Abstract
The growth and maturation of the ovarian follicle requires the coordinate function of somatic cells and the oocyte. Over the past three decades, numerous growth factors involved in the bidirectional signals between the somatic and germ cells have been identified. A possible function of epidermal growth factor (EGF) signaling at selected stages of follicle maturation had been proposed early on and is supported by many observations of in vitro effects of this growth factor on steroidogenesis, oocyte maturation, and cumulus expansion. However, attempts to link EGF levels in the follicular fluid with the state of follicle and oocyte maturation have been inconclusive. More recently, data generated using mouse genetic models perturbing ovulation and fertility indicate that EGF-like growth factors, rather than EGF itself, accumulate in the follicle at the time of ovulation. EGF-like growth factor mRNA is regulated by the luteinizing hormone surge, and corresponding proteins are detected in the follicle. The EGF-like growth factors amphiregulin, epiregulin, and betacellulin are potent stimulators of oocyte maturation and cumulus expansion, and perturbation of this EGF network in vivo impairs ovulation. Similar findings in species other than the mouse confirm an important physiological role for this network at the time of ovulation. Whether this network also plays a critical role in humans and whether it can be used as a biological marker of follicle development or for the improvement of fertility remains to be determined. This review summarizes the most recent findings on the EGF network during ovulation and the potential clinical applications of manipulating this intercellular communication pathway in the control of fertility.
Keywords: Epidermal growth factor (EGF), EGF-like factors, oocyte, follicular fluid
The development and maturation of an oocyte competent to fertilize and develop into an embryo depends on extraovarian and intraovarian signals, as well as on a permissive ovarian follicular microenvironment. The oocyte is housed within the ovarian follicle where, depending on the stage of development, it is surrounded by one or more layers of granulosa cells that are separated from the theca cells by a basement membrane.1,2 The pituitary gonadotropin follicle-stimulating hormone (FSH) promotes the growth of immature follicles to the preovulatory stage.3 This period of follicular growth is marked by proliferation of somatic cells, oocyte growth and acquisition of competence to resume meiosis, and the development of an antral cavity filled with follicular fluid. At the preovulatory stage, the oocyte is surrounded by specialized granulosa cells called cumulus cells that are functionally distinct from the mural granulosa cells that line the antrum.4 Following the surge in luteinizing hormone (LH), several critical steps are activated in the preovulatory follicle in preparation for ovulation of a mature cumulus-oocyte complex (COC): granulosa cells are reprogrammed to express specific genes required for their terminal differentiation, cumulus cells produce an extracellular matrix on which the cells move away from the oocyte, a process called cumulus expansion, and oocytes traverse two critical maturational steps, termed nuclear and cytoplasmic maturations.5–7 Oocyte nuclear maturation involves oocyte reentry into the cell cycle, completion of the first meiotic division, and upon ovulation, progression to metaphase II (MII).8,9 Although poorly defined, changes that are essential for embryo development also occur in the cytoplasm of the oocyte. These changes, collectively termed cytoplasmic maturation or acquisition of developmental competence, may include a carefully executed epigenetic program of recruitment of maternal mRNAs to the polysomes for the synthesis of selected proteins, post-translational modification of proteins likely involved in the reassembly of cell cycle machinery that will be required for the transition from the meiotic to mitotic cell cycle, and organelle modifications and relocation throughout the cytoplasm of the mature oocyte.10
Whereas the development of the follicle is regulated by bidirectional signals between the oocyte and surrounding somatic cells, the LH-induced maturation of the oocyte is under the control of signals from the follicular somatic cells. Many of the factors involved in this bidirectional crosstalk are likely present in the ovarian follicular fluid. This fluid is an ultrafiltrate of serum that is modified in composition by products secreted by or released from the somatic cells of the preovulatory follicle.11–15 Indeed numerous bioactive factors have been identified in the follicular fluid of humans (Table 1) as well as large farm animals, including insulin-like growth factor (IGF) 1 and IGF2,16–20 vascular endothelial growth factor (VEGF),21–26,87 and brain-derived neurotrophic growth factor (BDNF),27,28 as well as the oocyte-derived factors growth differentiation factor 9 (GDF-9) (in sheep; J.L. Juengel, unpublished data) and bone morphogenetic protein 15 (BMP-15).29 Briefly, BMP-15, a member of the transforming growth factor beta (TGF-β) superfamily, is required for folliculogenesis, granulosa cell proliferation, and fertility in mammals.30,31 In a recent study by Wu et al29 human follicular fluid BMP-15 levels monitored by Western blot were found to correlate positively with fertilization rates and embryo quality, suggesting this factor may be one determinant of oocyte quality and embryo development. The related family member GDF-9 that is also necessary during folliculogenesis and differentiation32,33 has not yet been reported in the follicular fluid of humans but has been detected in primate34 and in sheep fluids (J.L. Juengel, unpublished data). BDNF, a neurotrophic factor important during follicular development and likely at the time of ovulation, also has been described in human follicular fluid,27,28 although the role of this protein in oocyte developmental competence remains to be determined. Several studies report the presence of VEGF in human as well as mare and pig follicular fluids,21–26 but whether the role of VEGF in oocyte maturation is simply to increase the availability of serum factors, such as FSH or LH, for the developing follicle via enhanced vascularization and whether relative levels of VEGF correlate with other markers of oocyte health remain unclear. The IGF system that is also well documented in the follicular fluid is summarized elsewhere in this issue (Kwintkiewicz and Giudice).
Table 1.
Concentrations of Selected Growth Factors in the Human Follicular Fluid
| sGrowth Factor | Concentration in Human Follicular Fluid (from selected references) |
|---|---|
| EGF | <10 pg/mL53 4.6 ± 0.4 pg/mL85 |
| TGF-α | 306 ± 124 pg/mL53 16.1 ± 1.0 pg/mL85 |
| AREG | 108 ± 6.3 ng/mL85 |
| BDNF | 645 ± 23.6 pg/mL27 |
| BMP-15 | * Protein present29 |
| IGF-I | 110.9 ± 29 ng/mL16 100–215 ng/mL17 |
| IGF-II | 75–83.7 ng/mL16 486–759 ng/mL17 |
| VEGF | 2822 ± 356 pg/mL21 2269 ± 460 pg/mL87 |
| FGF | 8.7 ± 4.2 ng/mL88 128 ± 66 pg/mL89 |
| LIF | 13 ± 1.1 pg/mL90 |
| Leptin | 10–20 ng/mL91 |
Detected only by Western blot analysis.
