Abstract
BACKGROUND
Acute lung injury following trauma remains a significant source of morbidity and mortality. Though multiple trauma studies have used hypoxemia without radiographic adjudication as a surrogate for identifying acute respiratory distress syndrome (ARDS) cases, the differences between patients with hypoxemia alone and those with radiographically-confirmed ARDS are not well-described in the literature. We hypothesized that non-hypoxemic, hypoxemic, and ARDS patients represent distinct groups with unique characteristics and predictors.
METHODS
Laboratory, demographic, clinical, and outcomes data were prospectively collected from 621 intubated, critically-injured patients at an urban Level 1 Trauma Center from 2005-2013. Hypoxemia was defined as PaO2:FiO2 ratio ≤ 300. ARDS was adjudicated using Berlin criteria, with blinded two-physician consensus review of chest radiographs (CXR). Group comparisons were performed by hypoxemia and ARDS status. Logistic regression analyses were performed to separately assess predictors of hypoxemia and ARDS.
RESULTS
Of the 621 intubated patients, 64% developed hypoxemia. 46% of these hypoxemic patients developed ARDS by CXR. Across the three groups (no hypoxemia, hypoxemia, ARDS), there were no significant differences in age, gender, or comorbidities. However, there was an increase in severity of shock, injury, and chest injury by group, with corresponding trends in transfusion requirements and volume of early fluid administration. Outcomes followed a similar stepwise pattern, with pneumonia, multi-organ failure, length of ICU stay, number of ventilator days, and overall mortality highest in ARDS patients. In multiple logistic regression, early plasma transfusion, delayed crystalloid administration, body mass index (BMI), and head and chest injury were independent predictors of hypoxemia, while head and chest injury, early crystalloid infusion, and delayed platelet transfusion were independent predictors of ARDS.
CONCLUSIONS
Hypoxemia and ARDS exist on a spectrum of respiratory dysfunction following trauma, with increasing injury severity profiles and resuscitation requirements. However, they also represent distinct clinical states with unique predictors, which require directed research approaches and targeted therapeutic strategies.
Keywords: ARDS, lung injury, hypoxemia, platelet transfusion, crystalloid
INTRODUCTION
Trauma is the leading cause of death in the young worldwide (1), with a characteristic distribution of immediate, early, and delayed mortality (2, 3). Late mortality is characterized by dysregulated systemic inflammation leading to multiple organ failure, and lung dysfunction has been shown to play a central role in this process (4). Though some measure of post-traumatic lung injury may be mitigated by the implementation of lung-protective ventilation strategies and the adoption of more judicious transfusion practices (5-7), the incidence of acute respiratory distress syndrome (ARDS) in severely-injured trauma patients remains significant (8, 9). Independent of underlying illness severity, the development of ARDS has been associated with major increases in morbidity, and up to a nearly three-fold increase in mortality (10).
Since its initial description by Ashbaugh and colleagues in 1967, ARDS has been defined and redefined several times, with diagnostic criteria evolving to accommodate improved understanding of both underlying pathophysiologic processes and broader epidemiological trends (11, 12). Through multiple iterations, the definition of ARDS has included a combination of both acute hypoxemia and radiographic evidence of pulmonary edema, in the absence of cardiogenic causes (13). While several studies have investigated risk factors for the development of ARDS in trauma, the differences between trauma patients with hypoxemia alone and those who develop radiographically-confirmed ARDS have not been well studied (14-17). In fact, multiple recent trauma studies have intimated that hypoxemia alone is an appropriate surrogate for ARDS, utilizing PaO2:FiO2 ratios drawn from clinical databases, with no correlation to radiographic findings (8, 18-20). Given the multiple possible etiologies of post-traumatic hypoxemia, and the deliberate specificity with which consensus definitions of ARDS have been developed, such a practice may lead to misleading conclusions based on expanded and imprecise inclusion criteria. Whether hypoxemia is indeed a useful surrogate for ARDS remains an unanswered question in trauma epidemiology research.
We sought to describe the differences in demographics, injury profiles, clinical characteristics, and outcomes between patients with hypoxemia alone and those with adjudicated ARDS; we also sought to delineate the differences between patients with hypoxemia and those who required intubation but never developed hypoxemia. We aimed to identify independent predictors of both hypoxemia and ARDS. We hypothesized that in a critically-injured trauma cohort requiring intubation, non-hypoxemic, hypoxemic, and ARDS patients represent distinct groups with unique characteristics and predictors.
METHODS
Comprehensive demographic, injury, clinical, and outcomes data were prospectively collected on 621 critically injured highest-level trauma activation patients at an urban Level 1 trauma center between 2005 and 2013. These patients required intubation and mechanical ventilation, and survived at least six hours from time of admission; data was collected out to 28 days following admission. Data was collected under a protocol approved by the University of California, San Francisco Committee on Human Research. Hypoxemia was defined as a PaO2:FiO2 ratio ≤ 300 during the first 8 days of admission, and ARDS was determined using the Berlin definition, during the same acute time period (21). Radiographs obtained for clinical indications were reviewed by two expert physicians blinded to clinical data, and were assessed for the presence of bilateral pulmonary opacities; those deemed positive by consensus were designated as adjudicated ARDS patients.
