Abstract
Objectives
To evaluate associations of pre-ART CD4 with peripheral neuropathy (PN) and estimate the prevalence of PN in HIV positive patients starting modern combination antiretroviral therapy (cART) regimens.
Methods
ART-naïve subjects initiating cART were followed longitudinally and screened for signs/symptoms of PN.
Results
Lower pre-ART CD4 count was associated with post-ART PN. After 7 years (n=117), the prevalence (95% CI) of PN and SPN were 31% (23%, 40%) and 5% (2%, 11%) with pre-ART CD4 count > 250 copies/µL.
Conclusion
PN continues to be identified in HIV-infected individuals on modern cART by targeted assessment, but is generally without symptoms.
Keywords: peripheral neuropathy, symptomatic peripheral neuropathy, risk factors, HIV, modern non-neurotoxic ART, higher pre-ART CD4 count
INTRODUCTION
The advent of combination antiretroviral therapy (cART) has decreased the incidence of HIV-associated neurologic disorders, but distal sensory peripheral neuropathy (PN) remains problematic.1,2,3,4,5 Two common etiologies of PN, ART-toxic neuropathy (ATN) and primary HIV-associated polyneuropathy (HIV-PN), together account for approximately 30–67% of the advanced HIV infected population.6,7,8 HIV-PN is the most common form of PN in HIV infected population with a reported 1-year incidence in an advanced patient cohort selected for neurologic disease risk of 36% and 21% in the pre-cART and cART eras, respectively.9 In the early cART era, ATN contributed to the high prevalence of PN, primarily due to stavudine and to a lesser extent didanosine use.10,11 However, with the infrequent use of these agents in the current era and the emphasis on early treatment of HIV prior to advanced immunodeficiency, the prevalence of PN has not been well-defined.
Clinical manifestations of HIV-PN and ATN are indistinguishable and resemble the signs and symptoms of neuropathies due to other etiologies such as diabetes and alcohol. Common signs include reduced or absent ankle reflexes relative to patellar reflexes, reduced or absent vibration sensation in the toes, and decreased pin and temperature sensation in a stocking/glove distribution. Common symptoms include numbness, paresthesias, a burning sensation, and stabbing pain, especially in the feet. Currently FDA-approved therapies do not exist for HIV-associated PN, and only treatments of modest efficacy are available to alleviate symptoms.12
Since stavudine and didanosine use were found to be a risk factors for PN, the examination of HIV-infected patients not exposed to these agents reflects the anticipated future prevalence of neuropathy.1,3 In addition, pre-ART (nadir) CD4 count has been shown to be a strong predictor of PN.3 As HIV-infected patients are initiated on ART at higher CD4 counts13,14, this trend may further reduce the incidence of PN.
In this study, we investigated the prevalence of PN and symptomatic PN (SPN) in ART-naïve HIV-positive subjects who had initiated non-neurotoxic cART (nART) in three randomized AIDS Clinical Trials Group (ACTG) trials.
METHODS
Participants were selected from the ACTG Longitudinal Linked Randomized Trials (ALLRT), a prospective observational study of participants who continued follow-up after the completion of their randomized clinical trials of cART regimens within the ACTG.15 Study participants had variable length of follow-up and variable timing (relative to start of new regimens) for their first neuropathy evaluations due to the time when they enrolled in ALLRT (while the study participants may have started in the ACTG parent study at week 0, the same participants may not have enrolled in ALLRT until later weeks).
Participants from three ACTG randomized trials were analyzed: A5095 (enrolled from 2001–2002)16, A5142 (enrolled 2003–2004)17, and A5202 (enrolled 2005–2007)18. The included participants were who had initial cART and first neurological evaluation without nART (stavudine or didanosine).
Initial cART for each of the cohort was as follows: A5095 (lamivudine(3TC)+zidovudine(ZDV)+efavirenz(EFV), abacavir(ABC)+3TC+ZDV, or ABC+3TC+ZDV+EFV), A5142 (3TC+tenofovir(TDF)+EFV, 3TC+TDF+lopinavir/ritonavir(LPV/r), 3TC+ZDV+EFV, 3TC+ZDV+LPV/r, or EFV+LPV/r), A5202 (ABC+3TC+EFV, ABC+3TC+atazanavir/ritonavir(ATV/r), emtricitibine(FTC)+TDF+EFV, or FTC+TDF+ATV/r).
Neuropathy Data
The Brief Peripheral Neuropathy Screen (BPNS) was administered in ALLRT at entry, then at every 48 weeks afterwards by trained non-neurologist site personnel. The BPNS assessed signs (vibration sensation at the feet and ankle reflexes) and symptoms (pain, “pins and needles” sensation, and numbness). The performance characteristics of the BPNS have been reported elsewhere.19,20
PN was defined as absent or hypoactive ankle reflexes or at least mild loss of vibration sensation in both of the great toe joints. SPN was defined as PN plus any bilateral symptoms. Painful neuropathy was defined as PN plus symptoms of pain in both of the feet or legs. Neuropathic outcomes included PN, SPN, painful neuropathy, loss of vibration, absent reflexes, loss of vibration and reflexes, symptom of pain, “pins and needles” sensation, and numbness.
Objectives
The objectives of this study were:
To evaluate the association between pre-ART CD4 count and neuropathic outcomes.
To estimate the prevalence of PN and SPN in HIV-treatment naïve patients whose pre-ART CD4 count is greater than 250 cells/µl at initiation of non-neurotoxic cART.
