Abstract
Norovirus infections are notoriously difficult to prevent and control, owing to their low infectious dose, high shedding titre, and environmental stability. The virus can spread through multiple transmission routes, of which person-to-person and foodborne are the most important. Recent advances in molecular diagnostics have helped to establish norovirus as the most common cause of sporadic gastroenteritis and the most common cause of outbreaks of acute gastroenteritis across all ages. In this article, we review the epidemiology and virology of noroviruses, and prevention and control guidelines, with a focus on the principles of disinfection and decontamination. Outbreak management relies on sound infection control principles, including hand hygiene, limiting exposure to infectious individuals, and thorough environmental decontamination. Ideally, all infection control recommendations would rely on empirical evidence, but a number of challenges, including the inability to culture noroviruses in the laboratory and the challenges of outbreak management in complex environments, has made it difficult to garner clear evidence of efficacy in certain areas of infection control. New experimental data on cultivable surrogates for human norovirus and on environmental survivability and relative resistance to commonly used disinfectants are providing new insights for further refinining disinfection practices. Finally, clinical trials are underway to evaluate the efficacy of vaccines, which may shift the current infection control principles to more targeted interventions.
Keywords: Disinfection, epidemiology, infection control, norovirus, nosocomial
Introduction
Norovirus is a leading cause of acute gastroenteritis in people of all ages and settings. Approximately 19–21 million norovirus illnesses occur each year in the USA [1]. A high titre of shedding by infected persons, a low infectious dose and environmental stability are some of the attributes that facilitate effective norovirus transmission through a variety of modes (person-to-person, food, water, and environment) [2–5]. These attributes present an array of challenges for prevention and control, in particular in institutional settings [4,6]. Specialists involved with infection and environmental control use a range of strategies aimed at preventing and controlling norovirus outbreaks [7–9]. However, some of these measures, such as ward/unit closures in hospitals, can place a substantial burden on institutions and personnel; a UK study estimated a loss of c. $1 million for every 1000 beds [10–12]. Ideally, outbreak management guidelines would be supported by high-quality empirical evidence. However, generating high-quality evidence for efficacy is difficult, as the evidence for outbreak management is largely empirical, and there are challenges associated with a non-cultivable virus. Here, we review the current knowledge of norovirus outbreak epidemiology and virology, and infection control guidelines, with a focus on disinfection and decontamination, and highlight areas for future research.
Norovirus Outbreaks
Settings
Outbreaks provide an opportunity to study norovirus epidemiology, including how these viruses spread and what control measures are effective. Outbreaks occur in the diverse range of settings where humans congregate. In the USA, outbreaks in restaurants and on cruise ships are frequently picked up by the media. However, one would have a skewed sense of the distribution of norovirus outbreak patterns from media reports alone. Data from broad-based surveillance in high-income countries show that the majority of outbreaks occur in healthcare facilities; however, the specific types of facility reporting outbreaks can differ between countries. In the USA, >60% of all norovirus outbreaks occur in long-term-care facilities [13,14]. This contrasts with the settings reported in Europe, Japan, and other high-income settings, where outbreaks in acute-care hospitals are common and roughly equal in number to outbreaks in long-term-care facilities (Fig. 1) [15]. In the USA, acute-care outbreaks are relatively uncommon, constituting c. 5% of norovirus outbreaks [13,16]. Whether the lower frequency of outbreaks reported from US hospitals represents a real difference in epidemiology or infection control, or an artefact of reporting bias, is not well understood.
FIG. 1.
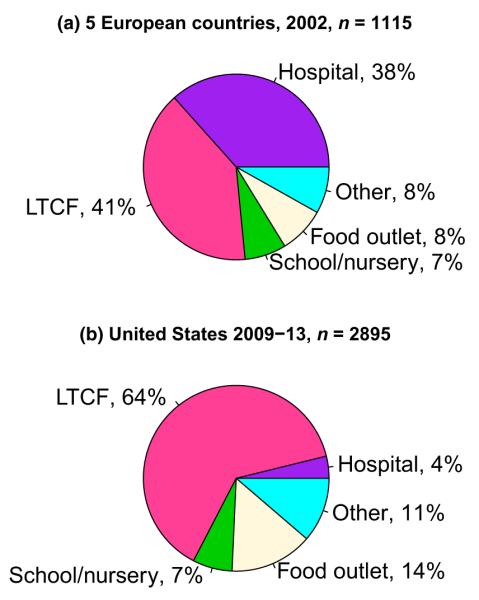
Setting of (a) norovirus outbreaks reported in five European countries with broad-based surveillance, 2002, n = 1115, and (b) the USA, 2009–2013, n = 2895. Long Term Care Facility (LTCF). Adapted from Lopman et al. [15] and Vega et al. [13].
Modes of transmission
Although noroviruses have been detected in bovines, mice, and canines, these virus strains appear to be highly species-specific, and zoonotic transmission does not seem be common. In humans, the virus typically spreads directly via person-to-person transmission (faecal–oral and vomit–oral) or indirectly through foodborne, waterborne and environmental transmission. Direct person-to-person transmission is reported in >90% of the norovirus outbreaks in healthcare facilities [6,13,17]. Food-borne, waterborne and environmental transmission have some features in common, in the sense that a food product, water source or fomite may become contaminated by an infected person, and another individual then ingests virus after coming into contact with that object. In the USA, norovirus is estimated to be the most common aetiological cause of foodborne illness, which accounts for 7–24% of norovirus outbreaks worldwide [13,14,18–20]. Although food may become contaminated at any point in the ‘farm to fork continuum’, the majority of foodborne norovirus illness is a result of contamination by infected food-handlers during preparation [21]. Ready-to-eat foods (such as leafy greens) and foods handled after cooking are the most frequently identified products associated with outbreaks [21]. Each of these transmission modes presents specific challenges in terms of infection prevention and control, as discussed below.
The high levels of virus shed in faeces and vomit [2], the low infectious dose [3] and the environmental stability of the virus [4] all contribute to the ability of noroviruses to utilize various modes of transmission (Table 1). Furthermore, transmission has been reported to occur before the onset of symptoms [22], in the post-symptomatic period, and during subclinical infections [23]. However, the currently available evidence suggests that individuals are less infectious when they are asymptomatic, and that vomiting [23] is strongly associated with transmission [24].
TABLE 1.