Massive transfusion was defined as ≥ 10 units of packed red blood cells (pRBCs) transfused in 24 hours. To account for survivor bias, those patients who did not survive 24 hours were counted as receiving massive transfusion if they were transfused ≥ 5 units of pRBCs in 12 hours or ≥ 2.5 units of pRBCs in 6 hours, as described previously (22). Multi-organ failure was defined using the Denver Postinjury Multiple Organ Failure Score (23).
Data are presented as mean (standard deviation), median (interquartile range), or percentage; univariate and group comparisons were made using Student's t test or one-way analysis of variance for normally distributed data, Wilcoxon rank sum or Kruskal Wallis testing for skewed data, and Fisher's exact test for proportions. An α < 0.05 was considered significant. For group comparisons, differences between multiple groups were assessed if the overall across-group comparison test was significant (α < 0.05); Bonferroni correction was then made for multiple between-group comparisons (α<0.017 for comparisons between three groups). The depicted N in figures and tables represents the total number of patients in the respective group. Logistic regression was used to assess predictors of both hypoxemia and ARDS. To preclude confounding by timing of exposures and outcomes, we excluded from the models patients who developed hypoxemia or ARDS in the first 24 hours of admission; in this way we could ascertain that predictors truly preceded their respective outcomes. Patients who died in the first 24 hours were also excluded, since they by definition could not develop the outcome in question. As a sensitivity analysis, additional models were subsequently constructed including patients who died or developed hypoxemia or ARDS in the first 24 hours, using as predictors only variables that preceded hospital arrival (eg demographics, injury profile); these identified no substantive differences in predictors from the main regression analysis presented here (data not shown). All analysis was performed by the authors using Stata version 12 (StataCorp, College Station, TX).
RESULTS
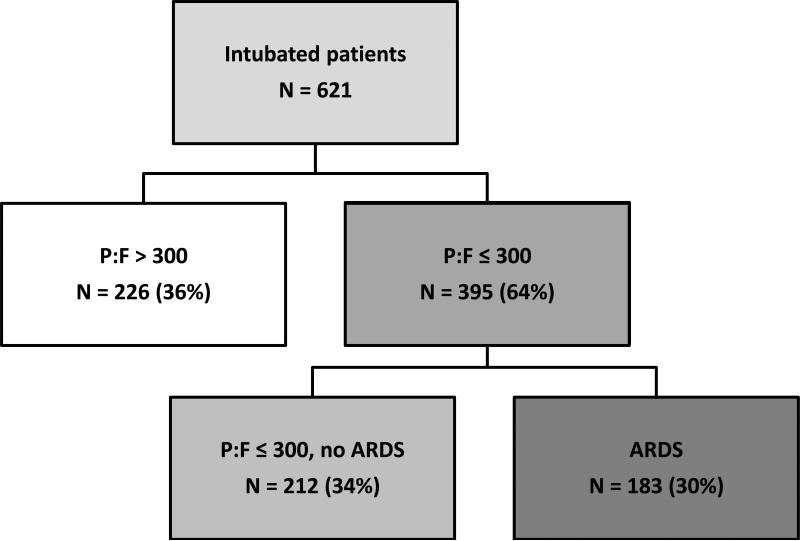
Of the 621 critically-injured trauma patients requiring intubation, 395 (64%) developed hypoxemia in the first eight days of admission, with PaO2:FiO2 ≤ 300; the other 226 intubated patients (36%) never developed hypoxemia (Figure 1). Among the hypoxemic patients, 183 (46%, or 30% of total cohort) were diagnosed with ARDS based on blinded two-physician review, while 212 (54%, or 34% of total cohort) never developed radiographic findings consistent with ARDS.
Figure 1.
Study Population
The demographic, injury, clinical, and outcome data by group is depicted in Table 1. Across the three respective groups (non-hypoxemic, hypoxemic with PaO2:FiO2 ratio ≤ 300, adjudicated ARDS) there were no significant differences in age or gender; demographics were consistent with those expected in an urban trauma population. Patients who did not develop hypoxemia or ARDS had a lower mean BMI. ARDS patients had a higher rate of blunt mechanism of injury (83%) than non-hypoxemic or hypoxemic non-ARDS patients (72%). There were no significant differences between groups with respect to underlying comorbidities, including chronic obstructive pulmonary disease, asthma, diabetes mellitus, or underlying cardiac disease (data not shown).
Table 1.