Statistical Methods
Descriptive statistics were used to summarize the study sample. Univariable and multivariable logistic models were used to evaluate the association [odds ratios (ORs) and associated confidence intervals (CIs)] between pre-ART CD4 and pre-ART (baseline) neuropathic outcomes in the entire study sample as well as in the subset of participants with a pre-ART CD4 > 250 cells/µL. Univariable and multivariable logistic regression models (logistic generalized estimating equation (GEE) with AR1 working covariance matrix) were used to estimate the association between pre-ART CD4 and post-ART (post-baseline) neuropathic outcomes. Prevalence with 95% CI of pre-ART neuropathic outcomes was plotted as a moving average function of pre-ART CD4. Longitudinal plots display the prevalence of PN and SPN over time since ART initiation.
Multivariable models were fit to obtain ORs adjusted for other variables. Variables included were demographics, HIV disease characteristics, protease inhibitor (PI) use at the time of the evaluation, concomitant therapy use, and other patient characteristics. Demographic variables included: age at the time of evaluation (scaled such that ORs are interpreted for a 10 year increment), race [white (reference), black, Hispanic, other], sex (reference = male), and height at parent study entry (scaled such that odds ratios are interpreted for a 5 cm increment). HIV disease characteristics variables included: log10(HIV-1 RNA) at baseline (pre-ART), HIV-1 RNA at the time of evaluation [categories: ≤400 (reference), >400 copies/ml], and CD4 at the time of evaluation: [categories: ≤200, 201–350, 351–500, ≥501 cells/µL(reference)]. Concomitant therapy variables included: the use a statin drug, a non-statin lipid-lowering drug, insulin, and a non-insulin glucose-lowering drug, all within the previous 21 days of the evaluation. Other patient characteristics included: reported history of diabetes, HCV seropositivity and history of IV drug use.
RESULTS
Association between pre-ART CD4+ counts and pre-ART neuropathic outcomes
The evaluation of the associations between pre-ART CD4 and pre-ART neuropathic outcomes is based on data from 698 patients (86% male, 46% white, 30% black, 42% were 30–39 years of age, median pre-ART log10 HIV-1 RNA = 4.70 copies/ml, and median pre-ART CD4 count = 238 cell/µL) (Table 1). In univariable logistic regression models, pre-ART CD4 was significantly associated with the following pre-ART neuropathic outcomes: SPN (OR=0.69 per 100 pre-ART CD4 increment, 95% CI=(0.52, 0.91), p=0.01) and numbness (OR=0.80; 95% CI=0.65, 0.98; p=0.03) (Table 2). In multivariable logistic regression models, these neuropathic outcomes were no longer significantly associated (p-values>0.05) with pre-ART CD4 (Table 2). *(Of note: Table 2 summarizes the results for full-range of pre-ART CD4 counts but not for pre-ART CD4 > 250 copies/µL).
Table 1.
Demographics & Baseline Characteristics
| Characteristic | Model 1* (N=698) |
Model 2t (N=2092) |
Model 3p (N=1929) |
Model 4** (N=996) |
|
|---|---|---|---|---|---|
| Age at Baseline (pre-ART) | < 20 | 5 (1%) | 13 (1%) | 10 (1%) | 11 (1%) |
| 20–29 | 125 (18%) | 373 (18%) | 340 (18%) | 207 (21%) | |
| 30–39 | 293 (42%) | 787 (38%) | 720 (37%) | 388 (39%) | |
| 40–49 | 198 (28%) | 653 (31%) | 609 (32%) | 280 (28%) | |
| 50–59 | 67 (10%) | 227 (11%) | 212 (11%) | 97 (10%) | |
| Over 60 | 10 (1%) | 39 (2%) | 38 (2%) | 13 (1%) | |
| Sex | Male | 598 (86%) | 1,713 (82%) | 1,579 (82%) | 832 (84%) |
| Female | 100 (14%) | 379 (18%) | 350 (18%) | 164 (16%) | |
| Race/Ethnicity | White | 321 (46%) | 899 (43%) | 852 (44%) | 482 (48%) |
| Black | 207 (30%) | 685 (33%) | 602 (31%) | 273 (27%) | |
| Hispanic | 150 (21%) | 443 (21%) | 412 (21%) | 206 (21%) | |
| Asian | 11 (2%) | 40 (2%) | 38 (2%) | 24 (2%) | |
| Native Indian/Alaskan | 4 (1%) | 9 (0%) | 9 (0%) | 3 (0%) | |
| Other | 5 (1%) | 11 (1%) | 11 (1%) | 6 (1%) | |
| Unknown/missing | 0 (0%) | 5 (0%) | 5 (0%) | 2 (0%) | |
| IV drug history | No | 637 (91%) | 1,905 (91%) | 1,757 (91%) | 904 (91%) |
| Yes | 61 (9%) | 187 (9%) | 172 (9%) | 92 (9%) | |
| HCV seropositivity | Positive ever | 79 (11%) | 209 (10%) | 190 (10%) | 89 (9%) |
| Negative | 597 (86%) | 1,883 (90%) | 1,739 (90%) | 896 (90%) | |
| Not available | 22 (3%) | 0 (0%) | 0 (0%) | 11 (1%) | |
| Baseline (pre-ART) CD4 Count (cells/ul) | N | 698 | 2,092 | 1,929 | 996 |
| Median | 238 | 226 | 229 | 350 | |
| Q1, Q3 | 109, 362 | 89, 335 | 94, 335 | 299, 440 | |
| Baseline (pre-ART) Log10(HIV RNA Viral Load),(cp/ml) | N | 698 | 2,092 | 1,929 | 996 |
| Median | 4.70 | 4.68 | 4.68 | 4.52 | |
| Q1, Q3 | 4.34, 5.20 | 4.36, 5.15 | 4.36, 5.13 | 4.15, 4.80 |
Model 1: Multivariable logistic regression model used to estimate association between pre-ART CD4 (full range) and neuropathy outcome
Model 2: Multivariable logistic GEE model used to estimate association between pre-ART CD4 (full range) and neuropathy outcome
Model 3: Same as Model 2 but restricted to sub-population (HIV-RNA VL ≤50cp/ml)
Model 4: ART-naïve subjects with pre-ART CD4 > 250 cells/µL
Table 2.
