Abstract
The diagnostic utility of obtaining chest and abdomen CT evaluating for invasive fungal infection (IFI) pre-and post-HSCT remains unclear. The study was conducted as a Quality Improvement project. Chest and abdomen CT of patients who underwent an allogeneic HSCT over a 13 month period were reviewed. Scans included those done pre-transplant in all patients, and day 0–100 post-transplant in selected patients. There were 66 patients with chest and abdomen CT scans pre-transplant. Chest CT was suggestive of IFI in 9 (13.6%) patients including 3 patients with prior history of IFI. After transplant, 37 patients had initial chest CT, and 14 patients had initial abdominal CT. The first chest CT post-transplant was suggestive of IFI in 3 patients; all had an abnormal CT pre-transplant. Following the initial post-transplant evaluation 15 patients had 28 additional CT scans of the chest, and 12 patients had 19 additional CT scans of the abdomen. An abnormal chest CT with proven evidence of IFI was seen in only one patient. None of the 99 abdominal CT scans performed pre-or post-transplant had evidence of IFI. There is little benefit in obtaining abdominal CT scans in HSCT patients for detecting IFI either pre-or post-transplant.
Keywords: abdominal, computed tomography, allogeneic, stem cell transplantation
INTRODUCTION
Radiation risk from computed tomography (CT) imaging is cumulative and in an unselected population increases the risk of subsequent solid tumors particularly in younger patients, girls, and in those who undergo CT imaging of the abdomen and pelvis [1]. A radiation-induced solid tumor is projected to result from every 300 to 390 abdomen/pelvis scans for girls [1]. The risk of leukemia in children is estimated at 0.8 to 1.0 cases per 10,000 abdomen/pelvis CT scans [1]. The Childhood Cancer Survivor Study (CCSS) reported the 30-year cumulative incidence of secondary malignant neoplasms in 14,359 5-year survivors of childhood cancer to be 20.5% [2]. The radiation risk of CT imaging may be higher in those who have undergone an allogeneic hematopoietic stem cell transplant (HSCT) due to more frequent imaging, exposure to chemotherapy, and radiation therapy in some patients.
In 1982, Bartley et al. reported the results of CT scanning to diagnose hepatic and systemic invasive fungal infections (IFI) in children undergoing treatment for leukemia at St. Jude Children’s Research Hospital (SJCRH), Memphis, TN [3]. Subsequently CT imaging of the abdomen was incorporated as part of routine screening in patients with unexplained prolonged fever with neutropenia, and as part of pre-HSCT evaluation studies. In the present era of prophylactic antifungal therapy, we hypothesize that the diagnostic benefit of this approach is limited, may be outweighed by the radiation risk of CT imaging, and when considered together with the risks of sedation and contrast administration, represents poor utilization of resources with limited benefit for the patient.
METHODS
The study was conducted as a Quality Improvement project and approved by the SJCRH Institutional Review Board. Two senior pediatric radiologists at our institution (S.C.K and R.A.K) reviewed the CT scans of chest and abdomen (CAF) to look for evidence of IFI, in all children who underwent allogeneic HSCT between September 1, 2012 and September 30, 2013. Scans reviewed included those done pre-HSCT in all patients as part of routine pre-transplant evaluation, and day 0–100 post-HSCT in those with signs and symptoms suggestive of IFI or with unexplained prolonged fever with neutropenia, obtained at the discretion of the requesting physician.
Standardized CAF scans were obtained using 3.75 mm (children up to 11.5 kg) and 5 mm (children over 11.5 kg) collimated helical images through the chest and abdomen, reconstructed in standard (soft tissue) and lung algorithm, and reformatted in coronal and sagittal planes. All scans were reviewed at lung, soft tissue and bone window/levels. Weight-based dose modulation was utilized for all patients weighing at least 31.5 kg; patients under 31.5 kg were imaged at 100 kVp while patients ≥ 31.5 kg were imaged at 120 kVp; the noise index for patients weighing up to 11.5 kg was 12.35 (chest) and 9.88 (abdomen), and for patients weighing over 11.5 kg was 11.57 and 9.83, respectively. Forty percent adaptive statistical iterative reconstruction (ASiR™) was employed for all studies on all patients. All CAF studies were performed during intravenous administration of 2.0 mL/kg iodixanol 270 mg-I/mL to a maximum of 150 mL. All studies were performed on GE LightSpeed VCT XTe 64 detector (General Electric Healthcare, Waukesha, WI) scanner.