Demographics, Clinical Characteristics, and Outcomes by Hypoxemia/ARDS Status
| P:F > 300 N = 226 | P:F ≤ 300, no ARDS N = 212 | ARDS N = 183 | p-value | |
|---|---|---|---|---|
| Age (years) | 37 (27 – 54) | 39 (25 – 57.5) | 39 (27 – 54) | 0.660 |
| Male | 80 | 75 | 81 | 0.307 |
| Body Mass Index (kg/m2) | 25.5 +/− 4.7 | 27.3 +/− 5.3 | 27.6 +/− 6.1 | <0.001 |
| Blunt Mechanism | 72 | 72 | 83 | 0.007 |
| Blood alcohol (mg/dL) | 203 (0 - 321) | 152 (0 - 245) | 68 (0 – 284) | 0.033 |
| Smoking history | 58 | 69 | 73 | 0.062 |
| Injury Severity Score | 16 (5 - 26) | 26 (17 - 34) | 30 (25 – 41) | 0.002* |
| Chest Injury (any) | 29 | 39 | 58 | <0.001 |
| Severe Chest Injury (AIS>3) | 22 | 29 | 49 | <0.001 |
| Rib fracture (any) | 15 | 24 | 41 | <0.001* |
| GCS | 9.5 (5 - 14) | 8 (4 - 14) | 7 (3 - 13) | 0.041 |
| Head injury (any) | 60 | 74 | 83 | <0.001 |
| Admit heart rate | 93.2 +/− 24.9 | 98.3 +/− 28.9 | 106.7 +/− 25.4 | <0.001 |
| Admit systolic BP (mmHg) | 127.9 +/− 32.3 | 128.8 +/− 35.7 | 127 +/− 35.2 | 0.879 |
| Admit base excess (mEq/L) | −3.3 +/− 5.5 | −5.4 +/− 5.6 | −7.4 +/−6.3 | <0.001* |
| Pre-hospital crystalloid (mL) | 100 (0 - 250) | 150 (50 - 300) | 100 (0 - 300) | 0.047 |
| IV crystalloid 0-6h (mL) | 2085 (915 - 3180) | 3000 (1500 - 4500) | 3590 (2075 - 6095) | <0.001* |
| IV crystalloid 7-24h (mL) | 2265 (1590 - 3180) | 2895 (2150 - 4020) | 2995 (2200 - 4670) | <0.001 |
| IV colloid 0-6h (mL) | 0 (0 - 0) | 0 (0 - 0) | 0 (0 - 0) | 0.163 |
| Transfusion (any) at 24h | 44 | 59 | 75 | <0.001* |
| Massive Transfusion | 6 | 13 | 23 | <0.001* |
| Ventilator-free days (in 28d) | 26 (25 - 27) | 19 (0 - 25) | 2 (0 - 16) | <0.001* |
| VAP | 0.4 | 5 | 28 | <0.001* |
| Multi-Organ Failure | 0.4 | 9 | 43 | <0.001* |
| Total hospital days | 5 (2 - 12) | 10 (3 - 25) | 21 (9 - 44) | <0.001* |
| Total ICU days | 2 (2 - 4) | 5 (2 - 12) | 14 (7 - 24) | <0.001* |
| Mortality at 24 hours | 4 | 10 | 3 | 0.006 |
| Mortality at discharge | 14 | 27 | 35 | <0.001 |
Legend: Data presented as percentage unless stated, mean +/− SD, or median (IQR). Statistical testing performed with analysis of variance or Student's t test, Kruskal Wallace or Wilcoxon rank sum test, or Fisher's exact test. p values shown are for across-group statistical tests
denotes significance between all groups following Bonferroni correction (α<0.017).
AIS, Abbreviated Injury Scale; GCS, Glasgow Coma Scale; ICU, intensive care unit.
In this intubated cohort, non-hypoxemic patients had higher median blood alcohol levels than hypoxemic patients, who in turn had higher median blood alcohol than patients diagnosed with ARDS (203 vs. 152 vs. 67.5 mg/dL, p=0.033 across groups). Reported history of smoking increased from non-hypoxemic to hypoxemic to ARDS patients, though this did not reach statistical significance (58% vs. 69% vs. 74%, p=0.062 across groups). There were no significant differences in the use of illicit drugs, either by toxicology screening data or by history (data not shown).
Injury profiles differed between groups, with a stepwise increase in median injury severity score (ISS) from non-hypoxemic to hypoxemic to ARDS patients (16 vs. 26 vs. 30, p<0.001 across and between groups); rate of chest injury (29% vs. 39% vs. 58%, p<0.001 across groups) and rate of severe chest injury (abbreviated injury scale (AIS) score >3) (22% vs. 29% vs. 58%, p<0.001 across groups) demonstrated similar stepwise increases. Rate of rib fractures also increased across groups (15% vs. 24% vs. 41%, p<0.001 across groups). Median Glasgow Coma Scale (GCS) decreased across the groups, from 9.5 to 8 to 7 (p=0.041 across groups), and rate of head injury (AIS≥1) increased from 60% to 74% to 83% (p<0.001 across groups).
Corresponding physiologic parameters on admission also demonstrated stepwise trends in severity, with increasing mean heart rate across groups (93 vs. 98 vs. 107, p<0.001 across groups) as well as increasing mean base deficit (−3.3 vs. −5.4 vs. −7.4 mEq/L, p<0.003 across and between groups). There were no significant differences in admission temperature or systolic blood pressure.
