Abstract
IMPORTANCE
Apolipoprotein E (APOE) ε4 is an established risk factor for cognitive decline and the development of dementia, but other factors may help to minimize its effects.
OBJECTIVE
Using APOE ε4 as an indicator of high risk, we investigated factors associated with cognitive resilience among black and white older adults who are APOE ε4 carriers.
DESIGN, SETTING, AND PARTICIPANTS
Participants included 2487 community-dwelling older (aged 69–80 years at baseline) black and white adults examined at 2 community clinics in the prospective cohort Health, Aging, and Body Composition (Health ABC) study. The baseline visits occurred from May 1997 through June 1998. Our primary analytic cohort consisted of 670 APOE ε4 carriers (329 black and 341 white participants) who were free of cognitive impairment at baseline and underwent repeated cognitive testing during an 11-year follow-up (through 2008) using the Modified Mini-Mental State Examination.
MAIN OUTCOMES AND MEASURES
We stratified all analyses by race. Using the Modified Mini-Mental State Examination scores, we assessed normative cognitive change in the entire cohort (n = 2487) and classified the APOE ε4 carriers as being cognitively resilient vs nonresilient by comparing their cognitive trajectories with those of the entire cohort. We then conducted bivariate analyses and multivariable random forest and logistic regression analyses to explore factors predictive of cognitive resilience in APOE ε4 carriers.
RESULTS
Among white APOE ε4 carriers, the strongest predictors of cognitive resilience were, in relative order of importance, no recent negative life events, a higher literacy level, advanced age, a higher educational level, and more time spent reading. Among black APOE ε4 carriers, the strongest predictors of cognitive resilience were, in relative order of importance, a higher literacy level, a higher educational level, female sex, and the absence of diabetes mellitus. In follow-up logistic regression models, higher literacy level (adjusted odds ratio [OR], 9.50 [95%CI, 2.67–60.89]), a higher educational level (adjusted OR for college graduate vs less than high school, 3.81 [95%CI, 1.13–17.56]), and age (adjusted OR for 73–76 vs 69–72 years, 2.01 [95%CI, 1.13–3.63]) had significant independent effects in predicting cognitive resilience among white APOE ε4 carriers. Among black APOE ε4 carriers, a higher literacy level (adjusted OR, 2.27 [95%CI, 1.29–4.06]) and a higher educational level (adjusted OR for high school graduate/some college vs less than high school, 2.86 [95%CI, 1.54–5.49]; adjusted OR for college graduate vs less than high school, 2.52 [95%CI, 1.14–5.62]) had significant independent effects in predicting cognitive resilience.
CONCLUSIONS AND RELEVANCE
Although APOE ε4 carriers are at high risk for cognitive decline, our findings suggest possible intervention targets, including the enhancement of cognitive reserve and improvement of other psychosocial and health factors, to promote cognitive resilience among black and white APOE ε4 carriers.
Carriers of apolipoprotein E (APOE[OMIM107741]) ε4 are at high risk for cognitive decline and Alzheimer disease.1–3 However, the risk associated with APOE ε4 varies with other factors. The ε4 allele is more common among black than white individuals,4,5 and evidence6–12 suggests that, although the ε4 allele is a cognitive risk factor in both races,7,8 its effects are weaker in black persons.11,12 The cognitive impact of the ε4 allele also appears to be weaker in advanced older age6,13,14; the ε4 allele is associated with a risk for cognitive decline and dementia among young-old individuals, but this association is no longer present among the oldest-old individuals, such as those 90 years or older studied by Corrada et al.14 A proportion of APOE ε4 carriers survives to 90 years or older still free of dementia,14 therefore appearing to be resilient to the deleterious effects of the allele. What factors might promote cognitive resilience among APOE ε4 carriers?
Previous work suggests that certain factors, such as higher educational levels, help to protect older adults with the neuropathologic features of Alzheimer disease from exhibiting cognitive decline.15 Such findings have been interpreted as supporting “cognitive reserve” theory, which posits that individuals with higher levels of reserve are better able to with stand aging and neuropathologic changes without showing cognitive decline.16 A similar phenomenon may hold in the context of APOE ε4; modifiable factors may promote cognitive resilience among APOE ε4 carriers despite their high genetic risk. Identifying such factors could inform interventions for preventing or slowing cognitive decline among these high-risk individuals.
Using APOE ε4 as an indicator of high risk, we sought to identify factors that promote cognitive resilience in black and white carriers of the ε4 allele. We aimed to examine whether protective factors are different in black and white individuals because racial differences in the frequency and impact of APOE ε4 suggest racial differences may exist in what contributes to cognitive resilience to the ε4 allele. Among a comprehensive set of potential predictors, we specifically aimed to identify the factors most strongly predictive of cognitive resilience in black and white carriers of the ε4 allele to determine which factors may be most important to target in interventions for individuals at high genetic risk.
Methods
Population
This study was approved by the institutional review boards at the University of Pittsburgh, Pittsburgh, Pennsylvania; the University of Tennessee, Memphis; and the University of California, San Francisco. All participants gave written informed consent.
