Abstract
Objective
Antidepressants targeting monoaminergic neurotransmitter systems, despite their immediate effects at the synaptic level, usually require several weeks of administration to achieve clinical efficacy. The authors propose a strategy of adding creatine monohydrate (creatine) to a selective serotonin reuptake inhibitor (SSRI) in the treatment of patients with major depressive disorder. Such augmentation may lead to a more rapid onset of antidepressant effects and a greater treatment response, potentially by restoring brain bioenergetics at the cellular level.
Method
Fifty-two women with major depressive disorder were enrolled in an 8-week double-blind placebo-controlled clinical trial and randomly assigned to receive escitalopram in addition to either creatine (5 g/day, N=25) or placebo (N=27). Efficacy was primarily assessed by changes in the Hamilton Depression Rating Scale (HAM-D) score.
Results
In comparison to the placebo augmentation group, patients receiving creatine augmentation showed significantly greater improvements in HAM-D score, as early as week 2 of treatment. This differential improvement favoring creatine was maintained at weeks 4 and 8. There were no differences between treatment groups in the proportion of patients who discontinued treatment prematurely (creatine: N=8, 32.0%; placebo: N=5, 18.5%) or in the overall frequency of all reported adverse events (creatine: 36 events; placebo: 45 events).
Conclusions
The current study suggests that creatine augmentation of SSRI treatment may be a promising therapeutic approach that exhibits more rapid and efficacious responses in women with major depressive disorder.
The standard antidepressants, including selective serotonin reuptake inhibitors (SSRIs), are generally regarded as safe and effective treatments. However, more than a few weeks may be required to achieve reliable antidepressant responses (1, 2). From a clinical perspective, a more rapid treatment response would be important in treating depressed patients not only because of the high morbidity during the latent period before the onset of antidepressant effects (2–4) but also because of the association between faster response and better long-term outcome (5). Moreover, in light of the results from a large-scale trial indicating that the response and remission rates of depressed patients treated with standard antidepressants, such as citalopram, were relatively modest (6), efforts to find novel therapeutic approaches that can yield greater efficacy, as well as more rapid responses, would be valuable.
Creatine monohydrate (creatine) has been established as a safe natural product and has been available as a dietary supplement for more than a decade (7, 8). We found that oral supplementation with creatine increased the cerebral reservoir of phosphocreatine (9). An increased brain creatine reservoir may cause a shift in brain creatine kinase activity and ultimately be used to produce adenosine triphosphate (ATP) from phosphocreatine in response to energy demand (9–11). Previous studies using magnetic resonance spectroscopy with phosphorus-31 (31P-MRS) have suggested that subjects with major depressive disorder have abnormal brain bioenergetics (12–15) and that as symptoms improve with treatment, altered turnover of ATP to phosphocreatine is normalized (12–16). Furthermore, a high phosphocreatine level and low level of β-nucleoside triphosphate, resonance of which comes mainly from ATP in the brain, at baseline were associated with a subsequent positive treatment response to an SSRI and triiodothyronine augmentation (13). This suggests that the proenergetic effect of creatine supplementation, including efficient regeneration of intracellular high-energy phosphate, may contribute to an earlier and greater response to standard antidepressants.
Recent preclinical evidence from an animal model of depression also provides support for the potential antidepressant effects of creatine (17). This antidepressant-like response induced by creatine supplementation was observed in female, but not male, rodents. Although it is not clear why the antidepressant efficacy of creatine supplementation was predominantly in female rodents, sexual dimorphism in expression of creatine kinase levels (18) and beneficial effects of estrogen on mitochondrial function (19, 20)—for example, greater energy-producing capacity and lower reactive oxygen species production—could play a role in these sex-dependent creatine effects in animal models. Furthermore, abnormal cerebral metabolism associated with depression has been reported to be more common in women than in men (12), suggesting that creatine supplementation may be more beneficial in women. On the basis of these preliminary findings, we focused on women in what we believe is the first double-blind placebo-controlled study of creatine augmentation for major depressive disorder.
In this clinical trial, the efficacy, safety, and tolerability of creatine augmentation of escitalopram were assessed in individuals with major depression. Given the nature of the proof-of-concept study, only depressed women were selected as study subjects because creatine's antidepressant-like effects have been shown preclinically only in female animals. We expected that creatine augmentation would be associated with earlier and greater improvements in depressive symptoms than placebo augmentation.