Association between neuropathy outcomes and full range pre-ART CD4 counts
| Association between neuropathy outcomes and pre-ART CD4 (per 100 higher cells/µL) | ||||||
| Univariable Logistic Regression GEE Models | ||||||
| At Baseline (pre-ART) | Post-Baseline (post-ART) | Post-Baseline (post-ART), HIV-RNA VL ≤ 50 cp/ml | ||||
| Dependent Variable: | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value |
| PN | 0.99 (0.90, 1.09) | 0.85 | 0.93 (0.89, 0.98) | 0.01 | 0.94 (0.88, 0.99) | 0.01 |
| SPN | 0.69 (0.52, 0.91) | 0.01 | 0.94 (0.86, 1.02) | 0.13 | 0.93 (0.85, 1.02) | 0.13 |
| Painful neuropathy | 0.75 (0.52, 1.08) | 0.12 | 0.95 (0.85, 1.05) | 0.29 | 0.95 (0.85, 1.06) | 0.33 |
| Loss of vibration (Sign) | 0.90 (0.79, 1.04) | 0.15 | 0.92 (0.87, 0.97) | 0.003 | 0.91 (0.85, 0.97) | 0.002 |
| Absent reflexes (Sign) | 1.01 (0.91, 1.12) | 0.85 | 0.94 (0.88, 0.99) | 0.02 | 0.94 (0.88, 1.00) | 0.04 |
| Loss of vibration and reflexes (Sign) | 0.88 (0.72, 1.07) | 0.19 | 0.89 (0.82, 0.97) | 0.003 | 0.86 (0.79, 0.95) | <.001 |
| Pain (Symptom) | 0.94 (0.80, 1.12) | 0.50 | 0.98 (0.91, 1.05) | 0.57 | 0.98 (0.90, 1.06) | 0.62 |
| Symptomatic (Symptom) | 0.88 (0.77, 1.01) | 0.07 | 0.97 (0.91, 1.03) | 0.30 | 0.97 (0.91, 1.03) | 0.31 |
| Pins and needles (Symptom) | 0.83 (0.67, 1.02) | 0.07 | 0.98 (0.91, 1.06) | 0.62 | 0.97 (0.89, 1.05) | 0.39 |
| Numbness (Symptom) | 0.80 (0.65, 0.98) | 0.03 | 0.92 (0.84, 0.99) | 0.03 | 0.91 (0.83, 0.99) | 0.02 |
| Multivariable Logistic Regression GEE Models | ||||||
| At Baseline (pre-ART) | Post-Baseline (post-ART) | Post-Baseline (post-ART), HIV-RNA VL ≤ 50 cp/ml | ||||
| Dependent Variable: | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value |
| PN | 1.03 (0.93, 1.14) | 0.58 | 0.98 (0.92, 1.04) | 0.49 | 0.99 (0.93, 1.06) | 0.82 |
| SPN | 0.75 (0.55, 1.03) | 0.08 | 0.97 (0.88, 1.07) | 0.59 | 0.98 (0.89, 1.09) | 0.76 |
| Painful neuropathy | 0.84 (0.57, 1.25) | 0.39 | 0.93 (0.83, 1.04) | 0.18 | 0.94 (0.83, 1.06) | 0.31 |
| Loss of vibration (Sign) | 0.98 (0.85, 1.13) | 0.78 | 0.95 (0.88, 1.02) | 0.15 | 0.95 (0.88, 1.03) | 0.19 |
| Absent reflexes (Sign) | 1.05 (0.93, 1.17) | 0.44 | 0.99 (0.92, 1.07) | 0.83 | 1.00 (0.93, 1.08) | 0.95 |
| Loss of vibration and reflexes (Sign) | 0.97 (0.79, 1.19) | 0.80 | 0.96 (0.87, 1.05) | 0.35 | 0.94 (0.85, 1.04) | 0.23 |
| Pain (Symptom) | 1.07 (0.90, 1.27) | 0.48 | 0.98 (0.90, 1.06) | 0.62 | 0.99 (0.90, 1.09) | 0.85 |
| Symptomatic (Symptom) | 0.96 (0.83, 1.11) | 0.58 | 0.99 (0.93, 1.07) | 0.85 | 1.00 (0.93, 1.08) | 0.99 |
| Pins and needles (Symptom) | 0.89 (0.70, 1.12) | 0.31 | 0.98 (0.90, 1.08) | 0.74 | 0.97 (0.88, 1.07) | 0.56 |
| Numbness (Symptom) | 0.84 (0.67, 1.05) | 0.13 | 0.91 (0.83, 1.00) | 0.054 | 0.90 (0.81, 1.00) | 0.049 |
The study sample was then restricted to individuals with pre-ART CD4 > 250 copies/µL, and the evaluation of the associations between pre-ART CD4 and pre-ART neuropathic outcomes is based on data from 336 patients (87% male, 49% white, 24% black, 43% were 30–39 years of age, median pre-ART log10 HIV-1 RNA = 4.53 cp/ml, and median pre-ART CD4 count = 368 cell/ mm3 at cART initiation). In univariable logistic regression models, pre-ART CD4 was significantly associated with: pre-ART PN (OR=1.17 per 100 Pre-ART CD4 increment; 95% CI=1.01, 1.35; p=0.04); this was no longer significant in a multivariable model.