Transplant-related variables were abstracted from a prospectively collected database that included patient demographics, underlying diagnosis, remission status, donor and product type, conditioning regimen, symptoms and signs suggestive of IFI if present, grade II-IV graft-versus-host disease (GVHD), and pre-HSCT absolute neutrophil count (ANC). Screening on whole blood for galactomannan was performed pre-HSCT, and once weekly post-HSCT on all patients. Galactomannan index value of ≥ 0.5 was considered positive. Microbiologic records were reviewed to identify patients with IFI defined according to accepted criteria [4]. Changes to anti-fungal therapy based on CT scan findings were noted.
Statistical analysis
The first evaluation date of CT scan before and after transplant was used for analysis. CT scans whose evaluation date exceeded 100 days post-transplant were not considered. Descriptive statistics such as frequencies/percentages for categorical variables and mean, median, range, and standard deviation for continuous variables were provided for pre and post-transplant data. Fisher’s exact test (for categorical variables) and Wilcoxon rank sum test (for quantitative variables) were used to compare patients with and without IFI as suggested by chest CT pre-and post-transplant. McNemar’s exact test was used to compare patients with and without IFI as suggested by chest CT pre-and post-transplant. Clinical variables tested included age at transplant, gender, underlying disease, remission status, transplant and product type, receipt of total body irradiation (TBI), presence of symptoms and signs of IFI, ANC, and galactomannan test if positive. All the reported p-values are 2-sided and are considered significant if < 0.05. Statistical analyses were performed with SAS software version 9.3.
RESULTS
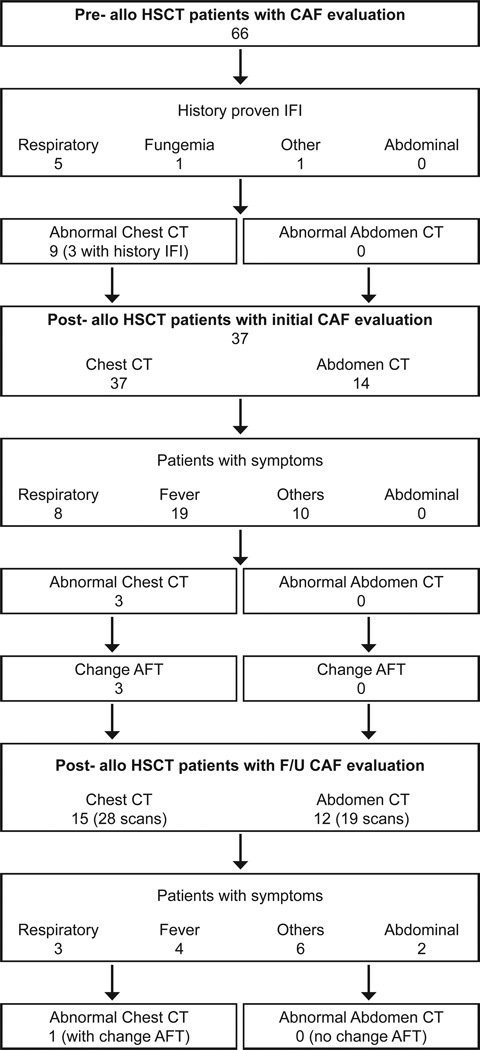
There were 66 patients who had CAF imaging pre-transplant. Demographics of these patients are presented in Table 1. There were 7 patients with history of proven IFI in the lung (4), extremity (1), sinus (1) and blood (1); (Figure 1). All patients tested negative for galactomannan in whole blood pre-transplant. Of these 66 patients pre-transplant chest CT was abnormal in a patient who had a focal nodule in the right lower lobe, which was completely resected with positive culture for Aspergillus fumigatus (Unique Patient Number /UPN 1), in a patient with a nodular opacity in the left upper lobe with history of Candida parapsilosis fungemia (UPN 2), and in a third patient with proven Aspergillus spp infection in a previous HSCT with resolving opacities prior to the current transplant (UPN 3). The remaining 4 patients had complete resolution of their IFI pre-transplant.
Table 1.
