Abstract
Melanoma originated from melanocytes is the most aggressive type of skin cancer with limited treatment options. New targeted therapeutic options with the discovery of BRAF and MEK inhibitors have shown significant survival benefits. Despite the recent progress, development of chemoresistance and systemic toxicity remains a challenge for treating metastatic melanoma. While the response from the first line of treatment against melanoma using dacarbazine remains only 5–10%, the prolonged use of targeted therapy against mutated oncogene BRAF develops chemoresistance. In this review, we will discuss the nanoparticle-based strategies for encapsulation and conjugation of drugs to the polymer for maximizing their tumor distribution through enhanced permeability and retention effect. We will also highlight photodynamic therapy and design of melanoma-targeted nanoparticles.
Keywords: : active targeting, metastatic melanoma, multidrug resistance, nanoparticles, photodynamic therapy, polymer–drug conjugate, tumor initiating cells
Melanoma is the cancer of melanocytes which are present as single cells within the basal layer of epidermis. Melanoma is a highly aggressive skin cancer with a potential of metastasis and responsible for the majority of skin-related deaths. The 5-year survival of metastasized melanoma is around 10% [1]. Systemic chemotherapy is the mainstay for the treatment of melanoma and dacarbazine (DTIC) was standard of care for melanoma till the approval of new targeted kinase inhibitors. DTIC monotherapy is associated with a poor response rate of around 7.2–7.5% and does not extend survival benefits [2].
About 60% of melanoma patients carry activating mutations in the gene encoding the serine–threonine protein kinase B-RAF (BRAF). Discovery of these activating mutations paved the way for developing small molecule inhibitors targeting mitogen-activated protein (MAP) kinase pathway. BRAF inhibitors (vemurafenib, dabrafenib), MEK inhibitor (trametinib) and the combination of dabrafenib with trametinib are the newest small molecules approved by the US FDA approved for treating BRAF-mutated melanoma. However, response to potent BRAF inhibitors is short lived and disease progression is seen at a median of 5–7 months [3,4]. Hence, there is a need for improved treatment options for effective treatment of melanoma. Tubulin-binding agents (TBA), platinum analogs, anthracyclines and nitrosoureas have been clinically prescribed in melanoma as combination therapy [5] and exhibit comparable response rate to DTIC. However, multiple drug resistance (MDR) mechanisms, poor delivery and dose-limiting systemic toxicity restrict the therapeutic application of these chemotherapeutic drugs. Thus, there is a need to improve the biodistribution of chemotherapeutics to tumor with minimum peripheral exposure and thereby minimize the systemic toxicity. Advent of nanoparticle-based drug delivery resulted in tumor selective delivery of drugs by enhanced permeability and retention (EPR) effect with reduced peripheral exposure. Furthermore, interaction between receptors overexpressed by tumor and their ligands has been exploited to enhance the cellular uptake of nanoparticles. However, formulation of nanoparticles with enhanced stability in plasma and limited premature drug release are potential pitfalls in harnessing the improved biodistribution by EPR effect. Hence, we have reviewed the different polymer modifications to enhance the noncovalent interaction between polymer backbone and drug as well as polymer conjugation to design kinetically stable nanoparticles with enhanced drug loadings. In addition, nanoparticle-based photodynamic therapy (PDT) presents exciting avenues for detection, treatment and evasion from possible mechanism of resistance from melanoma. In this review, we summarize the different mechanisms of resistance, polymer modification for preparation of nanoparticles, nanoparticles for PDT, targeted nanocarriers and future outlook of nanoparticle-based therapy for melanoma.
Factors influencing melanoma therapy
Melanocytes respond to hormones and environmental factors to produce colored pigments. Physiologically, ionizing ultraviolet radiation stalls the cell cycle division or kills most of the primary cells; however melanocytes proliferate and secrete melanin. Melanin serves as a photoreceptor for the skin and protects keratinocytes and other epidermal cells from damage [6,7]. This tendency of melanocytes to survive and proliferate under stress programs them to survive. Besides these features, cells in the neighborhood also stimulate their proliferation by paracrine mechanism. Fibroblast stimulates the growth of melanocytes by secreting FGF [8,9]. Keratinocytes maintain the homeostasis of melanocytes by affecting Bcl-2 expression by melanocytes. Secretion of NGF and SCF by keratinocytes promotes Bcl-2 expression and unchecked proliferation of melanocytes [10].
Melanocytes escape the homeostatic regulation of keratinocytes by differential expression of cadherins and altered paracrine signaling. Downregulation of receptors for cross-talk with keratinocytes and upregulation of receptors for paracrine signaling with fibroblast lead to proliferation of melanocytes [11]. Melanocytes migrate through the basement membrane into the dermis through altered extracellular matrix (ECM) expression [12]. Asymptomatic metastasis to multiple organs is the primary reason for the poor prognosis of melanoma. Early detection of melanoma and surgical excision of the primary tumor have significant success rate in melanoma; however detection of malignant lesions and circulating tumor cells (CTCs) remains investigational. mRNA, protein and size-based techniques have been utilized for detection of CTCs but clinical potential of CTC detection remains unfulfilled [13]. Overexpression of melanin pigment as an intrinsic, spectrally specific cancer marker and signal amplifier allows the use of photoacoustic (PA) imaging as highly sensitive, label-free detection of CTCs. Advancement in the CTC detection can provide breakthrough in early detection of CTCs and prognostic tools for prediction of resistance [14,15]. In this section, we would review the potential mechanism of chemoresistance in melanoma.
Stromal microenvironment
Tumor formation is not only the malignant growth of cells but also associated changes in its microenvironment to support malignant proliferation and eventual metastasis. Stroma of melanoma is a compilation of cells including fibroblasts/myofibroblasts, endothelial cells, infiltrating immune cells, vascular and smooth muscle cells, soluble growth factors and ECM proteins. In general, quiescent fibroblasts get activated in response to the tissue damage and secrete growth factors to support tissue repair. Normal dermal fibroblasts repress the growth of early stage or metastatically incompetent primary lesion. However, small number of metastatically competent cells overcome this resistance and show proliferative advantage upon coculture with normal dermal fibroblasts. This discriminatory effect of fibroblasts on melanoma was mediated by paracrine signaling and release of soluble factors [16]. Secretion of proteases by senescent fibroblasts has been shown as the key factor involved in the growth and metastasis of melanoma [17]. This finding has been confirmed by the genome-wide microarray analysis of invasive human melanoma and benign nevi. Expression of Cathespin B, L, matrix metalloproteinase 1/9, urokinase and tissue-plasminogen type activator was unregulated and invasion of melanoma was inhibited by cell membrane permeable Cathespin B/L inhibitors.
Although there is no consensus on the phenotype characteristics of melanoma resident fibroblasts, there is a distinct difference in the phenotype of fibroblasts from normal skin or tumor. Fibroblast derived from the tumor is CD56 negative and expresses high level of fibroblasts-activated protein (FAP), whereas normal fibroblasts are CD56 positive and lower levels of FAP [18]. FAP overexpressing fibroblasts represent activated fibroblasts that degrade ECM and promote invasion of primary melanoma. Secretion of growth factors upon malignant transformation of melanocytes promoted the expression of FAP by fibroblasts and FAP-driven migration and invasion [19]. Among various growth factors, secretion of PDGF by melanoma is crucial as it activates expression of IGF-1 by fibroblasts. Fibroblasts secreted IGF-1 is crucial for the early progression of melanoma as it promotes survival and growth of melanoma cells from the early stage. IGF-1 phosphorylates Erk 1 and 2 of MAP kinase pathway and also activates AKT pathway [20].
In addition to paracrine stromal support to metastatic melanoma, stromal cells increase intratumoral pressure and thereby restrict delivery of drugs to tumor [21,22]. This suboptimal delivery of drugs into poorly perfused and fibrotic tumor contributes to the resistance to melanoma. Clinical studies have reported significant increase over the baseline intratumoral pressure of melanoma lesions in nonresponding patients. Intratumoral pressure increased significantly over time for nonresponding melanoma lesions from a baseline of 24.4–53.9 mm Hg after treatment and decreased in melanoma lesions that responded to treatment with the mean baseline and post-treatment intratumoral pressures were 12.2 and 0 mm Hg, respectively [23]. Antagonist targeting stromal proliferation and intratumoral pressure have been tested to improve drug delivery [22,24]. Targeted delivery of picogram levels of TNF-α-enhanced doxorubicin (DOX) penetration into melanoma by altering endothelial barrier function and intratumoral pressure. This enhanced tumor perfusion improved therapeutic efficacy of DOX by eight to tenfold [25]. Improvement in delivery of immunotherapeutic agent by targeting stroma has also been reported [26]. Coadministration of paclitaxel (PTX) increased the uptake of stroma targeted IL-2 in melanomas by increasing tumor perfusion and permeability. PTX also boosted the recruitment of IL-2-induced natural killer (NK) cells to the tumor. Importantly, this increased tumor accumulation of IL-2 reduced both the tumor burden and the number of pulmonary metastatic nodules. Targeting stromal paracrine signaling and improving drug delivery to tumor may present a solution to avoid tumor microenvironment-mediated resistance and achieve durable patient responses.
Drug efflux enzymes
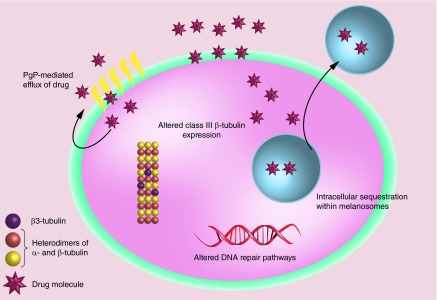
P-glycoprotein (Pgp) and other multidrug resistance associated protein (MRP) effectively efflux out the drug and thereby prevent accumulation of cytotoxic drugs (Figure 1). To resolve this, higher doses and frequency of chemotherapeutics are required to sustain intracellular concentration of drugs which is associated with toxicity to normal tissues. Physiologically, these MDR proteins act as an energy-dependent efflux transporter to maintain potential across the plasma membrane and involved in the xenobiotic of unwanted lipophilic compounds. Level of Pgp expression is a significant prognostic factor and has been shown to correlate well with progression-free survival and relapse in a variety of cancer [27–31]. Malignant melanoma intrinsically expresses MRP, the level of MRP expression increases upon treatment [32]. Increased expression of Pgp in melanoma has been associated with highly invasive and resistant melanoma [33]. However, analysis of more melanoma clinical samples is required for determination of correlations between the clinical outcome and expression level of MDR proteins. Overexpression of these MDR proteins due to genetic and epigenetic events during the oncogenesis has been suggested for generating predominant drug-resistant cell phenotypes [34]. Effect of chemotherapy on the levels of Pgp is debatable and requires further clinical evaluation [27,35–37].
Figure 1. . Potential multidrug resistance mechanisms of metastatic melanoma.
Development of resistance in metastatic melanoma can be attributed to inefficient intracellular drug accumulation, upregulation of expression of class III β-tubulin, intracellular sequestration and compensatory upregulation of DNA repair pathways.
Reversal of MDR efflux mechanism has been demonstrated by utilizing Pgp inhibitors in in vitro and preclinical models. Pgp inhibitors like verapamil and valspodar have been coadministered with PTX and DOX [38,39]. Although the administration of these inhibitors improved the pharmacokinetics of chemodrugs, there was lack of associated improvement in clinical outcome. This finding can be attributed to nonspecific inhibition of Pgp leading to nonselective increase in the plasma concentration of the chemotherapeutic drugs with little or insignificant increase in tumor accumulation [40]. Besides efflux mechanism of Pgp, alternative mechanism of resistance mediated by Pgp has been proposed to account for these findings. Development of intrinsic resistance has also been attributed to the expression of MDR proteins which is generally associated with the slow growing tumor initiating cells in the tumor [41]. Upregulation of signaling pathways, changes in cytoskeleton, reduced apoptosis and increased DNA repair by MDR transporters has been attributed to the development of resistance [33,41,42]. Additionally, intracellular location of MDR proteins isoforms has been suggested to be involved in the intracellular sequester of drugs in melanoma [43]. Multiple mechanisms of resistance by MDR proteins need to be studied in cohort and targeting MDR represents a promising therapeutic target to reverse chemoresistance.