Method
Participants
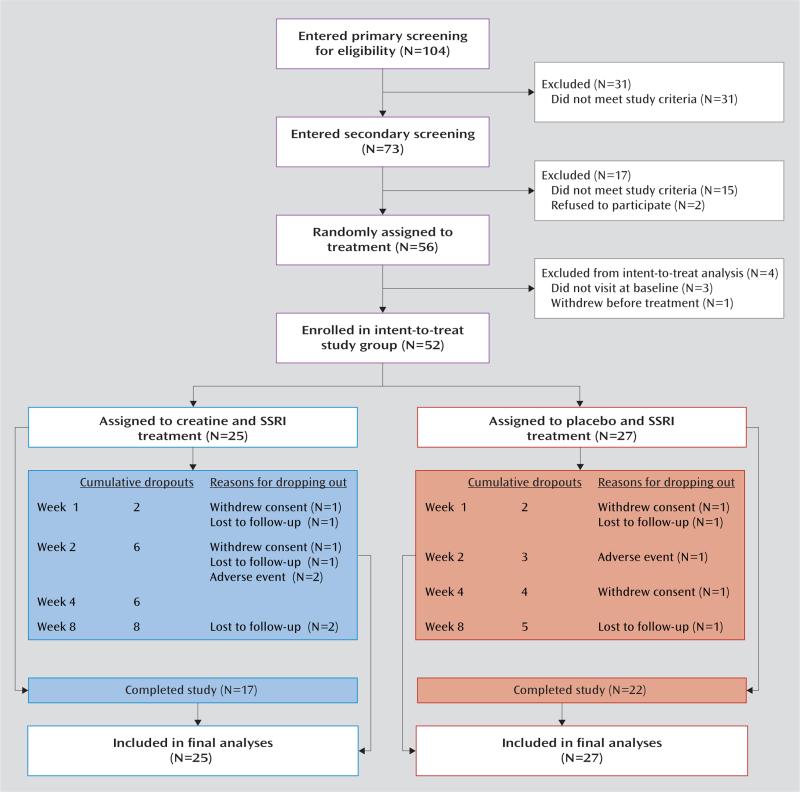
A total of 52 women completed baseline assessments and were enrolled in the study in an intention-to-treat study group (Figure 1). The inclusion criteria were 1) age between 19 and 65 years, 2) a diagnosis of major depressive disorder with a current major depressive episode as determined by the Structured Clinical Interview for DSM-IV (SCID-IV) (21), 3) a score of 16 or greater on the 17-item Hamilton Depression Rating Scale (HAM-D) (22), and 4) absence of any psychotropic medications for at least 8 weeks before the screening assessment. The exclusion criteria included a lifetime history of alcohol or other substance abuse, any clinically significant medical or psychiatric disease other than depression, a diagnosis of renal disease, an elevated serum creatinine level beyond the normal range (>1.1 mg/dL), a history of multiple adverse drug reactions or allergy to creatine or SSRIs, and pregnancy or breastfeeding.
FIGURE 1.
Screening, Randomization, and Disposition of Women With Major Depressive Disorder Randomly Assigned to Creatine Monohydrate or Placebo Augmentation of SSRI
The institutional review boards of the Seoul National University Hospital and the Catholic University of Korea College of Medicine approved the study protocol, and all participants provided written informed consent to participate in the study.
Study Design and Procedures
The enrolled subjects were randomly assigned in a 1:1 ratio to receive escitalopram plus creatine supplementation (the creatine group) or escitalopram plus placebo (the placebo group) according to a randomization log. The dose of creatine was 3 g/day for the first week and 5 g/day for another 7 weeks. Equivalent numbers of capsules were given to members of the placebo group (each placebo capsule contained dextrin and was identical in appearance to the 500-mg creatine monohydrate capsule). All individuals were prescribed escitalopram according to a schedule of 10 mg/day for the first week and then 20 mg/day for the following 7 weeks. However, the dose of escitalopram during the last 7 weeks could be adjusted according to the clinician's judgment of adverse events. Limited use of concomitant lorazepam (maximum daily dose of 1.5 mg) or zolpidem (maximum daily dose of 10 mg) was allowed during the study period for the control of anxiety or sleep disturbance, respectively. All other psychotropic medications were not permitted. Medication compliance was monitored by a self-rating visual analogue scale that rated medication administration from 0% to 100%. The scale was completed by the patient at each visit (23) and was also reviewed by an investigator during the interview at each visit. Individuals who took 80% or more of the assigned medications were considered compliant.
Outcome Measures
Participants were evaluated by the same board-certified psychiatrist (T.S.K), who was blinded to the assignment of treatment groups, at baseline and at 1, 2, 4, and 8 weeks after baseline. Reliability for each measurement is described in Appendix SA1, which appears in the data supplement accompanying the online version of this article. The primary outcome measures was the weekly change in the HAM-D score from baseline. The secondary outcome measures were weekly changes in scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) (24) and the Clinical Global Impressions Scale (CGI) severity of illness subscale (25). The response rate at each visit and the rate of remission after 8 weeks of treatment, based on the HAM-D score, were also measured as secondary outcomes. Treatment response was defined as a decrease of 50% or more in HAM-D score from baseline (26). Response within 4 weeks was considered to be an early treatment response (27). Remission was defined as a HAM-D score of 7 or less at week 8 (26).
Safety and tolerability of study medications were monitored and documented by the same board-certified psychiatrist (T.S.K) at every visit. The subjects’ spontaneously reported adverse events and specific inquiries regarding health problems that are frequently observed in individuals taking creatine or SSRIs (8, 28) were used for the safety assessments. Comprehensive physical and neurological examinations, electrocardiograms, and routine laboratory tests were performed at baseline and 8 weeks after baseline as safety measures. Height, weight, and vital signs were measured at every visit. Serum creatinine levels were checked at the screening visit and at 2 weeks and 8 weeks after baseline.
Statistical Analysis
The current study group of 52 subjects randomly assigned to creatine or placebo augmentation in a 1:1 ratio was large enough to permit detection of an effect size of 0.52 for the difference between groups in change from baseline in the HAM-D score (SD=20%) at each visit with a power of 0.92 at an alpha level of 0.05.