Demographics of patients who had evaluation of the chest and abdomen for fungus with computed tomography before and within 100 days post-HSCT
| Characteristic | CAF Pre-HSCT (n = 66) |
CAF Day 0–100 post-HSCT (n = 37) |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 9.1 (6.4) | 9.6 (6.1) |
| Median (Range) | 8.8 (0.2–20.4) | 9.1 (0.6–20.4) |
| Male sex | 36 (55) | 18 (49) |
| Diagnosis heme malignancy | 58 (87) | 32 (87) |
| Remission | 28 (42) | 20 (54) |
| Total body irradiation | 19 (29) | 13 (35) |
| Product Type | ||
| Bone marrow | 37 (56) | 18 (48) |
| Peripheral blood | 24 (36) | 15 (41) |
| Cord | 5 (8) | 4 (11) |
| History IFI | 7 (11) | 4 (11) |
| Positive galactomannan | 0 (0) | 3 (8) |
| ANC (cells/μL) | ||
| Mean (SD) | 1027 (1276) | 892 (1233) |
| Median (range) | 500 (0– 4400) | 500 (0–4400) |
| CT chest suggestive IFI | 9 (14) | 3 (8) |
| CT abdomen suggestive IFI | 0 (0) | 0 (0) |
| Transplant order | ||
| First transplant | 50 (76) | 27 (73) |
| Second transplant | 15 (23) | 10 (27) |
| Subsequent transplant | 1 (1) | 0 (0) |
Data are number (%) unless otherwise indicated.
Abbreviations : CAF, computed tomography of the chest and abdomen for fungus; HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; ANC, absolute neutrophil count; SD, standard deviation.
Figure 1.
Patients who had chest and abdominal CT for evaluation of fungus pre-transplant and subsequently post-transplant based on symptomatology.
Abbreviations: CAF, computed tomography of the chest and abdomen for fungus; HSCT, hematopoietic stem cell transplantation; IFI, invasive fungal infection; AFT, anti-fungal therapy.
There were 6 patients with no prior history of IFI who had small unilateral or bilateral pulmonary nodules not amenable to biopsy. Galactomannan in whole blood and testing for endemic mycoses were negative in all patients. The 7 patients with prior history of IFI and 6 asymptomatic patients with pulmonary nodules received empiric antifungal therapy post-transplant. None of the 66 patients studied had evidence of IFI on abdominal CT scan.
There were 37 patients who had CT imaging post-transplant. Demographics of these patients are presented in Table 1. Indications for performing scans for the first evaluation post-transplant included prior history of IFI or evidence thereof in pre-transplant CT with or without a history of unexplained fever with prolonged neutropenia (7 patients), unexplained fever with prolonged neutropenia (14 patients), increased respiratory rate and hypoxia (8 patients), abnormal pulmonary function tests (2 patients), C. albicans fungemia (1 patient), assessment of response in patients with solid tumor receiving an allogeneic HSCT (2 patients), and other causes including rising Epstein-Barr virus DNA in blood (3 patients). An abnormal chest CT suggestive of IFI was seen in 3 patients, all of whom had an abnormal chest CT pre-transplant; one patient, (UPN 2) had widespread nodular opacities post-transplant, 2 patients had nodules on chest CT with no prior history suggestive of IFI, and developed fever and more nodular opacities post-transplant. Galactomannan was negative and the nodules were not amenable to biopsy. Anti-fungal therapy was modified with addition of liposomal amphotericin for one patient (UPN 2) and voriconazole for the other 2 patients. Three patients had positive galactomannan post-transplant with a normal chest CT. Two of these patients had chest CT suggestive of IFI pre-transplant. An abdominal CT was performed with the chest CT in 14 patients. None had findings suggestive of IFI.
None of the variables including age (P = 0.90), gender (P =0.28), transplant product (P = 0.44), TBI conditioning (P = 1.00), or ANC (P = 0.62) were significant in predicting abnormalities suggestive of IFI in pre-transplant CT. None of the variables including age (P = 0.36), gender (P = 1.00), transplant product (P = 0.32), TBI conditioning (P = 1.00), or ANC (P = 0.26) were significant in predicting abnormalities suggestive of IFI in post-transplant CT. Abnormal pre- and post-transplant chest CT were not statistically discordant (P = 0.25).