Tumor initiating cells
Tumor initiating or cancer stem cells (CSCs) are the subpopulation of cells within the tumor that have high tumorigenicity, self-renewal potential and ability to undergo differentiation to reestablish the cellular composition of the parenteral tumor [44]. Expression of CD20, CD271, CD133 and ABCB5 has been reported as markers of melanoma stem cells [45]. CD271+ cells have been shown to coexpress SOX-2 and form fully grown phenotypical identical tumor upon transplantation in immune-compromised mice. Presence of CD271+ cells is essential for immune evasion and continuous growth of tumors. Clinically, analysis of patient tumor samples has shown that high frequency of CD271+ is associated with higher metastatic potential and poor prognosis [46–48]. Moreover, chemotherapy enriched the percentage of ABCB5+ cells which exhibited cross-resistance to drugs for melanoma [49]. Targeted ablation of ABCB5+ cells using ABCB5 antibody-dependent cell-mediated cytotoxicity inhibited tumor initiation and growth in preclinical models [49]. CD20, B-cell marker of melanoma tumor cells exhibited differentiation ability and tumor initiation potential. Treatment targeted at CD20+ cells results in long lasting eradication of melanoma lesions, whereas targeting of bulk tumor cell subset failed to arrest tumor progression [50]. Clinical application of targeting CD20-positive cells in a pilot study confirmed the potential therapeutic value of targeting CD20+ cell populations in melanoma as treatment with anti-CD20 antibody rituximab prevented the relapse [51]. All these studies indicate that elimination of melanoma stem cells is essential for preventing the recurrence and development of resistance. Targeting pathways and receptors overexpressed in CSCs using small molecules and/or using RNA interference may tackle the resistant melanoma.
Altered expression of β-tubulin
Cancer cells acquire resistance to microtubule-acting drugs mainly through the mutation in α- and β-tubulin, altered expression of β-tubulin isotypes or microtubule regulating proteins. Mutation of β-tubulin confers resistance either by altered binding of drugs to β-tubulin or by altering the dynamics of microtubule polymerization. PTX resistance in non-small-lung cancer patients has been attributed to the mutation in the exon 1 and exon 4 of β-tubulin [52]. However, subsequent clinical studies have shown that these mutations were polymorphic rather than somatic mutations and nonspecific design of PCR primers led to the amplification of nonfunctional β-tubulin pseudogenes [53]. PCR analysis of clinical samples of PTX-resistant cancer tissues have further ruled out β-tubulin mutation as the primary mechanism of development of resistance [54,55]. Mutations in the loop region of helix 6 or helix 7 of β-tubulin also alter the sensitivity to tubulin-acting drugs either by modifying the drug binding or by changing the dynamics of tubulin polymerization [56–60]. These studies involved in vitro site-directed mutagenesis with plasmids in cancer cell lines but there is a lack of clinical evidence for this mechanism.
β-tubulin is expressed by large multigene family with nine known isoforms which differ in their carboxyl terminal domain. Among these isoforms, overexpression of class III β-tubulin (TUBB3) is clinically associated with the taxane resistance and poor prognosis in varieties of cancer [61–64]. Modulation of resistance to different TBA by altering the expression level of TUBB3 using gene overexpression or silencing approach which lends further support to this hypothesis [65,66]. Overexpression of TUBB3 may develop resistance by modifying the drug binding site, tubulin dynamics or acting as cellular survival factor [67–69] (Figure 1). Interestingly, increase in TUBB3 level also mediated cross resistance to broad classes of nontubulin acting chemotherapeutics [70]. This broad spectrum of resistance can be attributed to the activation of prosurvival signaling by TUBB3 and protection of cells against apoptosis by chemotherapeutic drugs.
Melanocytes strongly express TUBB3 and its primary cultures are chemoresistant to PTX [71]. Exposure of melanocytes to α-MSH and activation of stress response kinase result in the expression of melanocortin receptor 1–TUBB3 isoforms and role of TUBB3 has been proposed in the transport of melanosomes [72,73]. TBA-resistant melanoma cell lines exhibit high expression of TUBB3 and downregulation of TUBB3 abrogates resistance to TBA [74]. However, clinical samples of melanoma demonstrated a decrease in TUBB3 expression with increasing stage of melanoma and associated with poor prognosis. To further unravel the resistance of melanoma to TBA and development of alternate therapeutic strategies, clinical studies are required to identify the regulatory mechanism of β-tubulin isotype expression and mechanistic basis for development of resistance by altered isotype expression of β-tubulin.
Intracellular sequestration of drugs
Mammalian cells are highly compartmentalized having membrane-bound organelles with distinct intraluminal properties from cytosol. Compartmentalization of drug within the cells restricts their intracellular diffusion leading to their low concentration at the target site [75–77]. Acidic pH of the endosomal organelles and their efflux as exosomes are the primary mechanisms responsible for intracellular entrapment and efflux of entrapped drugs [78,79]. Basic drugs especially with pKa near neutral pH are entrapped within the endosomal organelles and their entrapment can be explained by the pH partition theory. Intracellular sequester of cisplatin, DOX and vincristine has been widely studied as the prominent mechanism of resistance [80–82].
Resistance due to intracellular sequester of drugs is much more prominent in melanoma owing to the presence of intracellular structures known as melanosomes (Figure 1). Melanosomes are lysosomes alike intracellular organelles and provide an environment for the synthesis of melanin as the byproducts of melanin synthesis are cytotoxic. Melanosomes entrap these toxic intermediates of melanin biosynthesis to manage endogenous melanogenesis-related cytotoxicity [83]. Chen et al. have demonstrated that cisplatin is prominently sequestered in melanosomes, which significantly reduces its nuclear localization as compared with nonmelanoma carcinoma cells. Moreover, melanosomal accumulation of cisplatin further promoted melanogenesis and extracellular transport of melanosomes containing cisplatin [84]. Intracellular sequester of cisplatin and its subsequent efflux from cells as exosomes was further confirmed by analysis of entrapped cisplatin in the purified exosomes from the tumor cell culture supernatants. Exosomes purified from supernatants of melanoma cell cultures contained various amounts of cisplatin which correlated to the pH conditions of the culture medium. Pretreatment with a proton pump inhibitor blocked the acidification of melanosomes, and thereby intracellular sequester of cisplatin. Further analysis showed that pretreatment with a proton pump inhibitor induced a clear reduction in the plasma levels of tumor-derived exosomes containing lower levels of cisplatin [79]. Similarly, the proton inhibitor also improved nuclear accumulation of weakly basic DOX. Reduction in the intracellular entrapment of the drug increased the intratumoral concentration of free drugs and improved their distribution from blood vessels to the tumor [75].
Disruption of the cellular pathways involved in the melanosomal formation and melanin biosynthesis restored sensitivity of melanoma to the weakly basic drugs. Absence of the melanosomal structural protein, gp100/Pmel17 as well as mutations of Dtnbp1, Pldn, Vps33a genes which are involved in the melanosome biogenesis increased cisplatin sensitivity. In addition, mutation of the integral melanosomal protein tyrosinase affected the melanosomal formation and restored cisplatin sensitivity. Furthermore, alteration of the melanosomal formation also sensitized cells to vinblastine and etoposide [85]. Chen et al. also demonstrated the correlation between the dynamics of melanosomal formation and the sensitivity of cells. Cells which contain predominantly stage 4 melanosomes are sensitive to cisplatin whereas cells with melanosomes in stage 2, 3 are highly resistant [86]. Clinical samples of melanoma also confer this as potential mechanism of resistance. Patients exhibiting enhanced formation of melanosomes vesicles respond poorly to therapy and have shorter survival time [87,88]. These all preclinical and clinical studies demonstrate that melanosome formation, its trafficking or tyrosinase enzyme involved in melanin biosynthesis can be targeted for overcoming resistance and sensitizing the melanoma to conventional chemotherapeutic agents.
Nanotechnology for drug delivery
Lack of selectivity for tumor tissue, unfavorable physicochemical property and poor biopharmaceutical characteristics limit the therapeutic efficacy of chemotherapeutics. Free drugs circulate throughout the body and permeate the cell membrane through passive diffusion. This nonselective distribution and cellular permeability of anticancer drugs is the primary reason for dose-limiting toxicity [89,90]. Poor physicochemical and biopharmaceutical properties of anticancer drugs present additional challenges in their delivery. Drugs with low water solubility require cosolvent for their administration and cause systemic toxicity, whereas water-soluble drugs are rapidly eliminated through kidney restricting the plasma residence time for their partition to tumor.
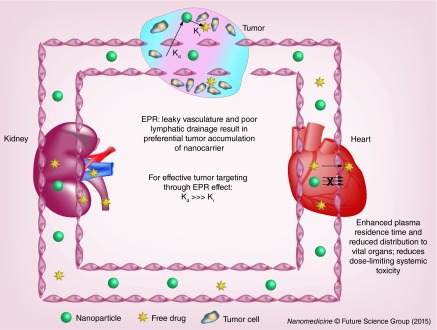
Nanotechnology has provided a platform to overcome these challenges and deliver drugs smartly to tumor [91,92]. Doxil and Abraxane are the two clinically approved nanoproducts of DOX and PTX with improved biodistribution and safety profiles, respectively. Micelles, nanoparticles, liposomes, dendrimers and polymer–drug conjugates are the most commonly used nanoparticles for drug delivery. Drugs loaded into nanoparticles exhibit altered biodistribution and pharmacokinetic profiles. Maeda et al. first have shown macromolecules preferentially accumulate at tumor site due to the enhanced vascular permeability and poor lymphatic drainage [93,94]. High molecular weight polymers (>50 kDa) showed significant accumulation at 6 h while low molecular weight polymers cleared rapidly due to rapid diffusion into the blood stream. Prolonged circulation of nanoparticles improves their accumulation at the tumor site for local release of drug and/or uptake of drug-loaded nanoparticles by cancer cells. To maximize the drug delivery, it is imperative that drug release rate constant (Kr) while nanoparticles are in the circulation should be miniscule as compared with distribution rate constant of nanoparticles to tumor (Kd) (Figure 2). Premature release of drugs from the nanoparticles would nullify the advantage of EPR effect and biodistribution of nanoparticle-associated drug would be identical to that of the free drug. However, drugs associated with the nanoparticles are pharmacologically inactive and require drug release at the tumor site to show therapeutic effect. Considering this, the nanoparticles should be designed to allow effective accumulation of its payload at the tumor site but should be released quick enough to maintain the concentration of free drug above the IC50 values. Broadly, drugs are either encapsulated within the nanoparticles or conjugated to a lipid or polymeric nanoparticles. In the following sections, we would review drug encapsulation and conjugation approach in terms of formulation design, interaction between drug and polymer backbone for enhanced drug loading, drug release and how the existing information can be utilized to design smart nanoparticles.
Figure 2. . Nanoparticle-mediated drug delivery.
Poor extravasation of nanoparticles across endothelium of vital organs and enhanced permeability and retention of nanoparticles at tumor site result in preferential tumor distribution of chemotherapeutics. To harness full potential of nanoparticle-based drug delivery, Kr from nanoparticles should be much smaller as compared with Kd preventing premature release of drug from nanoparticles while in circulation.
EPR: Enhanced permeability and retention; Kd: Distribution rate constant; Kr: Release rate constant.
Nanoparticles for drug delivery
Low solubility of anticancer drugs is the major obstacle for systemic therapy. Nanomedicines have been extensively explored for the delivery of lipophilic drugs. Hydrophobic core of an amphiphillic polymer improves solubilization of poorly soluble drugs and has reduced toxicity compared with solubilization of drug in cosolvent. Amphiphillic polymers encapsulate the drug based on noncovalent interactions like hydrophobic interaction, π–π stacking, hydrogen bonding and ionic interaction. Variety of PEG amphiphilic polyester and polyamide copolymers like poly(ethylene glycol)-b-poly-(aspartic acid) (PEG-b-PAA) [95], poly(ethylene glycol)-b-poly(lactide-co-glycolic acid) (PEG-b-PLGA) [96], poly(ethylene glycol)-b-poly(caprolactone) (PEG-b-PCL) [97] and poly(ethylene glycol)-b-poly(d,l-lactide) (PEG-b-PDLLA) [98] with biodegradable hydrophobic cores have been widely studied for formulation of poorly soluble drugs as nanoparticles. Key properties of these nanoparticles such as size, thermodynamic stability, drug loading and drug release kinetics have also been well reported [99–102]. These studies have shown that noncovalent entrapment of drugs into nanoparticles is restricted by the lack of kinetic stability of polymer nanoparticles upon dilution, poor drug loading and premature release. To improve the nanoparticle stability and drug loading, copolymers have been modified to enhance noncovalent interactions like hydrophobic interaction, π–π stacking, hydrogen bonding and ionic interaction between polymer and drug [102–104]. In this section, we would review the different modifications of the nanoparticle core based on the drug characteristics and the critical role of drug properties in designing the core of micelles.
