Abstract
Background
Little is known about the use and toxicity of anti-adhesion substances such as sodium hyaluronate-carboxymethylcellulose.
Objective
We analyzed the patterns of use and safety of sodium hyaluronate-carboxymethylcellulose in patients undergoing colectomy and gynecologic surgery.
Design
Retrospective cohort study.
Setting
Nationwide hospitals.
Patients
All patients in the Premier Perspective database who underwent colectomy or hysterectomy from 2000 to 2010 were included in the analyses.
Main Outcome Measure
Hyaluronate-carboxymethylcellulose use was determined by billing codes. For the primary outcome we used hierarchical mixed-effects logistic regression models to determine factors associated with use of hyaluronate-carboxymethylcellulose while a propensity score matched analysis was used to secondarily assess the association between hyaluronate-carboxymethylcellulose use and toxicity (abscess, bowel and wound complications, peritonitis).
Results
We identified 382,355 patients who underwent hysterectomy and 267,368 who underwent colectomy. For hysterectomy, hyaluronate-carboxymethylcellulose use was 5.0% overall increasing from 1.1% in 2000 to 9.8% in 2010. hyaluronate-carboxymethylcellulose was utilized in 8.1% of those who underwent colectomy and increased from 6.2% in 2000 to 12.4% in 2010. Year of diagnosis and procedure volume of the attending surgeon were the strongest predictors of hyaluronate-carboxymethylcellulose use. After matching and risk adjustment, hyaluronate-carboxymethylcellulose use was not associated with abscess formation (1.5% vs. 1.5%) (RR=0.97; 95% CI, 0.84–1.12) in those who underwent hysterectomy. A patient receiving hyaluronate-carboxymethylcellulose had a 13% increased risk of abscess (17.4% vs. 15.0%) (RR=1.13; 95% CI, 1.08–1.17) after colectomy.
Limitations
Observational study
Conclusion
Hyaluronate-carboxymethylcellulose use has increased over the last decade for colectomy and hysterectomy. While there is no association between hyaluronate-carboxymethylcellulose use and abscess following hysterectomy, hyaluronate-carboxymethylcellulose use was associated with a small increased risk of abscess after colectomy.
Keywords: Hyaluronate-carboxymethylcellulose, HA-CMC, adhesion, hysterectomy, colectomy
Introduction
Intraperitoneal adhesions cause significant morbidity in patients who have undergone surgery.1–5 Adhesions are associated with pain, bowel obstruction, infertility, and increase the risk of operative injury in future procedures.1–6 It has been estimated that adhesive disease is responsible for two-thirds of bowel obstructions in developed countries.1,2,4,5 Of greater concern, the symptoms that result from adhesions are unpredictable and often persist over a patient’s entire lifetime.1 Adhesions are particularly common after gynecologic and colorectal procedures with some studies suggesting that adhesions develop after 85% of gynecologic surgeries.1–6
Adhesions develop after surgical trauma and result from mesothelial regeneration between damaged serosal surfaces.1,6,7 Gentle tissue handling and attention to meticulous surgical technique may reduce, but cannot completely eliminate, the risk of postoperative adhesion formation. A number of interventions have been developed over the last two decades to reduce the risk of developing adhesions.1,6,8 While pharmacologic strategies such as corticosteroids and anticoagulants have met with only limited success, barrier agents have received greater enthusiasm.1,6,8 Barrier agents, either fluids or solid phase membranes, prevent contact between denuded serosal surfaces to reduce adhesions.1,6,8 A number of barrier substances, including hyaluronic acid, hyaluronic acid/carboxymethyl cellulose membrane (HA-CMC), oxidized regenerated cellulose, polytetrafluoroethylene, and 4% Icodextrin, have been marketed for general and gynecologic surgery.1,6,8
To date, hyaluronic acids and HA-CMC have received the greatest attention. Recent meta-analyses have suggested that these agents decrease adhesion formation in patients undergoing gynecologic (OR=0.31; 95% CI, 0.19–0.51) as well as non-gynecologic abdominal surgery (OR=0.15; 95% CI, 0.05–0.43).1,6 Despite potential efficacy in adhesion prevention, a number of potential safety concerns have arisen for HA-CMC including possible increased risk of abdominal-pelvic fluid collections and abscess formation.9–14 While anti-adhesion substances including HA-CMC are widely marketed, little is known about the patterns of use of these agents in practice or the factors that influence use and toxicity. We performed an analysis to determine the patterns of use and safety of HA-CMC in patients undergoing gynecologic and colorectal surgery.