Following the initial post-transplant evaluation 15 patients had 28 additional CT scans of the chest, and 12 patients had 19 additional CT scans of the abdomen. Indications for performing these scans included fever with increased CRP (4 patients), increased respiratory rate and hypoxia (3 patients), C. albicans fungemia (1 patient), assessment of response in patients with solid tumor (2 patients), abdominal pain and diarrhea (2 patients), and other causes (3 patients).
An abnormal chest CT suggestive of IFI was seen in one patient (UPN 4). He was a one-year old male with acute lymphoblastic leukemia who received a cord HSCT. The patient subsequently developed C. albicans fungemia 5 days post-HSCT which was broadly susceptible to antifungals; chest CT was normal. He responded poorly to a combination of micafungin and liposomal amphotericin and developed nodular lesions in both lungs on a follow-up chest CT with no abnormalities detected on abdominal CT (Figure 2). Biopsy of the nodules revealed budding yeast with pseudohyphae. Therapy was changed to micafungin with voriconazole to which he responded. He succumbed to a relapse of his leukemia. None of the 12 patients with abdominal CT obtained after the first post-transplant CT had findings suggestive of IFI.
Figure 2.
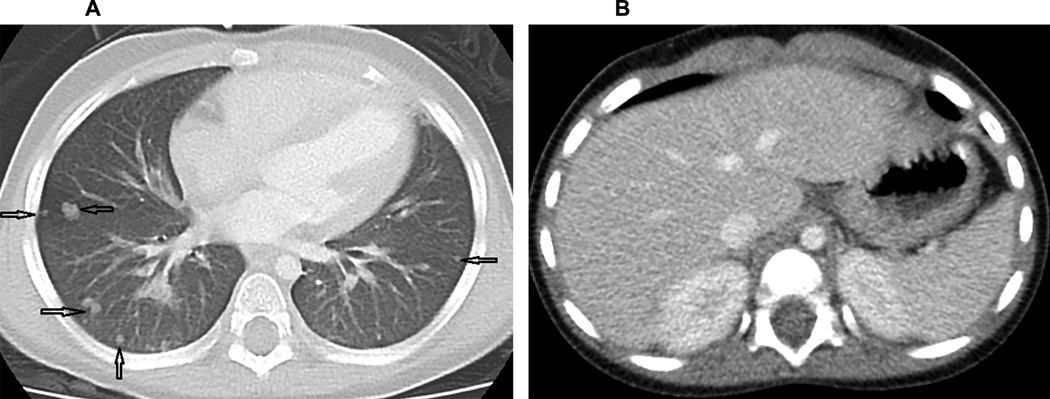
Axial CT images through the lung bases (A) and abdomen (B). A. The chest image is displayed at a lung window/level, demonstrating multiple, bilateral, non-calcified pulmonary nodules of various sizes (arrows) B. A representative image through the upper abdomen shows uniform solid organs and absence of lesions in the liver, spleen and kidneys.
DISCUSSION
Our study demonstrates that none of the patients who had an abdominal CT evaluating for IFI had any findings suggestive of the same. A total of 99 abdominal CT scans were done on 66 patients over 13 months exposing them to radiation risk without affecting change in anti-fungal therapy. Radiation-induced malignancies due to multiple radiation-based imaging studies have no known threshold dose, and may have additive effects with chemotherapy and TBI used as preparative regimen for transplant. Latency periods may span decades [5]. A third of our patients received TBI, and a quarter were undergoing a second HSCT. In addition, sedation if needed, and intravenous contrast administration for abdominal CT is not without risk.
The practice of obtaining a routine abdominal CT scan pre-transplant was started at our institution after recognition of the entity of hepato-splenic fungal abscesses by imaging children with leukemia undergoing therapy [3]. Mortality from this disease is substantial in the absence of early recognition and prompt therapy. Over a 11-year period between 1980–1990, a total of 35 children at our institution had CT findings compatible with IFI in the liver, spleen and /-or kidneys confirmed by culture, biopsy or autopsy [6]. With early diagnosis of this condition, the 3-month survival was 86% [6].