Hydrophobic interaction
Polymer/drug compatibility has been proposed as the key criteria affecting drug encapsulation within the core of micelles. Based on polymer/drug compatibility and thermodynamics, drug solubilization within micelles has been predicted which correlated well with the experimental findings [105]. Polymer/drug compatibility has been characterized by the Flory–Huggins interaction parameter (χFH) which accounts for the forces of interaction between the polymer and the drug; and low χFH values suggest that the polymer is thermodynamically a good solvent for the drug [106,107]. We have previously modified the core of PEG-PLA to decrease χFH between polymer and bicalutamide. Introduction of the carbonate monomer within the core enhanced the interaction between bicalutamide and the core, minimized the χFH resulting in improved bicalutamide loading and enhanced stability [108,109]. Similarly, encapsulation of embelin was enhanced inside the core of polymeric micelles by modifying the carbonate core with dodecanol. Hydrophobic interaction of the long aliphatic chains of embelin within the core was improved by insertion of long aliphatic chain dodecanol. Interaction between the aliphatic chains enhanced the thermodynamic stability by reducing the critical micellar concentration (CMC) values and slow release of embelin from the micelle core [110]. Mahmud et al. also reported chemical tailoring of the core with cholesteryl moieties to enhance the cholesterol-compatible cucurbitacin I in the polymeric micelles [111]. Increase in the drug/polymer compatibility also affects the rate of drug release as higher drug compatibility with the micelle core results in a considerable decrease in the drug release rate as evident from the sustained release of Amphotericin B from fatty acid modified core of PEG-b-poly (amino acid) [111]. Hydrophobic interaction can be critical for effective solubilization of a drug lacking hydrophilic groups for other noncovalent interactions and compatibility between drug and micelle core should be evaluated for effective delivery by nanoparticles.
π–π stacking
Interaction between π clouds of aromatic rings of DNA base pairs has been well studied and this π–π stacking has been utilized for design of nanoparticles. Polymeric core has also been designed to facilitate the π–π stacking between the core and drug as most of the poorly soluble drugs contain aromatic rings which can participate in the π stacking and thereby, improve the stability of polymeric micelles with enhanced drug loading and sustained release. Kataoka et al. have shown that dimers of DOX and DOX interacted with benzyl residue of poly (ethylene glycol)-poly(beta-benzyl-L-aspartate) block copolymer through π–π stacking. π–π stacking between the anthracycline moiety of DOX and benzyl side groups of polymer segment improved drug loading, exhibited sustained release and stabilized the micelles upon dilution [112]. Several other groups also have attributed improved drug loading and self-assembly of DOX to the presence of aromatic group in the hydrophobic block of polymers [113,114]. PTX loading of 34% has been reported with aromatic modification of N-(2 hydroxypropyl) methacrlyamide (HPMA) based micelles. Conjugation of benzoyl or naptholyl derivative to HPMA allowed π–π interaction with PTX which was confirmed by 1H solid-state NMR spectroscopy. π–π interaction between PTX and the aromatic core improved the drug retention within the core as evident from slow release [115].
π–π interaction also plays a critical role in enhancing the solubility of drugs by hydrotrophy. Hydrotropic molecules are believed to undergo aggregation by a stacking mechanism of the planar aromatic ring present in their chemical structures. Lee et al. designed amphiphillic polymers grafted with hydrotropic agents to the hydrophobic block of the copolymer. High local concentration of hydrotropic agents within the core allowed π–π stacking and solubilization of poorly soluble drugs within the core by hydrotrophy. Structural–activity relationship of 60 hydrotropic agents confirmed the presence of pyridine and benzene rings within the hydrotropes is essential for solubilization of drugs and thereby confirmed the role of π–π interaction in polymer hydrotropes [116]. Moreover, the presence of aromatic rings within the drug is also essential for solubility enhancement by hydrotropes which further confirmed the π–π stacking as the potential mechanism of the solubilization [117]. These studies reflect that core of the polymer can be modified to amplify the π–π interaction between the drug and the core for enhanced dynamic stability of nanoparticles, increased drug loading and sustained release.
Hydrogen bond & ionic interaction
Core of the micelles has been manipulated to improve the drug loading of weakly acidic and basic drugs. Micelle core containing carboxylic acid has been extensively reported for encapsulation of anticancer drugs DOX and cisplatin [95,100,118–120]. Ionic interaction between amino group of DOX (pKa = 8.25) and polymer with carboxylic group resulted in significantly high drug loading and pH-dependent DOX release. Strong ionic interaction between cationic drug and polymer is an enthalipically driven process and the encapsulated drug exhibited stoichiometry proportion of 1:1 to molar concentration of carboxyl group of polymer. Loading of polymer-bound drug by ionic interaction is further enhanced by stacking interactions among the drug molecule [121]. Similarly, Borsali et al. reported high loading capacity and pH-dependent drug release for weakly acidic drugs containing carboxylic functional groups. Core of PEG-poly acrylates was modified with the amino groups to design micelles with weakly basic core. Micelles with weakly basic core resulted in high drug loading of 50% w/w, which reversed upon esterification of carboxylic groups of these drugs. Role of the acid–base interaction was further confirmed by poor loading capacities with nonionic polyester block copolymers [122]. These studies indicate that weakly acidic and basic drugs can be effectively encapsulated at high loading with the modified core of the micelles.
Majority of drugs contain carbonyl groups, hydroxyl groups and amine group which could act as hydrogen-bonding sites. Polymers have been designed for hydrogen bond interaction between the micelle core and hydrogen-bonding sites of the drugs. Hamaguchi et al. reported enhanced drug loading and stable micelles of PTX by modification of the polyaspartate block by 4-phenol-1-butanol. Hydrogen bond interaction between 4-phenol-1-butanol and PTX significantly improved the micelles stability followed by sustained release. This enhanced stability improved the tumor accumulation by 25-fold as compared with free PTX and significantly reduced the associated neurotoxicity further improving the therapeutic profile of PTX [123]. Core of the poly-glutamate was grafted with aliphatic, aromatic hydrocarbons and polar side chains to evaluate the effect of hydrogen bond on micellar formulation of PTX. Although blank micelles containing polar groups were thermodynamically less stable, they showed enhanced drug loading, kinetic stability and sustained release of PTX. This enhanced stabilization is due to the additional stabilization by hydrogen bonding interaction between the polar groups grafted micelle core and the encapsulated PTX [124]. Hydrogen bond interaction has also been utilized to improve the kinetic stability of micelles. Modification of the core of micelles with the urea which is known to associate through bifurcated hydrogen bonds has been reported [125]. These urea-containing polymers stabilized micelles by hydrogen bonding Interactions leading to greater kinetic stability with narrow size distribution and high cargo loading capacity. These studies underline the significance of hydrogen bond and ionic interactions in formulation of kinetically stable nanoparticles with high drug loading capacity and sustained release.
Polymer–drug conjugate
Functional groups of certain drugs and their prodrugs allow covalent conjugation to the polymers for their formulation into nanoparticles. Molecular weight, conjugation chemistry and biodegradability are the key properties which affect the biodistribution and efficacy of polymer–drug conjugate [126,127]. For efficient plasma circulation and tumor accumulation, molecular weight of the polymer–drug conjugate should be ideally higher than 40 kDa as renal threshold for polymer elimination is approximately 30–40 kDa [128]. However, application of high molecular weight polymer may be limited by nondegradable nature of certain polymers and a balanced approach is required between renal elimination and enhanced tumor accumulation by EPR effect. Stability of polymer–drug conjugate in the circulation depends upon the covalent chemistry as well as molecular architecture of polymer–drug conjugate [128]. Ester, amide, hydrazone and enzyme-sensitive linkers have been reported for conjugation of numerous drugs. This different chemistry has been utilized to synthesize polymer–drug conjugate which are stable in plasma but would release the drug at the tumor site at a desired rate. In general, ester bonds undergo rapid hydrolysis contributing to poor stability in plasma whereas poor hydrolysis of amide bond restricts the release of drug at the tumor site. To resolve this, polymer drug linkers have been designed for intracellular or tumor-specific release of the drug. Among the linkers, tetrapeptide sequence Gly–Phe–Leu–Gly (GFLG) for cleavage by lysosomal proteases (especially cathepsin B) has been widely studied for intracellular drug release [129,130]. However, intracellular drug release from these conjugates is limited by poor cellular uptake of these hydrophilic polymer–drug conjugate which is reflected by higher in vitro IC50 of these conjugates [131,132]. An ideal polymer–drug conjugate should be biodegradable, nonimmunogenic, stable in plasma and exhibit rapid cell uptake followed by intracellular enzymatic hydrolysis or extracellular release at desired rate. In the following section, we would review the different polymers which have been extensively studied for polymer–drug conjugate.
PEG–drug conjugate
PEGylation of drugs and proteins is well established and has been extensively used in clinically approved products. Excellent biocompatibility and end group chemistry allows efficient conjugation of a drug to PEG (Figure 3). PEG with COOH, NH2, OH or SH reactive functional groups has been synthesized to conjugate drugs and proteins. Covalent conjugation of PTX, methotrexate (MTX), cisplatin, gemicitabine and camptothecin leading to the formation of ester, amide or disulphide bond has been reported. Enzon has reported the water-soluble PEG-PTX conjugate of 40 kDa with 4% loading and equivalent in vivo toxicity [133]. Renal threshold for clearance of PEG is approximately 20 kDa and the circulation half-life of PEG dramatically increases from 18 min to 16.5 h as the molecular weight is increased from 6 to 50 kDa [134]. However, the utilization of high molecular weight PEG is limited by its nondegradable nature. To overcome this potential challenge, PEG with heterobifunctional reactive groups has been synthesized to attach PEG to nanoparticles [135,136]. Polyester units of PEG copolymer increase the molecular weight of PEG–drug conjugate and are also biodegradable in nature. Unlike PEG–drug conjugate, PEG polyester copolymer–drug conjugate are generally amphiphillic and form nanoparticles.
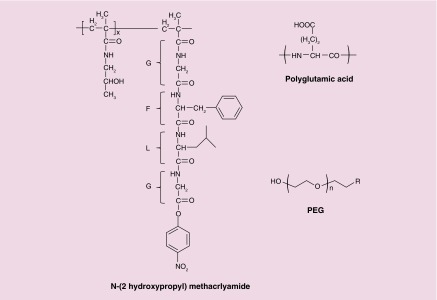
Figure 3. . Chemical structure of polymeric backbone used for formulation of polymer–drug conjugate-based nanoparticles.
Polyethylene glycol and N-(2 hydroxypropyl) methacrlyamide are nonbiodegradable. Polyglutamic acid contains degradable amide bond.
There are no reported studies to characterize and quantify kinetics of the cellular uptake of PEG–drug conjugate. Krtaz et al. have reported the synthesis of PEG–MTX conjugate of molecular weight ranging from 750 to 40,000 Da. All the conjugates exhibited 60–200-fold increase in IC50 values despite the similar inhibitory action on dihydrofolate reductase enzyme in cell free system [137]. This increase in IC50 value can be attributed to either inefficient cellular uptake or slow hydrolysis of free drug in the cytosol. Hydrolysis of drug as rate-limiting step can be ruled out as even rapid release of DOX from PEG–DOX conjugates with GFLG linker exhibited much higher IC50 as compared with free drug. These studies confirmed that cellular uptake rather than drug release from PEG–drug conjugate results in higher IC50 values of PEG–drug conjugate [138]. Veronese et al. studied poly(ethylene glycol) PEG-DOX conjugates of linear or branched architecture of different molecular weight and with different peptidyl linkers (GFLG, GLFG, GLG, GGRR and RGLG). Rapid hydrolysis of GFLG linker by lysosomal enzymes in vitro resulted in approximately 57% DOX release at 5 h, as compared with the other linkers (<16% release at 5 h). Highest molecular weight PEG had the longest plasma residence time and consequently the greatest tumor targeting with significantly lower anthracycline levels in heart which highlights the favorable biodistribution by PEG–drug conjugate [138]. PEG–drug conjugate has been preclinically tested for numerous anticancer drugs but challenges like limited capacity for drug conjugation, poor cellular uptake and nondegradable nature restricted further clinical development of PEG–drug conjugate.