Characteristics that facilitate norovirus transmission
| Characteristics | Description |
|---|---|
| Low infectious dose | Estimates of the infectious dose ranges from 18 to 103 virus particles [3] |
| High shedding titre | Peak shedding ranges from 105 to 109 particles/g of stool [2] |
| Prolonged shedding | Virus can be detected up to 8 weeks after symptom onset, with a median of 4 weeks; even longer durations of shedding may be detected in immunocompromised individuals [2,107] |
| Genetic diversity | Over 30 genotypes (nine GI and 22 GII) infect humans [5]. No long-lasting immunity [25,108]. Different genotypes can infect humans over their lifetime [25] |
| Environmental stability | Norovirus particles may be infectious for 2 weeks on environmental surfaces and for >2 months in water [67,109] |
| Resistant to common disinfectants |
Surrogates used to determine the efficacy of EPA-registered disinfectant products have different physiochemical properties; therefore, different disinfection profiles exist, and overestimate the efficacy of disinfectant products [29,87] |
| Vomiting | Vomiting appears to be a particularly effective route of norovirus spread. Vomiting events may occur and lead to direct transmission (when in public) as well as environmental contamination from vomit droplets [59,110] |
| Transmission through multiple routes |
Noroviruses are transmitted via the faecal–oral route and vomit–oral route, and through a number of specific modes, including foodborne, waterborne, environmental and direct person-to-person spread [6,13,21,57,64] |
Adapted from [4].
Importance of genotyping noroviruses for understanding transmission
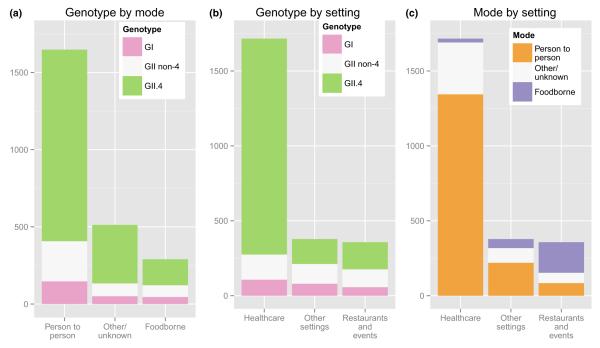
Noroviruses are a group of genetically diverse single-stranded RNA viruses. There are six known genogroups (G), two of which (I and II) commonly cause human disease, and can be further subdivided into nine and 22 genotypes, respectively [5]. Immunity appears to be largely restricted to homotypic genogroups or genotypes [25]. This genetic diversity has public health relevance, in that certain genotypes are associated with different modes of transmission and, perhaps, severity of disease outcomes. Genogroup I viruses are more often associated with food and waterborne outbreaks (Fig. 2). For example, the recently emerged GI.6 virus is more often associated with foodborne disease [26]. Conversely, GII.4 viruses are strongly associated with person-to-person transmission and healthcare settings [27]. Factors that may promote GII.4 transmission in closed settings include a possibly longer duration of shedding [25], more frequent vomiting [28], and different environmental survival and disinfection resistance profiles [29,30]. Moreover, GII.4 infections are likely to be of greater severity and result in more hospitalizations and deaths than those caused by other GII or GI viruses, even after accounting for the different case mix of the populations affected by the different viruses (that is, GII.4 viruses primarily cause outbreaks among the elderly in institutionalized healthcare settings) [31].
FIG. 2.
Distribution of norovirus genotype (GI, GII.4, GII non-4) by mode of transmission (a) and by outbreak setting (b), as well as mode of transmission by outbreak setting (c), from 2895 norovirus outbreaks reported to CaliciNet, 2009–2013. Adapted from Vega et al. [13].
Current Guidelines and the Evidence Base for their Efficacy
Outbreak management
Healthcare institutions provide services to vulnerable populations, and are the most common settings for norovirus outbreaks. For these reasons, these settings will constitute the focus of our discussions on infection control issues, but most of these principles also apply to other settings. Outbreak management is a multistage process: preparedness, identification, response, and evaluation [32]. An institutional structure conducive to organizing and timing the actions to prevent and control infection facilitates the containment of outbreaks [33]. The ability to identify a norovirus outbreak as early as possible is a key aspect in initiating infection control measures [8]. Although outbreak control measures are based on sound infection control principles [8,34], there are scant data to demonstrate that implementing specific infection control measures decrease the magnitude or duration of norovirus outbreaks [35]. Evaluating the effectiveness of infection control measures for norovirus outbreaks is an important part of the process for developing evidence-based guidelines [7,35,36], but there are ethical and scientific challenges to conducting such studies [37].
Guidelines for managing norovirus outbreaks have been issued by public health agencies in several countries, including Australia, Ireland, the UK, and the USA [7,8,36,38,39]. Some guidelines, such as those from the UK and the USA, used systematic literature reviews followed by grading the strength of recommendations. Guidelines from other countries based their recommendations on a more expert opinion-driven approach to assessing evidence. Regardless of the methods used, recent guidelines are generally consistent in the measures that they recommend. The main approaches to preventing and containing norovirus outbreaks that are common across several guidelines include implementing policies concerning hand hygiene, patient isolation (separation of symptomatic patients) and cohorting (grouping of patients based on symptoms), staff exclusion from work, visitor restrictions, enhanced environmental cleaning and disinfection, and ward closures (Table 2) [7,8,33,36,38,39].
TABLE 2.
Summary of infection control guidelines for the prevention and management of norovirus outbreaks in healthcare settings
| Infection control category |
Infection control strategy |
|---|---|
| Outbreak identification |
Define start of outbreak: enables the initiation of enhanced norovirus infection control measures Two or more associated patients with gastroenteritis onsets within 24–48 h of each other Use Kaplan’s clinical and epidemiological criteria to identify norovirus outbreak, if clinical laboratory testing is not available Stool negative for bacteria Mean duration of illness of 12–60 h Vomiting in >50% of cases Incubation period of 24–48 h |
| Hygiene | Wash hands with soap and warm running water for a minimum of 20 s before and after contact with patients, after using the lavatory, and/or before and after eating Wear appropriate personal protective equipment (PPE) Gloves: if directly contacting symptomatic patients Masks: if a potential risk of aerosolization, e.g. vomit, exists Gowns: if a potential risk of splashing exists Goggles/face shields: if a potential risk of splashing exists Change PPE frequently |
| Cleaning and disinfection |
Increase the frequency of cleaning and disinfection of high-traffic areas and implicated areas Clean and disinfect from unaffected to affected areas Clean areas of any organic material Disinfect all surfaces with freshly prepared 0.1% (1000 p.p.m.) sodium hypochlorite (bleach) Clean carpets with detergent and warm water, and follow this with steam cleaning Steam-clean all soft furnishings that may be damaged by bleach Discard all disposable cloths in biohazard bags Launder all non-disposable cloths, i.e. linens, blankets, towels, and clothing |
| Patient isolation/ cohorting and transfer |
Separate patients on the basis of symptomatic, exposed asymptomatic, or unexposed asymptomatic Limit movement and transfer of symptomatic patients |
| Staff exclusion and cohorting |
Exclude ill staff for at least 48 h after symptom resolution Assign staff to one patient cohort |
| Visitors | Limit visits to implicated wards Limit symptomatic visitors until 48 h after symptom resolution Provide educational material that describes the risks of norovirus transmission and measures to prevent infection |
| Ward closures | Consider closing the unit or ward to new admissions and transfers |
| Outbreak reporting | Notify appropriate local or state health departments, as per local and state public health regulations |
| Food safety | Discard exposed food Exclude ill staff for at least 48 h after symptom resolution Close communal dining areas Ensure proper food preparation, storage, and serving Eliminate bare-handed contact with ready-to-eat foods |
Hygiene
A diverse set of recommendations for the prevention and control of norovirus outbreaks are needed, given the various transmission modes by which norovirus spreads and the lack of a ‘magic bullet’ to curtail transmission. In general, hand hygiene adherence should be actively promoted among healthcare personnel, patients and visitors in patient-care areas affected by outbreaks of norovirus gastroenteritis. During outbreaks, hands should be washed with soap and running water for a minimum of 20 s after providing care for patients with suspected or confirmed infection [7,8]. Data from several studies suggest that this method of hand hygiene is an effective intervention for reducing norovirus risk [7,40–42]. Despite widespread use, there is inconclusive evidence for the effectiveness of alcohol-based hand sanitizers for norovirus [29,43–45]. Therefore, during outbreaks, they should be used as an adjunct to hand-washing [8]. Aerosolization of noroviruses and close, direct contact with an infected individual contribute to the high risk of transmission [46]. Therefore, the use of appropriate personal protective equipment, i.e. gloves and masks, especially when cleaning up vomit, is another measure for limiting the further spread of norovirus infection to staff in healthcare facilities [7]. During an outbreak, personal protective equipment should be disposable and single-use [7,39].