Participants were from the Health, Aging, and Body Composition (Health ABC) Study, a prospective cohort study of adults aged 69–80 years at baseline. Health ABC Study participants were recruited from a random sample of white older adults and every black older adult who were Medicare eligible, community dwelling, and living in Pittsburgh or Memphis. Three thousand seventy-five individuals were enrolled. The baseline (year 1) study visits occurred from May 1997 to June 1998. Individuals were required to be well functioning at baseline (no difficulty with activities of daily living, including the ability to walk 0.4 km and to climb 10 stairs without resting) to be included. Additional details about the study participants are included in prior Health ABC Study reports.17–19
Of the 3075 Health ABC Study participants, 2487 individuals (992 black and 1495 white)were free of prevalent cognitive impairment (defined as a baseline Modified Mini-Mental State Examination [3MS] score more than 1.5 SDs below the mean compared with peers of the same race) and underwent repeated cognitive testing (2 or more 3MS assessments) over 11 years (through 2008). Of these, 2349 individuals (924 black and 1425 white) underwent APOE genotype testing from standard single-nucleotide polymorphism analyses, with 670 (28.5%) being APOE ε4 carriers (329 black carriers [35.6%] and 341 white carriers [23.9%]). These 670 APOE ε4 carriers (ie, those possessing ≥1 ε4 allele) served as our primary analytic cohort.
Measures
Cognition and Cognitive Resilience
Cognitive functioning was assessed at years 1, 3, 5, 8, 10, and 11 with the 3MS.20 With scores ranging from0 to 100, the 3MS assesses orientation, attention, memory, construction, basic language, verbal fluency, and conceptualization and is more sensitive than the traditional Mini-Mental State Examination.21
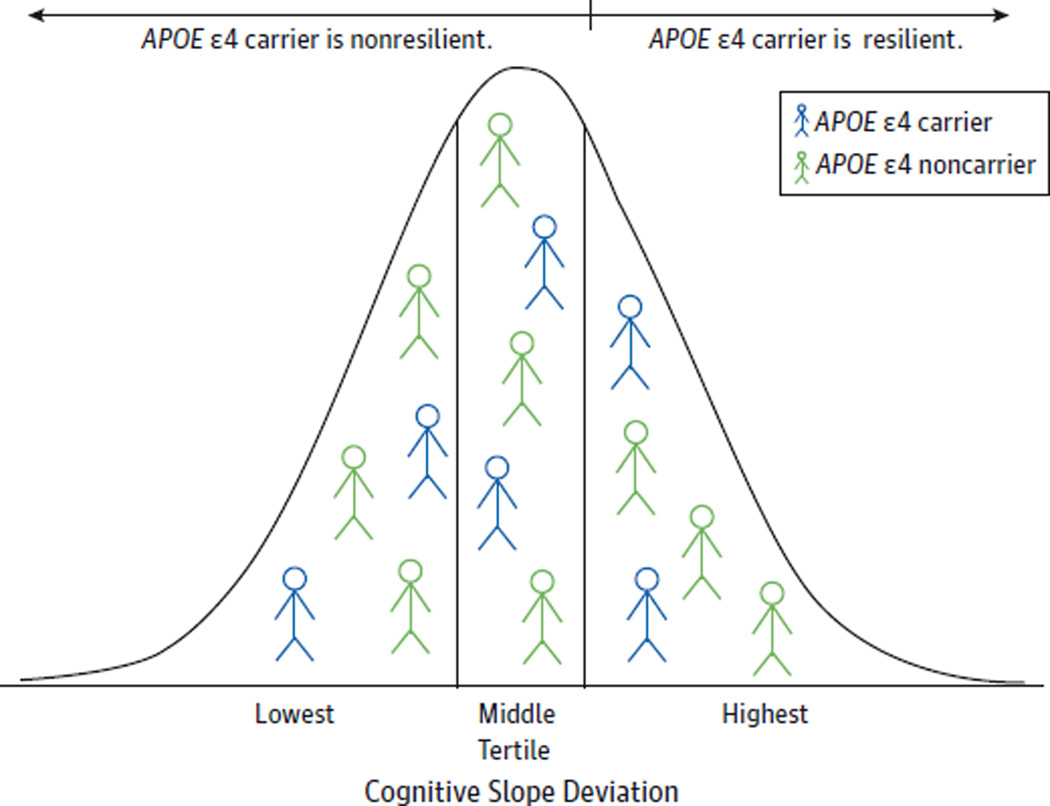
As depicted in Figure 1,we used data from the entire Health ABC Study cohort to classify APOE ε4 carriers as cognitively resilient vs nonresilient. We created our definition to investigate the following question: How do some ε4 carriers maintain cognitive health to the extent that their cognitive trajectories resemble those of the most cognitively healthy APOE ε4 noncarriers? First, we considered cognitive trajectory data from the entire Health ABC Study cohort to contextualize normative cognitive change for all participants during the follow-up period. Among the 2487 Health ABC Study participants without baseline cognitive impairment and with repeated 3MS testing, we constructed linear mixed-effects regression models to characterize individuals’ longitudinal cognitive trajectory using all their 3MS scores (baseline through the last score) stratified by race and adjusted for age group (69–72, 73–76, and 77–80 years) and sex. This analysis calculated each individual’s 3MS slope deviation from that of their demographically similar peers. Next, we compared the individual cognitive trajectories of the APOE ε4 carriers with those from the entire Health ABC Study cohort to define resilience. We classified APOE ε4 carriers as resilient if their cognitive trajectory fell within the highest tertile compared with demographically similar individuals in the entire cohort (ie, best retained cognition) and as nonresilient if their cognitive trajectory fell within the lowest 2 tertiles (ie, greatest decline in cognition). We reasoned that comparing APOE ε4 carriers with the entire cohort would yield a more representative classification of resilience than would basing our definition on the APOE ε4 carriers alone. We made this classification conservatively based on the highest tertile because no clinical cutoff has been established to define cognitive resilience. For descriptive purposes, eTable 1 in the Supplement lists 3MS change values that correspond to the top tertile cut point defining resilience for each demographic group.