EGF, epidermal growth factor; TGA-α, transforming growth factor alpha; AREG, amphiregulin; BDNF, brain-derived neurotrophic growth factor; IGF1, insulin-like growth factor 1; IGF2, insulin-like growth factor 2; VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; LIF, leukemia inhibitory factor.
Correlations between growth factors present in human follicular fluid and oocyte development and maturation have long been sought because a better understanding of the signals involved would have great potential clinical benefit for in vitro fertilization (IVF) and in vitro maturation (IVM) applications. The presence of epidermal growth factor (EGF) or EGF-like activity in human follicular fluid has also been described,35 as well as EGF effects on oocyte maturation and cumulus expansion.36 However, the source of this latter growth factor in the fluid and its role in oocyte developmental competence has been debated. In recent years, new insights into the physiological relevance of the EGF family of growth factors and related receptors in mediating LH effects during the periovulatory period have come into focus. Gonadotropin induction of the EGF-like growth factors amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC) has been reported in preovulatory follicles and granulosa cells of different species,37–42 and data from in vitro and in vivo studies support an essential role for these growth factors in mediating LH induction of ovulation.37,38,43 In light of these observations, this review focuses on the current understanding of the EGF-like growth factors and their role in the development and ovulation of mature oocytes. Emphasis is placed on human data and possible applications in assisted reproduction. The possible role of EGF-like growth factors during early follicle development and growth is not covered, and the reader is referred to one of the early reviews on this topic.44–46
EPIDERMAL GROWTH FACTOR IN FOLLICULAR FLUID
As just summarized, members of several different classes of growth factors have been identified in human follicular fluid. The majority of these studies analyzed fluid samples pooled from different follicles obtained from patients undergoing oocyte retrieval for IVF. Under conditions of ovarian stimulation there is rapid growth, division, and metabolic activity of the somatic cell compartment within the developing follicle. Therefore, it is not surprising that multiple growth factors and anti-apoptotic factors can be found in the follicular fluid in these studies. Whether these conditions of controlled ovarian hyperstimulation mimic what is occurring in the natural human menstrual cycle is unclear. Further, although oocyte-derived factors such as BMP-15 are known to be secreted and to impact cumulus cell functions, growth factors secreted from the somatic cells that impact oocyte development and maturation have been less well defined, although they play a role in oocyte amino acid and cholesterol uptake (this issue, Su et al).
One growth factor that has gained much attention in view of its effects on the follicle and the oocyte is EGF. It is the prototype of a family of ligands produced as transmembrane precursors that are shed from the cell surface by proteolytic processing by metalloproteases.47–50 The mature soluble factors are involved in autocrine and paracrine regulations via binding and activation of specific tyrosine kinase receptors (EGFR/ErbB1, ErbB2–4). This complex array of ligands and receptors is often referred to as the EGF network. The presence of EGF or EGF-like activity in follicular fluid of preovulatory follicles in women undergoing ovarian hyperstimulation and ovulation induction was reported almost two decades ago.35 Since then, numerous studies have focused on measuring EGF levels in follicular fluid. However, there is considerable controversy as to whether the presence of this growth factor reflects passive diffusion from serum or local secretion and whether its presence can be used as a predictor of follicle maturity and egg quality.51,52 Westergaard and Andersen35 found measurable concentrations of EGF in human follicular fluids of small and preovulatory follicles by radioimmunoassay (RIA) and reported a decline in EGF levels with increasing diameter of the follicles. Conversely, Reeka et al53 failed to detect EGF in the follicular fluid but detected, by immunostaining, EGF in oocytes of growing follicles, granulosa cells of preantral follicles, and theca cells of follicles from the preantral to preovulatory follicle stage. Hofmann et al54 suggested an inverse correlation between human follicular fluid EGF content and oocyte maturation. An inverse correlation between follicular fluid levels of EGF and IVF outcome was similarly observed by Ozornek et al.55 Of particular interest is the report of Hsu et al56 in which EGF-like activity was detected in porcine follicular fluid, but the EGF RIA was negative. Das et al57 proposed a stimulatory effect of EGF in human follicular fluid on mouse oocyte maturation and cumulus expansion because immunodepletion of EGF from the fluid resulted in a decrease in both processes compared with the effect of mature human follicular fluid. Given that many studies report little to no detectable EGF in the follicular fluid, the question is raised whether the activity observed is indeed due to EGF or to related molecules.
Another member of the EGF-like growth factor family reported in human follicular fluid is transforming growth factor alpha (TGF-α). In one study, TGF-α was detected in 90% of follicular fluid samples obtained from patients undergoing ovarian stimulation for IVF.58 However, no correlations were found between TGF-α levels and follicle maturity and fertilization outcome. In another study, TGF-α was present at detectable levels in only 37% of follicular fluid samples obtained from patients at IVF oocyte retrieval.53 By immunohistochemistry, TGF-α protein showed a similar distribution in human ovaries as EGF, as described earlier. Thus, although EGF-like biological activity has been detected in follicular fluid, there is controversy regarding the molecules responsible for this activity and its significance.