Cleaning and disinfection
Enhanced cleaning and disinfection protocols may control and prevent the spread of norovirus [47–49]. This includes increasing the frequency of cleaning and paying closer attention to high-traffic areas and frequently touched surfaces, including, for example, door handles and telephones [4,7,8]. For disinfection, a bleach solution at a minimum concentration of 1000 p.p.m. sodium hypochlorite prepared fresh daily is recommended [8]. The results from several studies have demonstrated that bleach effectively disinfects norovirus better than other products, i.e. quaternary ammonium-based products [50–53]. In areas where bleach is not available or is corrosive to materials, EPA-registered products, in particular List G, are available that can be effective against norovirus surrogates [54]. Cleaning and disinfection should proceed from unaffected areas to affected areas, with care being taken to clean from low-contamination areas to high-contamination areas [36]. Steam cleaning can be considered for soft furnishings, i.e. rugs, carpets, chairs, and other fabrics, that are adversely affected by bleach [7,36].
Isolation and cohorting
Isolation, cohorting (grouping of patients on the basis of symptoms) and exclusion of symptomatic staff, patients and visitors constitute another class of recommended strategies for infection control [7,8,33,36,38,39]. These strategies can prevent the amount of secondary transmission, and decrease the outbreak duration [55–59]. Although most guidelines recommend cohorting patients into groups on the basis of symptomatic, exposed asymptomatic and unexposed asymptomatic status [7,8,33,36,38,39], at a minimum, symptomatic patients should be isolated in a single ward or care unit in order to minimize secondary transmission [39]. Several guidelines stress that symptomatic patients should not be transferred to other wards/units within the facility or between facilities until at least 48 h after symptoms have been resolved, in order to reduce the spread of infection to unaffected areas or facilities [7,8,33,36,38]. To minimize the spread of norovirus between patient cohorts, healthcare institution staff should care for one patient cohort at a time, and movement of staff between patient cohorts should be lmited. In particular, staff assigned to symptomatic patients should strictly adhere to all enhanced infection control policies [7]. Exclusion of staff members from work during illness and for at least 48 h after resolution of symptoms can reduce transmission to patients during the symptomatic and post-symptomatic phases of infection [60]. Sick pay and sick leave policies in healthcare institutions that do not penalize ill workers may help to prevent staff from working while infectious [8] but these measures may also lead to unintended consequences, such as staff shortages [11,61]. Minimizing access of visitors and non-essential personnel to affected areas and the exclusion of symptomatic visitors is strongly recommended. As visitors may not be knowledgeable about norovirus, facilities can provide educational material describing the risks of norovirus transmission and measures to prevent infection [7,33,39]. Finally, and perhaps most controversially, some guidelines recommend closing units, or parts thereof, to new admissions or transfers [7,8,33,36,38,39]. Most data suggest that ward closure is effective in terms of reducing the number of cases and the duration of outbreaks [12,46,62].
Organizational structure and response
A common theme in several national guidelines is the value of an organizational structure within a healthcare institution that is capable of providing timely response to outbreaks [36,38,39]. One department that is accountable for identifying and implementing recommendations can streamline the initiation of protocols and infection control measures [63]. The reporting of norovirus outbreaks from healthcare institutions to appropriate public health authorities may assist in outbreak control and, ultimately, through collection of surveillance data, provide evidence supporting specific actions [7,8,33,36,38,39].
Food-handling
Foodborne outbreaks arise from a variety of contamination points, i.e. during production, processing, preparation, or service. Infected food-handlers contaminating ready-to-eat food is the most common source of foodborne norovirus outbreaks [21]. Leafy vegetables, fruits, and shellfish, all of which are commonly consumed raw or undercooked, are the food commodities most commonly reported as the cause of food-borne norovirus outbreaks [21,64]. Determining whether food is the cause of the outbreak as early as possible can facilitate the withdrawal of implicated food or the exclusion of infected food-handlers, hence limiting both primary food exposures and the secondary spread of norovirus infection [21,64]. Contaminated food and exposed utensils should be removed and appropriately disinfected, as should contaminated common areas such as dining halls [8,9,39]. Like healthcare workers, food-handlers should remain off work for at least 48 h after symptom resolution [65,66]. Ensuring that staff involved in food preparation, storage and serving adhere to the US Food and Drug Administration Food Code is important in preventing foodborne norovirus outbreaks [8,9,34]. Two key infection control measures specific to food-service settings include eliminating bare-handed contact with ready-to-eat foods and the presence of certified kitchen managers with food safety training [9].
Implications of Environmental Stability of Human Norovirus
The infectiousness of norovirus outside the human host is influenced by intrinsic characteristics of the virus, such as physiochemical properties (thermal and desiccation resistance) and extrinsic characteristics (surface types). Norwalk virus seeded into ground water for at least 61 days was still able to infect human volunteers [67]. Although human noroviruses cannot yet be cultured in vitro [68], cultivable viruses, i.e. coliphage MS2 (MS2), murine norovirus (MNV), and feline calicivirus (FCV), have been used widely to assess the use of physiochemical abilities to predict the infectivity of human norovirus [69–71]. Such surrogate-based studies have estimated that human norovirus could stay potentially infectious on frozen foods (less than or equal to −20°C), refrigerated foods (≤10°C) and fomites for up to 6 months [72,73], up to 7 days [74,75], and ≥7 days [76], respectively. Robust stability (<1 log10 of infectivity loss for 1 h of contact) of virus on hands was also demonstrated in an in vivo study with FCV and MNV [77]. Additionally, norovirus can be easily transferred between hands and surfaces through casual contact, which probably contributes to the spread of norovirus in the community [3,78,79].