Figure 1.
Defining Cognitive Resilience for Apolipoprotein E (APOE) ε4 Carriers
Graphic representation of how APOE ε4 carriers (n = 670) were classified as cognitively resilient vs nonresilient by comparing the cognitive trajectories of the carriers with those of demographically similar individuals (curve) in the entire Health, Aging, and Body Composition (Health ABC) Study cohort (n = 2487). The numbers of person-figures do not correspond directly to any real numbers.
Demographic Characteristics, Literacy, and Financial Status
Participants’ age, race, sex, and educational level (less than high school and high school graduate and/or some college vs college graduate)were collected at baseline. Participants were classified as having a higher financial status at baseline if they endorsed 2 or more of the following: the amount of money they have meets their needs very well; they have money left over at the end of the month; or they own their apartment or house. Literacy was assessed shortly after baseline with the Rapid Estimate of Adult Literacy in Medicine, a word-reading test that yields reading grade-level equivalents.22
Comorbidities and Health Factors
Self-report, medications, physician diagnoses, laboratory values, and clinical measurements were used to characterize baseline comorbidities, including hypertension, diabetes mellitus, history of stroke, and history of myocardial infarction. Individuals with a body mass index greater than 30 (calculated as the baseline weight in kilograms divided by height in meters squared)were considered obese. High cholesterol levels were defined as total cholesterol levels of at least 240 mg/dL (to convert to millimoles per liter, multiply by 0.0259). Individuals whose C-reactive protein and interleukin 6 values were greater than the median were classified as having high levels of inflammation.23 Participants completed the Center for Epidemiologic Studies–Depression Scale24; scores of at least 16 were considered to indicate depression. Participants self-reported the typical number of hours they slept per night (coded as ≤6, 7, and ≥8 hours).
Lifestyle and Psychosocial Factors
At baseline, participants reported their marital status, whether they lived alone, and whether they visited friends or family regularly (defined as at least twice per week). They answered yes/no questions25 assessing whether they had experienced major negative life events during the past year (ie, whether a close friend or family member had a serious accident or illness; whether a spouse or partner died; whether a child, grandchild, close friend, or relative died; whether a pet died; whether a relationship with a family member or close friend changed for the worse; whether the participant or a family member was assaulted or robbed; or whether a close friend or family member was arrested or had trouble with the law). Participants were coded as endorsing no negative life events vs any. Participants reported whether they were working or volunteering and how many hours per week they spend reading (classified as low, moderate, and avid reading based on tertiles). Participants self-reported physical activity; total weekly energy expenditure was calculated as kilocalories per kilogram per week and was used to classify participants as having low, moderate, or high energy expenditure based on tertiles. Participants reported their current alcohol use and smoking status.
Statistical Analysis
As shown in eTable 2 in the Supplement, the 2 racial groups differed by many characteristics, providing further justification for racial stratification of the following analyses. To examine predictors of cognitive resilience among APOE ε4 carriers, we first evaluated bivariate associations between participant characteristics and resilience using χ2 tests separately by race. Next, among all our potential predictors, we investigated which factors were most strongly predictive of resilience in a multivariable fashion using random forest analyses among race strata. Random forest analysis26 is a nonparametric approach capable of handling a large number of potential predictor variables. Random forest analysis uses a series of classification or regression trees to evaluate multiple potential predictors within the same analysis and to rank variables according to their importance in predicting the outcome. A set of classification trees are created, each based on a random sample of participants, and a random sample of potential predictor variables are evaluated a teach split in the tree. Results are combined across trees to produce a rank order list of important predictor variables, and aggregating tree results in this manner yields more robust findings than those produced by a single tree.26 We conducted random forest analyses in the R environment27 using the “party” package,28–30 which applies a conditional measure of variable importance to handle correlated predictor variables.26 Our forest consisted of5000trees, and at each potential split we evaluated a random sample of 10 predictors. Variables were ranked by an importance score, reflecting the relative strength of each variable in predicting the outcome. We followed the criteria recommended by Strobl et al26 for considering a variable to be an important predictor. As a complementary multivariable analysis, we constructed logistic regression models that included all variables considered to be important predictors in the random forest results. This analysis elucidates the effects of each of these predictors by quantifying the independent effect of each predictor on cognitive resilience.
Results
Verification of APOE ε4 Status as a Risk Factor in Both Races
Before conducting our primary analyses, we verified whether APOE ε4 status was a risk factor among both races in the Health ABC Study cohort by examining the effect of the ε4 allele on cognitive trajectory separately by race using linear mixed models. As anticipated, APOE ε4 carriers had worse cognitive change among black (β = −0.21; P < .001) and white (β = −0.33; P < .001) groups; these associations remained(P < .001 for both) after adjustment for all our examined variables.