HPMA drug conjugate
Application of copolymers of HPMA as drug conjugate was pioneered by Kopecek and coworkers [139]. Mainly, HPMA copolymer intermediates for conjugation are synthesized by free radical polymerization using HPMA and methacryloylated (MA)-peptidyl-nitrophenylester as comonomers (Figure 3). Facile synthesis of HPMA copolymer allowed incorporation of different functionalities with precise control of molecular weight and composition. HPMA polymer backbone is nondegradable limiting the molecular weight of polymer–drug conjugate below 40,000 kDa to ensure eventual renal elimination. HPMA copolymer-GFLG-DOX conjugate has been extensively studied clinically. HPMA-DOX conjugate demonstrated enhanced blood levels of DOX with four- to fivefold increase in the maximum tolerated dose [140]. By contrast, HPMA conjugates of PTX and camptothecin did not show significant improvement from parent molecules. This was attributed to poor plasma stability of ester conjugation of drugs to polymeric backbone which highlights the importance of optimization of conjugation chemistry [141,142]. Etrych et al. reported synthesis of HPMA-PTX conjugate through pH-sensitive hydrazone bond [143]. HPMA-PTX conjugate through hydrazone bond showed enhanced plasma stability with drug release at mild acidic conditions (pH = 5). However, further clinical translation of HPMA–drug conjugate is limited by rapid renal elimination with 50–75% elimination over 24 h [140]. To use high molecular weight HPMA–drug conjugate, Yang et al. reported synthesis of high molecular weight HPMA–drug conjugate which contained enzymatically degradable bonds in the polymer backbone. This high molecular weight multiblock HPMA copolymer with enzymatically degradable linker between HPMA blocks with molecular weight distributions below the renal threshold were reported for delivery of gemcitabine [144]. Increase in molecular weight of the second-generation enzyme degradable HPMA–drug conjugates resulted in distinct advantages as improved pharmacokinetics (three- to five-times half-life compared with the first generation), and dramatically enhanced tumor inhibition [145]. Future clinical translation of HPMA–drug conjugate would depend upon synthesis of second-generation long-circulating HPMA–drug conjugates and selective release of drug at the tumor site.
Polyglutamate–drug conjugate
Polyglutamate (PG) is composed of degradable amide bond unlike nondegradable carbon-carbon backbone of PEG and HPMA. PG is generated from hydrolytic removal of benzyl group of poly (γ- benzyl -L- glutamate) and carboxyl group of pendant-free PG is utilized for drug conjugation (Figure 3). PG–drug conjugates are water soluble and acquire α helix or random coil structure depending upon the pH of the surrounding media. PG is stable in plasma and long circulation time allows accumulation at tumors. PG like other macromolecules is extensively secreted by kidney and follows the renal threshold cut off for water-soluble polymers [146]. PG is not recognized by RES system and does not require PEG for stealth effect. However, high molecular weight PG has been shown to activate the fibrinolytic system and week immunogenic response depending when the PG–drug conjugates are administered [147]. Li et al. have done extensive work on PG–drug conjugates and extensively characterized PG–drug conjugate of numerous drugs [148].
PG–anthracycline conjugates have been reported using ester, amide, peptide and hydrazone linkers [149–151]. All of the PG conjugates have higher in vitro cytotoxicity compared with the free drug. This is attributed to the slow release of the free drug from PG–DOX conjugate. Preclinical data have shown that the conjugate with oligopeptide spacer were active, whereas conjugates without degradable spacers were completely inactive. Increase in the rate of DOX release with increase in the length of spacer improved in vitro and in vivo anticancer effect of PG–DOX conjugate [152]. Water-soluble PG–PTX conjugate was also synthesized through ester linkage of 2′ hydroxyl group of PTX. PG–PTX conjugate exhibited remarkable high loading of 37% and stability in plasma due to molecular architecture. Cellular uptake of radiolabeled PTX and PG backbone suggested that PTX is hydrolyzed extracellularly which was taken up by cells [131]. PG-PTX exhibited excellent efficacy in different cancer models as PG-PTX compared with free PTX exhibited enhanced circulation half-life and released the drug in the vicinity of tumor [153]. PG-PTX has shown excellent safety with equipotent efficacy in variety of cancer in Phase III trials and highlights the future potential of PG–drug conjugate-based nanoparticles.
Nanoparticles for PDT & detection
PDT is an exciting detection and treatment modality for melanoma. PDT is based on the excitation of photosensitizer at a specific wavelength of light after preferential tumor accumulation of photosensitizer. Nanoparticles for PDT can be designed for metastasis detection, destruction of bulk tumor and CSCs, for photoimmunothrepay and for improving drug delivery at both tumor and cellular level. Galanzha et al. reported a novel diagnostic and therapeutic platform with gold carbon nanotubes. Noninvasive in vivo detection and treatment of metastatic melanoma at single cell level was achieved after administration of gold carbon nanotubes by using a combination of PA imaging and photothermal (PT) therapy [14]. Nanoparticles labeled with CSC markers allowed detection and ablation of CSCs by multifunctional PAFC/PTFC nanoparticle-based platform. CD44 labeled gold carbon nanotubes enabled ultrasensitive detection of CSCs in vivo [154]. PT with antibody-labeled nanoparticles selectively targeted and eradicated CSCs [155,156]. These studies highlight the immense potential of nanotechnology-based PDT in detection and treatment of CTC and CSCs.
Destruction of primary tumor lesion with modulation of immunity against the tumor antigen is potential therapeutic option against metastatic melanoma [157,158]. Photoimmunotherapy, combination of PDT and immunomodulation using adjuvant has demonstrated significant benefits in late-stage metastatic melanoma [159,160]. PDT with indocyanine green (ICG) followed by local application of immunostimulant imiquimod resulted in beneficial systemic response against metastatic melanoma [159,160]. To further improve tumor selective delivery of both immunostimulant and photodynamic agents, nanoparticles have been formulated [161]. Bear et al. demonstrated PDT with gold nanoparticles in combination with adoptive T-cell transfer prevented primary tumor recurrence postablation, inhibited tumor growth at distant sites and abrogated the outgrowth of lung metastases [162]. Also, nanoparticles codelivering photodynamic agent and immunostimulant exhibited excellent anticancer effect against primary treated and distant untreated tumors. Chitosan-coating around hollow CuS nanoparticles allowed incorporation of the immunoadjuvants containing the cytosine-guanine (CpG) motifs. NIR irradiation triggered disintegration of CuS nanoparticles, allowing the complexation of chitosan and CpG motifs. Nanocomplexation of CpG motifs enhanced their tumor retention and uptake by plasmacytoid dendritic cells [161].
High tumor interstitial pressure and inefficient EPR effect can restrict the drug delivery by nanoparticles. PDT has been shown to improve the nanoparticle-mediated drug delivery by reducing interstitial pressure and enhancing tumor vessel permeability. PDT resulted in ˜2.5-fold higher tumor uptake at 3 h after administration of liposomal DOX by enlarging the endothelial gap of tumor vessels [163]. PDT selectively enhances tumor accumulation of nanoparticle-mediated chemotherapy as intratumoral accumulation of free DOX after PDT did not change. Tumor vessel targeted delivery of photosensitizer was achieved by arginine–glycine–aspartic acid (RGD)-modified ferritin. Ferritin encapsulating photosensitizer hexadecafluoro zinc phthalocyanine located to the endothelium of neoplastic vessels via RGD–integrin interactions. Photoradiation of tumor vessel located photosensitizer increased the vascular permeability of albumin, quantum dots and iron oxide nanoparticles by as much as 20-fold with no adverse effects to normal tissues. Enhanced tumor permeability by PDT improved the therapeutic efficacy of liposomal DOX by 75.3% highlighting the potential of PDT in improving the delivery of chemotherapeutics by nanoparticles [164].
Photochemical internilzation (PCI) was established at Norwegian Radium Hospital for cytosolic delivery of therapeutic agents entrapped in endocytic vesicles [165]. PCI has been extensively studied for cytosolic delivery of toxins, oligonucleotides, weakly basic drugs like DOX and cisplatin and can be therapeutically exploited for overcoming resistance by efflux transporters as well as intracellular sequesterization of drugs [166–168]. Light-mediated activation of endocytosed amphiphillic photosensitizer generates reactive oxygen species which damage membrane of endocytic vesicles and thereby lead to cytosolic delivery of therapeutic agent. Nanoparticles encapsulating phthalocyanine upon radiation exhibited PCI properties and facilitated DOX release from the endo-lysosomes to nuclei. This increase in intracellular concentration of DOX resulted in significant reduction in tumor volume [169]. Nanoparticles of weakly basic drugs, oligonucleotides and toxin are excellent candidates for combination therapy with PCI as they accumulate in endolysosomes compartment after cellular uptake [170,171]. PCI-mediated cytosolic delivery of immunotoxins saporin and gelonin have been studied in a melanoma xenograft model [167,172]. PCI improved the anticancer response effect of immunotoxins and complete regression was observed in 33% of tumor-bearing mice [167]. All these studies highlight the multifold potential of PDT and can be combined with chemo and immunotherapy for metastasis detection, destruction of bulk tumor and CSCs and for improving drug delivery in melanoma.
Active targeting of nanoparticles to melanoma
Passive targeting by EPR effect of nanoparticles improves tumor accumulation but does not enhance the cellular uptake and depends upon the convective transport of nanoparticles against high interstitial pressure [173]. To further improve the therapeutic efficacy of nanoparticles, interaction between receptor and ligands has been exploited for active targeting of nanoparticles [174]. Peptides, antibodies, small molecules and aptamers have been extensively studied to accomplish active targeting of nanoparticles [175]. Nanoparticles for active targeting against tumor vasculature, stromal microenvironments and cell surface receptors have been investigated in preclinical models of melanoma.
Endothelial cells of tumor neovasculature overexpress αvβ3 integrin receptor in comparison to low or negligible expression by normal vasculature [176]. αvβ3 integrin receptor not only promotes angiogenesis but also supports melanoma growth, adhesive, invasive and migratory properties of the melanoma tumor cells [177]. Tripeptide motif RGD, cell adhesion peptide sequence in ECM proteins and its derivatives have been extensively studied for design of αvβ3 targeted nanoparticles [178]. Benzera et al. reported significant increase in tumor-to-blood residence time ratios, and tumor-selective accumulation with cyclic RGD peptide ligands modified silica nanoparticles in melanoma xenograft mouse model [179]. Surface modification of DOX-loaded nanoparticles with DI17E6, a humanized monoclonal antibody against αv-integrins enhanced the integrin receptor specific cellular uptake in melanoma M21 cell line. Targeted nanoparticle reduced the integrin-mediated attachment of cells to ECM proteins with enhanced cell cytotoxicity. Enhanced alpha integrin mediated uptake of DOX-loaded nanoparticles significantly reduced the IC50 from 55 to 8 nm in integrin-positive melanoma cell line [180].
Progression of melanoma is associated with stromal changes contributing to uncontrolled growth and invasive behavior of melanocytes. These characteristic stromal changes have been exploited to target nanoparticles to tumor stroma. Secreted protein acidic and rich in cysteine (SPARC) a nonstructural matricellular protein has been well studied in the tumorigenicity and progression of melanoma [181–183]. Peptide sequence with high affinity and specificity for SPARC protein has been identified by phage display. SPARC targeting peptide modified nanoparticles exhibited high affinity with negligible binding in negative cell lines. Targeting of nanoparticles to SPARC did not alter their blood clearance but there was a 45-fold increase in tumor accumulation of SPARC-targeted nanoparticles as compared with the control group. Moreover, SPARC-labeled proteins allowed detection of metastatic growth in addition to primary tumors [184]. Albumin also exhibits SPARC-binding properties and enhances the tumor accumulation of albumin nanoparticles [185–187]. Albumin-bound PTX nanoparticles (nabPTX) exhibited enhanced response in SPARC overexpressing xenograft in comparison to wild-type PC3 tumor xenograft [186]. However, these results are largely correlative with a small sample size. Also, there are studies which demonstrated SPARC expression independent efficacy with nabPTX [188–190]. Tumor stroma also contains a mesh network of fibrin and fibronectin. Pentapetide CREKA has been reported to bind to this mesh-like network and further enhances the formation of clot-like mesh at the target site. CREKA labeled nanoparticles and liposomes showed enhanced accumulation in tumor vessels with induction of additional localized clotting and thereby producing new binding sites for more particles. This clotting-based amplification greatly enhanced tumor imaging with envision of the design of drug-carrying self-targeting nanoparticles [191].