Further considerations on norovirus interventions
The basis for recommending washing of hands with soap is that soap, in several in vivo experiments, has been demonstrated to be more effective in removing viruses from hands than topical agents (e.g. alcohol-based hand sanitizers) [41,45]. However, few data are available on the level and frequency of contamination on hands from infected individuals, and it therefore remains uncertain whether hand-washing alone is sufficient to reduce the risk [80]. Also, hand-washing compliance is a general issue, with implications for a range of healthcare-associated pathogens, and not only norovirus. Alcohol-based hand sanitizers may be used as an adjunct but not as a substitute for hand-washing during norovirus outbreaks [7,81]. A number of studies have supported the virucidal activity of alcohol-based hand sanitizers against human norovirus and multiple surrogates [29,43,52,77]; other active ingredients (e.g. benzalkonium chlorite (Quat), triclosan, or chlorhexidine) were ineffective [29]. However, the clinical value (i.e. effectiveness) of alcohol-based sanitizers is a function of both: (i) their ability to inactivate viruses (i.e. efficacy), which depends both on the formulation and on the way they are tested in vitro or in vivo [29,41,77,82,83]; and (ii) compliance, which includes both the frequency of use and proper application [34]. Overall, the lack of data on real-world effectiveness makes it difficult to generalize claims simply based on in vitro or in vivo experiments on a particular formulation. More comprehensive studies are warranted.
There is little information on the bio-burden of norovirus on hard surfaces, but recent data have shown that the surfaces of a few high-contact objects (i.e. doorknobs, toilet seats, and faucets) can be contaminated with up to 104 virus particles per object (unpublished data), which strongly suggests that reductions in levels of 3–4 log10 are required to eliminate norovirus contamination on high-contact surfaces [7,8]. The use of sodium hypochlorite solution (≥1000 p.p.m.) remains reliable for achieving a higher than 3 log10 reduction of human norovirus on surfaces, but pre-cleaning before its application is strongly recommended, to reduce the faecal organic load [7,8,48]. EPA-registered products claimed to have efficacy against human norovirus (e.g. List G) can be considered as alternative options. However, care should be taken, as FCV, which is officially used for claims of efficacy against human norovirus in EPA-registered products [54], is not the most resistant surrogate virus for predicting inactivation of human norovirus [29,71,86,87]. However, some recent EPA-registered products, which claimed norovirus antiviral activity, provided additional efficacy information against other norovirus surrogate viruses, such as MNV. The utilization of multiple norovirus surrogates demonstrating efficacy against norovirus can allow for a more conservative selection of appropriate disinfectants. In addition, the EPA test protocol allows for a longer duration of contact between disinfectant and inoculum (usually ≥5 min), whereas a shorter exposure time (1–3 min or shorter) is the practice more likely to be used in the field, potentially reducing the efficacy of these disinfectants [84,85]. Thus, strict compliance with the manufacturer’s instructions is strongly advised to achieve the claimed efficacy.
Contaminated hands and surfaces may both contribute to norovirus transmission via regular interactions between hands and their surroundings, and hand and surface interventions should therefore complement each other. It is important to note the limitations of these traditional hygiene interventions. In particular, if sufficient decontamination is achieved, surfaces in areas of high contamination risk (e.g. toilets) are susceptible to recontamination by contact with affected or asymptomatic carriers. However, the effects of surface disinfection or hand-washing are transient, because commercial chemical disinfectants do not have any residual antimicrobial activity [4]. In addition to having proven effectiveness against norovirus, chemical disinfectants must also satisfy other requirements, such as low toxicity for personnel, and a low risk of damaging contaminated surface materials [88]. Novel disinfection methods are being considered as alternatives or complements to traditional hygiene interventions, but further research is needed (Table 3) [89–94].
TABLE 3.
List of available alternative surface disinfection technologies for human noroviruses
| Disinfectant or disinfection process |
Proposed application | Efficacy |
|---|---|---|
| Fluorinated titanium dioxide film [95] |
Self-sanitizing surface | Antimicrobial activity of fluorinated titanium dioxide (TiO2)-coated coupons are activated by fluorescent light. After 60 min of exposure to fluorescent light (10 μW/cm2), fluorinated TiO2-coated coupons reduced the infectivity of MS2a, FCVb and MNV-1c by 1.7, 2.6 and 2.6 log10, respectively |
| Gaseous ozone [92] | Decontamination of larger surface areas |
Gaseous ozone at 20–25 p.p.m. inactivated FCV by 5 log10 after 20 min of exposure. The efficacy of gaseous ozone was not influenced by the test room size (34–47.6 m3), location of viral contamination, or surface type |
| Hydrogen peroxide gas [91] | Decontamination of larger surface areas |
Hydrogen peroxide gas (12% hydrogen peroxide) inactivated MNV-1 by >3 log10 in a test room (7 × 5 × 2.7 m3). The surface disinfection efficiency was not influenced by location of viral contamination or surface type |
| Super-oxidized water (hypochlorous acid) [90] |
Food contact sanitizer | Super-oxidized water (hypochlorous acid solution: 188 p.p.m. Cl2, pH between 5.5 and 6.2), generated electrolytically from a dilute NaCl solution, inactivated MS2 dried on coupons (stainless steel and ceramic tile) by ≥3 log10 after 1 min of contact time |
| Saturated steam vapour [93] | Food contact surfaces | Saturated steam vapour by VaporJet 2400 (Advanced Vapor Technologies, Seattle, WA, USA) inactivated MS2 dried on clay coupons by >3 log10 after 2 s of exposure |
| Steam–ultrasound [94] | Food contact surfaces | Steam (130°C) in combination with ultrasound (30–40 kHz) applied with the SonoSteam® technique inactivated FCV and MS2 by >4 log10 after 1 s, and MNV-1 by 3.7 log10 after 3 s |
Coliphage MS2, a non-enveloped, (+) single-stranded RNA virus, classified in family Leviviridae, genus Levivirus; a model strain for human enteric viruses [69].
Feline calicivirus, a non-enveloped, (+) single stranded RNA virus, classified in family Caliciviridae, genus Vesivirus; a surrogate for human norovirus [71].
Murine norovirus, a non-enveloped, (+) single stranded RNA virus, classified in family Caliciviridae, genus Norovirus; a surrogate for human norovirus [70].