Methods
Data Source
The Perspective database (Premier, Charlotte, North Carolina) was utilized. The Perspective database is a voluntary, fee-supported dataset that was originally developed to measure quality and resource use. The database includes patients from more than 600 acute-care hospitals throughout the U.S.15 In addition to demographics, disease characteristics, and procedures, the database collects information on all billed services including use of drugs and devices. The Perspective database has been previously validated and has been utilized in a number of outcomes studies.16–18 In 2006, Perspective recorded approximately 5.5 million hospital discharges that represent approximately 15% of nationwide hospitalizations.15,17 The study was deemed to be exempt by the Columbia University Institutional Review Board.
Cohort Selection
Our cohort included patients 18–90 years of age who underwent laparotomy and hysterectomy (ICD-9 68.3, 68.39, 68.4, 68.49, 68.6, 68.69, 68.9) or colectomy (ICD-9 45.7x, 45.82, 45.83) between January, 2000 and the March, 2010. Patients who underwent procedures coded as laparoscopic were not included in the analysis. We recorded concomitant procedures at the index operation including performance of adhesiolysis (ICD-9 54.5x, 59.1x, 65.8x, 59.02, 59.03) or small bowel resection (ICD-9 45.6x). For women who underwent hysterectomy, we also recorded performance of colectomy (codes as above) or rectosigmoid resection (ICD-9 48.5x, 48.6x). Use of HA-CMC was based on billing for any HA-CMC.
Clinical, Demographic, and Hospital Characteristics
Demographic data analyzed included: gender (male or female), age (<60 or ≥ 60 years of age), race (white, black or other), year of diagnosis, marital status (married or single), insurance (commercial, Medicare, Medicaid or uninsured), and presence of cancer (gynecologic cancer for women who underwent hysterectomy ICD-9 179-184.9 and colon cancer for patients who underwent primary colectomy ICD-9 153-153.9). Risk adjustment for comorbid conditions was performed using the Charlson comorbidity index.19 The ICD-9 coding to define the Charlson index as reported by Deyo and colleagues was utilized.20
A number of hospital characteristics were also analyzed including: area (metropolitan, or non-metropolitan), region (Eastern, Midwest, Southern or Western), size (<400, 400-600 or >600 beds), and teaching status (teaching, non-teaching). We also classified hospitals based on their individual case mix of patients who underwent hysterectomy and colectomy. Characteristics analyzed included: percentage of patients <60 years of age (<50 versus ≥50%), percentage of black patients (<20% versus ≥20%), percentage of Medicaid/uninsured patients (<10% versus ≥10%), percentage of patients with commercial insurance (<50% versus ≥50%), and percentage of patients with >1 comorbidity (<40% versus ≥40%).
For each surgeon and hospital, we determined the total number of procedures performed during the study period. As not all providers contributed data over the entire study period, we calculated annualized procedure volumes. The annualized procedure volume was estimated by dividing the total number of patients who underwent a procedure by the number of years a given surgeon or hospital contributed at least one procedure. The volumes were then divided to create three approximately equal tertiles of surgeon and hospital volume: low, intermediate, and high.21,22 Separate volume estimates were determined for hysterectomy and colectomy.
Outcomes
Outcomes of interest including perioperative complications potentially associated with use of adhesion barriers. The primary outcome was intraabdominal or retroperitoneal abscess formation (ICD-9 566.x, 567.22, 567.31, 569.5x, 57.38, 682.9x, 998.59). We also examined bowel obstruction (ICD-9 560, 560.9, 560.81, 537.3), ileus (ICD-9 560.1, 536.2), peritonitis (ICD-9 567.0, 567.1, 567.2, 572.21, 567.23, 567.29, 567.89, 567.8, 567.89, 614.5), and wound complications (ICD-9 879.3, 879.5, 879.9, 998.13, 998.3x, 998.51, 998.6, 998.83) as secondary end points.
Statistical Analysis
An initial analysis was performed to determine the characteristics of the hospitals within our dataset that used HA-CMC. All hospitals that performed hysterectomy or colectomy were included in these analyses. Hospital characteristics were compared using χ2 tests for any vs. no HA-CMC use. A second analysis was then performed to determine the hospital characteristics associated with early uptake (first use of HA-CMC in or before 2004 compared to no use of HA-CMC or first use after 2004).
The initial hospital-level analysis identified 464 hospitals in which HA-CMC was used. As surgeons in hospitals that never used HA-CMC may not have had access to the product, we limited all subsequent patient-level analyses to only those patients treated at hospitals that had reported HA-CMC use in at least one case. Characteristics associated with HA-CMC use were compared using χ2 tests. To determine the predictors of HA-CMC use, we developed multivariate mixed-effects log-Binomial regression models.23 These models included all of the patient and hospital characteristics as well as a random intercept for each hospital. Results are reported as relative risks with 95% confidence intervals. Separate models were developed for colectomy and hysterectomy.