With regard to resuscitation, there were no clinically significant differences in the amount of pre-hospital crystalloid administered. However, there were significant stepwise differences in the median amount of crystalloid received in the six hours following presentation to the trauma center: non-hypoxemic patients received 2085 mL of crystalloid, versus 3000 mL for hypoxemic patients, and 3590 mL for patients who developed ARDS (p<0.001 across and between groups). This trend was also observed during the following time interval, from 7 hours to 24 hours (2265 mL vs. 2895 mL vs. 2995 mL, p<0.001 across groups).
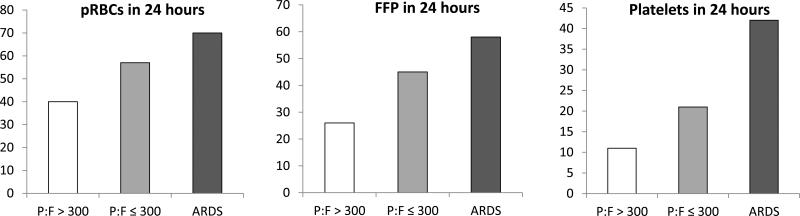
In parallel to the trend seen in crystalloid administration, the rates of blood product transfusion increased across the respective study groups. As shown in Table 1, the percentage of non-hypoxemic patients receiving any blood product in the first 24 hours was 44%, versus 59% for patients who had hypoxemia, and 75% for patients diagnosed with ARDS (p<0.001 across and between groups). A similar increase was seen with respect to massive transfusion, from 6% in non-hypoxemic patients up to 13% in those who developed hypoxemia, and 23% for ARDS patients (p<0.001 across and between groups). Significant increases were seen in the transfusion rates of all categories of blood products, including packed red blood cells, fresh frozen plasma, and platelets, as depicted in Figure 2.
Figure 2. Transfusion Requirements by Group.
Percentages of patients transfused in each group. All group and intergroup comparisons statistically significant, with Bonferroni correction. pRBCs, packed red blood cells; FFP, fresh frozen plasma; ARDS, acute respiratory distress syndrome.
The three groups demonstrated significant differences in ventilation requirements and critical care outcomes. Non-hypoxemic patients had significantly more ventilator-free days than hypoxemic or ARDS patients (26 vs. 19 vs. 2 ventilator-free days, p<0.001 across and between groups). Ventilator-associated pneumonia incidence was lowest in non-hypoxemic patients (0.4%) and increased in hypoxemic (5%) and ARDS patients (28%, p<0.001 across and between groups); multi-organ failure followed a similar pattern (0.4% vs. 9% vs. 43%, p<0.001 across and between groups).
ARDS patients had the longest hospital and ICU stays, while non-hypoxemic patients had the shortest stays (both p<0.001, across and between groups). Overall mortality was increased in the hypoxemia (27%) and ARDS (35%) groups, respectively, compared to non-hypoxemic patients (14%; p<0.001 across groups).
Logistic regression analyses were conducted to identify independent predictors of both hypoxemia and ARDS. To assess independent predictors of hypoxemia alone, ARDS patients were excluded from the hypoxemia models. In univariate logistic regression, body mass index (BMI), head injury (by AIS head), early (0-6 hours) transfusion of any blood product, late (7-24 hours) transfusion of packed red blood cells, and late administration of crystalloid or colloid were found to be significant predictors of hypoxemia (all p<0.05, Table 2). Incorporating these factors into a multiple logistic regression model including chest injury (AUC 0.76 with n of 272 and 91 patients who developed outcome), the significant predictors of hypoxemia were BMI (per 5 kg/m2, odds ratio [OR] 1.34, 95% confidence interval [CI] 1.02–1.79, p=0.037), head injury (OR 1.31, CI 1.13–1.53, p<0.001), chest injury (OR 1.28, CI 1.05–1.54, p=0.012), early FFP transfusion (by unit, OR 1.39, CI 1.11–1.74, p=0.005), and late crystalloid administration (by 500mL aliquot, OR 1.14, CI 1.03–1.25, p=0.008) [Table 2].
Table 2.
Logistic Regression: Predictors of Hypoxemia (P:F ≤ 300) after 24 hours
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| BMI (per 5 kg/m2) | 1.32 | 1.03 - 1.69 | 0.027 | 1.34 | 1.02 - 1.79 | 0.037 |
| Head Injury (AIS) | 1.25 | 1.10 - 1.41 | 0.001 | 1.31 | 1.13 - 1.53 | <0.001 |
| Chest Injury (AIS) | 1.13 | 0.97 - 1.33 | 0.122 | 1.28 | 1.05 - 1.54 | 0.012 |
| pRBCs 0-6h (units) | 1.08 | 1.03 - 1.14 | 0.004 | 0.88 | 0.75 - 1.04 | 0.129 |
| FFP 0-6h (units) | 1.17 | 1.07 - 1.27 | <0.001 | 1.39 | 1.11 - 1.74 | 0.005 |
| Platelets 0-6h (units) | 1.66 | 1.08 - 2.55 | 0.021 | 0.76 | 0.30 - 1.91 | 0.559 |
| Crystalloid 7-24h (500mL) | 1.22 | 1.12 - 1.32 | <0.001 | 1.14 | 1.03 - 1.25 | 0.008 |
| Colloid 7-24h (500mL) | 4.09 | 1.24 - 13.44 | 0.020 | 3.15 | 0.85 - 11.68 | 0.086 |
| pRBCs 7-24h (units) | 1.25 | 1.01 - 1.54 | 0.039 | 1.01 | 0.77 - 1.31 | 0.994 |
Legend: BMI, body mass index; AIS, abbreviated injury score; pRBC, packed red blood cells; FFP, fresh frozen plasma. Bold variables are those that remain significant predictors in multivariate analysis.
