DOI] [PubMed] [Google Scholar]
- 38.Leder K, Lau S, Leggat P. Innovative community-based initiatives to engage VFR travelers. Travel Med Infect Dis. 2011;9:258–61. doi: 10.1016/j.tmaid.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Chen LH, Wilson ME, Davis X, et al. GeoSentinel Surveillance Network. Illness in long-term travelers visiting GeoSentinel clinics. Emerg Infect Dis. 2009;15:1773–82. doi: 10.3201/eid1511.090945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartjes LB, Baumann LC, Henriques JB. Travel health risk perceptions and prevention behaviors of US study abroad students. J Travel Med. 2009;16:338–43. doi: 10.1111/j.1708-8305.2009.00322.x. [DOI] [PubMed] [Google Scholar]
- 41.Dahlgren AL, Deroo L, Avril J, Bise G, Loutan L. Health risks and risk-taking behaviors among International Committee of the Red Cross (ICRC) expatriates returning from humanitarian missions. J Travel Med. 2009;16:382–90. doi: 10.1111/j.1708-8305.2009.00350.x. [DOI] [PubMed] [Google Scholar]