Data analyses were conducted on an intent-to-treat basis. Analyses for the primary efficacy measure were performed by using a mixed-effects model repeated-measures analysis, which can use all available data and can handle missing data more appropriately (29). Treatment group, visit, and each visit-by-group interaction were included as fixed effects, and baseline scale scores and age were included as covariates. The within-subject factor was considered as a random effect.
For the secondary outcome measures, logistic regression modeling for the effect of treatment group on the outcome measure of treatment nonresponse or response at each visit was used with age as a covariate. The difference in remission rate between treatment groups was also estimated by using the logistic regression model including age as a covariate. For sensitivity analyses, we repeated the analyses under the assumption that all participants who dropped out (N=10) by 4 weeks neither responded to the treatment nor experienced remission.
Continuous and categorical demographic and clinical variables in the two treatment groups were compared by means of independent t tests and chi-square tests, respectively. Differences in the proportion of all reported adverse events (observed cases per person-visit) between groups were analyzed by using chi-square tests. Laboratory measures and body mass index in the two groups at baseline and week 8 were compared by using independent t tests.
An alpha value of less than 0.05 was considered significant for two-tailed tests. The data were analyzed with Stata SE, version 11.0 (Stata, College Station, Tex.).
Results
Study Group
Of the 52 enrolled women with major depression, 25 (48.1%) were randomly assigned to receive creatine augmentation of SSRI and 27 (51.9%) were assigned to placebo augmentation (Figure 1). Baseline demographic and clinical characteristics were similar in the two treatment groups, as shown in Table 1.
TABLE 1.
Baseline Characteristics of Women With Major Depressive Disorder Assigned to Creatine Monohydrate or Placebo Augmentation of SSRI
| Characteristic | Creatine Augmentation (N=25) | Placebo Augmentation (N=27) | Analysis | ||
|---|---|---|---|---|---|
| Demographic variables | |||||
| N | % | N | % | pa | |
| Perceived socioeconomic status | 0.31 | ||||
| Upper | 1 | 4.0 | 4 | 14.8 | |
| Middle | 19 | 76.0 | 16 | 59.3 | |
| Lower | 5 | 20.0 | 7 | 25.9 | |
| Marital status | 0.60 | ||||
| Married | 17 | 68.0 | 21 | 77.8 | |
| Never married | 4 | 16.0 | 2 | 7.4 | |
| Divorced, widowed, or separated | 4 | 16.0 | 4 | 14.8 | |
| Mean | SD | Mean | SD | pb | |
| Age (years) | 45.7 | 12.7 | 47.5 | 9.5 | 0.57 |
| Body mass index | 24.2 | 2.7 | 23.1 | 3.1 | 0.18 |
| Education (years) | 11.6 | 3.4 | 11.3 | 3.8 | 0.73 |
| Clinical variables | |||||
| Age at onset of major depressive episode (years) | 44.8 | 12.1 | 47.0 | 9.8 | 0.47 |
| Clinical rating scores | |||||
| Hamilton Depression Rating Scale | 27.0 | 3.9 | 26.7 | 4.3 | 0.85 |
| Montgomery-Åsberg Depression Rating Scale | 28.6 | 4.1 | 28.6 | 4.9 | 0.97 |
| CGI severity subscale | 4.08 | 0.49 | 4.04 | 0.52 | 0.76 |
| Beck Depression Inventory | 27.1 | 9.1 | 26.0 | 9.5 | 0.66 |
| N | % | N | % | pc | |
| Current major depressive episode | 0.46 | ||||
| Single episoded | 21 | 84.0 | 24 | 88.9 | |
| Recurrent episode | 4 | 16.0 | 3 | 11.1 | |
| Psychotropic medication history | 0.21 | ||||
| Medication naive | 18 | 72.0 | 23 | 85.2 | |
| Medication freee | 7 | 28.0 | 4 | 14.8 | |
Calculated by chi-square analysis.
Calculated by t test.
Calculated by Fisher's exact test.
Current episode was the first ever.
For at least 2 months before enrollment; the mean duration without psychoactive medication was 37.2 months (SD=47.0, range=2–120).
A total of 39 patients (75.0%) completed the trial. There was no significance difference in the study dropout rates between treatment groups: 32.0% in the creatine group (N=8) and 18.5% in the placebo group (N=5) (χ2=1.26, df=1, p=0.26). Reasons for discontinuation were withdrawal of consent and loss to follow-up (Figure 1). Two patients in the creatine group and one patient in the placebo group discontinued treatment at week 2 because of adverse events.
Adherence to treatment medication, which was measured by the self-administered visual analogue scale at each visit, was comparably high in the two groups (self-reported average adherence rate, creatine: 95.1%; placebo: 96.0%) (p=0.62). The dosage of escitalopram did not differ between treatment groups at any visit; the overall mean dose was 15.0 mg/day (SD=3.5) in the creatine group and 15.7 mg/day (SD=2.9) in the placebo group (p=0.43). Concomitant use of lorazepam was reported for 10 patients with creatine augmentation (40.0%) and 12 patients with placebo augmentation (44.4%) (p=0.75). Zolpidem was prescribed for six patients in the creatine group (24.0%) and nine patients in the placebo group (33.3%) (p=0.46).