Patients excluded if hypoxemia diagnosed in first 24 hours of admission, or if patient died before 24 hours. Total n for this model 272, with 91 developing outcome of interest (hypoxemia).
In similar fashion, logistic regression was conducted to analyze predictors of ARDS in the study population. In univariate logistic regression, blunt mechanism, head injury (by AIS score), chest injury (by AIS score), early (0-6 hours) or late (7-24 hours) administration of crystalloid, and early or late transfusion of all blood products were significant predictors of ARDS (all p<0.05, Table 3). When adjusting for these variables in a multiple logistic regression model (AUC 0.80, with total model n of 483, and 105 patients who developed ARDS outcome), the remaining significant predictors of ARDS were head injury (OR 1.47, CI 1.27–1.71, p<0.001), chest injury (OR 1.36, CI 1.17–1.58, p<0.001), early administration of crystalloid (by 500 mL, OR 1.08, CI 1.02–1.15, p=0.006), and late transfusion of platelets (by unit, OR 5.55, CI 2.37–12.99, p<0.001) [Table 3].
Table 3.
Logistic Regression: Predictors of ARDS after 24 hours
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Blunt Mechanism | 2.05 | 1.17 - 3.59 | 0.013 | 1.76 | 0.82 - 3.74 | 0.145 |
| Head Injury (AIS) | 1.44 | 1.27 - 1.63 | <0.001 | 1.47 | 1.27 - 1.71 | <0.001 |
| Chest Injury (AIS) | 1.33 | 1.17 - 1.51 | <0.001 | 1.36 | 1.17 - 1.58 | <0.001 |
| Crystalloid 0-6h (by 500mL) | 1.07 | 1.03 - 1.11 | 0.001 | 1.08 | 1.0235 - 1.15 | 0.006 |
| pRBCs 0-6h (units) | 1.05 | 1.01 - 1.09 | 0.010 | 0.94 | 0.83 - 1.06 | 0.297 |
| FFP 0-6h (units) | 1.08 | 1.03 - 1.14 | 0.001 | 1.03 | 0.89 - 1.18 | 0.729 |
| Platelets 0-6h (units) | 1.41 | 1.07 - 1.87 | 0.016 | 1.46 | 0.94 - 2.27 | 0.091 |
| Crystalloid 7-24h (by 500mL) | 1.11 | 1.05 - 1.17 | <0.001 | 1.05 | 0.98 - 1.12 | 0.203 |
| pRBCs 7-24h (units) | 1.34 | 1.17 - 1.55 | <0.001 | 0.99 | 0.76 - 1.28 | 0.924 |
| FFP 7-24h (units) | 1.36 | 1.14 - 1.61 | <0.001 | 0.93 | 0.67 - 1.27 | 0.640 |
| Platelets 7-24h (units) | 5.53 | 2.82 - 10.85 | <0.001 | 5.55 | 2.37 - 12.99 | <0.001 |
Legend: AIS, abbreviated injury score; pRBC, packed red blood cells; FFP, fresh frozen plasma. Bold variables are those that remain significant predictors in multivariate analysis.
Patients excluded if ARDS diagnosed in first 24 hours of admission, or if patient died before 24 hours. Total n for this model 483, with 105 developing outcome of interest (ARDS).
DISCUSSION
Since its earliest description nearly half a century ago, ARDS has been a recognized and dire complication of severe trauma. The incidence and severity of ARDS, from causes both traumatic and non-traumatic, represents a major concern in both critical care and public health (24). Recognizing that diverse traumatic and non-traumatic insults lead to a similar pathophysiological process and endpoint, investigators have worked to arrive at consensus definitions of this acute lung injury, in order to facilitate broad epidemiological characterizations, evaluate outcomes across populations, and assess potential therapeutic approaches (25). Though these consensus definitions have evolved with improved understanding of lung injury at both the population and molecular level, they have consistently included both the acute onset of hypoxemia and a corresponding radiographic finding indicative of pulmonary infiltration (21, 26).
Due to limitations in available datasets that lack radiographic findings, several recent investigations in the trauma literature have resorted to using hypoxemia alone to determine pulmonary dysfunction from presumed ARDS (8, 20); others have deferred actual review of radiographs to utilize billing codes or secondary radiology reports (18, 19). Using such protocols is less resource intensive, and allows investigators to analyze findings from large clinical databases. However, little is known as to the effects of conflating hypoxemia with rigorously adjudicated ARDS, or what potentially erroneous conclusions might be drawn from such an approach.