EPIDERMAL GROWTH FACTOR EFFECTS ON THE OOCYTE AND CUMULUS EXPANSION
Despite the discordant results on the presence and significance of EGF in follicular fluid, the effects of EGF on oocyte maturation and cumulus expansion are well documented. Dekel and Sherizly59 were the first to demonstrate that EGF could induce the maturation of rat follicle-enclosed oocytes. Downs et al60 went on to show that EGF promoted germinal vesicle breakdown (GVBD) in cumulus cell–enclosed mouse oocytes maintained in meiotic arrest in vitro with purines, dibutyryl cyclic adenosine monophosphate (dbcAMP), 3-isobutyl-1-methylxanthine (IBMX), or hypoxanthine (HX), and that this effect required the presence of cumulus cells. In addition, Downs61 reported that EGF triggered cumulus cell expansion more effectively than FSH, suggesting a role for EGF-like factors in maturation of the COC. Since these first reports, numerous studies have confirmed that in vitro exposure of COCs to EGF promotes cumulus expansion and improves the progression of the oocyte through MI and MII. This is true for rodents57,62–66 as well as for many other species, including horses, pigs, and cows.67–72 Even in zebrafish, EGF and TGF-α promote oocyte maturation, possibly by regulation of activin.73
In addition to an effect on nuclear maturation, developmental competence or cytoplasmic maturation is improved in oocytes exposed to EGF. As an example, exposure of bovine oocytes to EGF during maturation allows them to develop to blastocysts in a chemically defined medium74 and promotes protein synthesis.75 In an IVF system using porcine oocytes, the addition of EGF significantly increased the number of oocytes reaching the MII stage, increased the proportion of monospermic oocytes forming normal two pronuclei embryos, and also promoted protein synthesis.76 In cumulus-enclosed oocytes from gonadotropin-primed mice, both nuclear maturation to MII and cytoplasmic maturation, as determined by development to the blastocyst stage, were enhanced with EGF.62 Such a biological action of EGF has also been documented in humans. Human germinal vesicle (GV) stage oocytes exposed to EGF reach the MII stage at a significantly higher rate than controls.36 In addition, significantly more oocytes with an intact cumulus undergo normal intracytoplasmic sperm injection (ICSI) fertilization in the EGF-supplemented versus unsupplemented group, indicating a higher rate of oocyte cytoplasmic maturation. Thus, addition of EGF to the culture medium and retention of the cumulus during culture improve the nuclear and cytoplasmic maturation of human oocytes in vitro.36 These effects may be direct on the oocyte or indirect and mediated by EGF activation of its cognate receptors on cumulus cells.
EGF-LIKE GROWTH FACTORS RATHER THAN EGF ARE REQUIRED FOR OVULATION
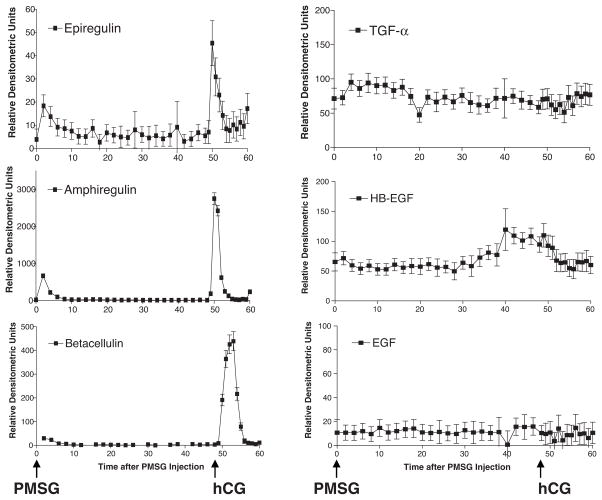
Recent biochemical, genetic and pharmacological evidence from rodent models support the hypothesis that paracrine actions of EGF-like growth factors, rather than EGF, mediate LH effects during the periovulatory period.37,38,43,77 Expression of the EGF-like factor genes Areg, Ereg, and Btc is rapidly and transiently increased in preovulatory follicles in response to LH/human chorionic gonadotropin (hCG) stimulation.37–39,41,42,78 Transcripts for these growth factors are localized specifically to mural granulosa cells of preovulatory follicles but not to the cumulus cells that surround the oocyte (with the exception of betacellulin).37,39 Subsequent studies by Shimada et al have shown that Areg, Ereg, and Btc mRNAs appear in cumulus cells 4 to 8 hours after hCG, indicating that at a certain time before ovulation, the cumulus cells become autonomous in the production of these growth factors and no longer rely on mural granulosa cells.79 AREG protein was detected in mouse ovaries by Western blot,79 whereas EREG was detected by both immunological means and metabolic labeling of mouse granulosa cells isolated after hCG stimulation.37 It should be noted that that a genome-wide analysis of the genes induced by hCG in the mouse ovary indicates dramatic upregulation of Areg, Ereg, and Btc mRNAs, whereas mRNAs coding for Egf, Tgf-α, and Hb-Egf are not affected (Fig. 1). This finding suggests that the physiological activators of EGFRs in the follicle at the time of ovulation are likely neither EGF nor TGF-α.
Figure 1.
Pattern of epidermal growth factor (EGF)-like growth factor mRNA expression during follicle growth and ovulation. Microarray analysis was performed to evaluate the expression of EGF-like growth factor mRNAs in immature wild-type mouse ovaries primed with pregnant mare serum gonadotropin (PMSG) to promote follicle development to the preovulatory stage, and 48 hours later by human chorionic gonadotropin (hCG) to promote differentiation of follicular cells and ovulation. Ereg, Areg, and Btc mRNAs were rapidly and transiently increased in mouse ovaries in response to an ovulatory dose of hCG, whereas Tgf-α, Hb-Egf, and Egf mRNAs were not affected by this stimulus.