Cancer cells overexpress receptors for cellular uptake of essential nutrients to meet their high proliferation rate. Transferrin receptor, a type II transmembrane glycoprotein is overexpressed by cancer cells to meet growing demands of iron [192]. Targeting overexpression of transferrin receptor with its ligand, transferrin has been extensively studied for targeted delivery of nanoparticles [192]. Davis et al. reported the first-in-human clinical trial with transferrin-targeted nanoparticles [193]. Targeted nanoparticles exhibited dose-dependent localization within transferrin-positive melanoma tissue with little localization in the adjacent epidermis. Intracellular delivery of nanoparticles was observed only in the transferrin-targeted nanoparticles which highlights the need of targeted nanoparticles for intracellular delivery [194,195]. Altered glycosylation patterns are a hallmark of the malignant growth. Proliferation of cancer cells is associated with enhanced sialylation of N-linked glycans and this altered glycosylation enabled the design of sialic acid targeted nanoparticles [196,197]. Phenylboronic acid (PBA) reversibly binds with 1,2- or 1.3-diols, which are major constituent of glycan. PBA–sugar complexes are unstable at plasma pH, however PBA forms extremely stable complex with sialic acid at physiological pH [198]. This tumor sialic acid recognition at physiological pH with controlled pKa of PBA, have been studied as a molecular basis for the design of targeted nanoparticles. Deshayes et al. reported the design of PBA-modified nanoparticles for enhanced delivery to melanoma [199]. PBA-targeted nanoparticles exhibited higher tumor accumulation level indicating interaction of PBA-targeted nanoparticles with the sialic acid moieties on the surface of cancer cells improved the retention of nanoparticles at the tumor site. PBA-targeted nanoparticles demonstrated improved efficacy as compared with nontargeted nanoparticles correlating with their enhanced accumulation in tumors.
Progression of melanoma is associated with expression of high levels of the melanocortin type-1 receptor (MC1R) making it one of the melanoma-specific targets for highly sensitive detection and therapy of metastatic melanoma [200]. α-MSH, a tridecapeptide and its derivatives have been investigated for melanoma-specific targeting [201]. Conjugation of MSH derivative as targeting moiety significantly reduced the tumor burden upon PDT with gold nanoparticles. Receptor-mediated uptake of targeted nanoparticles resulting into the enhanced tumor nanoparticle levels caused significantly greater necrotic response than nontargeted nanoparticles. Targeting improved the nanoparticle distribution within the tumor matrix as suggested by their presence at more than 200 μm away from the nearest blood vessels whereas nontargeted were scattered only adjacent to tumor vasculature. Enhanced retention and uptake was confirmed by colocalization of targeted nanoparticles and MC1R [202]. Similarly, targeting MC1R raised the sensitivity and specificity of PA imaging with gold nanocages by 300%. Interaction between MC1R and targeting ligand enhanced nanoparticle per tumor mass by 360% highlighting that targeted systems can detect early-stage melanomas metastatic lymph nodes, and can be potentially used to treat the melanomas [203]. Besides MC1R, chondroitin sulphate proteoglycan 4 (CSPG4) is a highly specific marker of the nevomelanocyte lineage and has been utilized for targeting melanoma. Ep1 monoclonal antibody to the human melanoma-specific antigen CSPG4 targeted nanoparticles showed a 25-fold preference for melanoma as compared with nontarget cells. Selectivity for melanoma was also confirmed in xenograft models where targeted nanoparticles significantly inhibited growth of melanoma xenograft with little effect in breast carcinoma xenograft [204]. These all studies highlight the potential of active targeting to improve the outcome of nanoparticle-based therapy. However, tumor heterogeneity and translation of preclinical xenograft data to human tumor are the major roadblocks.
Conclusion
Numerous anticancer drugs have been discovered through advancement in the molecular biology and drug discovery. All these anticancer drugs exhibit excellent efficacy in petri dishes but fail during translation to clinic. BRAF and MEK inhibitors show remarkable response in initial therapy but resistance develops invariably. Moreover, nonspecific distribution of BRAF inhibitors leads to malignant and benign growth including squamous cell carcinomas and other severe dermatological side effects [205,206]. Combination therapy to overcome the potential resistance mechanism is the way forward for preventing the relapse. However, unfavorable physicochemical properties and pharmacokinetic profiles limit the clinical application of conventional chemotherapeutics as combination therapy. Nanoparticle-based formulation approach to minimize peripheral exposure and to maximize tumor accumulation can be exploited to overcome these potential roadblocks. Significant progress has been made since the idea of polymer-based delivery was first conceived [207]. Active targeting strategies using stimuli sensitive and receptor-targeted nanoparticles have been devised to further augment the application of nanoparticle-based delivery of chemotherapeutics. Despite of all these efforts, there are limited numbers of approved nanoparticle-based products.
Future perspective
Clinical translation of nanoparticulate drug delivery systems is limited by their kinetic instablility, rapid uptake by the RES and failure to scale-up at commercial setting. A concerted effort between drug discovery and formulation scientists will help in selecting lead drug candidates which can be effectively loaded into the nanoparticles with sustained drug release. Additional data on their biodistribution and pharmacokinetic profiles in larger animal models are needed to fully understand the pros and cons of nanoparticle therapeutics. Canine and swine with similar anatomic and physiological characteristics to human should be used to study the nanoparticle-based drug delivery in metastatic melanoma [208,209]. Additionally, successful scale-up of nanoparticle formulation with batch-to-batch content uniformity, absence of residual organic solvent and surfactants will ensure their quick approval from regulatory agencies. Thus, the priority is to scale-up the existing nanoparticle-based formulations to establish their safety and pharmacokinetic profiles in human subjects. Finally, combination therapy of chemotherapeutics, photodynamic agents, antigens and immunoadjuvants will be a potential avenue for the treatment and detection of melanoma.
Executive summary.
Metastatic melanoma is highly aggressive skin cancer with 5-year survival rate of around of 10%.
Resistance develops invariably to current treatment options and tubulin-binding agents promise to be potential chemotherapeutic agent in combination therapy for treatment of metastatic melanoma.
Tumor initiating cells, altered expression of class III β-tubulin and inefficient intracellular accumulation of drugs often lead to resistance and relapse of metastatic melanoma.
To maximize tumor distribution through enhanced permeability and retention effect and reduce dose-limiting toxicity, micelles, nanoparticles and polymer–drug conjugates have been designed.
Nanoparticles designed to enhance noncovalent interactions like hydrophobic interaction, π–π stacking, hydrogen and ionic interaction between micelle core and drug molecules improved kinetic stability, high drug loading and sustained release of drugs.
Molecular weight, conjugation chemistry and biodegradability are the key properties which affect the biodistribution and efficacy of polymer–drug conjugate.
Nanoparticle-based photodynamic therapy can be designed for metastasis detection, destruction of bulk tumor and cancer stem cells, for photoimmunotherapy and for improving drug delivery at both tumor and cellular level.
Interaction between receptor and ligands has been exploited to enhance the endocytosis-mediated cellular uptake of nanoparticles and increase the intracellular concentration of drugs.
Footnotes
Financial & competing interests disclosure
This work is supported by NIH/NCI grant R01CA148706 to W Li. The authors also duly acknowledge the Buffett Cancer Center at the University of Nebraska Medical Center for financial support. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erdei E, Torres SM. A new understanding in the epidemiology of melanoma. Expert Rev. Anticancer Ther. 2010;10(11):1811–1823. doi: 10.1586/era.10.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508(7494):118–122. doi: 10.1038/nature13121. [DOI] [PubMed] [Google Scholar]
- 4.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23(6):488–496. [PMC free article] [PubMed] [Google Scholar]
- 6.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445(7130):843–850. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- 7.Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008;84(3):539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halaban R, Langdon R, Birchall N, et al. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J. Cell Biol. 1988;107(4):1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alanko T, Rosenberg M, Saksela O. FGF expression allows nevus cells to survive in three-dimensional collagen gel under conditions that induce apoptosis in normal human melanocytes. J. Invest. Dermatol. 1999;113(1):111–116. doi: 10.1046/j.1523-1747.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 10.Marconi A, Terracina M, Fila C, et al. Expression and function of neurotrophins and their receptors in cultured human keratinocytes. J. Invest. Dermatol. 2003;121(6):1515–1521. doi: 10.1111/j.1523-1747.2003.12624.x. [DOI] [PubMed] [Google Scholar]
- 11.Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J. Investig. Dermatol. Symp. Proc. 2005;10(2):153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukunaga-Kalabis M, Santiago-Walker A, Herlyn M. Matricellular proteins produced by melanocytes and melanomas: in search for functions. Cancer Microenviron. 2008;1(1):93–102. doi: 10.1007/s12307-008-0009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoja L, Lorigan P, Dive C, Keilholz U, Fusi A. Circulating tumour cells as tumour biomarkers in melanoma: detection methods and clinical relevance. Ann. Oncol. 2015;26(1):33–39. doi: 10.1093/annonc/mdu207. [DOI] [PubMed] [Google Scholar]
- 14.Galanzha EI, Zharov VP. Circulating tumor cell detection and capture by photoacoustic flow cytometry in vivo and ex vivo . Cancers (Basel) 2013;5(4):1691–1738. doi: 10.3390/cancers5041691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanzha EI, Shashkov EV, Spring PM, Suen JY, Zharov VP. In vivo noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 2009;69(20):7926–7934. doi: 10.1158/0008-5472.CAN-08-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel RS. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc. Natl Acad. Sci. USA. 1991;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim E, Rebecca V, Fedorenko IV, et al. Senescent fibroblasts in melanoma initiation and progression: an integrated theoretical, experimental, and clinical approach. Cancer Res. 2013;73(23):6874–6885. doi: 10.1158/0008-5472.CAN-13-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl Acad. Sci. USA. 2009;106(49):20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waster P, Rosdahl I, Gilmore BF, Seifert O. Ultraviolet exposure of melanoma cells induces fibroblast activation protein-alpha in fibroblasts: implications for melanoma invasion. Int. J. Oncol. 2011;39(1):193–202. doi: 10.3892/ijo.2011.1002. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Satyamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seeds meet soil. Oncogene. 2003;22(20):3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- 21.Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62(19):5476–5484. [PubMed] [Google Scholar]
- 22.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat. Rev. Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 23.Curti BD, Urba WJ, Alvord WG, et al. Interstitial pressure of subcutaneous nodules in melanoma and lymphoma patients: changes during treatment. Cancer Res. 1993;53(10 Suppl.):2204–2207. [PubMed] [Google Scholar]
- 24.Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 25.Curnis F, Sacchi A, Corti A. Improving chemotherapeutic drug penetration in tumors by vascular targeting and barrier alteration. J. Clin. Invest. 2002;110(4):475–482. doi: 10.1172/JCI15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschetta M, Pretto F, Berndt A, et al. Paclitaxel enhances therapeutic efficacy of the F8-IL2 immunocytokine to EDA-fibronectin-positive metastatic human melanoma xenografts. Cancer Res. 2012;72(7):1814–1824. doi: 10.1158/0008-5472.CAN-11-1919. [DOI] [PubMed] [Google Scholar]