To analyze the outcomes of interest and minimize selection bias, we performed a propensity score matched analysis. The propensity score is the conditional probability that a patient will receive a given intervention, in this case HA-CMC. Once estimated, the propensity score can be used to reduce bias through matching.24–26 A propensity score was generated for each patient using logistic regression models that included all of the clinical and demographic variables. The probability this analysis were used to generate a propensity score ranging from 0 to 1 for each patient. Separate models were developed for patients who underwent hysterectomy and colectomy. Based on the propensity score, matched groups (2 controls to 1 case) were generated using a matching algorithm with a caliper of 0.005.24 The caliper is the largest distance allowed between two propensity scores to define a match. Sensitivity analyses were performed matching different numbers of controls to cases, as well as using different caliper settings. Separate PS models were developed for patients who underwent hysterectomy and colectomy.
The characteristics of patients who received HA-CMC and those who did not after propensity matching were compared using χ2 tests. Univariable regression was then performed to determine the association between HA-CMC use and the outcomes of interest. Despite propensity matching for the use of HA-CMC, a number of factors, such as perioperative complications that occur after HA-CMC placement, may also influence the outcomes of interest, such as abscess formation and bowel complications. To control for these confounding factors, multivariable logistic regression models were developed to determine the influence of HA-CMC use on the primary and secondary outcomes in the propensity matched cohort. In addition to use of HA-CMC, these models included the occurrence of intraoperative complications (bladder, ureteral, intestinal, bowel or vascular injury or other operative injury), infectious complications (pneumonia, bacteremia, sepsis), medical complications (venous thromboembolism, myocardial infarction, cardiopulmonary arrest, renal failure, respiratory failure, shock, stroke), hemorrhage and transfusion, as well as factors that predispose patients to complications (age, comorbidity). All analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). All statistical tests were two-sided.
Results
A total of 649,723 patients including 382,355 who underwent hysterectomy and 267,368 who underwent colectomy were identified. HA-CMC was utilized at least once by 464 (75.8%) of the 612 hospitals that performed procedures in our dataset (Table 1). Hospitals with a higher concentration of elderly patients, a lower concentration of black patients, a lower concentration of Medicaid/uninsured patients, a higher concentration of patients with commercial insurance, and a higher concentration of patients with ≥1 medical comorbidity were more likely to use HA-CMC (p<0.05 for all). Similarly, teaching hospitals, hospitals in metropolitan areas, larger hospitals, and centers in the western U.S. were more likely to use HA-CMC (p<0.05). Similar trends were noted for early uptake (use in or before 2004) of HA-CMC, hospitals with a higher concentration of elderly patients, a lower concentration of Medicaid/uninsured patients, a higher concentration of patients with commercial insurance, teaching hospitals, large hospitals and centers in the south and west were more likely to incorporate HA-CMC early (p<0.05).
Table 1.
Association between hospital characteristics and use of intraoperative HA-CMC.
| Any use of HA-CMC | Early uptake of HA-CMC (on or before 2004) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||||
|
|
||||||||||
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | p-value | |
| 148 | (24.2) | 464 | (75.8) | 317 | (51.8) | 295 | (48.2) | |||