In this study, using the gold standard of blinded two-physician review of chest radiographs to adjudicate ARDS in intubated trauma patients, we found significant differences in clinical characteristics and outcomes between patients with no hypoxemia, those with hypoxemia but no ARDS, and those with confirmed ARDS. Though basic demographics like age, gender, and comorbidities were similar across these three groups, differences in severity of injury, degree of shock, fluid resuscitation and transfusion requirements, and overall mortality followed a stepwise trend, increasing from the non-hypoxemic to the hypoxemic to the ARDS group. As such, these groups may be considered to be different in terms of degree, with more severely injured patients in the hypoxemic group, and most severely injured patients in the ARDS group.
We used logistic regression analysis to assess independent risk factors for both hypoxemia and ARDS, adjusting for injury severity and transfusion parameters. This analysis revealed that, in addition to the above-mentioned differences in degree, these groups were actually different in kind as well. In multiple logistic regression, the significant risk factors for hypoxemia after 24 hours included severity of head injury, severity of chest injury, BMI, amount of late (7 to 24 hours) crystalloid administration, and amount of early (0 to 6 hours) fresh frozen plasma transfusion. A similar analysis for ARDS after 24 hours identified severity of both head and chest injury, amount of early crystalloid administration, and amount of late platelet transfusion as independent risk factors.
Our finding of unique risk factors for both hypoxemia and ARDS underscores the clinical differences between these groups, and corresponds to clinical intuition: patients may develop hypoxemia for a host of reasons, from mucus plugging to shunting to atelectasis, whereas ARDS likely represents a specific severe biological syndrome. One shared risk factor for both hypoxemia and ARDS, degree of head injury, likewise makes clinical sense, and resonates with prior literature linking traumatic brain injury (TBI) to acute lung injury (27, 28); the exact mechanisms of this correlation in ARDS are not well-characterized, and may reflect both systemic inflammation and the employment of TBI-specific ventilator management strategies (29). The correlation of chest injury and hypoxemia also corresponds to clinical experience, while the finding of chest trauma severity as an independent predictor of ARDS supports direct lung injury (including pulmonary contusion) as a predisposing factor for acute lung injury, corroborating the trauma literature to date (17, 30-32). That BMI predicts hypoxemia resonates clinically as well, in that injured patients with larger habitus may have higher risk of atelectasis, along with pulmonary mechanics that impair adequate oxygenation.
The hydrostatic and pro-inflammatory effects of intravenous crystalloid on the lung have been described for decades (33-35), and a fluid-restrictive strategy has been shown in a multi-center randomized clinical trial to improve clinical outcomes in ARDS (36); as such, our finding that early crystalloid administration independently predicts ARDS is not surprising. While the data provides no obvious mechanistic explanation for delayed crystalloid as a risk factor for hypoxemia, it may well represent a surrogate of ongoing aggressive resuscitation (justified or not), which may in turn correlate to incidence of hypoxemia after 24 hours.
Delayed platelet administration (7 to 24 hours after presentation) was the strongest independent predictor of ARDS after 24 hours in our cohort. This resonates with early theories on ARDS pathophysiology and platelet-related pulmonary damage (37), as well as with more recent clinical investigations (8, 38), and animal model data implicating platelet sequestration as a key precipitating factor in neutrophil-mediated lung injury (39). The exact mechanism of this relationship cannot be determined from our clinical data, and could reflect either a direct insult from ongoing platelet transfusion, or a secondary effect of early pulmonary platelet sequestration, prompting delayed platelet transfusion by clinicians in response to a low peripheral platelet count. Similarly, the association between early FFP administration and hypoxemia after 24 hours may represent either a true biological effect, as alluded to in prior studies (40), or may be a surrogate for injury severity and resuscitation requirements in our population.
This study has notable strengths: it draws on prospectively-collected data from a relatively large set of intubated critically-injured patients, utilizes blinded physician review of radiographs to confirm ARDS diagnosis, and by careful patient selection enables identification of clinical predictors that clearly precede the outcomes in question. It is of course limited in that it is a single-center study, though our findings should be applicable and relevant to other major urban trauma centers and similar patient populations. Also, as noted, despite the prospective collection of data in this trauma cohort, the granular details of minute-by-minute timing of potential exposures in the first hours of resuscitation could not be determined with certainty in this analysis; thus we limited our regression analyses to assessing predictors of hypoxemia and ARDS after 24 hours. We acknowledge that the independent predictors of ARDS in the first 24 hours after injury may differ from those reported here, especially given the potential phenotypic heterogeneity of ARDS in trauma patients by time of onset (41-43). Of note, performing our regression analyses including all patients, and excluding process-of-care variables in order to prevent confounding of exposure-outcome timing, we found no major differences from the predictors reported here.