Associations Between Participant Characteristics and Cognitive Resilience in APOE ε4 Carriers
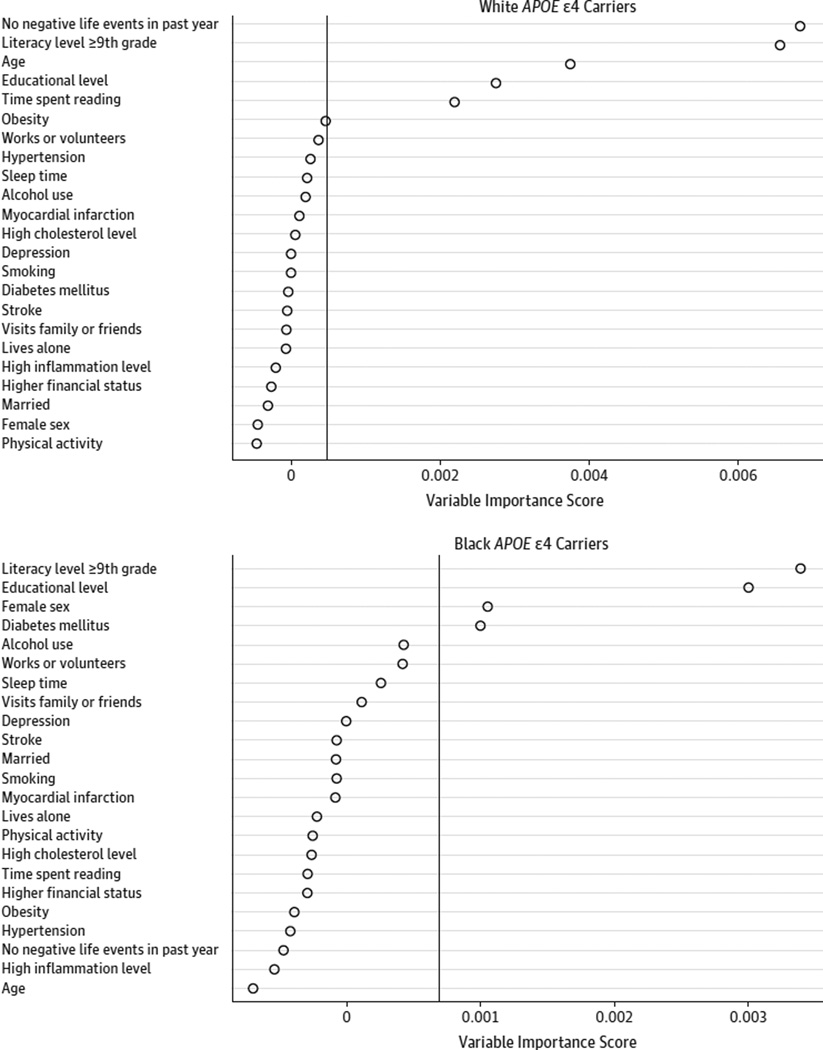
Among white APOE ε4 carriers, the following characteristics were associated with being cognitively resilient in bivariate analyses (Table 1): older age, a higher educational level, a higher literacy level, the absence of diabetes mellitus, the absence of obesity, having no negative life events in the past year, and more time spent reading (P < .05 for all).The multivariable random forest analysis for white APOE ε4 carriers showed very strong model classification performance (C statistic,0.86), and the strongest predictors of resilience in rank order were (1) no negative life events, (2) higher literacy levels, (3) older age, (4) higher educational level, and (5) more time spent reading (Figure 2).
Table 1.
Bivariate Associations: Potential Predictors of Cognitive Resilience Among White APOE ε4 Carriers
| Characteristic | Study Participants, %a | P Value | ||
|---|---|---|---|---|
| All (N = 341) |
Resilient (n = 89) |
Nonresilient (n = 252) |
||
| Age, y | ||||
| 69–72 | 40.8 | 29.2 | 44.8 | .03b |
| 73–76 | 44.0 | 55.1 | 40.1 | |
| 77–80 | 15.2 | 15.7 | 15.1 | |
| Female sex | 47.5 | 52.8 | 45.6 | .24 |
| Educational level | ||||
| Did not graduate high school | 11.1 | 3.4 | 13.9 | <.001b |
| High school graduate and/or some college | 58.4 | 47.2 | 62.3 | |
| College graduate | 30.5 | 49.4 | 23.8 | |
| Literacy level ≥9th grade | 84.2 | 97.8 | 79.4 | <.001b |
| Higher financial status | 75.9 | 82.6 | 73.6 | .09 |
| Hypertension | 49.0 | 44.9 | 50.4 | .38 |
| Diabetes mellitus | 12.9 | 5.6 | 15.5 | .02b |
| Stroke | 8.0 | 7.9 | 8.0 | .96 |
| Myocardial infarction | 12.2 | 15.9 | 10.9 | .22 |
| Obesity | 15.8 | 7.9 | 18.7 | .02a |
| High cholesterol levelc | 19.5 | 21.4 | 18.8 | .60 |
| High inflammation level | 20.8 | 18.0 | 21.8 | .44 |
| Depressiond | 4.7 | 4.6 | 4.8 | .93 |
| Sleep time, h/night | ||||
| ≤6 | 33.7 | 28.4 | 35.6 | .46 |
| 7 | 29.9 | 33.0 | 28.7 | |
| ≥8 | 36.4 | 38.6 | 35.6 | |
| Married | 66.1 | 67.1 | 65.8 | .84 |
| Living with someone | 75.9 | 79.6 | 74.6 | .35 |
| Regularly visits family or friendse | 83.2 | 84.3 | 82.9 | .76 |
| Lack of negative life events in past year | 32.8 | 42.7 | 29.4 | .02b |
| Works or volunteers | 60.4 | 68.5 | 57.5 | .07 |
| Time spent reading, h/wk | ||||
| <7 (Low) | 26.9 | 15.7 | 31.0 | .02a |
| 7–14 (Moderate) | 49.2 | 56.2 | 46.7 | |
| >14 (Avid) | 23.9 | 28.1 | 22.3 | |
| Physical activity, kcal/kg/wk | ||||
| <45.7 (Low) | 24.9 | 21.4 | 26.2 | .32 |
| 45.7–88.9 (Moderate) | 39.9 | 37.1 | 40.9 | |
| >88.9 (High) | 35.2 | 41.6 | 32.9 | |
| Current alcohol usef | 61.1 | 67.1 | 59.0 | .18 |
| Current nonsmoker | 94.7 | 97.8 | 93.7 | .14 |
Abbreviation: APOE, apolipoprotein E.