- 27.Penson RT, Oliva E, Skates SJ, et al. Expression of multidrug resistance-1 protein inversely correlates with paclitaxel response and survival in ovarian cancer patients: a study in serial samples. Gynecol. Oncol. 2004;93(1):98–106. doi: 10.1016/j.ygyno.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 28.Kim YH, Ishii G, Goto K, et al. Expression of breast cancer resistance protein is associated with a poor clinical outcome in patients with small-cell lung cancer. Lung Cancer. 2009;65(1):105–111. doi: 10.1016/j.lungcan.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Mignogna C, Staibano S, Altieri V, et al. Prognostic significance of multidrug-resistance protein (MDR-1) in renal clear cell carcinomas: a five year follow-up analysis. BMC Cancer. 2006;6:293. doi: 10.1186/1471-2407-6-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pratt S, Shepard RL, Kandasamy RA, Johnston PA, Perry W, 3rd, Dantzig AH. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol. Cancer Ther. 2005;4(5):855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 31.Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. Int. J. Breast Cancer. 2011;2011:967419. doi: 10.4061/2011/967419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ichihashi N, Kitajima Y. Chemotherapy induces or increases expression of multidrug resistance-associated protein in malignant melanoma cells. Br. J. Dermatol. 2001;144(4):745–750. doi: 10.1046/j.1365-2133.2001.04129.x. [DOI] [PubMed] [Google Scholar]
- 33.Colone M, Calcabrini A, Toccacieli L, et al. The multidrug transporter P-glycoprotein: a mediator of melanoma invasion? J. Invest. Dermatol. 2008;128(4):957–971. doi: 10.1038/sj.jid.5701082. [DOI] [PubMed] [Google Scholar]
- 34.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin. Cancer Res. 2012;18(7):1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triller N, Korosec P, Kern I, Kosnik M, Debeljak A. Multidrug resistance in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54(2):235–240. doi: 10.1016/j.lungcan.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 36.Calatozzolo C, Gelati M, Ciusani E, et al. Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GST-pi in human glioma. J. Neurooncol. 2005;74(2):113–121. doi: 10.1007/s11060-004-6152-7. [DOI] [PubMed] [Google Scholar]
- 37.Baker EK, Johnstone RW, Zalcberg JR, El-Osta A. Epigenetic changes to the MDR1 locus in response to chemotherapeutic drugs. Oncogene. 2005;24(54):8061–8075. doi: 10.1038/sj.onc.1208955. [DOI] [PubMed] [Google Scholar]
- 38.Berg SL, Tolcher A, O'Shaughnessy JA, et al. Effect of R-verapamil on the pharmacokinetics of paclitaxel in women with breast cancer. J. Clin. Oncol. 1995;13(8):2039–2042. doi: 10.1200/JCO.1995.13.8.2039. [DOI] [PubMed] [Google Scholar]
- 39.Advani R, Fisher GA, Lum BL, et al. A Phase I trial of doxorubicin, paclitaxel, and valspodar (PSC 833), a modulator of multidrug resistance. Clin. Cancer Res. 2001;7(5):1221–1229. [PubMed] [Google Scholar]
- 40.Kelly RJ, Draper D, Chen CC, et al. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin. Cancer Res. 2011;17(3):569–580. doi: 10.1158/1078-0432.CCR-10-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo Y, Ellis LZ, Dallaglio K, et al. Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance. J. Invest. Dermatol. 2012;132(10):2440–2450. doi: 10.1038/jid.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wouters J, Stas M, Gremeaux L, et al. The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLoS ONE. 2013;8(10):e76550. doi: 10.1371/journal.pone.0076550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen KG, Szakacs G, Annereau JP, et al. Principal expression of two mRNA isoforms (ABCB 5alpha and ABCB 5beta) of the ATP-binding cassette transporter gene ABCB 5 in melanoma cells and melanocytes. Pigment Cell Res. 2005;18(2):102–112. doi: 10.1111/j.1600-0749.2005.00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 45.Lee N, Barthel SR, Schatton T. Melanoma stem cells and metastasis: mimicking hematopoietic cell trafficking? Lab. Invest. 2014;94(1):13–30. doi: 10.1038/labinvest.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Civenni G, Walter A, Kobert N, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71(8):3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 47.Reid AL, Millward M, Pearce R, et al. Markers of circulating tumour cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br. J. Dermatol. 2013;168(1):85–92. doi: 10.1111/bjd.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chartrain M, Riond J, Stennevin A, et al. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS ONE. 2012;7(5):e36762. doi: 10.1371/journal.pone.0036762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl Acad. Sci. USA. 2011;108(6):2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinc A, Somasundaram R, Wagner C, et al. Targeting CD20 in melanoma patients at high risk of disease recurrence. Mol. Ther. 2012;20(5):1056–1062. doi: 10.1038/mt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monzo M, Rosell R, Sanchez JJ, et al. Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J. Clin. Oncol. 1999;17(6):1786–1793. doi: 10.1200/JCO.1999.17.6.1786. [DOI] [PubMed] [Google Scholar]
- 53.Kelley MJ, Li S, Harpole DH. Genetic analysis of the beta-tubulin gene, TUBB, in non-small-cell lung cancer. J. Natl Cancer Inst. 2001;93(24):1886–1888. doi: 10.1093/jnci/93.24.1886. [DOI] [PubMed] [Google Scholar]
- 54.Mesquita B, Veiga I, Pereira D, et al. No significant role for beta tubulin mutations and mismatch repair defects in ovarian cancer resistance to paclitaxel/cisplatin. BMC Cancer. 2005;5:101. doi: 10.1186/1471-2407-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sale S, Sung R, Shen P, et al. Conservation of the class I beta-tubulin gene in human populations and lack of mutations in lung cancers and paclitaxel-resistant ovarian cancers. Mol. Cancer Ther. 2002;1(3):215–225. [PubMed] [Google Scholar]
- 56.Wang Y, Yin S, Blade K, Cooper G, Menick DR, Cabral F. Mutations at leucine 215 of beta-tubulin affect paclitaxel sensitivity by two distinct mechanisms. Biochemistry. 2006;45(1):185–194. doi: 10.1021/bi051207d. [DOI] [PubMed] [Google Scholar]
- 57.Yin S, Cabral F, Veeraraghavan S. Amino acid substitutions at proline 220 of beta-tubulin confer resistance to paclitaxel and colcemid. Mol. Cancer Ther. 2007;6(10):2798–2806. doi: 10.1158/1535-7163.MCT-06-0791. [DOI] [PubMed] [Google Scholar]
- 58.Hari M, Loganzo F, Annable T, et al. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol. Cancer Ther. 2006;5(2):270–278. doi: 10.1158/1535-7163.MCT-05-0190. [DOI] [PubMed] [Google Scholar]
- 59.Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant beta-tubulins that exhibit impaired paclitaxel-driven polymerization. J. Biol. Chem. 1997;272(27):17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- 60.Hari M, Wang Y, Veeraraghavan S, Cabral F. Mutations in alpha- and beta-tubulin that stabilize microtubules and confer resistance to colcemid and vinblastine. Mol. Cancer Ther. 2003;2(7):597–605. [PubMed] [Google Scholar]
- 61.Mozzetti S, Ferlini C, Concolino P, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005;11(1):298–305. [PubMed] [Google Scholar]
- 62.Leskela S, Leandro-Garcia LJ, Mendiola M, et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr. Relat. Cancer. 2010;18(1):85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 63.Ploussard G, Terry S, Maille P, et al. Class III beta-tubulin expression predicts prostate tumor aggressiveness and patient response to docetaxel-based chemotherapy. Cancer Res. 2010;70(22):9253–9264. doi: 10.1158/0008-5472.CAN-10-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roque DM, Bellone S, English DP, et al. Tubulin-beta-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer. 2013;119(14):2582–2592. doi: 10.1002/cncr.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamath K, Wilson L, Cabral F, Jordan MA. BetaIII-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J. Biol. Chem. 2005;280(13):12902–12907. doi: 10.1074/jbc.M414477200. [DOI] [PubMed] [Google Scholar]
- 66.Kavallaris M, Burkhart CA, Horwitz SB. Antisense oligonucleotides to class III beta-tubulin sensitize drug-resistant cells to Taxol. Br. J. Cancer. 1999;80(7):1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stengel C, Newman SP, Leese MP, Potter BV, Reed MJ, Purohit A. Class III beta-tubulin expression and in vitro resistance to microtubule targeting agents. Br. J. Cancer. 2010;102(2):316–324. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levallet G, Bergot E, Antoine M, et al. High TUBB3 expression, an independent prognostic marker in patients with early non-small cell lung cancer treated by preoperative chemotherapy, is regulated by K-Ras signaling pathway. Mol. Cancer Ther. 2012;11(5):1203–1213. doi: 10.1158/1535-7163.MCT-11-0899. [DOI] [PubMed] [Google Scholar]
- 69.Narvi E, Jaakkola K, Winsel S, et al. Altered TUBB3 expression contributes to the epothilone response of mitotic cells. Br. J. Cancer. 2013;108(1):82–90. doi: 10.1038/bjc.2012.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gan PP, Pasquier E, Kavallaris M. Class III beta-tubulin mediates sensitivity to chemotherapeutic drugs in non small cell lung cancer. Cancer Res. 2007;67(19):9356–9363. doi: 10.1158/0008-5472.CAN-07-0509. [DOI] [PubMed] [Google Scholar]
- 71.Akasaka K, Maesawa C, Shibazaki M, et al. Loss of class III beta-tubulin induced by histone deacetylation is associated with chemosensitivity to paclitaxel in malignant melanoma cells. J. Invest. Dermatol. 2009;129(6):1516–1526. doi: 10.1038/jid.2008.406. [DOI] [PubMed] [Google Scholar]
- 72.Locher H, de Rooij KE, de Groot JC, et al. Class III beta-tubulin, a novel biomarker in the human melanocyte lineage. Differentiation. 2013;85(4–5):173–181. doi: 10.1016/j.diff.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Dalziel M, Kolesnichenko M, das Neves RP, Iborra F, Goding C, Furger A. Alpha-MSH regulates intergenic splicing of MC1R and TUBB3 in human melanocytes. Nucleic Acids Res. 2011;39(6):2378–2392. doi: 10.1093/nar/gkq1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mhaidat NM, Thorne RF, de Bock CE, Zhang XD, Hersey P. Melanoma cell sensitivity to Docetaxel-induced apoptosis is determined by class III beta-tubulin levels. FEBS Lett. 2008;582(2):267–272. doi: 10.1016/j.febslet.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Patel KJ, Lee C, Tan Q, Tannock IF. Use of the proton pump inhibitor pantoprazole to modify the distribution and activity of doxorubicin: a potential strategy to improve the therapy of solid tumors. Clin. Cancer Res. 2013;19(24):6766–6776. doi: 10.1158/1078-0432.CCR-13-0128. [DOI] [PubMed] [Google Scholar]
- 76.Gotink KJ, Broxterman HJ, Labots M, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin. Cancer Res. 2011;17(23):7337–7346. doi: 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalayda GV, Wagner CH, Buss I, Reedijk J, Jaehde U. Altered localisation of the copper efflux transporters ATP7A and ATP7B associated with cisplatin resistance in human ovarian carcinoma cells. BMC Cancer. 2008;8 doi: 10.1186/1471-2407-8-175. 175–2407–8–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swietach P, Hulikova A, Patiar S, Vaughan-Jones RD, Harris AL. Importance of intracellular pH in determining the uptake and efficacy of the weakly basic chemotherapeutic drug, doxorubicin. PLoS ONE. 2012;7(4):e35949. doi: 10.1371/journal.pone.0035949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Federici C, Petrucci F, Caimi S, et al. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS ONE. 2014;9(2):e88193. doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fan S, Niu Y, Tan N, et al. LASS2 enhances chemosensitivity of breast cancer by counteracting acidic tumor microenvironment through inhibiting activity of V-ATPase proton pump. Oncogene. 2013;32(13):1682–1690. doi: 10.1038/onc.2012.183. [DOI] [PubMed] [Google Scholar]
- 81.Chapuy B, Koch R, Radunski U, et al. Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia. 2008;22(8):1576–1586. doi: 10.1038/leu.2008.103. [DOI] [PubMed] [Google Scholar]
- 82.Ouar Z, Lacave R, Bens M, Vandewalle A. Mechanisms of altered sequestration and efflux of chemotherapeutic drugs by multidrug-resistant cells. Cell Biol. Toxicol. 1999;15(2):91–100. doi: 10.1023/a:1007521430236. [DOI] [PubMed] [Google Scholar]
- 83.Chen KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell. Melanoma Res. 2009;22(6):740–749. doi: 10.1111/j.1755-148X.2009.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen KG, Valencia JC, Lai B, et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc. Natl Acad. Sci. USA. 2006;103(26):9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie T, Nguyen T, Hupe M, Wei ML. Multidrug resistance decreases with mutations of melanosomal regulatory genes. Cancer Res. 2009;69(3):992–999. doi: 10.1158/0008-5472.CAN-08-0506. [DOI] [PubMed] [Google Scholar]