In sum, we have demonstrated that in a cohort of intubated trauma patients at a high-volume urban trauma center in the United States, hypoxemia and ARDS exist on a spectrum of injury severity profile and resuscitation requirements, yet patients with adjudicated ARDS represent a distinct group from those with hypoxemia alone. While ARDS is a well-described pathophysiologic syndrome, hypoxemia may result from various stimuli, and may not be a meaningful indicator in the absence of acute lung injury. Hypoxemia does not appear to be an appropriate surrogate for lung injury, and future epidemiologic studies should thus incorporate precise, adjudicated ascertainment of ARDS.
ACKNOWLEDGEMENTS
We appreciate the participation of the ICU nursing staff and of the patients and their families.
Funding: Supported by HL110969 (CSC), DoD W911NF-10-1-0384 (MJC)
Footnotes
Meetings: Presented at the 2014 Thomas L. Petty Aspen Lung Conference, “Rebuilding the Injured Lung”, June 4, 2014, Aspen, Colorado.
AUTHOR CONTRIBUTIONS:
BMH, LZK, CMH, RAC, CSC, and MJC contributed to study design, data collection, data analysis, data interpretation, writing, and critical revision.
BJR, ASC, MFN, contributed to study design, data collection, and critical revision.
REFERENCES
- 1.Norton R, Kobusingye O. Injuries. The New England journal of medicine. 2013;368(18):1723–30. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 2.Trunkey DD. Trauma. Accidental and intentional injuries account for more years of life lost in the U.S. than cancer and heart disease. Among the prescribed remedies are improved preventive efforts, speedier surgery and further research. Scientific American. 1983;249(2):28–35. [PubMed] [Google Scholar]
- 3.Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, Gruzinski G, Chan L. Trauma deaths in a mature urban trauma system: is “trimodal” distribution a valid concept? Journal of the American College of Surgeons. 2005;201(3):343–8. doi: 10.1016/j.jamcollsurg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Ciesla DJ, Moore EE, Johnson JL, Burch JM, Cothren CC, Sauaia A. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138(4):749–57. doi: 10.1016/j.surg.2005.07.020. discussion 57-8. [DOI] [PubMed] [Google Scholar]
- 5.Ciesla DJ, Moore EE, Johnson JL, Cothren CC, Banerjee A, Burch JM, Sauaia A. Decreased progression of postinjury lung dysfunction to the acute respiratory distress syndrome and multiple organ failure. Surgery. 2006;140(4):640–7. doi: 10.1016/j.surg.2006.06.015. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 6.Martin M, Salim A, Murray J, Demetriades D, Belzberg H, Rhee P. The decreasing incidence and mortality of acute respiratory distress syndrome after injury: a 5-year observational study. The Journal of trauma. 2005;59(5):1107–13. doi: 10.1097/01.ta.0000188633.94766.d0. [DOI] [PubMed] [Google Scholar]
- 7.Plurad D, Martin M, Green D, Salim A, Inaba K, Belzberg H, Demetriades D, Rhee P. The decreasing incidence of late posttraumatic acute respiratory distress syndrome: the potential role of lung protective ventilation and conservative transfusion practice. The Journal of trauma. 2007;63(1):1–7. doi: 10.1097/TA.0b013e318068b1ed. discussion 8. [DOI] [PubMed] [Google Scholar]
- 8.Robinson BR, Cotton BA, Pritts TA, Branson R, Holcomb JB, Muskat P, Fox EE, Wade CE, del Junco DJ, Bulger EM, et al. Application of the Berlin definition in PROMMTT patients: the impact of resuscitation on the incidence of hypoxemia. The journal of trauma and acute care surgery. 2013;75(1 Suppl 1):S61–7. doi: 10.1097/TA.0b013e31828fa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. American journal of respiratory and critical care medicine. 2011;183(12):1660–5. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah CV, Localio AR, Lanken PN, Kahn JM, Bellamy S, Gallop R, Finkel B, Gracias VH, Fuchs BD, Christie JD. The impact of development of acute lung injury on hospital mortality in critically ill trauma patients. Critical care medicine. 2008;36(8):2309–15. doi: 10.1097/CCM.0b013e318180dc74. [DOI] [PubMed] [Google Scholar]
- 11.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–23. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 12.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342(18):1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JL, Haenel JB. Chapter 57: Respiratory Insufficiency. In: Mattox KL, Moore EE, Feliciano DV, editors. Trauma. 7th ed. McGraw-Hill Medical; New York: 2013. pp. 1055–72. [Google Scholar]
- 14.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 15.Garber BG, Hebert PC, Yelle JD, Hodder RV, McGowan J. Adult respiratory distress syndrome: a systemic overview of incidence and risk factors. Critical care medicine. 1996;24(4):687–95. doi: 10.1097/00003246-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Critical care medicine. 2005;33(6):1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 17.Miller PR, Croce MA, Kilgo PD, Scott J, Fabian TC. Acute respiratory distress syndrome in blunt trauma: identification of independent risk factors. The American surgeon. 2002;68(10):845–50. discussion 50-1. [PubMed] [Google Scholar]