Abdominal fungal disease occurs in patients with known fungemia [3]. Improved culture methodologies increasing the sensitivity of diagnostic fungal culture allowing rapid initiation of therapy, and decline in incidence of IFI with prophylactic anti-fungals, may explain the low yield of routine abdominal CT imaging for IFI. In a review of 355 autopsies performed between 1990–1994 on HSCT patients, fluconazole prophylaxis resulted in a decrease of Candida spp infections from 27% to 8% while Aspergillus spp infections increased from 18% to 29% [7]. With the advent of voriconazole prophylaxis in the 2000s the incidence of invasive aspergillosis at our institution has dramatically declined [8]. In a prior retrospective study of allogeneic HSCT patients at our institution, routine imaging of the abdomen in asymptomatic patients pre-transplant did not reveal any previously unknown abnormalities requiring changes in anti-fungal therapy [9]. Our current study validates this finding.
Our patients were followed up to 100 days post-transplant to study the evolution of imaging findings in those with evidence of IFI pre-transplant, and utility of obtaining chest and abdominal CT scans in patients with unexplained fever with neutropenia. Typhlitis and hepatosplenic fungal infection are often listed as the reasons for performing an abdominal CT examination in this setting. However typhlitis on imaging generally is preceded by symptoms, in the absence of which, is extremely unlikely. Abdominal ultrasound has been shown to be a sensitive modality for diagnosis of typhlitis at our institution [10], and by others [11]. Ultrasound is suggested as a sensitive, cost-effective and safe modality for detection of hepatosplenic candidiasis [12]. Abdominal ultrasound is the recommended modality for diagnostic imaging in patients with prolonged fever and neutropenia by some oncology groups [13].
In a review of cases of aspergillosis over a 34-year period at our institution, no cases of isolated abdominal or pelvic disease were noted; the lung was the most common organ involved with concurrent abdominal disease [14]. Of the autopsy proven cases of aspergillosis in the liver, spleen and kidney, CT imaging showed evidence of abscesses in 25% of patients with hepatic and splenic disease, and in none of the patients with renal involvement. Our patient (UPN 4) had no evidence of abdominal involvement despite proven IFI with lung nodules on chest CT.
In patients with prior history of IFI, with unexplained fever and neutropenia, a chest CT may be useful. Other indications for obtaining a chest CT may include increased respiratory rate or hypoxia, and serially increasing galactomannan. Empiric chest CT imaging for unexplained fever and neutropenia did not result in alteration of anti-fungal therapy, and may not be indicated in the absence of localizing signs or symptoms. In a retrospective study of 52 children with malignancies excluding HSCT patients, with 68 unique episodes of fever and neutropenia who underwent routine CAF imaging after 4 days of unexplained fever, positive findings in chest CT seen in 25 (37%) of episodes were not clinically significant, and did not lead to changes in anti-fungal therapy [15]. There were no patients with occult evidence of IFI in abdominal CT [15].
The presence of circulating galactomannan was predictive of invasive aspergillosis with a sensitivity of 66% at our institution [16]. None of our patients had a positive test for galactomannan pre-transplant. Galactomannan and chest CT-based preemptive antifungal therapy have reduced anti-fungal drug use in patients at high risk for IFI [17]. A recently published meta-analysis which included more than 1,770 patients with hematologic malignancies showed that the performance of 2 consecutive β-glucan tests has an excellent specificity (98.9%) but a low sensitivity (49.6%) for the diagnosis of IFI [18]. The use of PCR, and other molecular approaches, such as matrix-assisted laser desorption ionization (MALDI), and fluorescence in situ hybridization (FISH), have proved promising in clinical trials but still need to undergo standardization before their clinical use can become widespread [19].
Children and adolescents are at higher risk from exposure to ionizing radiation from CT for all types of cancer [20, 21]. In keeping with “As low as reasonably achievable radiation” (ALARA) principles, we recommend reconsideration of routine CAF imaging of HSCT patients by omitting CT imaging of the abdomen pre-transplant and replacing it with ultrasound or limited magnetic resonance imaging post-transplant when clinically indicated. Future studies may evaluate the utility of CT imaging of the sinuses both pre-transplant, and in the setting of unexplained fever and neutropenia post-transplant.
ACKNOWLEDGMENTS
The authors thank Kimberly Bailey-Johnson, Clinical Research Associate, Department of Radiological Sciences, SJCRH for assistance in data collection.