These newly identified EGF-like factors mimic many of the LH effects in vitro, in a manner similar to that shown for EGF. In a follicle culture model that recapitulates most of the events that occur in vivo, exogenously applied AREG or EREG induced oocyte meiotic resumption to the same extent as LH but with a faster time course of induction.37 BTC was partially effective in promoting oocyte maturation. These findings are reminiscent of early studies showing that in vitro treatment with EGF promotes oocyte maturation and is a potent stimulus of cumulus expansion.60,61,64 The three growth factors stimulated cumulus expansion in intact follicles, as well as the expression of Ptgs2, Has2, and Tnfaip6 genes that are associated with this event.37,38 In cultures of isolated COCs where LH no longer has an effect, AREG, EREG, or BTC were effective in promoting cumulus expansion and oocyte maturation.37 Given that the presence of EGF-like bioactivity in follicular fluid has been previously reported, AREG, EREG, and BTC are most likely the physiological ligands for the EGFR in the follicle and appear to function downstream of the LH signal. Notably, these factors did not induce maturation of denuded oocytes, indicating a requirement for interactions between the oocyte and cumulus cells.37 A similar absence of direct effects on the isolated oocyte have been reported when using EGF.60,61,64
A more in-depth analysis of the regulation of the EGF network during the periovulatory period by in vitro follicle culture and in vivo genetic disruptions of the EGF network in mice revealed that this signaling system is important not only for the early and rapid propagation of the LH signal, but also for long-lasting effects on the oocyte and embryo development. LH induced EGFR phosphorylation within 30 minutes of stimulation of mouse preovulatory follicles, and this transactivation was sensitive to metalloprotease inhibitors, indicating a requirement for shed EGF-like growth factors.80 In addition, LH-induced but not AREG-induced EGFR phosphorylation and oocyte maturation were prevented when preovulatory follicles were cultured in the presence of a protein synthesis inhibitor.80 Thus transactivation of EGFR signaling occurs either through the rapid de novo synthesis and release of growth factors or by the processing of a small preformed pool of EGF-like growth factors. LH-induced EGFR phosphorylation precedes the onset of oocyte meiotic resumption by at least 2 hours, indicating a compatible role for EGF-like growth factors in the induction of oocyte maturation. The early transactivation of the EGF network, then, likely serves to amplify the initial LH signal and appears indispensable for LH-stimulated oocyte maturation. Indeed, the onset of oocyte maturation was delayed in Areg-null mice, and with increased disruption of the EGF signaling network, significantly reduced EGFR phosphorylation induced by LH was associated with impaired oocyte meiotic resumption and cumulus expansion in hCG-primed Areg−/− Egfrwa2/wa2 mouse ovaries in vivo.43
Most striking is the finding that disruption of the EGF signaling network in mice results in impaired LH-induced ovulation.43 Similar results were observed in rats, where injection of the EGFR tyrosine kinase inhibitor AG1478 into rat ovarian bursas resulted in a significant decrease in LH-induced ovulation in the treated ovary, compared with the untreated contralateral ovaries and vehicle-treated ovaries.38 Histological analyses of these AG1478-treated ovaries revealed most large antral follicles contained entrapped oocytes, many that were still immature. The late effects of the EGF network during the periovulatory period are potentially important. For instance, EREG expression in the follicle decreases 9 hours after LH but then increases again around the time of ovulation, suggesting that this growth factor serves an additional function after ovulation.37 Shimada et al also observed that AREG expression in cumulus cells persists in COCs retrieved from the ampulla after ovulation; ovulated, expanded COCs cultured in the presence of AREG retained their structural integrity, whereas COCs cultured in the absence of AREG disintegrated.79 Thus it is likely that the function of these growth factors extends to fertilization and beyond.
EGF-LIKE GROWTH FACTORS IN HUMAN FOLLICLES
In light of the studies just summarized demonstrating an indispensable role of EGF-like growth factors as mediators of gonadotropin signaling and initiation of oocyte nuclear maturation in rodent models of ovulation, the EGF activity detected in the follicular fluid of different species may be due not to EGF but to EGF-like growth factors. This would explain the discrepancy between the bioactivity and RIA results previously reported in porcine follicular fluid.56 Thus a question to answer is whether AREG, EREG, or BTC accumulate in human follicular fluid.
Recent studies have documented the LH/hCG-induced expression of Areg and Ereg mRNAs in primary cultures of human granulosa cells.40,81,82 To our knowledge, induction of Btc expression in human follicles has not yet been reported. However, in granulosa cells obtained from rhesus macaques undergoing controlled ovarian stimulation protocols, Areg and Ereg mRNAs increased 3 and 12 hours post-hCG, respectively, whereas Btc expression did not change.42 This is in contrast to the gonadotropin-induced increase in Btc mRNAs observed in rodent ovaries.37,38 In primary human granulosa cells obtained from IVF patients, Rimon et al83 reported a >200-fold increase in the mRNA coding Areg by LH, as well as by the direct adenylyl cyclase activator forskolin. Furthermore, in human granulosa cells, it has been demonstrated that LH, forskolin, FSH, and prostaglandin E2 (PGE2) induce the expression of not only Areg mRNA but also AREG protein.40,81 Feuerstein et al84 reported that in patients undergoing ovarian stimulation, elevated Areg mRNA levels in cumulus cells isolated from COCs before ICSI were positively associated with oocyte nuclear maturation. Conversely, lower Areg transcript levels were detected in cumulus cells from oocytes that developed to the blastocyst stage, compared with levels in cumulus cells from oocytes that failed to develop beyond the embryo stage. Taken together, these studies document the hormone-regulated expression of EGF-like growth factors in human follicular somatic cells, with emphasis on Areg.
Recently, Inoue et al85 reported the presence of AREG protein at abundant levels in human follicular fluid collected at the time of IVF oocyte retrieval. The mean concentration of AREG in the follicular fluid measured by a commercial enzyme-linked immunosorbent assay was found to be 108.4 ± 6.3 ng/mL, which was significantly higher when compared with the low levels measured for EGF and TGF-α (in the pg/mL range). Supporting some of the previous reports, EGF levels were below the lower limit of detection in >90% of samples examined. Our laboratory has measured immunoreactive AREG in human follicular fluid from single follicles at levels in a similar range as those reported by Inoue et al85 (A.M. Zamah et al, unpublished data). More importantly, the mean AREG concentration was three orders of magnitude higher in follicular fluid than in what has been published for serum, suggesting local production of this growth factor (A.M. Zamah et al, unpublished data).85,86 In addition, biological activity associated with AREG in these human follicular fluid samples could be demonstrated using a sensitive COC assay (A.M. Zamah et al., unpublished data).
In the study of Inoue et al,85 follicular fluid was pooled from multiple follicles and then analyzed, which makes correlating any results to individual oocyte outcomes difficult. With these limitations in mind, no significant correlation between levels of AREG and embryo quality was detected. On the contrary, high levels of AREG appeared to be correlated with lower fertilization rates even though statistical significance could not be reached. Interestingly, an inverse correlation between the intrafollicular levels of hCG and AREG was also observed, which is somewhat surprising because it is assumed that the hCG trigger causes increased AREG expression. Ideally, similar studies using discrete follicular fluid samples will ideally more clearly identify a role for EGF-like ligands in relevant clinical outcomes.
CONCLUSIONS AND FUTURE PERSPECTIVES
The recent findings summarized here demonstrate that the LH surge causes activation of complex paracrine and autocrine regulations in the follicle that include activation of the EGF network. These studies, mostly conducted in mice, show that activation of this network is necessary and sufficient for oocyte maturation, cumulus expansion, and ovulation. Thus, in this species, the EGF-like growth factors function as mediators of the biological actions of LH (Fig. 2). Although not as extensive, studies in species other than the mouse support a similar conclusion. Based on numerous data with EGF, it is also safe to conclude that exposure to EGF-like factors improves oocyte developmental competence. This conclusion needs to be confirmed with the EGF-like growth factors, but a correlation of these growth factors with egg quality is likely.