Association between pre-ART CD4+ counts and post-ART neuropathic outcomes
The evaluation of the associations between pre-ART CD4 and post-ART neuropathic outcomes is based on data from 2,092 patients (82% male, 43% white, 33% black, 38% were 30–39 years of age, median pre-ART log10 HIV-1 RNA =4.68 copies/ml, and median pre-ART CD4 count = 226 cell/ mm3 at cART initiation), consisting of 7,487 patient-visits (Table 1). In univariable logistic regression models, pre-ART CD4 was significantly associated with the following post-ART neuropathic outcomes: PN (OR=0.93 per 100 pre-ART CD4 increment; 95% CI=0.89, 0.98, p=0.01), loss of vibration (OR=0.92; 95% CI=0.87, 0.97; p=0.003), absent reflexes (OR=0.94; 95% CI=0.88, 0.99; p=0.02), loss of vibration and reflexes (OR=0.89; 95% CI=0.82, 0.97; p=0.003), and numbness (OR=0.92; 95%CI=0.84, 0.99; p=0.03). In multivariable logistic GEE model, these neuropathic outcomes were no longer significantly associated (p-values > 0.05) with pre-ART CD4 (Table 2).
The study sample was then restricted to individuals with pre-ART CD4 > 250 cells/µL, and the evaluation of the associations between pre-ART CD4 and post-ART neuropathic outcomes and is based on data from 937 patients (83% male, 48% white, 27% black, 38% were 30–39 years of age, median pre-ART log10 HIV-1 RNA =4.51 cp/ml, and median pre-ART CD4 count = 349 cells/µL at cART initiation), consisting of 3,382 patient-visits. In univariable and multivariable logistic GEE models, post-ART neuropathic outcomes were not significantly associated (p-values > 0.05) with pre-ART CD4.
Association between pre-ART CD4+ counts and post-ART neuropathy among virally suppressed subjects
The following sub-analysis included the full range of pre-ART CD4 counts but only those subjects with HIV RNA VL ≤ 50 cp/ml at neuropathy assessments, and is based on data from 1,929 patients (82% male, 44% white, 31% black, 37% were 30–39 years of age, median pre-ART log10 HIV-1 RNA =4.68 copies/ml, and median pre-ART CD4 count = 229 cells/ µL at cART initiation), consisting of 6,315 patient-visits (Table 1). In univariable logistic GEE models, pre-ART CD4 was significantly associated with the following post-ART neuropathic outcomes: PN (OR=0.94 per 100 pre-ART CD4 increment; 95%CI=0.88, 0.99; p=0.01), loss of vibration (OR=0.91; 95% CI=0.85, 0.97; p=0.002), absent reflexes (OR=0.94; 95% CI= 0.88, 1.00; p=0.04), loss of vibration and reflexes (OR=0.86; 95% CI=0.79, 0.95; p<0.001), and numbness (OR=0.91; 95% CI=0.83, 0.99; p=0.02). In multivariable logistic GEE models, pre-ART CD4 was significantly associated numbness (OR=0.90 per 100 pre-ART CD4 increment; 95% CI=0.81, 1.00; p=0.049) (Table 2).
This study sample was then restricted to pre-ART CD4 > 250 copies/µL, and the evaluation of the associations between pre-ART CD4 and post-baseline neuropathic outcomes is based on appropriate data from 882 patients (83% male, 49% white, 26% black, 38% were 30–39 years of age, median pre-ART log10 HIV-1 RNA =4.51 cp/ml, and median pre-ART CD4 count = 347 cell/ mm3 at cART initiation), consisting of 2,881 patient-visits. In univariable and multivariable logistic GEE models, pre-ART CD4 was not significantly associated with any of the post-ART neuropathic outcomes.
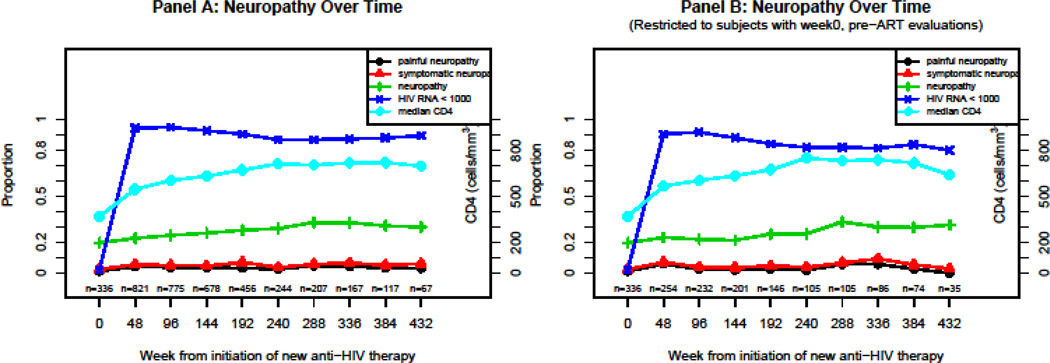
Prevalence of PN and SPN
To estimate the prevalence of PN and SPN, we restricted the analysis to 996 participants whose pre-ART CD4 was greater than 250 cells/µl (84% male, 48% white, 27% black, 39% were 30–39 years of age, median pre-ART log10 HIV-1 RNA =4.52 copies/ml, and median pre-ART CD4 count = 350 cells/ µL at cART initiation) (Table 1). Prior to the initiation of cART, the prevalence (95% CI) of PN and SPN were 19.6% (15.5%, 24.3%) and 2.4% (1.0%, 4.6%). While the prevalence of PN initially increased and then remained stable to about 30%, SPN was rarely encountered in this population (Figure 1A). When the study sample was restricted to subjects with week 0 (pre-ART) neuropathy evaluations, the prevalence of PN and SPN remained similar to the non-restricted study sample (Figure 1B). Among those with concurrent PN, the prevalence of SPN (95% CI) at cART initiation and after 432 weeks of follow-up after cART initiation were 12.1% (5.4%, 22.5%) and 20.0% (5.7%, 43.7%) respectively.
Figure 1.