Percentages have been rounded and may not total 100.
Indicates significance at P < .05.
Indicates total cholesterol level of 240 mg/dL or greater (to convert to millimoles per liter, multiply by 0.0259).
Indicates Center for Epidemiologic Studies–Depression Scale score of 16 or greater.
Indicates at least twice per week.
Indicates at least 1 drink per week.
Figure 2.
Random Forest Analyses to Identify the Strongest Predictors of Cognitive Resilience
Random forest results identify the strongest predictors of cognitive resilience among 272 white and 258 black carriers of apolipoprotein E (APOE) ε4 who had complete data for all examined variables. Variables are ranked by their relative importance in predicting resilience using a variable importance score.26 Variables to the right of the vertical line are considered important predictors.
Among black APOE ε4 carriers, female sex, higher educational level, higher literacy level, fewer hours of sleep per night, regularly visiting friends or family, and working or volunteering were associated with being cognitively resilient in bivariate analyses (P < .05 for all; Table 2). The random forest analysis for black APOE ε4 carriers showed very strong model classification performance (C statistic, 0.83), and the strongest predictors of resilience in rank order were (1) higher literacy levels, (2) higher educational level, (3) female sex, and (4) absence of diabetes mellitus (Figure 2).
Table 2.
Bivariate Associations: Potential Predictors of Cognitive Resilience Among Black APOE ε4 Carriers
| Characteristics | Study Participants, %a | P Value | ||
|---|---|---|---|---|
| All (N = 329) |
Resilient (n = 98) |
Nonresilient (n = 231) |
||
| Age, y | ||||
| 69–72 | 45.9 | 40.8 | 48.1 | .19 |
| 73–76 | 38.9 | 38.8 | 39.0 | |
| 77–80 | 15.2 | 20.4 | 13.0 | |
| Female sex | 62.9 | 71.4 | 59.3 | .04b |
| Educational level | ||||
| Did not graduate high school | 37.8 | 18.4 | 46.1 | <.001b |
| High school graduate and/or some college | 46.0 | 61.2 | 39.6 | |
| College graduate | 16.2 | 20.4 | 14.4 | |
| Literacy level ≥9th grade | 54.1 | 74.5 | 45.5 | <.001b |
| Higher financial status | 45.1 | 52.6 | 41.9 | .08 |
| Hypertension | 66.3 | 64.3 | 67.1 | .62 |
| Diabetes mellitus | 24.3 | 18.4 | 26.8 | .10 |
| Stroke | 8.3 | 9.4 | 7.9 | .65 |
| Myocardial infarction | 10.7 | 12.2 | 10.0 | .55 |
| Obesity | 37.7 | 35.7 | 38.5 | .63 |
| High cholesterol levelc | 20.1 | 23.7 | 18.6 | .29 |
| High inflammation level | 33.1 | 28.6 | 35.1 | .25 |
| Depressiond | 3.4 | 2.0 | 4.0 | .38 |
| Sleep time, h/night | ||||
| ≤6 | 50.0 | 59.4 | 46.0 | .02b |
| 7 | 20.4 | 21.9 | 19.8 | |
| ≥8 | 29.6 | 18.8 | 34.2 | |
| Married | 38.7 | 42.6 | 37.1 | .36 |
| Living with someone | 64.9 | 68.4 | 63.4 | .39 |
| Regularly visits family or friendse | 84.8 | 91.8 | 81.8 | .02b |
| No negative life events in past year | 31.0 | 28.6 | 32.0 | .53 |
| Works or volunteers | 53.2 | 63.9 | 48.7 | .01b |
| Time spent reading, h/wk | ||||
| <7 (Low) | 42.5 | 36.6 | 45.1 | .13 |
| 7–14 (Moderate) | 42.8 | 43.0 | 42.7 | |
| >14 (Avid) | 14.7 | 20.4 | 12.2 | |
| Physical activity, kcal/kg/wk | ||||
| <45.7 (Low) | 35.0 | 28.6 | 37.7 | .29 |
| 45.7–88.9 (Moderate) | 32.8 | 35.7 | 31.6 | |
| >88.9 (High) | 32.2 | 35.7 | 30.7 | |
| Current alcohol usef | 36.8 | 36.7 | 36.8 | .99 |
| Current nonsmoker | 84.5 | 89.8 | 82.3 | .08 |
Abbreviation: APOE, apolipoprotein E.