Figure 2.
Epidermal growth factor (EGF)-like growth factors in the human follicular fluid. Luteinizing (LH) induces the expression of Areg in the preovulatory follicle, and AREG (amphiregulin) acts in an autocrine and paracrine manner to mediate LH effects throughout the follicle, including the promotion of oocyte meiotic resumption and cumulus expansion. Areg expression is also upregulated in granulosa cells by prostaglandin E2 (PGE2);79,81 at a time preceding ovulation, AREG can also induce the expression of its mRNA in cumulus cells by the PGE2-PGE2 receptor subtype (PTGER2) pathway.81 AREG protein is present in abundant levels in the human follicular fluid85(A.M. Zamah et al, unpublished data). EGF and transforming growth factor alpha (TGF-α) protein levels are very low or undetectable in the fluid and higher in serum, indicating that the presence of these factors in the follicular fluid is likely serum derived. Whether epiregulin (EREG) and betacellulin (BTC) are also present in the fluid remains to be determined.
The obvious question that needs to be addressed is whether the EGF network plays a comparable function in humans. In the in vitro models using cultured human granulosa cells, LH produces identical effects to those described for the mouse. Specifically, LH induces the expression of EGF-like growth factors.40 Thus activation of the EGF network in human granulosa cells is likely (Fig. 2). This conclusion is consistent with the observation of Inoue et al85 and our own: High levels of AREG are detected in the follicular fluid of patients after hCG stimulation. Nevertheless, the significance of the activation of this network in humans remains elusive. One would expect that on the basis of the numerous observations in animal models, LH activation of the EGF network should be a positive factor in ovulation. However, the recent report discussed here shows either no correlation or an inverse relationship between AREG and fertilization rate. With the caveat of the confounding factor that fluids from different follicles were pooled, it is difficult to reconcile this conclusion with the data in other species. An issue that will need to be addressed is the timing of the release of these growth factors after the endogenous LH stimulation or the hCG injection. Although difficult to obtain in humans, this information will be critical to interpret the human data correctly. Ideally it should be possible to detect these changes in the follicular fluid throughout the periovulatory period. Moreover, it is possible there is a threshold effect, and ovarian hyperstimulation removes any potential correlation between accumulation of growth factors in the follicular fluid and oocyte quality.
A better understanding of the growth factors involved in oocyte development and maturation would be of great potential clinical benefit. According to the most recent SART (Society for Assisted Reproductive Technology; http://www.sart.org/) data for all IVF cycles in 2006, the average pregnancy rate per cycle in women ≤ 40 years of age was roughly 38.5%. In terms of ovarian stimulation, understanding the relevant intrafollicular growth factors could allow for the selection of stimulation protocols that optimize the intrafollicular environment. After oocyte retrieval, the in vitro media may be supplemented with optimal growth factor concentrations to allow for further oocyte maturation, which could theoretically improve fertilization rates and possibly lessen the use of additional techniques such as ICSI, which was used in 62% of all IVF cycles in 2006. As with any manipulation of the germ line, long-term studies on safety and efficacy are warranted.
Our knowledge of the intrafollicular environment is advancing at a rapid pace. Challenges remain in being able to define which factors are critical for proper oocyte maturation and in our ability to manipulate the follicular environment to define if it is indeed possible to improve oocyte health and quality in this manner. Understanding the physiological signals responsible for human oocyte development and maturation will allow for safer and more effective fertility treatments, along with potentially improved pregnancy rates and outcomes.
Acknowledgments
The work done in the authors’ laboratory was supported by NIH grants HD 1R01HD058939, 5R01HD020788, and 5R01GM080527, and a grant from Organon. The authors thank Dr. Marcelle Cedars for helpful discussions, and S.M. Mulders and M.D. Sollewign Gelpke at N.V. Organon for making available the microarray data.
References
- 1.Eppig JJ. Oocyte-somatic cell communication in the ovarian follicles of mammals. Semin Dev Biol. 1994;5(1):51–59. [Google Scholar]
- 2.Tsafriri A, Dekel N. Molecular mechanisms in ovulation. In: Findlay JK, editor. Molecular Biology of the Female Reproductive System. San Diego, CA: Academic Press; 1994. pp. 207–258. [Google Scholar]
- 3.Adashi EY. Endocrinology of the ovary. Hum Reprod. 1994;9(5):815–827. doi: 10.1093/oxfordjournals.humrep.a138602. [DOI] [PubMed] [Google Scholar]
- 4.Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120(Pt 8):1330–1340. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 5.Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. Bioessays. 1991;13(11):569–574. doi: 10.1002/bies.950131105. [DOI] [PubMed] [Google Scholar]
- 6.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 7.Tsafriri A, Reich R. Molecular aspects of mammalian ovulation. Exp Clin Endocrinol Diabetes. 1999;107(1):1–11. doi: 10.1055/s-0029-1212066. [DOI] [PubMed] [Google Scholar]
- 8.Conti M, Andersen CB, Richard F, et al. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187(1–2):153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- 9.Dekel N. Protein phosphorylation/dephosphorylation in the meiotic cell cycle of mammalian oocytes. Rev Reprod. 1996;1(2):82–88. doi: 10.1530/ror.0.0010082. [DOI] [PubMed] [Google Scholar]
- 10.Schultz RM. From egg to embryo: a peripatetic journey. Reproduction. 2005;130(6):825–828. doi: 10.1530/rep.1.00902. [DOI] [PubMed] [Google Scholar]