Neuropathy over time
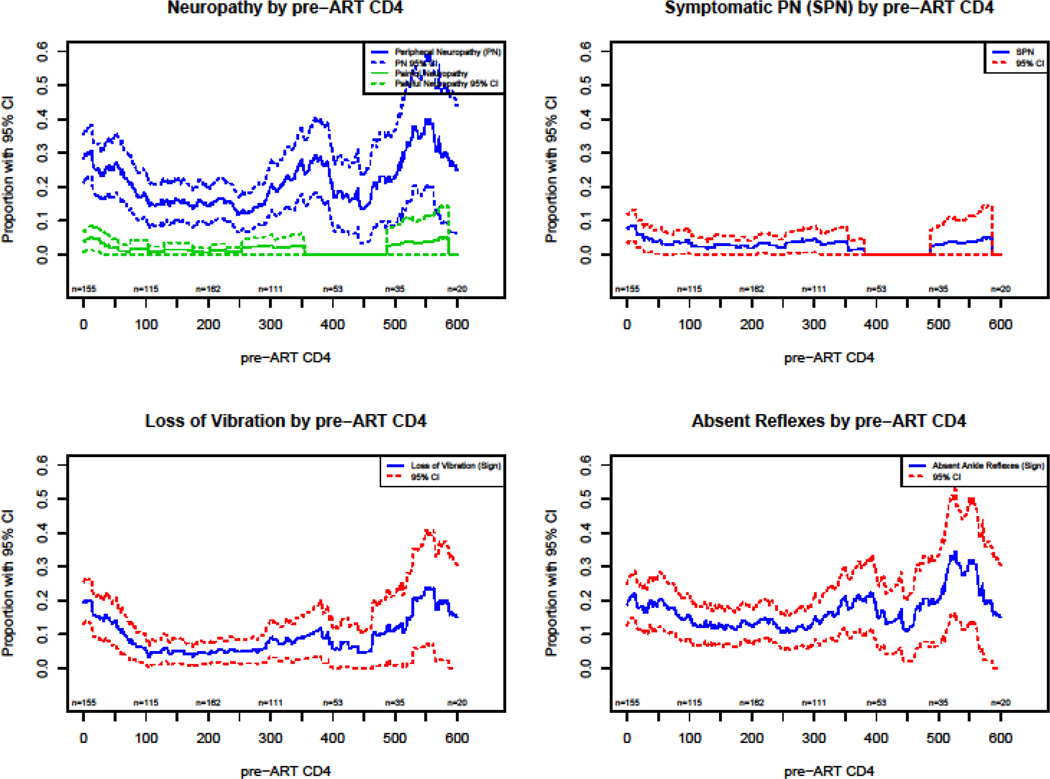
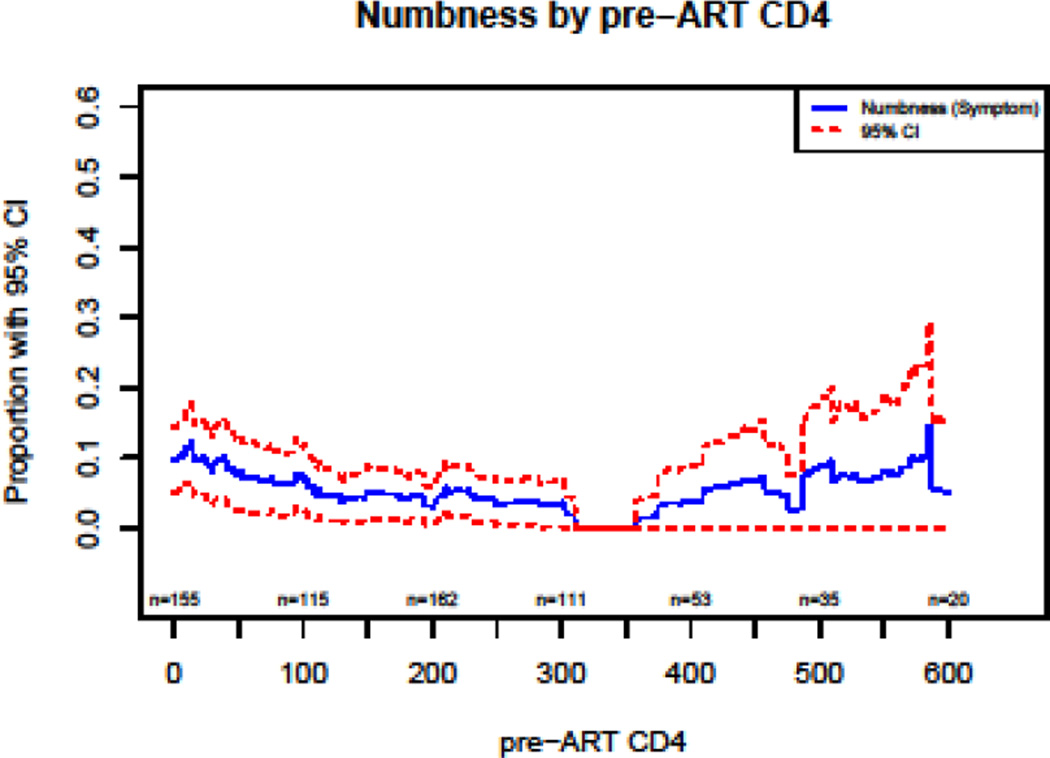
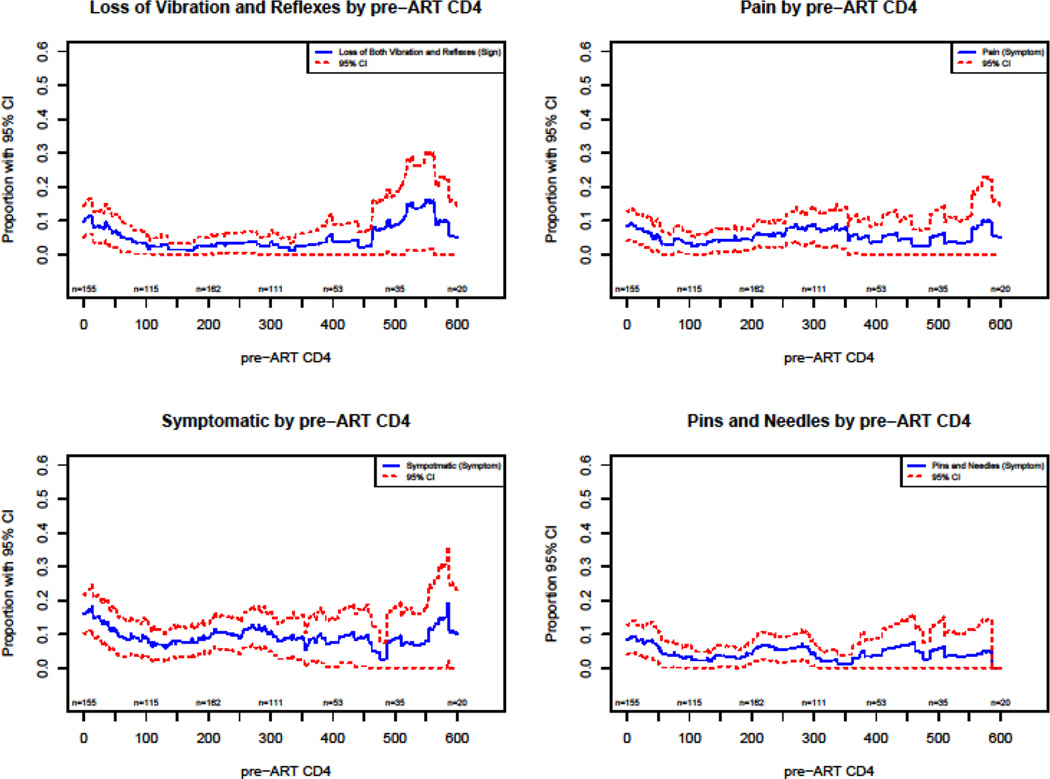
Plots of the prevalence of neuropathic variables as a function of pre-ART CD4 show a decrease in the prevalence of PN with an increase of CD4 count in the regions of 0 to 250 CD4 count (Figures 2–4).
Figure 2.
Pre-ART neuropathy by pre-ART CD4
DISCUSSION
Signs of peripheral nerve injury persist in HIV-infected patients despite improved immunologic function and virologic control associated with earlier cART initiation and decreased nART use.3 However, recent studies evaluating interventions for painful peripheral neuropathy suffered from slow enrollment, and clinicians anecdotally note decreasing complaints of neuropathy in their HIV clinics. The data in this study suggest that despite the presence of signs associated with peripheral nerve dysfunction, symptomatic PN is uncommon and does not increase over a period of as long as eight years after cART initiation in the absence of neurotoxic cART.
Our large database of HIV-infected participants, who were evaluated regularly during and after their participation in randomized treatment initiation studies in the ACTG, is a valuable means of describing peripheral nerve involvement in HIV-infected populations in the current treatment era. Peripheral nerves are delicate and easily injured cells that may be damaged by inflammatory responses during chronic infection including HIV, and thus early studies emphasized the relationship between CD4 counts and viral loads and PN.6,9 PN became more problematic in HIV clinics during the era when neurotoxic nucleosides were commonly used,3,21 and current clinics continue to follow patients who suffered combined HIV and neurotoxic injuries and have persistent neuropathic symptoms. Additionally, metabolic abnormalities in HIV including diabetes and hyperlipidemia may also lead to nerve injury.22 Neurotoxic chemotherapy or alcohol use, sometimes