Primary Outcome Measure
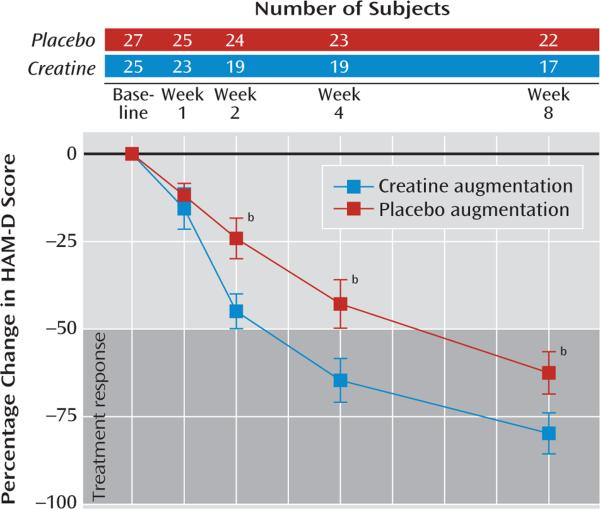
In relation to the patients who received placebo augmentation, those who received creatine augmentation showed greater improvement of depressive symptoms as early as week 2. This differential improvement between treatment groups was maintained until the end of treatment. Percentage changes and individual trajectories of HAM-D scores are presented in Figure 2 and online supplemental Figure SF1, respectively.
FIGURE 2.
Percentage Change in Hamilton Depression Rating Scale (HAM-D) Score for Women With Major Depressive Disorder Assigned to Creatine Monohydrate or Placebo Augmentation of SSRIa
For the HAM-D scores, significant interactions of visit and treatment group were observed at week 2, week 4, and week 8 (Table 2 and Figure 2).
TABLE 2.
Changes in Score on Hamilton Depression Rating Scale (HAM-D) for Women With Major Depressive Disorder Assigned to Creatine Monohydrate or Placebo Augmentation of SSRI
| Creatine Augmentation |
Placebo Augmentation |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAM-D Score |
Percent Change in Score |
HAM-D Score |
Percent Change in Score |
Between-Group Effect Sizea |
|||||||||
| Time | N | Mean | SD | Mean | SD | N | Mean | SD | Mean | SD | Cohen's d | 95% CI | z |
| Baseline | 25 | 26.9 | 3.9 | 27 | 26.7 | 4.3 | |||||||
| Week 1 | 23 | 22.8 | 5.3 | –15.6 | 13.6 | 25 | 23.5 | 4.2 | –11.9 | 8.2 | 0.33 | –0.19, 0.86 | –0.88 |
| Week 2 | 19 | 14.7 | 3.2 | –44.9 | 10.3 | 24 | 20.3 | 5.5 | –24.1 | 14.0 | 1.28 | 0.98, 1.59 | –4.89b |
| Week 4 | 19 | 9.4 | 3.6 | –64.6 | 12.9 | 23 | 15.1 | 4.8 | –42.8 | 16.0 | 1.20 | 0.86, 1.54 | –5.00b |
| Week 8 | 17 | 5.4 | 3.0 | –79.7 | 11.4 | 22 | 9.8 | 3.5 | –62.5 | 13.6 | 1.13 | 0.75, 1.52 | –3.84b |
For percent change in HAM-D score based on the pooled standard deviation.
Significant interaction (p<0.001) between treatment group and visit for change in the HAM-D score, according to a mixed-effects model repeated-measures analysis. Main effects for treatment group and visit as well as their interaction terms were included in the model. Age and baseline HAM-D score were also included as covariates in the model.
Secondary Outcome Measures
Similarly, changes in MADRS scores showed significant differences in symptom improvement between treatment groups at week 2 (z=–4.87, p<0.001), week 4 (z=–4.73, p<0.001), and week 8 (z=–3.55, p<0.001). Significant improvements from baseline with creatine relative to placebo augmentation were also observed in the CGI severity subscale at week 2 (z=–2.84, p=0.005), week 4 (z=–4.24, p<0.001), and week 8 (z=–3.89, p<0.001).
The treatment response rates based on the HAM-D score in the creatine group (N=25) were 0% (N=0), 32.0% (N=8), 68.0% (N=17), and 64.0% (N=16) at weeks 1, 2, 4, and 8, respectively, while the placebo group (N=27) showed treatment response rates of 0% (N=0), 3.7% (N=1), 29.6% (N=8), and 63.0% (N=17) at weeks 1, 2, 4, and 8, respectively, as shown in online supplemental Figure SF2. There were significant effects of treatment group, favoring creatine augmentation, on the change in treatment response rate at week 2 (odds ratio=16.05, SE=18.11, p=0.02) and week 4 (odds ratio=16.54, SE=14.61, p=0.001) but not at week 8 (odds ratio=5.01, SE=5.83, p=0.17).
According to the HAM-D score, 13 (52.0%) of the patients with creatine augmentation achieved remission at week 8, while remission was achieved in seven (25.9%) of the patients in the placebo group. The remission rate at end point was higher in the creatine group than in the placebo group (odds ratio=6.92, SE=5.07, p=0.008) (online supplemental Figure SF2).