- 18.Afshar M, Smith GS, Terrin ML, Barrett M, Lissauer ME, Mansoor S, Jeudy J, Netzer G. Blood alcohol content, injury severity, and adult respiratory distress syndrome. The journal of trauma and acute care surgery. 2014;76(6):1447–55. doi: 10.1097/TA.0000000000000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park PK, Cannon JW, Ye W, Blackbourne LH, Holcomb JB, Beninati W, Napolitano LM. Transfusion strategies and development of acute respiratory distress syndrome in combat casualty care. The journal of trauma and acute care surgery. 2013;75(2 Suppl 2):S238–46. doi: 10.1097/TA.0b013e31829a8c71. [DOI] [PubMed] [Google Scholar]
- 20.Sharpe JP, Weinberg JA, Magnotti LJ, Fabian TC, Croce MA. Does plasma transfusion portend pulmonary dysfunction? A tale of two ratios. The journal of trauma and acute care surgery. 2013;75(1):32–6. doi: 10.1097/TA.0b013e318294672d. discussion 6. [DOI] [PubMed] [Google Scholar]
- 21.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Cripps MW, Kutcher ME, Daley A, McCreery RC, Greenberg MD, Cachola LM, Redick BJ, Nelson MF, Cohen MJ. Cause and timing of death in massively transfused trauma patients. The journal of trauma and acute care surgery. 2013;75(2 Suppl 2):S255–62. doi: 10.1097/TA.0b013e31829a24b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauaia A, Moore EE, Johnson JL, Ciesla DJ, Biffl WL, Banerjee A. Validation of postinjury multiple organ failure scores. Shock. 2009;31(5):438–47. doi: 10.1097/SHK.0b013e31818ba4c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 25.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. The Journal of clinical investigation. 2012;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994;149(3 Pt 1):818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 27.Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, Erickson VR, Pittet JF. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. The Journal of trauma. 2003;55(1):106–11. doi: 10.1097/01.TA.0000071620.27375.BE. [DOI] [PubMed] [Google Scholar]
- 28.Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, McBride W, Moussouttas M, Bell R, Ratliff JK, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery. 2012;71(4):795–803. doi: 10.1227/NEU.0b013e3182672ae5. [DOI] [PubMed] [Google Scholar]
- 29.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocritical care. 2009;11(3):417–26. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 30.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. American journal of surgery. 1982;144(1):124–30. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 31.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC. ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. The Journal of trauma. 2001;51(2):223–8. doi: 10.1097/00005373-200108000-00003. discussion 9-30. [DOI] [PubMed] [Google Scholar]
- 32.Watkins TR, Nathens AB, Cooke CR, Psaty BM, Maier RV, Cuschieri J, Rubenfeld GD. Acute respiratory distress syndrome after trauma: development and validation of a predictive model. Critical care medicine. 2012;40(8):2295–303. doi: 10.1097/CCM.0b013e3182544f6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staub NC. Pulmonary edema: physiologic approaches to management. Chest. 1978;74(5):559–64. doi: 10.1378/chest.74.5.559. [DOI] [PubMed] [Google Scholar]
- 34.Cotton BA, Guy JS, Morris JA, Jr., Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115–21. doi: 10.1097/01.shk.0000209564.84822.f2. [DOI] [PubMed] [Google Scholar]
- 35.Calfee CS, Gallagher D, Abbott J, Thompson BT, Matthay MA, Network NA. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Critical care medicine. 2012;40(6):1731–7. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 37.Blaisdell FW, Lewis FR., Jr. Respiratory distress syndrome of shock and trauma: post-traumatic respiratory failure. Major problems in clinical surgery. 1977;21:95–7. [PubMed] [Google Scholar]
- 38.Harr JN, Moore EE, Johnson J, Chin TL, Wohlauer MV, Maier R, Cuschieri J, Sperry J, Banerjee A, Silliman CC, et al. Antiplatelet therapy is associated with decreased transfusion-associated risk of lung dysfunction, multiple organ failure, and mortality in trauma patients. Critical care medicine. 2013;41(2):399–404. doi: 10.1097/CCM.0b013e31826ab38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. The Journal of clinical investigation. 2009;119(11):3450–61. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inaba K, Branco BC, Rhee P, Blackbourne LH, Holcomb JB, Teixeira PG, Shulman I, Nelson J, Demetriades D. Impact of plasma transfusion in trauma patients who do not require massive transfusion. Journal of the American College of Surgeons. 2010;210(6):957–65. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Dicker RA, Morabito DJ, Pittet JF, Campbell AR, Mackersie RC. Acute respiratory distress syndrome criteria in trauma patients: why the definitions do not work. The Journal of trauma. 2004;57(3):522–6. doi: 10.1097/01.ta.0000135749.64867.06. discussion 6-8. [DOI] [PubMed] [Google Scholar]
- 42.Croce MA, Fabian TC, Davis KA, Gavin TJ. Early and late acute respiratory distress syndrome: two distinct clinical entities. The Journal of trauma. 1999;46(3):361–6. doi: 10.1097/00005373-199903000-00001. discussion 6-8. [DOI] [PubMed] [Google Scholar]
- 43.Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, Kaplan S, Holena DN, May AK, Ware LB, et al. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Annals of the American Thoracic Society. 2014;11(5):728–36. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]