- 11.Clarke HG, Hope SA, Byers S, Rodgers RJ. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 2006;132(1):119–131. doi: 10.1530/rep.1.00960. [DOI] [PubMed] [Google Scholar]
- 12.Shalgi R, Kraicer PF, Soferman N. Gases and electrolytes of human follicular fluid. J Reprod Fertil. 1972;28(3):335–340. doi: 10.1530/jrf.0.0280335. [DOI] [PubMed] [Google Scholar]
- 13.Shalgi R, Kraicer P, Rimon A, Pinto M, Soferman N. Proteins of human follicular fluid: the blood-follicle barrier. Fertil Steril. 1973;24(6):429–434. [PubMed] [Google Scholar]
- 14.Manarang-Pangan S, Menge AC. Immunologic studies on human follicular fluid. Fertil Steril. 1971;22(6):367–372. [PubMed] [Google Scholar]
- 15.Andersen MM, Kroll J, Byskov AG, Faber M. Protein composition in the fluid of individual bovine follicles. J Reprod Fertil. 1976;48(1):109–118. doi: 10.1530/jrf.0.0480109. [DOI] [PubMed] [Google Scholar]
- 16.Wang TH, Chang CL, Wu HM, et al. Insulin-like growth factor-II (IGF-II), IGF-binding protein-3 (IGFBP-3), and IGFBP-4 in follicular fluid are associated with oocyte maturation and embryo development. Fertil Steril. 2006;86(5):1392–1401. doi: 10.1016/j.fertnstert.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 17.Thierry van Dessel HJ, Chandrasekher Y, Yap OW, et al. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81(3):1224–1231. doi: 10.1210/jcem.81.3.8772603. [DOI] [PubMed] [Google Scholar]
- 18.Beg MA, Ginther OJ. Follicle selection in cattle and horses: role of intrafollicular factors. Reproduction. 2006;132(3):365–377. doi: 10.1530/rep.1.01233. [DOI] [PubMed] [Google Scholar]
- 19.Spicer LJ, Santiago CA, Davidson TR, Bridges TS, Chamberlain CS. Follicular fluid concentrations of free insulin-like growth factor (IGF)-I during follicular development in mares. Domest Anim Endocrinol. 2005;29(4):573–581. doi: 10.1016/j.domaniend.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Hammond JM, Hsu CJ, Klindt J, Tsang BK, Downey BR. Gonadotropins increase concentrations of immunoreactive insulin-like growth factor-I in porcine follicular fluid in vivo. Biol Reprod. 1988;38(2):304–308. doi: 10.1095/biolreprod38.2.304. [DOI] [PubMed] [Google Scholar]
- 21.Lee A, Christenson LK, Stouffer RL, Burry KA, Patton PE. Vascular endothelial growth factor levels in serum and follicular fluid of patients undergoing in vitro fertilization. Fertil Steril. 1997;68(2):305–311. doi: 10.1016/s0015-0282(97)81520-8. [DOI] [PubMed] [Google Scholar]
- 22.Ferrari B, Pezzuto A, Barusi L, Coppola F. Follicular fluid vascular endothelial growth factor concentrations are increased during GnRH antagonist/FSH ovarian stimulation cycles. Eur J Obstet Gynecol Reprod Biol. 2006;124(1):70–76. doi: 10.1016/j.ejogrb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Artini PG, Monti M, Fasciani A, et al. Correlation between the amount of follicle-stimulating hormone administered and plasma and follicular fluid vascular endothelial growth factor concentrations in women undergoing in vitro fertilization. Gynecol Endocrinol. 1998;12(4):243–247. doi: 10.3109/09513599809015596. [DOI] [PubMed] [Google Scholar]
- 24.Kawano Y, Zeineh Hasan K, Fukuda J, Mine S, Miyakawa I. Production of vascular endothelial growth factor and angiogenic factor in human follicular fluid. Mol Cell Endocrinol. 2003;202(1–2):19–23. doi: 10.1016/s0303-7207(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 25.Ginther OJ, Gastal EL, Gastal MO, Checura CM, Beg MA. Dose-response study of intrafollicular injection of insulin-like growth factor-I on follicular fluid factors and follicle dominance in mares. Biol Reprod. 2004;70(4):1063–1069. doi: 10.1095/biolreprod.103.024844. [DOI] [PubMed] [Google Scholar]
- 26.Barboni B, Turriani M, Galeati G, et al. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod. 2000;63(3):858–864. doi: 10.1095/biolreprod63.3.858. [DOI] [PubMed] [Google Scholar]
- 27.Seifer DB, Feng B, Shelden RM, Chen S, Dreyfus CF. Brain-derived neurotrophic factor: a novel human ovarian follicular protein. J Clin Endocrinol Metab. 2002;87(2):655–659. doi: 10.1210/jcem.87.2.8213. [DOI] [PubMed] [Google Scholar]
- 28.Seifer DB, Lambert-Messerlian G, Schneyer AL. Ovarian brain-derived neurotrophic factor is present in follicular fluid from normally cycling women. Fertil Steril. 2003;79(2):451–452. doi: 10.1016/s0015-0282(02)04669-1. [DOI] [PubMed] [Google Scholar]
- 29.Wu YT, Tang L, Cai J, et al. High bone morphogenetic protein-15 level in follicular fluid is associated with high quality oocyte and subsequent embryonic development. Hum Reprod. 2007;22(6):1526–1531. doi: 10.1093/humrep/dem029. [DOI] [PubMed] [Google Scholar]
- 30.Su YQ, Wu X, O’Brien MJ, et al. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276(1):64–73. doi: 10.1016/j.ydbio.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 31.Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Albertini DF, Nishimori K, et al. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383(6600):531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 33.Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13(6):1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 34.Duffy DM. Growth differentiation factor-9 is expressed by the primate follicle throughout the periovulatory interval. Biol Reprod. 2003;69(2):725–732. doi: 10.1095/biolreprod.103.015891. [DOI] [PubMed] [Google Scholar]
- 35.Westergaard LG, Andersen CY. Epidermal growth factor (EGF) in human preovulatory follicles. Hum Reprod. 1989;4(3):257–260. doi: 10.1093/oxfordjournals.humrep.a136883. [DOI] [PubMed] [Google Scholar]
- 36.Goud PT, Goud AP, Qian C, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13(6):1638–1644. doi: 10.1093/humrep/13.6.1638. [DOI] [PubMed] [Google Scholar]
- 37.Park JY, Su YQ, Ariga M, et al. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 38.Ashkenazi H, Cao X, Motola S, et al. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146(1):77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- 39.Sekiguchi T, Mizutani T, Yamada K, et al. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33(1):281–291. doi: 10.1677/jme.0.0330281. [DOI] [PubMed] [Google Scholar]