The higher response and remission rates in the creatine group remained significant in sensitivity analyses after imputation of the results from individuals who had stopped taking the study medication at week 4 for any reason (N=10), who were considered nonresponders and nonremitters.
Sensitivity analyses for the responders showed earlier improvement in the HAM-D score; odds ratios for the creatine group relative to the placebo group were significant at week 2 (odds ratio=11.68, SE=13.04, p=0.03) and week 4 (odds ratio=4.99, SE=3.04, p=0.008). Sensitivity analysis for remission (at end point) also showed a higher remission rate in the creatine group (odds ratio=3.62, SE=2.25, p=0.04).
Safety and Tolerability
All reported adverse events by week are presented in Table 3. The overall frequencies of adverse events in the two treatment groups were similar: 43.4% (36 events) and 46.4% (45 events) of the person-examinations for creatine and placebo, respectively (χ2=0.16, df=1, p=0.69). The most common adverse events in both treatment groups were tension headache, nausea and/or vomiting, and sleep difficulties. Most of the adverse events occurred at an early phase of treatment and improved without specific interventions. No serious adverse events were observed during the treatment period in either group. Two patients with creatine augmentation (8.0%) and one with placebo (3.7%) discontinued use of study medications at week 2 because of adverse events. The symptom clusters of nausea, tension headache, restlessness, and insomnia, which may be related to the use of SSRIs (28), were the major reasons for intolerance of the study medications.
TABLE 3.
Adverse Events in Women With Major Depressive Disorder Assigned to Creatine Monohydrate or Placebo Augmentation of SSRI
| Creatine Augmentation (N=25) |
Placebo Augmentation (N=27) |
||||
|---|---|---|---|---|---|
| Adverse Event and Week | N | %a | N | %a | pb |
| Tension headache | |||||
| Week 1 | 5 | 20.8 | 7 | 26.9 | |
| Week 2 | 1 | 4.3 | 3 | 12.0 | |
| Week 4 | 0 | 0.0 | 2 | 8.3 | |
| Week 8 | 0 | 0.0 | 1 | 4.5 | |
| Cumulative | 6 | 7.2 | 13 | 13.4 | 0.18c |
| Nausea and/or vomiting | |||||
| Week 1 | 8 | 33.3 | 5 | 19.2 | |
| Week 2 | 1 | 4.3 | 0 | 0.0 | |
| Week 4 | 0 | 0.0 | 1 | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 9 | 10.8 | 6 | 6.2 | 0.26c |
| Sleep difficulties, insomnia, or somnolence | |||||
| Week 1 | 3 | 12.5 | 3 | 11.5 | |
| Week 2 | 1 | 4.3 | 4 | 16.0 | |
| Week 4 | 2 | 10.5 | 1 | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 6 | 7.2 | 8 | 8.2 | 0.80c |
| Dizziness | |||||
| Week 1 | 4 | 16.7 | 2 | 7.7 | |
| Week 2 | 1 | 4.3 | 0 | 0.0 | |
| Week 4 | 0 | 0.0 | 1 | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 5 | 6.0 | 3 | 3.1 | 0.47 |
| Agitation and/or restlessness | |||||
| Week 1 | 2 | 8.3 | 2 | 7.7 | |
| Week 2 | 0 | 0.0 | 2 | 8.0 | |
| Week 4 | 0 | 0.0 | 1 | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 2 | 2.4 | 5 | 5.2 | 0.46 |
| Difficulty in concentration | |||||
| Week 1 | 1 | 4.2 | 2 | 7.7 | |
| Week 2 | 0 | 0.0 | 1 | 4.0 | |
| Week 4 | 0 | 0.0 | 0 | 0.0 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 1 | 1.2 | 3 | 3.1 | 0.63 |
| Increased sweating | |||||
| Week 1 | 1 | 4.2 | 0 | 0.0 | |
| Week 2 | 0 | 0.0 | 1 | 4.0 | |
| Week 4 | 1 | 5.3 | 1 | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 2 | 2.4 | 2 | 2.1 | 1.00 |
| Decreased appetite | |||||
| Week 1 | 1 | 4.2 | 1 | 3.8 | |
| Week 2 | 0 | 0.0 | 1 | 4.0 | |
| Week 4 | 0 | 0.0 | 0 | 0.0 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 1 | 1.2 | 2 | 2.1 | 1.00 |
| Otherd | |||||
| Week 1 | 3e | 12.5 | 2f | 7.7 | |
| Week 2 | 1g | 4.3 | 0 | 0.0 | |
| Week 4 | 0 | 0.0 | 1h | 4.2 | |
| Week 8 | 0 | 0.0 | 0 | 0.0 | |
| Cumulative | 4 | 4.8 | 3 | 3.1 | 0.71 |
The rate at each week was calculated by dividing the number of observed cases by the number of individuals who underwent examination of side effects at that week in each treatment group. The cumulative rate was calculated by dividing the total cumulative observed cases of that side effect by person-examinations (the total number of side effect examinations during the treatment period; 83 for the creatine group and 97 for the placebo group) in each treatment group.
Calculated by Fisher's exact test unless otherwise specified.
Calculated by chi-square test.