- 86.Chen KG, Leapman RD, Zhang G, et al. Influence of melanosome dynamics on melanoma drug sensitivity. J. Natl Cancer Inst. 2009;101(18):1259–1271. doi: 10.1093/jnci/djp259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma XH, Piao S, Wang D, et al. Measurements of tumor cell autophagy predict invasiveness, resistance to chemotherapy, and survival in melanoma. Clin. Cancer Res. 2011;17(10):3478–3489. doi: 10.1158/1078-0432.CCR-10-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hertzman Johansson C, Azimi A, Frostvik Stolt M, et al. Association of MITF and other melanosome-related proteins with chemoresistance in melanoma tumors and cell lines. Melanoma Res. 2013;23(5):360–365. doi: 10.1097/CMR.0b013e328362f9cd. [DOI] [PubMed] [Google Scholar]
- 89.O'Brien ME, Wigler N, Inbar M, et al. Reduced cardiotoxicity and comparable efficacy in a Phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann. Oncol. 2004;15(3):440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 90.Rademaker-Lakhai JM, Crul M, Zuur L, et al. Relationship between cisplatin administration and the development of ototoxicity. J. Clin. Oncol. 2006;24(6):918–924. doi: 10.1200/JCO.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 91.Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 92.Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov. Today. 2003;8(6):259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- 93.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 94.Noguchi Y, Wu J, Duncan R, et al. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89(3):307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yokoyama M, Miyauchi M, Yamada N, et al. Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res. 1990;50(6):1693–1700. [PubMed] [Google Scholar]
- 96.Yoo HS, Park TG. Biodegradable polymeric micelles composed of doxorubicin conjugated PLGA-PEG block copolymer. J. Control. Release. 2001;70(1–2):63–70. doi: 10.1016/s0168-3659(00)00340-0. [DOI] [PubMed] [Google Scholar]
- 97.Liu J, Zeng F, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur. J. Pharm. Biopharm. 2007;65(3):309–319. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 98.Ramaswamy M, Zhang X, Burt HM, Wasan KM. Human plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers. J. Pharm. Sci. 1997;86(4):460–464. doi: 10.1021/js960333n. [DOI] [PubMed] [Google Scholar]
- 99.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70(1–2):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 100.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control. Release. 2005;109(1–3):169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 101.Gaucher G, Marchessault RH, Leroux JC. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release. 2010;143(1):2–12. doi: 10.1016/j.jconrel.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 102.Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug Deliv. 2010;7(1):49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 103.Lavasanifar A, Samuel J, Kwon GS. Poly(ethylene oxide)-block-poly(L-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002;54(2):169–190. doi: 10.1016/s0169-409x(02)00015-7. [DOI] [PubMed] [Google Scholar]
- 104.Liu J, Xiao Y, Allen C. Polymer-drug compatibility: a guide to the development of delivery systems for the anticancer agent, ellipticine. J. Pharm. Sci. 2004;93(1):132–143. doi: 10.1002/jps.10533. [DOI] [PubMed] [Google Scholar]
- 105.Latere Dwan'Isa JP, Rouxhet L, Preat V, Brewster ME, Arien A. Prediction of drug solubility in amphiphilic di-block copolymer micelles: the role of polymer-drug compatibility. Pharmazie. 2007;62(7):499–504. [PubMed] [Google Scholar]
- 106.Forrest ML, Zhao A, Won CY, Malick AW, Kwon GS. Lipophilic prodrugs of Hsp90 inhibitor geldanamycin for nanoencapsulation in poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J. Control. Release. 2006;116(2):139–149. doi: 10.1016/j.jconrel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 107.Marsac PJ, Shamblin SL, Taylor LS. Theoretical and practical approaches for prediction of drug-polymer miscibility and solubility. Pharm. Res. 2006;23(10):2417–2426. doi: 10.1007/s11095-006-9063-9. [DOI] [PubMed] [Google Scholar]
- 108.Danquah M, Fujiwara T, Mahato RI. Self-assembling methoxypoly(ethylene glycol)-b-poly(carbonate-co-L-lactide) block copolymers for drug delivery. Biomaterials. 2010;31(8):2358–2370. doi: 10.1016/j.biomaterials.2009.11.081. [DOI] [PubMed] [Google Scholar]
- 109.Mundra V, Lu Y, Danquah M, Li W, Miller DD, Mahato RI. Formulation and characterization of polyester/polycarbonate nanoparticles for delivery of a novel microtubule destabilizing agent. Pharm. Res. 2012;29(11):3064–3074. doi: 10.1007/s11095-012-0881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li F, Danquah M, Mahato RI. Synthesis and characterization of amphiphilic lipopolymers for micellar drug delivery. Biomacromolecules. 2010;11(10):2610–2620. doi: 10.1021/bm100561v. [DOI] [PubMed] [Google Scholar]
- 111.Mahmud A, Patel S, Molavi O, Choi P, Samuel J, Lavasanifar A. Self-associating poly(ethylene oxide)-b-poly(alpha-cholesteryl carboxylate-epsilon-caprolactone) block copolymer for the solubilization of STAT-3 inhibitor cucurbitacin I. Biomacromolecules. 2009;10(3):471–478. doi: 10.1021/bm800846a. [DOI] [PubMed] [Google Scholar]
- 112.Kataoka K, Matsumoto T, Yokoyama M, et al. Doxorubicin-loaded poly(ethylene glycol)-poly(beta-benzyl-L-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J. Control. Release. 2000;64(1–3):143–153. doi: 10.1016/s0168-3659(99)00133-9. [DOI] [PubMed] [Google Scholar]
- 113.Deng X, Xu X, Lai Y, He B, Gu Z. Novel nanoparticles generated by polymeric amphiphiles with pi-pi conjugated small molecules for anti-tumor drug delivery. J. Biomed. Nanotechnol. 2013;9(8):1336–1344. doi: 10.1166/jbn.2013.1626. [DOI] [PubMed] [Google Scholar]
- 114.Lai Y, Long Y, Lei Y, et al. A novel micelle of coumarin derivative monoend-functionalized PEG for anti-tumor drug delivery: in vitro and in vivo study. J. Drug Target. 2012;20(3):246–254. doi: 10.3109/1061186X.2011.639023. [DOI] [PubMed] [Google Scholar]
- 115.Shi Y, van Steenbergen MJ, Teunissen EA, et al. Pi-pi stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules. 2013;14(6):1826–1837. doi: 10.1021/bm400234c. [DOI] [PubMed] [Google Scholar]
- 116.Lee J, Lee SC, Acharya G, Chang CJ, Park K. Hydrotropic solubilization of paclitaxel: analysis of chemical structures for hydrotropic property. Pharm. Res. 2003;20(7):1022–1030. doi: 10.1023/a:1024458206032. [DOI] [PubMed] [Google Scholar]
- 117.Kim JY, Kim S, Papp M, Park K, Pinal R. Hydrotropic solubilization of poorly water-soluble drugs. J. Pharm. Sci. 2010;99(9):3953–3965. doi: 10.1002/jps.22241. [DOI] [PubMed] [Google Scholar]
- 118.Eckman AM, Tsakalozou E, Kang NY, Ponta A, Bae Y. Drug release patterns and cytotoxicity of PEG-poly(aspartate) block copolymer micelles in cancer cells. Pharm. Res. 2012;29(7):1755–1767. doi: 10.1007/s11095-012-0697-5. [DOI] [PubMed] [Google Scholar]
- 119.Kim JO, Kabanov AV, Bronich TK. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J. Control. Release. 2009;138(3):197–204. doi: 10.1016/j.jconrel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ye L, Letchford K, Heller M, et al. Synthesis and characterization of carboxylic acid conjugated, hydrophobically derivatized, hyperbranched polyglycerols as nanoparticulate drug carriers for cisplatin. Biomacromolecules. 2011;12(1):145–155. doi: 10.1021/bm101080p. [DOI] [PubMed] [Google Scholar]
- 121.Tian Y, Ravi P, Bromberg L, Hatton TA, Tam KC. Synthesis and aggregation behavior of pluronic F87/poly(acrylic acid) block copolymer in the presence of doxorubicin. Langmuir. 2007;23(5):2638–2646. doi: 10.1021/la060780a. [DOI] [PubMed] [Google Scholar]
- 122.Giacomelli C, Schmidt V, Borsali R. Specific interactions improve the loading capacity of block copolymer micelles in aqueous media. Langmuir. 2007;23(13):6947–6955. doi: 10.1021/la700337s. [DOI] [PubMed] [Google Scholar]
- 123.Hamaguchi T, Matsumura Y, Suzuki M, et al. NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel. Br. J. Cancer. 2005;92(7):1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao X, Poon Z, Engler AC, Bonner DK, Hammond PT. Enhanced stability of polymeric micelles based on postfunctionalized poly(ethylene glycol)-b-poly(gamma-propargyl L-glutamate): the substituent effect. Biomacromolecules. 2012;13(5):1315–1322. doi: 10.1021/bm201873u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim SH, Tan JP, Nederberg F, et al. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31(31):8063–8071. doi: 10.1016/j.biomaterials.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 126.Duncan R, Vicent MJ. Do HPMA copolymer conjugates have a future as clinically useful nanomedicines? A critical overview of current status and future opportunities. Adv. Drug Deliv. Rev. 2010;62(2):272–282. doi: 10.1016/j.addr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 127.Etrych T, Kovar L, Strohalm J, Chytil P, Rihova B, Ulbrich K. Biodegradable star HPMA polymer-drug conjugates: biodegradability, distribution and anti-tumor efficacy. J. Control. Release. 2011;154(3):241–248. doi: 10.1016/j.jconrel.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 128.Fox ME, Szoka FC, Frechet JM. Soluble polymer carriers for the treatment of cancer: the importance of molecular architecture. Acc. Chem. Res. 2009;42(8):1141–1151. doi: 10.1021/ar900035f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peterson JJ, Meares CF. Cathepsin substrates as cleavable peptide linkers in bioconjugates, selected from a fluorescence quench combinatorial library. Bioconjug. Chem. 1998;9(5):618–626. doi: 10.1021/bc980059j. [DOI] [PubMed] [Google Scholar]
- 130.Dubowchik GM, Firestone RA, Padilla L, et al. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug. Chem. 2002;13(4):855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 131.Oldham EA, Li C, Ke S, Wallace S, Huang P. Comparison of action of paclitaxel and poly(L-glutamic acid)-paclitaxel conjugate in human breast cancer cells. Int. J. Oncol. 2000;16(1):125–132. [PubMed] [Google Scholar]
- 132.Liu J, Bauer H, Callahan J, Kopeckova P, Pan H, Kopecek J. Endocytic uptake of a large array of HPMA copolymers: elucidation into the dependence on the physicochemical characteristics. J. Control. Release. 2010;143(1):71–79. doi: 10.1016/j.jconrel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Greenwald RB, Gilbert CW, Pendri A, Conover CD, Xia J, Martinez A. Drug delivery systems: water soluble taxol 2′-poly(ethylene glycol) ester prodrugs-design and in vivo effectiveness. J. Med. Chem. 1996;39(2):424–431. doi: 10.1021/jm950475e. [DOI] [PubMed] [Google Scholar]
- 134.Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J. Pharm. Sci. 1994;83(4):601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- 135.Zhang X, Li Y, Chen X, et al. Synthesis and characterization of the paclitaxel/MPEG-PLA block copolymer conjugate. Biomaterials. 2005;26(14):2121–2128. doi: 10.1016/j.biomaterials.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 136.Xie Z, Guan H, Chen X, et al. A novel polymer-paclitaxel conjugate based on amphiphilic triblock copolymer. J. Control. Release. 2007;117(2):210–216. doi: 10.1016/j.jconrel.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 137.Riebeseel K, Biedermann E, Loser R, et al. Polyethylene glycol conjugates of methotrexate varying in their molecular weight from MW 750 to MW 40000: synthesis, characterization, and structure-activity relationships in vitro and in vivo . Bioconjug. Chem. 2002;13(4):773–785. doi: 10.1021/bc010098m. [DOI] [PubMed] [Google Scholar]
- 138.Veronese FM, Schiavon O, Pasut G, et al. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjug. Chem. 2005;16(4):775–784. doi: 10.1021/bc040241m. [DOI] [PubMed] [Google Scholar]
- 139.Kopecek J. Polymer-drug conjugates: origins, progress to date and future directions. Adv. Drug Deliv. Rev. 2013;65(1):49–59. doi: 10.1016/j.addr.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vasey PA, Kaye SB, Morrison R, et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: first member of a new class of chemotherapeutic agents-drug-polymer conjugates. Cancer Research Campaign Phase I/II Committee. Clin. Cancer Res. 1999;5(1):83–94. [PubMed] [Google Scholar]
- 141.Meerum Terwogt JM, ten Bokkel Huinink WW, Schellens JH, et al. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs. 2001;12(4):315–323. doi: 10.1097/00001813-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 142.Schoemaker NE, van Kesteren C, Rosing H, et al. A Phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Br. J. Cancer. 2002;87(6):608–614. doi: 10.1038/sj.bjc.6600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Etrych T, Sirova M, Starovoytova L, Rihova B, Ulbrich K. HPMA copolymer conjugates of paclitaxel and docetaxel with pH-controlled drug release. Mol. Pharm. 2010;7(4):1015–1026. doi: 10.1021/mp100119f. [DOI] [PubMed] [Google Scholar]
- 144.Yang J, Luo K, Pan H, Kopeckova P, Kopecek J. Synthesis of biodegradable multiblock copolymers by click coupling of RAFT-generated heterotelechelic polyHPMA conjugates. React. Funct. Polym. 2011;71(3):294–302. doi: 10.1016/j.reactfunctpolym.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang R, Yang J, Sima M, Zhou Y, Kopecek J. Sequential combination therapy of ovarian cancer with degradable N-(2-hydroxypropyl)methacrylamide copolymer paclitaxel and gemcitabine conjugates. Proc. Natl Acad. Sci. USA. 2014;111(33):12181–12186. doi: 10.1073/pnas.1406233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Akamatsu K, Yamasaki Y, Nishikawa M, Takakura Y, Hashida M. Development of a hepatocyte-specific prostaglandin E(1) polymeric prodrug and its potential for preventing carbon tetrachloride-induced fulminant hepatitis in mice. J. Pharmacol. Exp. Ther. 1999;290(3):1242–1249. [PubMed] [Google Scholar]