associated with nutritional deficiencies, may also contribute to the prevalence of PN in HIV clinics. However, looking at patients treated in the current era who never develop severe immunodeficiency predicts a more optimistic future. It should be emphasized that symptomatic PN is the fundamental concern, given its association with functional impairment and disability.23,24
In this well-treated population, we show that painful and symptomatic PN is uncommon, and show little sign of increasing prevalence over 8 years of follow-up. While signs compatible with peripheral nerve damage increased slightly over time, we did not have a control group (HIV-negative population) to assess the impacts of aging or other co-morbid conditions. In our rigorously monitored observed cohort with pre-ART evaluations (Figure 1, Panel B), the prevalence of painful and symptomatic PN is rare and does not develop over prolonged observation.
Pre-ART CD4 may predict peripheral nervous system damage and resulting in PN or SPN. Within our large study population, univariable analyses showed pre-ART CD4 to be associated with SPN and numbness, but multivariable analysis reveals only a marginal association between pre-ART CD4 and sensory neuropathy. Based on this, we conclude that HIV infection causes, at most, a very modest clinical nerve damage before advanced immunodeficiency develops. In Figure 2, the vibratory loss, the most reliable sign of nerve injury, appears to show damage with pre-ART CD4 <100 cells which is consistent with earlier observations of nerve damage at very low CD4 counts.25,26 Our analysis, designed to isolate the impact of early advanced immunodeficiency on later development of neuropathy independent of viral load also supports the impact of advanced immune injury as the critical factor in manifestations of PN dysfunction. However, even so, with exception to the association between pre-ART CD4 and numbness, all other neuropathic outcomes were not significant in multivariable models.