Observed no more than twice per person during the treatment period.
Urinary difficulty, dry mouth, and constipation, respectively.
Urinary difficulty and palpitation, respectively.
Constipation.
Increased dreaming.
There were no differences in laboratory measures or BMI during the treatment period between groups (see online supplemental Table ST1). Two patients in the creatine group and one in the placebo group showed slight increases in the levels of liver transaminases beyond the upper normal limit at week 8.
As expected, mild elevation of the serum creatinine level was observed exclusively in patients receiving creatine augmentation (z=2.30, p=0.03), although there was no individual whose serum creatinine level increased beyond the normal range (online supplemental Table ST1).
Discussion
The current study is, to our knowledge, the first randomized, double-blind placebo-controlled trial to demonstrate more rapid antidepressant efficacy of the SSRI escitalopram when augmented with creatine for women with major depressive disorder.
This proof-of-concept study suggests that depression symptoms, as measured by three different scales (HAM-D, MADRS, and CGI severity subscale), may improve to a greater extent in escitalopram-treated depressed patients receiving creatine than in those receiving placebo as early as 2 weeks after the initiation of treatment. It is also noteworthy that more patients with creatine augmentation achieved early treatment response, defined a priori, during the study period.
In addition to the more rapid onset of antidepressant efficacy, a significantly higher remission rate was observed in the creatine augmentation group. The remission rate (25.9%) in our placebo group was comparable with the result (28%) from a previous large-scale study evaluating clinical outcomes of citalopram treatment for depression by the same measurement we used (6), but the remission rate was substantially increased when creatine was added, to 52.0% (13 of the 25 patients in the creatine group), which is twice the rate observed for the group receiving the SSRI plus placebo.
Although the exact intracellular mechanism underlying adjunctive creatine's bolstering of SSRI antidepressant efficacy remains unclear, the involvement of creatine in enhancing the phosphocreatine energy pool, thereby improving cellular bioenergetics (10, 11), is a possible mechanism. A series of 31P-MRS studies have shown that abnormal brain bioenergetics related to major depressive disorder are likely to improve with successful treatment (12–16). It is intriguing that a higher baseline phosphocreatine level has been suggested as a biological marker for a better treatment response (13). S-Adenosyl-l-methionine, which is a methyl donor in the production of creatine (10) and exerts robust antidepressant efficacy, particularly in SSRI-resistant major depression (30), may be similar to creatine in terms of increasing phosphocreatine levels in the brain (31). A recent preliminary trial also showed that creatine augmentation improves depressive symptoms in adolescents with SSRI-resistant major depressive disorder by increasing brain phosphocreatine levels (32). Emerging evidence suggests that in addition to modulating cellular energy metabolism, creatine exerts neuroprotective effects through mechanisms involving antiaptotic and antioxidant effects on mitochondrial functioning (11). Future 31P-MRS studies in depressed patients treated with creatine would provide important information regarding the neurobiological basis for its effects on brain bioenergetics.
Creatine augmentation was relatively well tolerated in this study. Aside from one case report of idiosyncratic renal effects with a large dose (20 g/day) for a period of 4 weeks (33), creatine supplementation has not been associated with major health risks (34). Mild adverse effects, including increased body mass, muscle cramps, diarrhea, and gastrointestinal pain, have been reported in athletes who consumed 10–20 g/day of creatine (34). Although a significant increase in serum creatinine levels from baseline was observed in patients in our creatine group, as in other previous trials (35, 36), serum creatinine and blood urea nitrogen levels, both of which represent overall renal function, were all within normal limits during the study. According to the profile of creatine-related side effects (34), the most frequently observed adverse events in both treatment groups, such as tension headache, nausea/vomiting, and sleep difficulties, seemed to be more directly related to the SSRI rather than to creatine (28).
Although the difference was not significant, the discontinuation rate was higher in the creatine group (N=8, 32.0%) than in the placebo group (N=5, 18.5%). Six patients receiving creatine left the trial within 2 weeks, while three patients with placebo augmentation dropped out during the same time. The tendency toward a higher discontinuation rate with creatine augmentation, particularly during the early treatment period, may be attributed to intolerance, lack of efficacy, or both.
Although we did not find an overall higher rate of creatine-specific side effects and there appear to be no proven pharmacokinetic drug interactions with creatine (7), intolerance of the study medication could increase if there are interactions between creatine and escitalopram. Further pharmacokinetic and pharmacodynamic studies may be necessary to examine whether creatine augmentation could alter the metabolism of SSRIs.
To consider inefficacy, we recalculated the odds ratios for remission under the assumption that the patients who dropped out prematurely did not achieve remission. The remission rate based on the HAM-D scores was still higher in the creatine group.