- 147.Sumi H, Kawabe K, Nakajima N. Effect of various polyamino acids and D- and L-amino acids on the blood fibrinolytic system. Comp. Biochem. Physiol. B. 1992;102(1):159–162. doi: 10.1016/0305-0491(92)90289-4. [DOI] [PubMed] [Google Scholar]
- 148.Li C. Poly(L-glutamic acid)–anticancer drug conjugates. Adv. Drug Deliv. Rev. 2002;54(5):695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 149.Hurwitz E, Wilchek M, Pitha J. Soluble macromolecules as carriers for daunorubicin. J. Appl. Biochem. 1980;2(1):25–35. [Google Scholar]
- 150.Zunino F, Pratesi G, Micheloni A. Poly(carboxylic acid) polymers as carriers for anthracyclines. J. Control. Release. 1989;10(1):65–73. [Google Scholar]
- 151.Hoes CJT, Grootoonk J, Duncan R, et al. Biological properties of adriamycin bound to biodegradable polymeric carriers. J. Control. Release. 1993;23(1):37–53. [Google Scholar]
- 152.Hoes CJT, Potman W, van Heeswijk WAR, et al. Optimization of macromolecular prodrugs of the antitumor antibiotic adriamycin. J. Control. Release. 1985;2(0):205–213. [Google Scholar]
- 153.Li C, Yu DF, Newman RA, et al. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58(11):2404–2409. [PubMed] [Google Scholar]
- 154.Kim JW, Galanzha EI, Zaharoff DA, Griffin RJ, Zharov VP. Nanotheranostics of circulating tumor cells, infections and other pathological features in vivo . Mol. Pharm. 2013;10(3):813–830. doi: 10.1021/mp300577s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 156.Barth BM, I Altinoglu E, Shanmugavelandy SS, et al. Targeted indocyanine-green-loaded calcium phosphosilicate nanoparticles for in vivo photodynamic therapy of leukemia. ACS Nano. 2011;5(7):5325–5337. doi: 10.1021/nn2005766. [DOI] [PubMed] [Google Scholar]
- 157.Qiang YG, Yow CM, Huang Z. Combination of photodynamic therapy and immunomodulation: current status and future trends. Med. Res. Rev. 2008;28(4):632–644. doi: 10.1002/med.20121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Almeida JP, Drezek RA, Foster AE. Controlling melanoma at local and systemic levels: is a combination of ablative therapy and immunotherapy the way forward? Immunotherapy. 2014;6(2):109–111. doi: 10.2217/imt.13.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br. J. Dermatol. 2006;155(6):1287–1292. doi: 10.1111/j.1365-2133.2006.07514.x. [DOI] [PubMed] [Google Scholar]
- 160.Li X, Naylor MF, Le H, et al. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol. Ther. 2010;10(11):1081–1087. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guo L, Yan DD, Yang D, et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8(6):5670–5681. doi: 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bear AS, Kennedy LC, Young JK, et al. Elimination of metastatic melanoma using gold nanoshell-enabled photothermal therapy and adoptive T cell transfer. PLoS ONE. 2013;8(7):e69073. doi: 10.1371/journal.pone.0069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Snyder JW, Greco WR, Bellnier DA, Vaughan L, Henderson BW. Photodynamic therapy: a means to enhanced drug delivery to tumors. Cancer Res. 2003;63(23):8126–8131. [PubMed] [Google Scholar]
- 164.Zhen Z, Tang W, Chuang YJ, et al. Tumor vasculature targeted photodynamic therapy for enhanced delivery of nanoparticles. ACS Nano. 2014;8(6):6004–6013. doi: 10.1021/nn501134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Selbo PK, Weyergang A, Hogset A, et al. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release. 2010;148(1):2–12. doi: 10.1016/j.jconrel.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 166.Lou PJ, Lai PS, Shieh MJ, Macrobert AJ, Berg K, Bown SG. Reversal of doxorubicin resistance in breast cancer cells by photochemical internalization. Int. J. Cancer. 2006;119(11):2692–2698. doi: 10.1002/ijc.22098. [DOI] [PubMed] [Google Scholar]
- 167.Selbo PK, Rosenblum MG, Cheung LH, Zhang W, Berg K. Multi-modality therapeutics with potent anti-tumor effects. photochemical internalization enhances delivery of the fusion toxin scFvMEL/rGel. PLoS ONE. 2009;4(8):e6691. doi: 10.1371/journal.pone.0006691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mathews MS, Vo V, Shih EC, et al. Photochemical internalization-mediated delivery of chemotherapeutic agents in human breast tumor cell lines. J. Environ. Pathol. Toxicol. Oncol. 2012;31(1):49–59. doi: 10.1615/jenvironpatholtoxicoloncol.v31.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lu HL, Syu WJ, Nishiyama N, Kataoka K, Lai PS. Dendrimer phthalocyanine-encapsulated polymeric micelle-mediated photochemical internalization extends the efficacy of photodynamic therapy and overcomes drug-resistance in vivo . J. Control. Release. 2011;155(3):458–464. doi: 10.1016/j.jconrel.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 170.Lai PS, Pai CL, Peng CL, Shieh MJ, Berg K, Lou PJ. Enhanced cytotoxicity of saporin by polyamidoamine dendrimer conjugation and photochemical internalization. J. Biomed. Mater. Res. A. 2008;87(1):147–155. doi: 10.1002/jbm.a.31760. [DOI] [PubMed] [Google Scholar]
- 171.Lai PS, Lou PJ, Peng CL, et al. Doxorubicin delivery by polyamidoamine dendrimer conjugation and photochemical internalization for cancer therapy. J. Control. Release. 2007;122(1):39–46. doi: 10.1016/j.jconrel.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 172.Bostad M, Olsen CE, Peng Q, Berg K, Hogset A, Selbo PK. Light-controlled endosomal escape of the novel CD133-targeting immunotoxin AC133-saporin by photochemical internalization – a minimally invasive cancer stem cell-targeting strategy. J. Control. Release. 2015;206:37–48. doi: 10.1016/j.jconrel.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 173.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pirollo KF, Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake? Trends Biotechnol. 2008;26(10):552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 175.Gu FX, Karnik R, Wang AZ, et al. Targeted nanoparticles for cancer therapy. Nano Today. 2007;2(3):14–21. [Google Scholar]
- 176.Weis SM, Cheresh DA. alphaV integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 2011;1(1):a006478. doi: 10.1101/cshperspect.a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Felding-Habermann B, Fransvea E, O'Toole TE, Manzuk L, Faha B, Hensler M. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin. Exp. Metastasis. 2002;19(5):427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- 178.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 179.Phillips E, Penate-Medina O, Zanzonico PB, et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014;6(260):260ra149. doi: 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wagner S, Rothweiler F, Anhorn MG, et al. Enhanced drug targeting by attachment of an anti alphav integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials. 2010;31(8):2388–2398. doi: 10.1016/j.biomaterials.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 181.Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL. The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J. Invest. Dermatol. 1997;108(2):210–214. doi: 10.1111/1523-1747.ep12334263. [DOI] [PubMed] [Google Scholar]
- 182.Ikuta Y, Nakatsura T, Kageshita T, et al. Highly sensitive detection of melanoma at an early stage based on the increased serum secreted protein acidic and rich in cysteine and glypican-3 levels. Clin. Cancer Res. 2005;11(22):8079–8088. doi: 10.1158/1078-0432.CCR-05-1074. [DOI] [PubMed] [Google Scholar]
- 183.Robert G, Gaggioli C, Bailet O, et al. SPARC represses E-cadherin and induces mesenchymal transition during melanoma development. Cancer Res. 2006;66(15):7516–7523. doi: 10.1158/0008-5472.CAN-05-3189. [DOI] [PubMed] [Google Scholar]
- 184.Thomas S, Waterman P, Chen S, et al. Development of secreted protein and acidic and rich in cysteine (SPARC) targeted nanoparticles for the prognostic molecular imaging of metastatic prostate cancer. J. Nanomed. Nanotechnol. 2011;2(112) doi: 10.4172/2157-7439.1000112. 2157–7439–2–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sheng Z, Hu D, Zheng M, et al. Smart human serum albumin-indocyanine green nanoparticles generated by programmed assembly for dual-modal imaging-guided cancer synergistic phototherapy. ACS Nano. 2014;8(12):12310–12322. doi: 10.1021/nn5062386. [DOI] [PubMed] [Google Scholar]
- 186.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl. Oncol. 2009;2(2):59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Desai NP, Trieu V, Hwang LY, Wu R, Soon-Shiong P, Gradishar WJ. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anticancer Drugs. 2008;19(9):899–909. doi: 10.1097/CAD.0b013e32830f9046. [DOI] [PubMed] [Google Scholar]
- 188.Schneeweiss A, Seitz J, Smetanay K, et al. Efficacy of nab-paclitaxel does not seem to be associated with SPARC expression in metastatic breast cancer. Anticancer Res. 2014;34(11):6609–6615. [PubMed] [Google Scholar]
- 189.Shao H, Tang H, Salavaggione OE, et al. Improved response to nab-paclitaxel compared with cremophor-solubilized paclitaxel is independent of secreted protein acidic and rich in cysteine expression in non-small cell lung cancer. J. Thorac. Oncol. 2011;6(6):998–1005. doi: 10.1097/JTO.0b013e318217b739. [DOI] [PubMed] [Google Scholar]
- 190.Neesse A, Frese KK, Chan DS, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–983. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Simberg D, Duza T, Park JH, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl Acad. Sci. USA. 2007;104(3):932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin. Immunol. 2006;121(2):159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 193.Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pun SH, Tack F, Bellocq NC, et al. Targeted delivery of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther. 2004;3(7):641–650. doi: 10.4161/cbt.3.7.918. [DOI] [PubMed] [Google Scholar]
- 195.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol. Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 196.Dube DH, Bertozzi CR. Glycans in cancer and inflammation – potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005;4(6):477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 197.Matsumoto A, Cabral H, Sato N, Kataoka K, Miyahara Y. Assessment of tumor metastasis by the direct determination of cell-membrane sialic acid expression. Angew. Chem. Int. Ed. Engl. 2010;49(32):5494–5497. doi: 10.1002/anie.201001220. [DOI] [PubMed] [Google Scholar]
- 198.Matsumoto A, Sato N, Kataoka K, Miyahara Y. Noninvasive sialic acid detection at cell membrane by using phenylboronic acid modified self-assembled monolayer gold electrode. J. Am. Chem. Soc. 2009;131(34):12022–12023. doi: 10.1021/ja902964m. [DOI] [PubMed] [Google Scholar]
- 199.Deshayes S, Cabral H, Ishii T, et al. Phenylboronic acid-installed polymeric micelles for targeting sialylated epitopes in solid tumors. J. Am. Chem. Soc. 2013;135(41):15501–15507. doi: 10.1021/ja406406h. [DOI] [PubMed] [Google Scholar]
- 200.Siegrist W, Solca F, Stutz S, et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49(22):6352–6358. [PubMed] [Google Scholar]
- 201.Ghanem GE, Libert A, Arnould R, Vercammen A, Lejeune F. Human melanoma targeting with alpha-MSH-melphalan conjugate. Melanoma Res. 1991;1(2):105–114. doi: 10.1097/00008390-199106000-00005. [DOI] [PubMed] [Google Scholar]
- 202.Lu W, Xiong C, Zhang G, et al. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009;15(3):876–886. doi: 10.1158/1078-0432.CCR-08-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim C, Cho EC, Chen J, et al. In vivo molecular photoacoustic tomography of melanomas targeted by bioconjugated gold nanocages. ACS Nano. 2010;4(8):4559–4564. doi: 10.1021/nn100736c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Falvo E, Tremante E, Fraioli R, et al. Antibody-drug conjugates. targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale. 2013;5(24):12278–12285. doi: 10.1039/c3nr04268e. [DOI] [PubMed] [Google Scholar]
- 205.Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib. a study of 42 patients. Ann. Oncol. 2013;24(6):1691–1697. doi: 10.1093/annonc/mdt015. [DOI] [PubMed] [Google Scholar]
- 206.Anforth R, Fernandez-Penas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncol. 2013;14(1):e11–e18. doi: 10.1016/S1470-2045(12)70413-8. [DOI] [PubMed] [Google Scholar]
- 207.Duncan R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer. 2006;6(9):688–701. doi: 10.1038/nrc1958. [DOI] [PubMed] [Google Scholar]
- 208.Cook N, Jodrell DI, Tuveson DA. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today. 2012;17(5–6):253–260. doi: 10.1016/j.drudis.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 209.Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis. Model. Mech. 2008;1(2–3):78–82. doi: 10.1242/dmm.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]