Future Research
Advances in disinfection technology for environmental and food safety use may direct updated guidelines for infection control practices [95,96]. A successful technology, such as high hydrostatic pressure, may have the potential for use in food safety [97–100]. Short of developing a norovirus cell culture system, norovirus surrogates such as Tulane virus, porcine enteric calicivirus, MNV and FCV may help in better assessment of the efficacy of cleaning and disinfection practices [98,100]. Future studies should be directed towards quantitative assessment of norovirus contamination at each stage of the infection transmission cycle. Carefully designed observational studies or, preferably, intervention trials may help to answer the question of whether cohorting and/or unit closures alone or in conjunction with other strategies, i.e. cleaning and disinfection, are effective at controlling norovirus outbreaks. Progress is also being made in the development of a norovirus vaccine [101–105]. Accordingly, there are a number of possible strategies (e.g. vaccinating healthcare workers or nursing home residents) that will require careful evaluation. None of these developments in infection prevention will happen in isolation, so the costs and benefits of both individual interventions and combinations should be assessed [10,106].
Footnotes
Disclaimer
The findings and conclusions in this article are those of the authors, and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Transparency Declaration
None of the authors have any conflict of interests to declare.
References
- 1.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013;19:1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atmar RL, Opekun AR, Gilger MA, et al. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14:1553–1557. doi: 10.3201/eid1410.080117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teunis PF, Moe CL, Liu P, et al. Norwalk virus: how infectious is it? J Med Virol. 2008;80:1468–1476. doi: 10.1002/jmv.21237. [DOI] [PubMed] [Google Scholar]
- 4.Lopman B, Gastanaduy P, Park GW, Hall AJ, Parashar UD, Vinje J. Environmental transmission of norovirus gastroenteritis. Curr Opin Virol. 2012;2:96–102. doi: 10.1016/j.coviro.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Green K. Caliciviridae: the noroviruses. In: Knipe DM, Howley PM, editors. Fields’ virology. 6th edn Lippincott Williams & Wilkins; Philadelphia, PA: 2013. pp. 583–609. [Google Scholar]
- 6.Kroneman A, Verhoef L, Harris J, et al. Analysis of integrated virological and epidemiological reports of norovirus outbreaks collected within the Foodborne Viruses in Europe network from 1 July 2001 to 30 June 2006. J Clin Microbiol. 2008;46:2959–2965. doi: 10.1128/JCM.00499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacCannell T, Umscheid CA, Agarwal RK, et al. Guideline for the prevention and control of norovirus gastroenteritis outbreaks in healthcare settings. Infect Control Hosp Epidemiol. 2011;32:939–969. doi: 10.1086/662025. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Updated norovirus outbreak management and disease prevention guidelines. MMWR Recomm Rep. 2011;60:1–18. [PubMed] [Google Scholar]
- 9.US Food and Drug Administration . Food code 2013. US Health and Human Services; College Park, MD: [last accessed 13 April 2014]. 2013. Available at: http://www.fda.gov/downloads/Food/GuidanceRegulation/RetailFoodProtection/FoodCode/UCM374510.pdf. [Google Scholar]
- 10.Lee BY, Wettstein ZS, McGlone SM, et al. Economic value of norovirus outbreak control measures in healthcare settings. Clin Microbiol Infect. 2011;17:640–646. doi: 10.1111/j.1469-0691.2010.03345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danial J, Cepeda JA, Cameron F, Cloy K, Wishart D, Templeton KE. Epidemiology and costs associated with norovirus outbreaks in NHS Lothian, Scotland 2007–2009. J Hosp Infect. 2011;79:354–358. doi: 10.1016/j.jhin.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Lopman BA, Reacher MH, Vipond IB, et al. Epidemiology and cost of nosocomial gastroenteritis, Avon, England, 2002–2003. Emerg Infect Dis. 2004;10:1827–1834. doi: 10.3201/eid1010.030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinje J. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol. 2014;52:147–155. doi: 10.1128/JCM.02680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall AJ, Wikswo ME, Manikonda K, Roberts VA, Yoder JS, Gould LH. Acute gastroenteritis surveillance through the national outbreak reporting system, United States. Emerg Infect Dis. 2013;19:1305–1309. doi: 10.3201/eid1908.130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- 16.Wikswo ME, Hall AJ, Centers for Disease Control and Prevention Outbreaks of acute gastroenteritis transmitted by person-to-person contact—United States, 2009–2010. MMWR Surveill Summ. 2012;61:1–12. [PubMed] [Google Scholar]
- 17.Lopman BA, Adak GK, Reacher MH, Brown DW. Two epidemiologic patterns of norovirus outbreaks: surveillance in England and Wales, 1992–2000. Emerg Infect Dis. 2003;9:71–77. doi: 10.3201/eid0901.020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scallan E, Hoekstra RM, Angulo FJ, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopman B, van Duynhoven Y, Hanon FX, et al. Laboratory capability in Europe for foodborne viruses. Euro Surveill. 2002;7:61–65. doi: 10.2807/esm.07.04.00323-en. [DOI] [PubMed] [Google Scholar]
- 20.Verhoef L, Vennema H, van Pelt W, et al. Use of norovirus genotype profiles to differentiate origins of foodborne outbreaks. Emerg Infect Dis. 2010;16:617–624. doi: 10.3201/eid1604.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall AJ, Eisenbart VG, Etingue AL, Gould LH, Lopman BA, Parashar UD. Epidemiology of foodborne norovirus outbreaks, United States, 2001–2008. Emerg Infect Dis. 2012;18:1566–1573. doi: 10.3201/eid1810.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozawa K, Oka T, Takeda N, Hansman GS. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol. 2007;45:3996–4005. doi: 10.1128/JCM.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sukhrie FH, Teunis P, Vennema H, et al. Nosocomial transmission of norovirus is mainly caused by symptomatic cases. Clin Infect Dis. 2012;54:931–937. doi: 10.1093/cid/cir971. [DOI] [PubMed] [Google Scholar]
- 24.O’Neill PD, Marks PJ. Bayesian model choice and infection route modelling in an outbreak of norovirus. Stat Med. 2005;24:2011–2024. doi: 10.1002/sim.2090. [DOI] [PubMed] [Google Scholar]
- 25.Saito M, Goel-Apaza S, Espetia S, et al. Multiple norovirus infections in a birth cohort in a Peruvian periurban community. Clin Infect Dis. 2014;58:483–491. doi: 10.1093/cid/cit763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leshem E, Barclay L, Wikswo M, et al. Genotype GI.6 norovirus, United States, 2010–2012. Emerg Infect Dis. 2013;19:1317–1320. doi: 10.3201/eid1908.130445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leshem E, Wikswo M, Barclay L, et al. Effects and clinical significance of GII.4 Sydney norovirus, United States, 2012–2013. Emerg Infect Dis. 2013;19:1231–1238. doi: 10.3201/eid1908.130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huhti L, Szakal ED, Puustinen L, et al. Norovirus GII-4 causes a more severe gastroenteritis than other noroviruses in young children. J Infect Dis. 2011;203:1442–1444. doi: 10.1093/infdis/jir039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park GW, Barclay L, Macinga D, Charbonneau D, Pettigrew CA, Vinje J. Comparative efficacy of seven hand sanitizers against Murine norovirus, Feline calicivirus, and GII.4 norovirus. J Food Prot. 2010;73:2232–2238. doi: 10.4315/0362-028x-73.12.2232. [DOI] [PubMed] [Google Scholar]
- 30.Gentry J, Vinje J, Lipp EK. A rapid and efficient method for quantitation of genogroups I and II norovirus from oysters and application in other complex environmental samples. J Virol Methods. 2009;156:59–65. doi: 10.1016/j.jviromet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Desai R, Hembree CD, Handel A, et al. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: a systematic literature review. Clin Infect Dis. 2012;55:189–193. doi: 10.1093/cid/cis372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connolly MA, World Health Organization, editor. Communicable disease control in emergencies: a field manual. WHO; Geneva: [accessed 13 April 2014]. 2005. Available at: http://whqlibdoc.who.int/publications/2005/9241546166_eng.pdf?ua=1. [Google Scholar]
- 33.New Zealand Ministry of Health [accessed 13 April 2014];Guidelines for the management of norovirus outbreaks in hospitals and elderly care institutions, 2009. Available at: http://www.health.govt.nz/system/files/documents/publications/guidelines-management-norovirus_0.pdf.