Our analysis suggests that given current trends in therapy for HIV worldwide which emphasize early treatment with non-neurotoxic cART, SPN is not likely to be a frequent complication. Although it is important to consider other recognized PN risk factors (including poor nutritional status, diabetes, and alcohol, which may contribute to development or exacerbation of SPN), our observations are consistent with the declining importance of painful and symptomatic PN in association with treated HIV infection. However, the risk of PN has been very consistently associated with increasing age, so the impact of the aging HIV population may in some measure blunt the benefits of earlier, non-toxic ARR.1,3,27,28
Limitations of this study include its observational nature with the potential for informative (non-random) drop-out/in, self-selection issues in ART and concomitant medication use, and the potential for selection bias since the participants analyzed are those who volunteered and were able to return for follow-up visits. Non-significant p-values should not be interpreted as “no association”. Instead the confidence intervals should be used to “rule out” associations with reasonable confidence. Some effect estimates, although not significant, cannot rule out potentially large associations. Also, the neurological examinations were performed by non-neurologists who had received training, but for some parts of the assessments, such as distal ankle jerk, reliability is difficult to establish between examiners and may result in imprecision in some observations.
In summary, this analysis of ART-naïve patients starting non-neurotoxic cART showed a low prevalence of symptomatic PN. Lower pre-ART CD4 counts were associated with asymptomatic and also symptomatic PN (numbness) after cART initiation but not when pre-ART CD4 was > 250 cells/µL, providing additional evidence to support early initiation of ART.
Table 3.
Prevalence of PN and SPN (restricted to pre-ART CD4 > 250 cells/ul)
| PN | SPN | SPN among those with PN | ||||
|---|---|---|---|---|---|---|
| Week since cART initiation |
N | Neuropathy % (95%CI) | N | Neuropathy % (95%CI) | N | Neuropathy % (95%CI) |
| 0 | 336 | 19.6 (15.5, 24.3) | 336 | 2.4 (1.0, 4.6) | 66 | 12.1 (5.4, 22.5) |
| 48 | 821 | 22.7 (19.8, 25.7) | 821 | 5.6 (4.1, 7.4) | 186 | 24.7 (18.7, 31.6) |
| 96 | 775 | 24.6 (21.6, 27.8) | 775 | 5.0 (3.6, 6.8) | 191 | 20.4 (14.9, 26.8) |
| 144 | 678 | 26.0 (22.7, 29.4) | 678 | 4.7 (3.3, 6.6) | 176 | 18.2 (12.8, 24.7) |
| 192 | 456 | 27.9 (23.8, 32.2) | 456 | 7.0 (4.8, 9.8) | 127 | 25.2 (17.9, 33.7) |
| 240 | 244 | 29.1 (23.5, 35.2) | 244 | 3.7 (1.7, 6.9) | 71 | 12.7 (6.0, 22.7) |
| 288 | 207 | 32.9 (26.5, 39.7) | 207 | 5.8 (3.0, 9.9) | 68 | 17.6 (9.5, 28.8) |
| 336 | 167 | 32.9 (25.9, 40.6) | 167 | 6.6 (3.3, 11.5) | 55 | 20.0 (10.4, 33.0) |
| 384 | 117 | 30.8 (22.6, 40.0) | 117 | 5.1 (1.9, 10.8) | 36 | 16.7 (6.4, 32.8) |
| 432 | 67 | 29.9 (19.3, 42.3) | 67 | 6.0 (1.7, 14.6) | 20 | 20.0 (5.7, 43.7) |
ACKNOWLEDGEMENTS
This work was supported by National Institute of Health (NIH) grants including the Neurologic AIDS Research Consortium grant NS32228 from NINDS, the AIDS Clinical Trials Grant U01AI068636 from NIAID, and the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant 1 U01 068634. The clinical trials registration unique identifier is NCT00001137. The authors acknowledge the generous dedication of the many participants volunteering for the ALLRT study, and for the contributions of the contributing AIDS Clinical Trials Units, their investigators and staffs, that collected the samples and clinical data used for this analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health
Sources of Support:
This work was supported by National Institute of Health (NIH) grants including the Neurologic AIDS Research Consortium grant NS32228 from NINDS, the AIDS Clinical Trials Grant AI068636 from NIAID, and the Statistical and Data Management Center of the Adult AIDS Clinical Trials Group grant AI 068634.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Evans SR, Lee AJ, Ellis RJ, Chen H, Wu K, Bosch RJ, Clifford DB. HIV peripheral neuropathy progression: protection with glucose-lowering drugs? J Neurovirol. 2012;18(5):428–433. doi: 10.1007/s13365-012-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Clifford DB, Deng L, Wu K, Lee AJ, Bosch RJ, Riddler SA, Ellis RJ, Evans SR. Peripheral neuropahty in ART-experienced patients: prevalence and risk factors. J Neurovirol. 2013;19(6):557–564. doi: 10.1007/s13365-013-0216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans SR, Ellis RJ, Chen H, Yeh T, Lee AJ, Schifitto G, Wu K, Bosch RJ, McArthur JC, Simpson DM, Clifford DB. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25:919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 5.Ellis R, Rosario D, Clifford D, McArthur JC, Simpson D, Alexander T, Marra C, Gelman BB, Grant I. CROI. Montreal, Canada: 2009. Persisting high prevalence of HIV distal sensory peripheral neuropathy in the era of cART: correlates in the CHARTER study [Paper #461] [Google Scholar]
- 6.Wulff EA, Wang AK, Simpson DM. HIV-associated peripheral neuropathy: Epidemiology, pathophysiology and treatment. Drugs. 2000;59:1251–1260. doi: 10.2165/00003495-200059060-00005. [DOI] [PubMed] [Google Scholar]
- 7.Cornblath DR, McArthur JC. Predominantly sensory neuropathy in patients with AIDS and AIDS-related complex. Neurology. 1988;38:794–796. doi: 10.1212/wnl.38.5.794. [DOI] [PubMed] [Google Scholar]