- 40.Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324(2):829–834. doi: 10.1016/j.bbrc.2004.09.129. [DOI] [PubMed] [Google Scholar]
- 41.Lindbloom SM, Farmerie TA, Clay CM, Seidel GE, Jr, Carnevale EM. Potential involvement of EGF-like growth factors and phosphodiesterases in initiation of equine oocyte maturation. Anim Reprod Sci. 2008;103(1–2):187–192. doi: 10.1016/j.anireprosci.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22(5):1247–1252. doi: 10.1093/humrep/del519. [DOI] [PubMed] [Google Scholar]
- 43.Hsieh M, Lee D, Panigone S, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27(5):1914–1924. doi: 10.1128/MCB.01919-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richards JS, Russell DL, Ochsner S, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 45.Matzuk MM. Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol. 2000;163(1–2):61–66. doi: 10.1016/s0303-7207(99)00241-5. [DOI] [PubMed] [Google Scholar]
- 46.Hsueh AJ. Paracrine mechanisms involved in granulosa cell differentiation. Clin Endocrinol Metab. 1986;15(1):117–134. doi: 10.1016/s0300-595x(86)80045-7. [DOI] [PubMed] [Google Scholar]
- 47.Sanderson MP, Dempsey PJ, Dunbar AJ. Control of ErbB signaling through metalloprotease mediated ectodomain shedding of EGF-like factors. Growth Factors. 2006;24(2):121–136. doi: 10.1080/08977190600634373. [DOI] [PubMed] [Google Scholar]
- 48.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 49.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 50.Prenzel N, Zwick E, Daub H, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402(6764):884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 51.Eden JA, Jones J, Carter GD, Alaghband-Zadeh J. Follicular fluid concentrations of insulin-like growth factor 1, epidermal growth factor, transforming growth factor-alpha and sex-steroids in volume matched normal and polycystic human follicles. Clin Endocrinol (Oxf) 1990;32(4):395–405. doi: 10.1111/j.1365-2265.1990.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 52.Barreca A, Minuto F, Volpe A, et al. Insulin-like growth factor-I (IGF-I) and IGF-I binding protein in the follicular fluids of growth hormone treated patients. Clin Endocrinol (Oxf) 1990;32(4):497–505. doi: 10.1111/j.1365-2265.1990.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 53.Reeka N, Berg F, Brucker C. Presence of transforming growth factor alpha and epidermal growth factor in human ovarian tissue and follicular fluid. Hum Reprod. 1998;13(8):2199–2205. doi: 10.1093/humrep/13.8.2199. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann GE, Scott RT, Jr, Brzyski RG, Jones HW., Jr Immunoreactive epidermal growth factor concentrations in follicular fluid obtained from in vitro fertilization. Fertil Steril. 1990;54(2):303–307. [PubMed] [Google Scholar]
- 55.Ozornek MH, Bielfeld P, Krussel JS, et al. Epidermal growth factor and leukemia inhibitory factor levels in follicular fluid. Association with in vitro fertilization outcome. J Reprod Med. 1999;44(4):367–369. [PubMed] [Google Scholar]
- 56.Hsu CJ, Holmes SD, Hammond JM. Ovarian epidermal growth factor-like activity. Concentrations in porcine follicular fluid during follicular enlargement. Biochem Biophys Res Commun. 1987;147(1):242–247. doi: 10.1016/s0006-291x(87)80112-2. [DOI] [PubMed] [Google Scholar]
- 57.Das K, Phipps WR, Hensleigh HC, Tagatz GE. Epidermal growth factor in human follicular fluid stimulates mouse oocyte maturation in vitro. Fertil Steril. 1992;57(4):895–901. doi: 10.1016/s0015-0282(16)54977-2. [DOI] [PubMed] [Google Scholar]
- 58.McWilliam R, Leake RE, Coutts JR. Growth factors in human ovarian follicle fluid and growth factor receptors in granulosaluteal cells. Int J Biol Markers. 1995;10(4):216–220. doi: 10.1177/172460089501000405. [DOI] [PubMed] [Google Scholar]
- 59.Dekel N, Sherizly I. Epidermal growth factor induces maturation of rat follicle-enclosed oocytes. Endocrinology. 1985;116(1):406–409. doi: 10.1210/endo-116-1-406. [DOI] [PubMed] [Google Scholar]
- 60.Downs SM, Daniel SA, Eppig JJ. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: evidence for a positive stimulus of somatic cell origin. J Exp Zool. 1988;245(1):86–96. doi: 10.1002/jez.1402450113. [DOI] [PubMed] [Google Scholar]
- 61.Downs SM. Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41(2):371–379. doi: 10.1095/biolreprod41.2.371. [DOI] [PubMed] [Google Scholar]
- 62.De La Fuente R, O’Brien MJ, Eppig JJ. Epidermal growth factor enhances preimplantation developmental competence of maturing mouse oocytes. Hum Reprod. 1999;14(12):3060–3068. doi: 10.1093/humrep/14.12.3060. [DOI] [PubMed] [Google Scholar]
- 63.Smitz J, Cortvrindt R, Hu Y. Epidermal growth factor combined with recombinant human chorionic gonadotrophin improves meiotic progression in mouse follicle-enclosed oocyte culture. Hum Reprod. 1998;13(3):664–669. doi: 10.1093/humrep/13.3.664. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Yosef D, Galiani D, Dekel N, Shalgi R. Rat oocytes induced to mature by epidermal growth factor are successfully fertilized. Mol Cell Endocrinol. 1992;88(1–3):135–141. doi: 10.1016/0303-7207(92)90018-2. [DOI] [PubMed] [Google Scholar]
- 65.Boland NI, Gosden RG. Effects of epidermal growth factor on the growth and differentiation of cultured mouse ovarian follicles. J Reprod Fertil. 1994;101(2):369–374. doi: 10.1530/jrf.0.1010369. [DOI] [PubMed] [Google Scholar]
- 66.Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol. 1990;138(1):16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- 67.Lorenzo PL, Liu IK, Carneiro GF, Conley AJ, Enders AC. Equine oocyte maturation with epidermal growth factor. Equine Vet J. 2002;34(4):378–382. doi: 10.2746/042516402776249065. [DOI] [PubMed] [Google Scholar]