- 34.Said MA, Perl TM, Sears CL. Healthcare epidemiology: gastrointestinal flu: norovirus in health care and long-term care facilities. Clin Infect Dis. 2008;47:1202–1208. doi: 10.1086/592299. [DOI] [PubMed] [Google Scholar]
- 35.Harris JP, Lopman BA, O’Brien SJ. Infection control measures for norovirus: a systematic review of outbreaks in semi-enclosed settings. J Hosp Infect. 2010;74:1–9. doi: 10.1016/j.jhin.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Health Protection Agency. British Infection Association. Healthcare Infection Society. Infection Prevention Society. National Concern for Healthcare Infections. National Health Service Confederation [accessed 13 April 2014];Guidelines for the management of norovirus outbreaks in acute and community health and social care settings. 2012 Available at: http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317131639453.
- 37.Stone SP, Cooper BS, Kibbler CC, et al. The ORION statement: guidelines for transparent reporting of outbreak reports and intervention studies of nosocomial infection. Lancet Infect Dis. 2007;7:282–288. doi: 10.1016/S1473-3099(07)70082-8. [DOI] [PubMed] [Google Scholar]
- 38.National Disease Surveillance Centre Ireland [accessed 13 April 2014];National guidelines on the management of outbreaks of norovirus infection in healthcare settings. 2004 Available at: https://www.hpsc.ie/hpsc/A-Z/Gastroenteric/Viral-Gastroenteritis/Publications/File,1194,en.pdf.
- 39.Communicable Disease Network Australia [accessed 13 April 2014];Guidelines for the public health management of gastroenteritis outbreaks due to norovirus or suspected viral agents in Australia. 2010 Available at: https://www.health.gov.au/internet/main/publishing.nsf/Content/cda-cdna-norovirus.htm/$File/norovirus-guidelines.pdf.
- 40.Cheng VC, Wong LM, Tai JW, et al. Prevention of nosocomial transmission of norovirus by strategic infection control measures. Infect Control Hosp Epidemiol. 2011;32:229–237. doi: 10.1086/658330. [DOI] [PubMed] [Google Scholar]
- 41.Liu P, Yuen Y, Hsiao HM, Jaykus LA, Moe C. Effectiveness of liquid soap and hand sanitizer against Norwalk virus on contaminated hands. Appl Environ Microbiol. 2010;76:394–399. doi: 10.1128/AEM.01729-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blaney DD, Daly ER, Kirkland KB, Tongren JE, Kelso PT, Talbot EA. Use of alcohol-based hand sanitizers as a risk factor for norovirus outbreaks in long-term care facilities in Northern New England: December 2006 to March 2007. Am J Infect Control. 2011;39:296–301. doi: 10.1016/j.ajic.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Macinga DR, Sattar SA, Jaykus LA, Arbogast JW. Improved inactivation of nonenveloped enteric viruses and their surrogates by a novel alcohol-based hand sanitizer. Appl Environ Microbiol. 2008;74:5047–5052. doi: 10.1128/AEM.00487-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolton SL, Kotwal G, Harrison MA, Law SE, Harrison JA, Cannon JL. Sanitizer efficacy against murine norovirus, a surrogate for human norovirus, on stainless steel surfaces when using three application methods. Appl Environ Microbiol. 2013;79:1368–1377. doi: 10.1128/AEM.02843-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sickbert-Bennett EE, Weber DJ, Gergen-Teague MF, Sobsey MD, Samsa GP, Rutala WA. Comparative efficacy of hand hygiene agents in the reduction of bacteria and viruses. Am J Infect Control. 2005;33:67–77. doi: 10.1016/j.ajic.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris JP, Lopman BA, Cooper BS, O’Brien SJ. Does spatial proximity drive norovirus transmission during outbreaks in hospitals? BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-003060. doi: 10.1136/bmjopen-2013-003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heijne JC, Teunis P, Morroy G, et al. Enhanced hygiene measures and norovirus transmission during an outbreak. Emerg Infect Dis. 2009;15:24–30. doi: 10.3201/1501.080299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park GW, Sobsey MD. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII.4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne Pathog Dis. 2011;8:1005–1010. doi: 10.1089/fpd.2010.0782. [DOI] [PubMed] [Google Scholar]
- 49.Sandora TJ, Shih MC, Goldmann DA. Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: a randomized, controlled trial of an infection-control intervention. Pediatrics. 2008;121:e1555–e1562. doi: 10.1542/peds.2007-2597. [DOI] [PubMed] [Google Scholar]
- 50.Feliciano L, Li J, Lee J, Pascall MA. Efficacies of sodium hypochlorite and quaternary ammonium sanitizers for reduction of norovirus and selected bacteria during ware-washing operations. PLoS One. 2012;7:e50273. doi: 10.1371/journal.pone.0050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Girard M, Ngazoa S, Mattison K, Jean J. Attachment of noroviruses to stainless steel and their inactivation, using household disinfectants. J Food Prot. 2010;73:400–404. doi: 10.4315/0362-028x-73.2.400. [DOI] [PubMed] [Google Scholar]
- 52.Tung G, Macinga D, Arbogast J, Jaykus LA. Efficacy of commonly used disinfectants for inactivation of human noroviruses and their surrogates. J Food Prot. 2013;76:1210–1217. doi: 10.4315/0362-028X.JFP-12-532. [DOI] [PubMed] [Google Scholar]
- 53.Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J Hosp Infect. 2004;58:42–49. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 54.US Environmental Protection Agency [accessed 14 April 2014];List G: EPA’s registered antimicrobial products effective against norovirus (Norwalk-like virus) 2009 Available at: http://www.epa.gov/oppad001/list_g_norovirus.pdf.