- 8.So YT, Holtzman DM, Abrams DI, Olney RK. Peripheral neuropathy associated with acquired immunodeficiency syndrome. Prevalence and clinical features from a population-based survey. Arch Neurol. 1988;45:945. doi: 10.1001/archneur.1988.00520330023005. [DOI] [PubMed] [Google Scholar]
- 9.Schifitto G, McDermott MP, McArthur JC, Marder K, Sacktor N, McClernon DR, Conant K, Cohen B, Epstein LG, Kieburtz K NEAD Consortium. Markers of immune activation and viral load in HIV-associated sensory neuropathy. Neurology. 2005;64:842–848. doi: 10.1212/01.WNL.0000152981.32057.BB. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein KA, Armon C, Baron A, et al. Modification of the incidence of drug-associated symmetrical peripheral neuropathy by host and disease factors in the HIV outpatient study cohort. Clin Infect Dis. 2005;40:148. doi: 10.1086/426076. [DOI] [PubMed] [Google Scholar]
- 11.Cherry CL, Skolasky RL, Lal L, et al. Antiretroviral use and other risks for HIV-associated neuropathies in an international cohort. Neurology. 2006;66:867. doi: 10.1212/01.wnl.0000203336.12114.09. [DOI] [PubMed] [Google Scholar]
- 12.Keswani SC, Pardo CA, Cherry CL, Hoke A, McArthur JC. HIV-associated sensory neuropathies. AIDS. 2002;16:2105–2117. doi: 10.1097/00002030-200211080-00002. [DOI] [PubMed] [Google Scholar]
- 13.Althoff KN, Gange SJ, Klein MB, Brooks JT, Hogg RS, Bosch RJ, Horberg MA, Saag MS, Kitahata MM, Justice AC, Gebo KA, Eron JJ, Rourke SB, Gill MJ, Rodriguez B, Sterling TR, Calzavara LM, Deeks SG, Martin JN, Rachlis AR, Napravnik S, Jacobson LP, Kirk GD, Collier AC, Benson CA, Silverberg MJ, Kushel M, Goedert JJ, McKaig RG, Van Rompaey SE, Zhang J, Moore RD. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50(11):1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lennox JL, Landovitz RJ, Ribaudo HJ, Ofotokun I, Na LH, Godfrey C, Kuritzkes DR, Sagar M, Brown TT, Cohn SE, McComsey GA, Aweeka F, Fichtenbaum CJ, Presti RM, Koletar SL, Haas DW, Patterson KB, Benson CA, Baugh BP, Leavitt RY, Rooney JF, Seekins D, Currier JS ACTG A5257 Team. Efficacy and Tolerability of 3 Nonnucleoside Reverse Transcriptase Inhibitor-Sparing Antiretroviral Regimens for Treatment-Naive Volunteers Infected With HIV-1: A Randomized, Controlled Equivalence Trial. Ann Intern Med. 2014;161(7):461–471. doi: 10.7326/M14-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, Benson CA. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): Rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulick RM, Ribaudo HJ, Shikuma CM, Lustgarten S, Squires KE, Meyer WA, 3rd, Acosta EP, Schackman BR, Pilcher CD, Murphy RL, Maher WE, Witt MD, Reichman RC, Snyder S, Klingman KL, Kuritzkes DR AIDS Clinical Trials Group Study A5095 Team. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350(18):1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 17.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, Garren KW, George T, Rooney JF, Brizz B, Lalloo UG, Murphy RL, Swindells S, Havlir D, Mellors JW AIDS Clinical Trials Group Study A5142 Team. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358(20):2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sax PE, Tierney C, Collier AC, Fischl MA, Mollan K, Peeples L, Godfrey C, Jahed NC, Myers L, Katzenstein D, Farajallah A, Rooney JF, Ha B, Woodward WC, Koletar SL, Johnson VA, Geiseler PJ, Daar ES AIDS Clinical Trials Group Study A5202 Team. Abacavir-lamivudine versus tenofovir-emtricitabine for initial HIV-1 therapy. N Engl J Med. 2009;361(23):2230–2240. doi: 10.1056/NEJMoa0906768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArthur JH. The reliability and validity of the subjective peripheral neuropathy screen. J Assoc Nurses AIDS Care. 1998;9:84–94. doi: 10.1016/S1055-3290(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 20.Simpson DM, Kitch D, Evans SR, McArthur JC, Asmuth DM, Cohen B, Goodkin K, Gerschenson M, So Y, Marra CM, Diaz-Arrastia R, Shriver S, Millar L, Clifford DB ACTG A5117 Study Group. HIV Neuropathy Natural History Cohort Study: Assessment Measures and Risk Factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 21.Moyle GJ, Sadler M. Peripheral neuropathy with nucleoside antiretrovirals: risk factors, incidence and management. Drug Saf. 1998;19(6):481–494. doi: 10.2165/00002018-199819060-00005. [DOI] [PubMed] [Google Scholar]
- 22.Calvo M, Martinez E. Update on metabolic issues in HIV patients. Curr Opin HIV AIDS. 2014;9(4):332–339. doi: 10.1097/COH.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 23.Ellis RJ, et al. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67(5):552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandya R, et al. HIV-related neurological syndromes reduce health-related quality of life. Can J Neurol Sci. 2005;32(2):201–204. doi: 10.1017/s0317167100003978. [DOI] [PubMed] [Google Scholar]
- 25.Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Paul R, Shikuma C, Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection--The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12(5):387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- 26.Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, Becker JT, Mellors J, McArthur JC. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52(3):607–613. doi: 10.1212/wnl.52.3.607. [DOI] [PubMed] [Google Scholar]
- 27.Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25(4):451–458. doi: 10.1080/09540121.2012.712669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR, Jr, Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P OAR Working Group on HIV and Aging. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–S18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]