Funding sources: This work was supported by NCI Cancer Center CORE Support Grant P30 CA 21765 and by the American Lebanese Syrian Associated Charities
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartley DL, Hughes WT, Parvey LS, Parham D. Computed tomography of hepatic and splenic fungal abscesses in leukemic children. Pediatr Infect Dis. 1982;1:317–321. doi: 10.1097/00006454-198209000-00007. [DOI] [PubMed] [Google Scholar]
- 4.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brody AS, Frush DP, Huda W, Brent RL. Radiation risk to children from computed tomography. Pediatrics. 2007;120:677–682. doi: 10.1542/peds.2007-1910. [DOI] [PubMed] [Google Scholar]
- 6.Flynn PM, Shenep JL, Crawford R, Hughes WT. Use of abdominal computed tomography for identifying disseminated fungal infection in pediatric cancer patients. Clin Infect Dis. 1995;20:964–970. doi: 10.1093/clinids/20.4.964. [DOI] [PubMed] [Google Scholar]
- 7.van Burik JH, Leisenring W, Myerson D, et al. The effect of prophylactic fluconazole on the clinical spectrum of fungal disease in bone marrow transplant recipients with special attention to hepatic candidiasis. An autopsy study of 355 patients. Medicine. 1998;77:246–254. doi: 10.1097/00005792-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Maron GM, Hayden RT, Rodriguez A, Rubnitz JE, Flynn PM, Shenep JL, et al. Voriconazole prophylaxis in children with cancer: changing outcomes and epidemiology of fungal infections. Pediatr Infect Dis J. 2013;32:e451–e455. doi: 10.1097/INF.0b013e3182a74233. [DOI] [PubMed] [Google Scholar]
- 9.Kasow KA, Krueger J, Srivastava DK, et al. Clinical utility of computed tomography screening of chest, abdomen and sinuses before hematopoietic stem cell transplantation: the St Jude experience. Biol Blood Marrow Transplant. 2009;15:490–495. doi: 10.1016/j.bbmt.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarville MB, Adelman CS, Li C, et al. Typhlitis in childhood cancer. Cancer. 2005;104:380–387. doi: 10.1002/cncr.21134. [DOI] [PubMed] [Google Scholar]
- 11.Ojala AE, Lanning FP, Lanning BM. Abdominal ultrasound findings during and after treatment of childhood acute lymphoblastic leukemia. Med Pediatr Oncol. 1997;29:266–271. doi: 10.1002/(sici)1096-911x(199710)29:4<266::aid-mpo6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Von Eiff M, Essink M, Roos N, Hiddemann W, Buchner T, van de Loo J. Hepatosplenic candidiasis, a late manifestation of candida septicemia in neutropenic patients with hematologic malignancies. Blut. 1990;60:242–248. doi: 10.1007/BF01728792. [DOI] [PubMed] [Google Scholar]
- 13.Ruhnke M, Böhme A, Buchheidt D, et al. Diagnosis of invasive fungal infections in hematology and oncology: Guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO) Ann Hematol. 2003;82:S141–S148. doi: 10.1007/s00277-003-0768-0. [DOI] [PubMed] [Google Scholar]
- 14.Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: A 34-year experience. Clin Infect Dis. 1999;29:1210–1219. doi: 10.1086/313445. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal AK, Saini N, Gildengorin G, Feusner JH. Is routine computed tomographic scanning justified in the first week of persistent febrile neutropenia in children with malignancies. Pediatr Blood Cancer. 2011;57:620–624. doi: 10.1002/pbc.22974. [DOI] [PubMed] [Google Scholar]
- 16.Hayden RT, Pounds S, Knapp K, et al. Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis. Pediatr Infect Dis J. 2008;27:815–819. doi: 10.1097/INF.0b013e31817197ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maertens J, Theunissen K, Verhoef G, et al. Galactomannan and computed tomography-based preemptive antifungal therapy in neutropenic patients at high risk for invasive fungal infection: a prospective feasibility study. Clin Infect Dis. 2005;41:1242–1250. doi: 10.1086/496927. [DOI] [PubMed] [Google Scholar]
- 18.Lamoth F, Cruciani M, Mengoli C, et al. Beta-glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3) Clin Infect Dis. 2012;54:633–643. doi: 10.1093/cid/cir897. [DOI] [PubMed] [Google Scholar]
- 19.Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clinical Microbiology Reviews. 2014;27:490–526. doi: 10.1128/CMR.00091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. Am J Roent. 2001;176:289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 21.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine. 2007;86:215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]