Percentages have been rounded and may not total 100.
Indicates significance at P < .05.
Indicates total cholesterol level of 240 mg/dL or greater (to convert to millimoles per liter, multiply by 0.0259).
Indicates Center for Epidemiologic Studies–Depression Scale score of 16 or greater.
Indicates at least twice per week.
Indicates at least 1 drink per week.
The independent effects of these variables on resilience in both races are presented in Table 3 and are based on a multivariable logistic regression model including the most important predictors identified in the random forest analyses. In sensitivity analyses, we repeated the random forest analyses excluding APOE ε4 homozygous carriers in our cohort (19white and 29 black participants), and results were similar to those of our primary analyses.
Table 3.
Results of Multivariable Logistic Regression Models Examining Most Important Predictors of Cognitive Resiliencea
| Predictor | Resilience, OR (95% CI) |
|---|---|
| Model 1: white APOE ε4 carriers (n = 331) | |
| No negative life events in past year | 1.75 (1.00–3.03) |
| Literacy level ≥9th grade | 9.50 (2.67–60.89) |
| Age group compared with 69–72 y | |
| 73–76 y | 2.01 (1.13–3.63) |
| 77–80 y | 1.87 (0.82–4.18) |
| Educational level compared with did not graduate high school | |
| High school graduate and/or some college | 1.77 (0.55–7.99) |
| College graduate | 3.81 (1.13–17.56) |
| Time spent reading compared with low reading levelb | |
| Moderate | 1.91 (0.96–4.00) |
| Avid | 1.36 (0.62–3.07) |
| Model 2: black APOE ε4 carriers (n = 328) | |
| Literacy level ≥9th grade | 2.27 (1.29–4.06) |
| Educational level compared with did not graduate high school | |
| High school graduate and/or some college | 2.86 (1.54–5.49) |
| College graduate | 2.52 (1.14–5.62) |
| Diabetes mellitus | 0.69 (0.36–1.26) |
| Female sex | 1.42 (0.83–2.47) |
Abbreviations: APOE, apolipoprotein E; OR, odds ratio.
Models included all variables that were identified in the random forest analyses as being important predictors of cognitive resilience. To be included in these analyses, participants were required to have complete data for all variables included in the model.
Levels of reading were defined as low (<7 h/wk), moderate (7–14 h/wk), and avid (≥14 h/wk).
Discussion
We identified a number of factors predictive of cognitive resilience among APOE ε4 carriers. The factors most strongly predictive of resilience among white APOE ε4 carriers included no recent negative life events, higher literacy levels, older age, higher educational level, and more time spent reading (in relative order of importance). Among black APOE ε4 carriers, the factors most strongly predictive of resilience included higher literacy level, higher educational level, female sex, and the absence of diabetes mellitus (in relative order of importance). These findings raise the possibility that interventions targeting the modifiable factors among these predictors could help promote cognitive resilience in APOE ε4 carriers.
By investigating cognitive resilience in black and white APOE ε4 carriers, we found similarities and differences between racial groups for the factors that best protect individuals against the deleterious effects of the ε4 allele. Among both races, factors thought to be markers of cognitive reserve16 stood out as strong predictors of resilience, including higher literacy and educational levels in both racial groups and greater cognitive activity (time spent reading) among white carriers. These findings provide further evidence of the beneficial role of cognitive reserve in aging, specifically that greater reserve is protective even among individuals at high genetic risk for decline.31
The pattern of predictors between racial groups diverged for other factors. Among white APOE ε4 carriers, we found an absence of negative life events to be the strongest predictor of resilience, but this relationship was not apparent among black carriers. These findings mirror those of a recent study32 that identified stress as a cognitive risk factor among white, but not black, older adults regardless of APOE ε4 status. Stress in aging can have negative effects on memory and the structure of the hippocampus,33 but mechanisms driving potential racial differences in the influence of stress on cognitive aging remain unclear. Future research might explore whether the following differ by race: (1) types of stressors experienced in aging, (2) older adults’ coping styles, and (3) the neurobiological effects of stress on the aging brain.
Another area where patterns appeared to differ by race was the relative strength of the influence of cardiovascular factors. Among black APOE ε4 carriers, the absence of diabetes mellitus was among the strongest predictors of resilience, whereas the association between cardiovascular factors and resilience was less clear in white APOE ε4 carriers (although the absence of diabetes mellitus and obesity was associated with resilience in bivariate analyses for white carriers, no cardiovascular health factors were identified as strong predictors in our multivariable analysis).Overall, results suggest possible complex racial differences in what promotes resilience among APOE ε4 carriers. Although our study suggests that the relative importance of factors in promoting resilience varies by race, the mechanisms behind racial differences remain unclear and should be explored in future research. Racial differences in participant characteristics may in turn influence racial differences in which factors contribute to cognitive resilience.
A few factors associated with cognitive resilience were counterintuitive. We found that older age was predictive of resilience in white APOE ε4 carriers, a pattern consistent with previous findings that the cognitive impact of the ε4 allele lessens once individuals reach advanced age.6,14 In contrast to evidence of the ε4 allele being a stronger risk factor for Alzheimer disease in women compared with men,34 we found that black female APOE ε4 carriers were more likely to be resilient than their male counterparts, a discrepancy that may be influenced by race effects. Among black carriers, we found a bivariate association between sleep and resilience, suggesting that shorter sleep duration was protective. Although the direction of this relationship is surprising, other studies have been mixed in identifying long and short sleep durations as cognitive risk factors.35,36 However, given that an association between sleep duration and resilience did not hold up in our multivariable analyses, the observed bivariate association may have been driven by other factors.