Our study has several limitations that merit consideration in interpreting the results. First, the study has the limit imposed by the small number of subjects to detect a relatively modest effect size from the difference between treatment groups. In addition, patient characteristics in this study were relatively homogenous, since the study included only women and had a large percentage of patients who had never taken psychotropic medications, since most were having their first major depressive episode. Although the homogeneity of this study group may have advantages in terms of statistical power, particularly in a proof-of-concept study, it limits the generalizability of the results to all patients with major depressive disorder. Third, a small portion of the current subjects, particularly those experiencing the first major depressive episode, may eventually convert to bipolar disorder even though they had no previous manic nor hypomanic episodes. Because of pilot data reporting the occurrence of a manic or hypomanic switch in patients with bipolar depression who were taking creatine (37), future studies ensuring the long-term safety of creatine are necessary. Fourth, the study included only women with major depressive disorder because of preclinical evidence of antidepressant-like effects of creatine in female rodents (17). Consequently, our study could not test whether the antidepressant effects of creatine are specific to women. Future studies that also include men are warranted. Fifth, given the ethnicity-based differences in the pharmacodynamics and pharmacokinetics of psychotropic medications (38, 39), the current results from an ethnically homogenous study group need replication in a larger pool with different ethnic groups.
The present results suggest that creatine, used to augment treatment with the SSRI escitalopram, provides a promising therapeutic approach for major depressive disorder in terms of its superior efficacy, relatively good tolerability, minimal side effects, and easy attainability. Replication of the current findings is required in larger study groups and with a longer period of observation. Furthermore, on the basis of previous findings indicating that creatine supplementation increases cerebral creatine levels (9, 32) and that changes in cerebral phosphocreatine levels may be related to antidepressant efficacy (13, 40), future studies are required in order to assess the possibility of the cerebral phosphocreatine level as a relevant biological marker for predicting creatine-induced antidepressant efficacy.
Supplementary Material
Acknowledgments
Dr. Lyoo has received research support from AstraZeneca, Boryung, Eli Lilly, GlaxoSmithKline, Lundbeck, and Organon. Dr. Renshaw has been a consultant for Kyowa Hakko, Novartis, Repligen, and Roche and has received research support from GlaxoSmithKline and Roche, and he is also an inventor on related patent applications assigned to McLean Hospital and the University of Utah; Dr. Renshaw did not participate in the assessment of study subjects in this project.
This study was supported by an independent investigator award from NARSAD (Dr. Lyoo), grant 2011K000273 from the Brain Research Center of the 21st Century Frontier Research Program (Dr. Lyoo), grant KRF-2008-220-E00021 from the Global Research Network Program, the Korea Research Foundation (Dr. Lyoo), the Mental Illness Research, Education and Clinical Center of Veterans Integrated Service Network 19 (Dr. Renshaw), National Institute on Drug Abuse grants DA-015116 and DA-031247 (Dr. Renshaw) and DA-024070 (Drs. Renshaw and Lyoo), and NIMH grant MH-058681 (Dr. Renshaw).
The sponsors of the study had no role in the study design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Preliminary data presented as a poster at Neuroscience 2011, Washington, D.C., Nov. 12–16, 2011.
ClinicalTrials.gov registration number, NCT00729755.
The other authors report no financial relationships with commercial interests.
References
- 1.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes:m a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):5–9. [PubMed] [Google Scholar]
- 2.Machado-Vieira R, Salvadore G, Luckenbaugh DA, Manji HK, Zarate CA., Jr Rapid onset of antidepressant action: a new paradigm in the research and treatment of major depressive disorder. J Clin Psychiatry. 2008;69:946–958. doi: 10.4088/jcp.v69n0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–343. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 4.Donovan S, Clayton A, Beeharry M, Jones S, Kirk C, Waters K, Gardner D, Faulding J, Madeley R. Deliberate self-harm and antidepressant drugs: investigation of a possible link. Br J Psychiatry. 2000;177:551–556. doi: 10.1192/bjp.177.6.551. [DOI] [PubMed] [Google Scholar]
- 5.Stassen HH, Delini-Stula A, Angst J. Time course of improvement under antidepressant treatment: a survival-analytical approach. Eur Neuropsychopharmacol. 1993;3:127–135. doi: 10.1016/0924-977x(93)90264-m. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. STAR*D Study Team: Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 7.Graham AS, Hatton RC. Creatine: a review of efficacy and safety. J Am Pharm Assoc (Wash) 1999;39:803–810. [PubMed] [Google Scholar]
- 8.Persky AM, Brazeau GA. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol Rev. 2001;53:161–176. [PubMed] [Google Scholar]