- 68.Prochazka R, Srsen V, Nagyova E, Miyano T, Flechon JE. Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: nuclear maturation, expansion, and F-actin remodeling. Mol Reprod Dev. 2000;56(1):63–73. doi: 10.1002/(SICI)1098-2795(200005)56:1<63::AID-MRD8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 69.Prochazka R, Kalab P, Nagyova E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell complexes in vitro. Biol Reprod. 2003;68(3):797–803. doi: 10.1095/biolreprod.102.005520. [DOI] [PubMed] [Google Scholar]
- 70.Lorenzo PL, Illera MJ, Illera JC, Illera M. Enhancement of cumulus expansion and nuclear maturation during bovine oocyte maturation in vitro by the addition of epidermal growth factor and insulin-like growth factor I. J Reprod Fertil. 1994;101(3):697–701. doi: 10.1530/jrf.0.1010697. [DOI] [PubMed] [Google Scholar]
- 71.Lonergan P, Carolan C, Van Langendonckt A, et al. Role of epidermal growth factor in bovine oocyte maturation and preimplantation embryo development in vitro. Biol Reprod. 1996;54(6):1420–1429. doi: 10.1095/biolreprod54.6.1420. [DOI] [PubMed] [Google Scholar]
- 72.Rieger D, Luciano AM, Modina S, et al. The effects of epidermal growth factor and insulin-like growth factor I on the metabolic activity, nuclear maturation and subsequent development of cattle oocytes in vitro. J Reprod Fertil. 1998;112(1):123–130. doi: 10.1530/jrf.0.1120123. [DOI] [PubMed] [Google Scholar]
- 73.Pang Y, Ge W. Epidermal growth factor and TGF{alpha} promote zebrafish oocyte maturation in vitro: potential role of the ovarian activin regulatory system. Endocrinology. 2002;143(1):47–54. doi: 10.1210/endo.143.1.8579. [DOI] [PubMed] [Google Scholar]
- 74.Park KW, Iga K, Niwa K. Exposure of bovine oocytes to EGF during maturation allows them to develop to blastocysts in a chemically-defined medium. Theriogenology. 1997;48(7):1127–1135. doi: 10.1016/s0093-691x(97)00345-2. [DOI] [PubMed] [Google Scholar]
- 75.Goff A, Yang Z, Cortvrindt R, Smitz J, Miron P. Protein synthesis during maturation of bovine oocytes, effect of epidermal growth factor. Reprod Domest Anim. 2001;36(1):19–24. doi: 10.1046/j.1439-0531.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 76.Singh B, Meng L, Rutledge JM, Armstrong DT. Effects of epidermal growth factor and follicle-stimulating hormone during in vitro maturation on cytoplasmic maturation of porcine oocytes. Mol Reprod Dev. 1997;46(3):401–407. doi: 10.1002/(SICI)1098-2795(199703)46:3<401::AID-MRD20>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 77.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20(4):715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 78.Espey LLRJ. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67(6):1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- 79.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 80.Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22(4):924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ben-Ami I, Freimann S, Armon L, et al. PGE2 up-regulates EGF-like growth factor biosynthesis in human granulosa cells: new insights into the coordination between PGE2 and LH in ovulation. Mol Hum Reprod. 2006;12(10):593–599. doi: 10.1093/molehr/gal068. [DOI] [PubMed] [Google Scholar]
- 82.Negishi H. Regulation of amphiregulin, EGFR-like factor expression by hCG in cultured human granulosa cells. Acta Obstet Gynecol Scand. 2007;86(6):706–710. doi: 10.1080/00016340701314959. [DOI] [PubMed] [Google Scholar]
- 83.Rimon E, Sasson R, Dantes A, Land-Bracha A, Amsterdam A. Gonadotropin-induced gene regulation in human granulosa cells obtained from IVF patients: modulation of genes coding for growth factors and their receptors and genes involved in cancer and other diseases. Int J Oncol. 2004;24(5):1325–1338. doi: 10.3892/ijo.24.5.1325. [DOI] [PubMed] [Google Scholar]
- 84.Feuerstein P, Cadoret V, Dalbies-Tran R, et al. Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod. 2007;22(12):3069–3077. doi: 10.1093/humrep/dem336. [DOI] [PubMed] [Google Scholar]
- 85.Inoue Y, Miyamoto S, Fukami T, et al. Amphiregulin is much more abundantly expressed than transforming growth factor-alpha and epidermal growth factor in human follicular fluid obtained from patients undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.01.014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 86.Lemos-Gonzalez Y, Rodriguez-Berrocal FJ, Cordero OJ, Gomez C, Paez de la Cadena M. Alteration of the serum levels of the epidermal growth factor receptor and its ligands in patients with non-small cell lung cancer and head and neck carcinoma. Br J Cancer. 2007;96(10):1569–1578. doi: 10.1038/sj.bjc.6603770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gutman G, Barak V, Maslovitz S, et al. Regulation of vascular endothelial growth factor-A and its soluble receptor sFlt-1 by luteinizing hormone in vivo: implication for ovarian follicle angiogenesis. Fertil Steril. 2008;89(4):922–926. doi: 10.1016/j.fertnstert.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 88.Artini PG, Monti M, Matteucci C, et al. Vascular endothelial growth factor and basic fibroblast growth factor in polycystic ovary syndrome during controlled ovarian hyperstimulation. Gynecol Endocrinol. 2006;22(8):465–470. doi: 10.1080/09513590600906607. [DOI] [PubMed] [Google Scholar]
- 89.Hammadeh ME, Fischer-Hammadeh C, Hoffmeister H, et al. Relationship between cytokine concentrations (FGF, sICAM-1 and SCF) in serum, follicular fluid and ICSI outcome. Am J Reprod Immunol. 2004;51(1):81–85. doi: 10.1046/j.8755-8920.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 90.Arici A, Oral E, Bahtiyar O, et al. Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Hum Reprod. 1997;12(6):1233–1239. doi: 10.1093/humrep/12.6.1233. [DOI] [PubMed] [Google Scholar]
- 91.Li MG, Ding GL, Chen XJ, et al. Association of serum and follicular fluid leptin concentrations with granulosa cell phosphorylated signal transducer and activator of transcription 3 expression in fertile patients with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2007;92(12):4771–4776. doi: 10.1210/jc.2007-0978. [DOI] [PubMed] [Google Scholar]