Similar to previous studies of predictors of cognitive outcome in APOE ε4 carriers,37,38 we found that multiple domains influence the cognitive trajectory of APOE ε4 carriers, including demographics, cognitive reserve, and other psychosocial and health factors. Limitations of prior studies include a lack of investigation of the potential for racial differences in what predicts cognitive outcome in APOE ε4 carriers or examination of a limited number of potential predictors. The strengths of our study include our ability to explore a comprehensive set of factors in a well-characterized prospective cohort of black and white older adults. Another strength is our application of a novel multivariable analysis to reveal the factors most strongly predictive of resilience and thus those most important to target in interventions. We were also able to use the entire Health ABC Study cohort to contextualize normative cognitive change across 11 years and use this context as a reference in defining cognitive resilience among APOE ε4 carriers. A main limitation of our study is that some of our predictors were based on self-report; thus, future studies would benefit from the use of objective measures of sleep and physical activity, for example. In addition, our study does not capture APOE ε4 carriers whose cognitive decline may have begun before 69 years of age (before our baseline); thus, we do not know how a survival effect may have influenced our findings.
Conclusions
A number of factors appear to help minimize the deleterious effect of the ε4 allele on cognition in aging, although APOE ε4 carriers are a high-risk group. Future directions include investigating interventions geared toward promoting cognitive resilience in APOE ε4 carriers by targeting the modifiable factors we found to be predictors of resilience. Enhancing cognitive reserve appears to be an important intervention target in black and white APOE ε4 carriers. Our study provides preliminary evidence that other top intervention targets may vary by racial group. For example, although an intervention designed for white APOE ε4 carriers may benefit from a greater focus on stress reduction or stress management, an intervention designed for black APOE ε4 carriers may benefit from a greater focus on cardiovascular health (eg, management of diabetes mellitus). Our results also suggest that interventions designed to target more than 1domain concurrently, such as those addressing cognitive reserve and psychosocial or health factors within the same program, could have greater cognitive benefit.
Supplementary Material
Acknowledgments
Dr Sink serves on 2 data safety and monitoring boards (DSMBs) for studies sponsored by the National Institutes of Health (NIH) and is the site principal investigator for an industry-sponsored clinical trial (Sponsor Navidea Biopharmaceuticals). Dr Yaffe is a consultant for Novartis and Pfizer; serves on DSMBs for Takeda, Inc, and a study sponsored by the National Institute on Aging (NIA); and serves on the Beeson Scientific Advisory Board.
Funding/Support: This study was supported by contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106 and grant R01-AG028050 from the NIA; by grant R01-NR012459 from the National Institute on Nursing Research; and in part by the Intramural Research Program of the NIA/NIH, all of which supported the design and conduct of the study; collection, management, analysis, and interpretation of the data; and review and approval of the manuscript. This study was also supported by grant K24AG031155 from the NIA (Dr Yaffe), which supported the design and conduct of the study, analysis and interpretation of the data, preparation and review of the manuscript, and decision to submit the manuscript for publication. Preparation of the manuscript was supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the San Francisco Veterans Affairs Medical Center, and the Sierra Pacific Mental Illness Research, Education, and Clinical Center.
Role of the Funder/Sponsor: The NIA was involved in the design and conduct of the study; data collection and management; and the review and approval of the manuscript.
Group Information
Principle investigators for the ABC Health Study included Tamara B. Harris, MD, National Institute on Aging (lead investigator); Melissa E. Garcia, MPH, National Institute on Aging; Anne B. Newman, MD, MPH, University of Pittsburgh, Pittsburgh, Pennsylvania; Suzanne Satterfield, MD, DrPH, University of Tennessee, Memphis; Steven R. Cummings, MD, University of California, San Francisco; and Michael C. Nevitt, PhD, University of California, San Francisco. Health ABC Study coordinators were Diane Ives, MPH, University of Pittsburgh, and Jan Elam, BS, University of Tennessee, Memphis. Health ABC project director was Susan M. Rubin, MPH, University of California, San Francisco.
Footnotes
Author Contributions: Dr Kaup had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kaup, Yaffe.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kaup, Yaffe.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Kaup, Nettiksimmons, Yaffe.
Obtained funding: Harris, Yaffe.
Administrative, technical, or material support: Satterfield, Ayonayon.