- 9.Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 10.Adhihetty PJ, Beal MF. Creatine and its potential therapeutic value for targeting cellular energy impairment in neurodegenerative diseases. Neuromolecular Med. 2008;10:275–290. doi: 10.1007/s12017-008-8053-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andres RH, Ducray AD, Schlattner U, Wallimann T, Widmer HR. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 12.Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, Frederick BdeB, Fava M, Hennen J, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- 13.Iosifescu DV, Bolo NR, Nierenberg AA, Jensen JE, Fava M, Renshaw PF. Brain bioenergetics and response to triiodothyronine augmentation in major depressive disorder. Biol Psychiatry. 2008;63:1127–1134. doi: 10.1016/j.biopsych.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Forester BP, Harper DG, Jensen JE, Ravichandran C, Jordan B, Renshaw PF, Cohen BM. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. Int J Geriatr Psychiatry. 2009;24:788–797. doi: 10.1002/gps.2230. [DOI] [PubMed] [Google Scholar]
- 15.Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- 16.Volz HP, Rzanny R, Riehemann S, May S, Hegewald H, Preussler B, Hübner G, Kaiser WA, Sauer H. 31P magnetic resonance spectroscopy in the frontal lobe of major depressed patients. Eur Arch Psychiatry Clin Neurosci. 1998;248:289–295. doi: 10.1007/s004060050052. [DOI] [PubMed] [Google Scholar]
- 17.Allen PJ, D'Anci KE, Kanarek RB, Renshaw PF. Chronic creatine supplementation alters depression-like behavior in rodents in a sex-dependent manner. Neuropsychopharmacology. 2010;35:534–546. doi: 10.1038/npp.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gledhill RF, Van der Merwe CA, Greyling M, Van Niekerk MM. Race-gender differences in serum creatine kinase activity: a study among South Africans. J Neurol Neurosurg Psychiatry. 1988;51:301–304. doi: 10.1136/jnnp.51.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razmara A, Duckles SP, Krause DN, Procaccio V. Estrogen suppresses brain mitochondrial oxidative stress in female and male rats. Brain Res. 2007;1176:71–81. doi: 10.1016/j.brainres.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 21.First MB, Gibbon M, Spitzer RL, Williams JBW. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version 2.0. New York State Psychiatric Institute, Biometrics Research Department; New York: 1996. [Google Scholar]
- 22.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfield S, Clifford S, Eliasson L, Barber N, Willson A. Suitability of measures of self-reported medication adherence for routine clinical use: a systematic review. BMC Med Res Methodol. 2011;11:149. doi: 10.1186/1471-2288-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 25.Guy W, editor. The Clinical Global Impressions Scale, in ECDEU Assessment Manual for Psychopharmacology–Revised: Publication ADM 76-338. US Department of Health, Education and Welfare, ADAMHA, NIMH Psychopharmacology Research Branch; Rockville, Md: 1976. pp. 218–222. [Google Scholar]
- 26.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003;289:3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 27.Trivedi MH, Morris DW, Grannemann BD, Mahadi S. Symptom clusters as predictors of late response to antidepressant treatment. J Clin Psychiatry. 2005;66:1064–1070. doi: 10.4088/jcp.v66n0816. [DOI] [PubMed] [Google Scholar]
- 28.Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors: serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. 1998;51:215–235. doi: 10.1016/s0165-0327(98)00221-3. [DOI] [PubMed] [Google Scholar]
- 29.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 30.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-Adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 31.Silveri MM, Parow AM, Villafuerte RA, Damico KE, Goren J, Stoll AL, Cohen BM, Renshaw PF. S-Adenosyl-L-methionine: effects on brain bioenergetic status and transverse relaxation time in healthy subjects. Biol Psychiatry. 2003;54:833–839. doi: 10.1016/s0006-3223(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 32.Kondo DG, Sung YH, Hellem TL, Fiedler KK, Shi X, Jeong EK, Renshaw PF. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: a 31-phosphorus magnetic resonance spectroscopy study. J Affect Disord. 2011;135:354–361. doi: 10.1016/j.jad.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient taking creatine. N Engl J Med. 1999;340:814–815. doi: 10.1056/NEJM199903113401017. [DOI] [PubMed] [Google Scholar]
- 34.Poortmans JR, Francaux M. Adverse effects of creatine supplementation: fact or fiction? Sports Med. 2000;30:155–170. doi: 10.2165/00007256-200030030-00002. [DOI] [PubMed] [Google Scholar]
- 35.Pline KA, Smith CL. The effect of creatine intake on renal function. Ann Pharmacother. 2005;39:1093–1096. doi: 10.1345/aph.1E628. [DOI] [PubMed] [Google Scholar]
- 36.Schedel JM, Tanaka H, Kiyonaga A, Shindo M, Schutz Y. Acute creatine ingestion in human: consequences on serum creatine and creatinine concentrations. Life Sci. 1999;65:2463–2470. doi: 10.1016/s0024-3205(99)00512-3. [DOI] [PubMed] [Google Scholar]
- 37.Roitman S, Green T, Osher Y, Karni N, Levine J. Creatine monohydrate in resistant depression: a preliminary study. Bipolar Disord. 2007;9:754–758. doi: 10.1111/j.1399-5618.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- 38.Meyer JM, Rosenblatt LC, Kim E, Baker RA, Whitehead R. The moderating impact of ethnicity on metabolic outcomes during treatment with olanzapine and aripiprazole in patients with schizophrenia. J Clin Psychiatry. 2009;70:318–325. doi: 10.4088/jcp.08m04267. [DOI] [PubMed] [Google Scholar]
- 39.Hong Ng C, Norman TR, Naing KO, Schweitzer I, Kong Wai Ho B, Fan A, Klimidis S. A comparative study of sertraline dosages, plasma concentrations, efficacy and adverse reactions in Chinese versus Caucasian patients. Int Clin Psychopharmacol. 2006;21:87–92. doi: 10.1097/01.yic.0000188214.46667.3f. [DOI] [PubMed] [Google Scholar]
- 40.Sonawalla SB, Renshaw PF, Moore CM, Alpert JE, Nierenberg AA, Rosenbaum JF, Fava M. Compounds containing cytosolic cho-line in the basal ganglia: a potential biological marker of true drug response to fluoxetine. Am J Psychiatry. 1999;156:1638–1640. doi: 10.1176/ajp.156.10.1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.