- 55.Wadl M, Scherer K, Nielsen S, et al. Food-borne norovirus-outbreak at a military base, Germany, 2009. BMC Infect Dis. 2010;10:30. doi: 10.1186/1471-2334-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinnard C, Lee I, Linkin D. Successful control of a norovirus outbreak among attendees of a hospital teaching conference. Am J Infect Control. 2012;40:73–74. doi: 10.1016/j.ajic.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 57.Centers for Disease Control and Prevention Norovirus outbreak in an elementary school—District of Columbia, February 2007. MMWR Morb Mortal Wkly Rep. 2008;56:1340–1343. [PubMed] [Google Scholar]
- 58.Illingworth E, Taborn E, Fielding D, Cheesbrough J, Diggle PJ, Orr D. Is closure of entire wards necessary to control norovirus outbreaks in hospital? Comparing the effectiveness of two infection control strategies. J Hosp Infect. 2011;79:32–37. doi: 10.1016/j.jhin.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 59.Cheng FW, Leung TF, Lai RW, Chan PK, Hon EK, Ng PC. Rapid control of norovirus gastroenteritis outbreak in an acute paediatric ward. Acta Paediatr. 2006;95:581–586. doi: 10.1080/08035250500449874. [DOI] [PubMed] [Google Scholar]
- 60.Vivancos R, Sundkvist T, Barker D, Burton J, Nair P. Effect of exclusion policy on the control of outbreaks of suspected viral gastroenteritis: analysis of outbreak investigations in care homes. Am J Infect Control. 2010;38:139–143. doi: 10.1016/j.ajic.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Zingg W, Colombo C, Jucker T, Bossart W, Ruef C. Impact of an outbreak of norovirus infection on hospital resources. Infect Control Hosp Epidemiol. 2005;26:263–267. doi: 10.1086/502537. [DOI] [PubMed] [Google Scholar]
- 62.Kanerva M, Maunula L, Lappalainen M, Mannonen L, von Bonsdorff CH, Anttila VJ. Prolonged norovirus outbreak in a Finnish tertiary care hospital caused by GII.4-2006b subvariants. J Hosp Infect. 2009;71:206–213. doi: 10.1016/j.jhin.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24:141–173. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bitler EJ, Matthews JE, Dickey BW, Eisenberg JN, Leon JS. Norovirus outbreaks: a systematic review of commonly implicated transmission routes and vehicles. Epidemiol Infect. 2013;141:1563–1571. doi: 10.1017/S095026881300006X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Greig JD, Lee MB, Harris JE. Review of enteric outbreaks in prisons: effective infection control interventions. Public Health. 2011;125:222–228. doi: 10.1016/j.puhe.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Thornley CN, Hewitt J, Perumal L, et al. Multiple outbreaks of a novel norovirus GII.4 linked to an infected post-symptomatic food handler. Epidemiol Infect. 2013;141:1585–1597. doi: 10.1017/S0950268813000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seitz SR, Leon JS, Schwab KJ, et al. Norovirus infectivity in humans and persistence in water. Appl Environ Microbiol. 2011;77:6884–6888. doi: 10.1128/AEM.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Papafragkou E, Hewitt J, Park GW, Greening G, Vinje J. Challenges of culturing human norovirus in three-dimensional organoid intestinal cell culture models. PLoS One. 2013;8:e63485. doi: 10.1371/journal.pone.0063485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones MV, Bellamy K, Alcock R, Hudson R. The use of bacteriophage MS2 as a model system to evaluate virucidal hand disinfectants. J Hosp Infect. 1991;17:279–285. doi: 10.1016/0195-6701(91)90272-a. [DOI] [PubMed] [Google Scholar]
- 70.Wobus CE, Thackray LB, Virgin HW., 4th Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.US Environmental Protection Agency . Inital virucidal effectiveness test using feline calicivirus as surrogate for norovirus. EPA; Washington, DC: [accessed 14 April 2014]. 2001. Available at: http://www.epa.gov/oppad001/pdf_files/initial_virucidal_test.pdf. [Google Scholar]
- 72.Baert L, Uyttendaele M, Vermeersch M, Van Coillie E, Debevere J. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J Food Prot. 2008;71:1590–1597. doi: 10.4315/0362-028x-71.8.1590. [DOI] [PubMed] [Google Scholar]
- 73.Butot S, Putallaz T, Sanchez G. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int J Food Microbiol. 2008;126:30–35. doi: 10.1016/j.ijfoodmicro.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 74.Lamhoujeb S, Fliss I, Ngazoa SE, Jean J. Evaluation of the persistence of infectious human noroviruses on food surfaces by using real-time nucleic acid sequence-based amplification. Appl Environ Microbiol. 2008;74:3349–3355. doi: 10.1128/AEM.02878-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhaelen K, Bouwknegt M, Lodder-Verschoor F, Rutjes SA, de Roda Husman AM. Persistence of human norovirus GII.4 and GI.4, murine norovirus, and human adenovirus on soft berries as compared with PBS at commonly applied storage conditions. Int J Food Microbiol. 2012;160:137–144. doi: 10.1016/j.ijfoodmicro.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 76.D’Souza DH, Sair A, Williams K, et al. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int J Food Microbiol. 2006;108:84–91. doi: 10.1016/j.ijfoodmicro.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 77.Sattar SA, Ali M, Tetro JA. In vivo comparison of two human norovirus surrogates for testing ethanol-based handrubs: the mouse chasing the cat! PLoS One. 2011;6:e17340. doi: 10.1371/journal.pone.0017340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotwal G, Cannon JL. Environmental persistence and transfer of enteric viruses. Curr Opin Virol. 2014;4C:37–43. doi: 10.1016/j.coviro.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 79.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38:S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 80.Liu P, Escudero B, Jaykus LA, et al. Laboratory evidence of Norwalk virus contamination on the hands of infected individuals. Appl Environ Microbiol. 2013;79:7875–7881. doi: 10.1128/AEM.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. World Health Organization CDC/WHO Hand Hygiene Guidelines crosswalk. Jt Comm Perspect. 2008;28:4–7. [PubMed] [Google Scholar]
- 82.Edmonds SL, Mann J, McCormack RR, et al. SaniTwice: a novel approach to hand hygiene for reducing bacterial contamination on hands when soap and water are unavailable. J Food Prot. 2010;73:2296–2300. doi: 10.4315/0362-028x-73.12.2296. [DOI] [PubMed] [Google Scholar]
- 83.Liu P, Macinga DR, Fernandez ML, et al. Comparison of the activity of alcohol-based handrubs against human noroviruses using the finger-pad method and quantitative real-time PCR. Food Environ Virol. 2011;3:35–42. doi: 10.1007/s12560-011-9053-x. [DOI] [PubMed] [Google Scholar]
- 84.Rutala WA, Weber DJ. [last accessed 14 April 2014];Healthcare Infection Control Practices Advisory Committee HICPAC. Guideline for disinfection and sterilization in healthcare facilities. 2008 Available at: http://www.cdc.gov/hicpac/pdf/guidelines/disinfection_nov_2008.pdf.