Study supervision: Yaffe.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55(7):964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsztein DC, Easton DF. Apolipoprotein E genetic variation and Alzheimer’s disease: a meta-analysis. Dement Geriatr Cogn Disord. 1999;10(3):199–209. doi: 10.1159/000017120. [DOI] [PubMed] [Google Scholar]
- 3.Schiepers OJG, Harris SE, Gow AJ, et al. APOE ε4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17(3):315–324. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 4.Corbo R, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world: is APOE*4 a “thrifty” allele? Ann Hum Genet. 1999;63(4):301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- 5.Borenstein AR, Mortimer JA, Wu Y, et al. Apolipoprotein E and cognition in community-based samples of African Americans and Caucasians. Ethn Dis. 2006;16(1):9–15. [PubMed] [Google Scholar]
- 6.Farrer LA, Cupples LA, Haines JL, et al. APOE and Alzheimer Disease Meta Analysis Consortium. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 7.Fillenbaum GG, Landerman LR, Blazer DG, Saunders AM, Harris TB, Launer LJ. The relationship of APOE genotype to cognitive functioning in older African-American and Caucasian community residents. J Am Geriatr Soc. 2001;49(9):1148–1155. doi: 10.1046/j.1532-5415.2001.49230.x. [DOI] [PubMed] [Google Scholar]
- 8.Sawyer K, Sachs-Ericsson N, Preacher KJ, Blazer DG. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community-dwelling older adults. Gerontology. 2009;55(1):32–40. doi: 10.1159/000137666. [DOI] [PubMed] [Google Scholar]
- 9.Evans DA, Bennett DA, Wilson RS, et al. Incidence of Alzheimer disease in a biracial urban community: relation to apolipoprotein E allele status. Arch Neurol. 2003;60(2):185–189. doi: 10.1001/archneur.60.2.185. [DOI] [PubMed] [Google Scholar]
- 10.Tang MX, Stern Y, Marder K, et al. The APOE-ε4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. JAMA. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 11.Maestre G, Ottman R, Stern Y, et al. Apolipoprotein E and Alzheimer’s disease: ethnic variation in genotypic risks. Ann Neurol. 1995;37(2):254–259. doi: 10.1002/ana.410370217. [DOI] [PubMed] [Google Scholar]
- 12.Tang M-X, Maestre G, Tsai W-Y, et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- 13.Skoog I, Hesse C, Aevarsson O, et al. A population study of apoE genotype at the age of 85: relation to dementia, cerebrovascular disease, and mortality. J Neurol Neurosurg Psychiatry. 1998;64(1):37–43. doi: 10.1136/jnnp.64.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corrada MM, Paganini-Hill A, Berlau DJ, Kawas CH. Apolipoprotein E genotype, dementia, and mortality in the oldest old: the 90+ Study. Alzheimers Dement. 2013;9(1):12–18. doi: 10.1016/j.jalz.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris TB, Visser M, Everhart J, et al. Waist circumference and sagittal diameter reflect total body fat better than visceral fat in older men and women: the Health, Aging and Body Composition Study. Ann N Y Acad Sci. 2000;904(1):462–473. doi: 10.1111/j.1749-6632.2000.tb06501.x. [DOI] [PubMed] [Google Scholar]
- 18.Rooks RN, Simonsick EM, Miles T, et al. The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the Health, Aging, and Body Composition Study. J Gerontol B Psychol Sci Soc Sci. 2002;57(4):S247–S256. doi: 10.1093/geronb/57.4.s247. [DOI] [PubMed] [Google Scholar]
- 19.Yaffe K, Barnes D, Lindquist K, et al. Health ABC Investigators. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28(2):171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 21.McDowell I, Kristjansson B, Hill GB, Hebert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50(4):377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 22.Davis TC, Long SW, Jackson RH, et al. Rapid Estimate of Adult Literacy in Medicine: a shortened screening instrument. Fam Med. 1993;25(6):391–395. [PubMed] [Google Scholar]
- 23.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 24.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 25.Vogelzangs N, Beekman AT, Kritchevsky SB, et al. Psychosocial risk factors and the metabolic syndrome in elderly persons: findings from the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2007;62(5):563–569. doi: 10.1093/gerona/62.5.563. [DOI] [PubMed] [Google Scholar]
- 26.Strobl C, Malley J, Tutz G. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol Methods. 2009;14(4):323–348. doi: 10.1037/a0016973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team. R. A language and environment for statistical computing. R Foundation for Statistical Computing. [Accessed May 1, 2014];2013 http://www.R-project.org/. [Google Scholar]
- 28.Hothorn T, Buhlmann P, Dudoit S, Molinaro A, van der Laan MJ. Survival ensembles. Biostatistics. 2006;7(3):355–373. doi: 10.1093/biostatistics/kxj011. [DOI] [PubMed] [Google Scholar]
- 29.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8(1):25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strobl C, Boulesteix A-L, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinformatics. 2008;9(1):307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vemuri P, Lesnick TG, Przybelski SA, et al. Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol. 2014;71(8):1017–1024. doi: 10.1001/jamaneurol.2014.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheffler J, Moxley J, Sachs-Ericsson N. Stress, race, and APOE: understanding the interplay of risk factors for changes in cognitive functioning. Aging Ment Health. 2014;18(6):784–791. doi: 10.1080/13607863.2014.880403. [DOI] [PubMed] [Google Scholar]
- 33.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 34.Altmann A, Tian L, Henderson VW, Greicius MD Alzheimer’s Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13(7):886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16(9):990–997. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 37.Ferrari C, Xu W-L, Wang H-X, et al. How can elderly apolipoprotein E ε4 carriers remain free from dementia? Neurobiol Aging. 2013;34(1):13–21. doi: 10.1016/j.neurobiolaging.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Sachs-Ericsson NJ, Sawyer KA, Corsentino EA, Collins NA, Blazer DG. APOE ε4 allele carriers: biological, psychological, and social variables associated with cognitive impairment. Aging Ment Health. 2010;14(6):679–691. doi: 10.1080/13607860903292594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.