- 85.Sattar SA, Springthorpe VS, Adegbunrin O, Zafer AA, Busa M. A disc-based quantitative carrier test method to assess the virucidal activity of chemical germicides. J Virol Methods. 2003;112:3–12. doi: 10.1016/s0166-0934(03)00192-7. [DOI] [PubMed] [Google Scholar]
- 86.Park GW, Linden KG, Sobsey MD. Inactivation of murine norovirus, feline calicivirus and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett Appl Microbiol. 2011;52:162–167. doi: 10.1111/j.1472-765X.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 87.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinje J. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot. 2006;69:2761–2765. doi: 10.4315/0362-028x-69.11.2761. [DOI] [PubMed] [Google Scholar]
- 88.Russell AD, Hugo WB, Ayliffe GAJ, editors. Principles and practice of disinfection, preservation and sterilization. 3rd edn Blackwell Scientific; Oxford: 1999. [Google Scholar]
- 89.Park GW, Boston DM, Kase JA, Sampson MN, Sobsey MD. Evaluation of liquid- and fog-based application of sterilox hypochlorous acid solution for surface inactivation of human norovirus. Appl Environ Microbiol. 2007;73:4463–4468. doi: 10.1128/AEM.02839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tuladhar E, Terpstra P, Koopmans M, Duizer E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J Hosp Infect. 2012;80:110–115. doi: 10.1016/j.jhin.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 91.Hudson JB, Sharma M, Petric M. Inactivation of norovirus by ozone gas in conditions relevant to healthcare. J Hosp Infect. 2007;66:40–45. doi: 10.1016/j.jhin.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 92.Tanner BD. Reduction in infection risk through treatment of microbially contaminated surfaces with a novel, portable, saturated steam vapor disinfection system. Am J Infect Control. 2009;37:20–27. doi: 10.1016/j.ajic.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Schultz AC, Uhrbrand K, Norrung B, Dalsgaard A. Inactivation of norovirus surrogates on surfaces and raspberries by steam-ultrasound treatment. J Food Prot. 2012;75:376–381. doi: 10.4315/0362-028X.JFP-11-271. [DOI] [PubMed] [Google Scholar]
- 94.Cho M, Kim J, Vinjé J, Park GW. Development of fluorinated TiO2 film and evaluation of its potential application to control human norovirus on environmental surfaces. Am J Infect Control. 2010;38:e16. [Google Scholar]
- 95.Sattar SA. Promises and pitfalls of recent advances in chemical means of preventing the spread of nosocomial infections by environmental surfaces. Am J Infect Control. 2010;38:S34–S40. doi: 10.1016/j.ajic.2010.04.207. [DOI] [PubMed] [Google Scholar]
- 96.Li J, Predmore A, Divers E, Lou F. New interventions against human norovirus: progress, opportunities, and challenges. Annu Rev Food Sci Technol. 2012;3:331–352. doi: 10.1146/annurev-food-022811-101234. [DOI] [PubMed] [Google Scholar]
- 97.Tibollo S, Cesari C, Colucci ME, et al. High hydrostatic pressure activity on the disinfection of clams artificially contaminated with feline calicivirus. Ann Ig. 2013;25:201–208. doi: 10.7416/ai.2013.1922. [DOI] [PubMed] [Google Scholar]
- 98.Li X, Ye M, Neetoo H, Golovan S, Chen H. Pressure inactivation of Tulane virus, a candidate surrogate for human norovirus and its potential application in food industry. Int J Food Microbiol. 2013;162:37–42. doi: 10.1016/j.ijfoodmicro.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 99.Li X, Chen H, Kingsley DH. The influence of temperature, pH, and water immersion on the high hydrostatic pressure inactivation of GI.1 and GII.4 human noroviruses. Int J Food Microbiol. 2013;167:138–143. doi: 10.1016/j.ijfoodmicro.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 100.Hirneisen KA, Kniel KE. Inactivation of internalized and surface contaminated enteric viruses in green onions. Int J Food Microbiol. 2013;166:201–206. doi: 10.1016/j.ijfoodmicro.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 101.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk virus illness. N Engl J Med. 2011;365:2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blazevic V, Lappalainen S, Nurminen K, Huhti L, Vesikari T. Norovirus VLPs and rotavirus VP6 protein as combined vaccine for childhood gastroenteritis. Vaccine. 2011;29:8126–8133. doi: 10.1016/j.vaccine.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 103.Velasquez LS, Shira S, Berta AN, et al. Intranasal delivery of Norwalk virus-like particles formulated in an in situ gelling, dry powder vaccine. Vaccine. 2011;29:5221–5231. doi: 10.1016/j.vaccine.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tamminen K, Huhti L, Vesikari T, Blazevic V. Pre-existing immunity to norovirus GII-4 virus-like particles does not impair de novo immune responses to norovirus GII-12 genotype. Viral Immunol. 2013;26:167–170. doi: 10.1089/vim.2012.0082. [DOI] [PubMed] [Google Scholar]
- 105.Wang L, Cao D, Wei C, Meng XJ, Jiang X, Tan M. A dual vaccine candidate against norovirus and hepatitis E virus. Vaccine. 2014;32:445–452. doi: 10.1016/j.vaccine.2013.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine. 2012;30:7097–7104. doi: 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sukhrie FH, Siebenga JJ, Beersma MF, Koopmans M. Chronic shedders as reservoir for nosocomial transmission of norovirus. J Clin Microbiol. 2010;48:4303–4305. doi: 10.1128/JCM.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis. 2013;19:1260–1267. doi: 10.3201/eid1908.130472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheesbrough JS, Barkess-Jones L, Brown DW. Possible prolonged environmental survival of small round structured viruses. J Hosp Infect. 1997;35:325–326. doi: 10.1016/s0195-6701(97)90230-9. [DOI] [PubMed] [Google Scholar]
- 110.Thornley CN, Emslie NA, Sprott TW, Greening GE, Rapana JP. Recurring norovirus transmission on an airplane. Clin Infect Dis. 2011;53:515–520. doi: 10.1093/cid/cir465. [DOI] [PubMed] [Google Scholar]