| Age <60 | 0.05 | 0.01 | ||||||||
| <50% | 24 | (33.3) | 48 | (66.7) | 47 | (65.3) | 25 | (34.7) | ||
| ≥50% | 124 | (23.0) | 416 | (77.0) | 270 | (50.0) | 270 | (50.0) | ||
| Black patients | 0.006 | 0.37 | ||||||||
| <20% | 78 | (20.1) | 303 | (79.5) | 192 | (50.4) | 189 | (49.6) | ||
| ≥20% | 70 | (30.3) | 161 | (69.7) | 125 | (54.1) | 106 | (45.9) | ||
| Medicaid/uninsured population | 0.003 | 0.003 | ||||||||
| <10% | 58 | (19.1) | 246 | (80.9) | 139 | (45.7) | 165 | (54.3) | ||
| ≥10% | 90 | (29.2) | 218 | (70.8) | 178 | (57.8) | 130 | (42.2) | ||
| Commercial insurance | 0.05 | 0.008 | ||||||||
| <50% | 58 | (29.0) | 142 | (71.0) | 119 | (59.5) | 81 | (40.5) | ||
| ≥50% | 90 | (21.8) | 322 | (78.2) | 198 | (48.1) | 214 | (51.9) | ||
| Comorbidity ≥1 | 0.003 | 0.51 | ||||||||
| <40% | 62 | (33.7) | 122 | (66.3) | 99 | (53.8) | 85 | (46.2) | ||
| ≥40% | 86 | (20.1) | 342 | (80.0) | 218 | (50.9) | 210 | (49.1) | ||
| Teaching status | 0.009 | 0.001 | ||||||||
| Non-teaching | 121 | (26.9) | 329 | (73.1) | 251 | (55.8) | 199 | (44.2) | ||
| Teaching | 27 | (16.7) | 135 | (83.3) | 66 | (40.7) | 96 | (59.3) | ||
| Area of residence | 0.001 | 0.001 | ||||||||
| Metropolitan | 103 | (21.3) | 380 | (78.7) | 233 | (48.2) | 250 | (51.8) | ||
| Non-metropolitan | 45 | (34.9) | 84 | (65.1) | 84 | (65.1) | 45 | (34.9) | ||
| Region | 0.05 | 0.05 | ||||||||
| Eastern | 23 | (25.8) | 66 | (74.2) | 52 | (58.4) | 37 | (41.6) | ||
| Midwest | 47 | (30.7) | 106 | (69.3) | 88 | (57.5) | 65 | (42.5) | ||
| Southern | 64 | (23.0) | 214 | (77.0) | 127 | (45.7) | 151 | (54.3) | ||
| Western | 14 | (15.2) | 78 | (84.8) | 50 | (54.4) | 42 | (45.7) | ||
| Hospital size | 0.001 | <0.0001 | ||||||||
| <400 beds | 127 | (27.9) | 329 | (72.2) | 262 | (57.5) | 194 | (42.5) | ||
| 400-600 beds | 14 | (13.3) | 91 | (86.7) | 36 | (34.3) | 69 | (65.7) | ||
| >600 beds | 7 | (13.7) | 44 | (86.3) | 19 | (37.3) | 32 | (62.8) | ||
Tables 2 and 3 display the characteristics of the unmatched and propensity matched cohort of patients who underwent hysterectomy and colectomy respectively. HA-CMC was used in 5.0% (19,304) of all hysterectomies and increased from 1.1% in 2000 to 10.2% in 2009 and 9.8% in 2010 (p<0.0001) (Figure 1). In a multivariable model, year of diagnosis was the strongest predictor of use of HA-CMC (Table 4). Patients treated in non-metropolitan hospitals (RR=0.48; 95% CI, 0.28–0.80) were less likely to receive HA-CMC. Patients with cancer (RR=1.38; 95% CI, 1.32–1.45), ≥2 comorbidities (RR=1.26; 95% CI, 1.20–1.33), and patients treated by intermediate (RR=1.30; 95% CI, 1.24–1.36) and high (RR=2.40; 95% CI, 2.29–2.50) volume surgeons were more likely to receive HA-CMC (intracluster correlaction coefficient=0.04). Hospital procedural volume had no influence on use of HA-CMC.
Table 2.
Utilization of HA-CMC in patients who underwent hysterectomy.
| Hysterectomy (unmatched) | Hysterectomy (propensity score matched) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||||
|
|
||||||||||
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | p-value | |
| 363,051 | (95.0) | 19,304 | (5.0) | 38,608 | 19,304 | |||||
| Age | <0.0001 | 0.16 | ||||||||
| <60 | 317,033 | (87.3) | 14,684 | (76.1) | 29,165 | (75.5) | 14,684 | (76.1) | ||
| ≥60 | 46,018 | (12.7) | 4620 | (23.9) | 9443 | (24.5) | 4620 | (23.9) | ||
| Race | <0.0001 | <0.0001 | ||||||||
| White | 217,504 | (59.9) | 10,898 | (56.5) | 22,162 | (57.4) | 10,898 | (56.5) | ||
| Black | 68,554 | (18.9) | 3663 | (19.0) | 7595 | (19.7) | 3663 | (19.0) | ||
| Other | 76,993 | (21.2) | 4743 | (24.6) | 8851 | (22.9) | 4743 | (24.6) | ||
| Unknown | 0 | – | 0 | – | ||||||
| Year of diagnosis | <0.0001 | 0.55 | ||||||||
| 2000 | 6360 | (1.8) | 71 | (0.4) | 167 | (0.4) | 71 | (0.4) | ||
| 2001 | 11,689 | (3.2) | 139 | (0.7) | 279 | (0.7) | 139 | (0.7) | ||
| 2002 | 32,037 | (8.8) | 316 | (1.6) | 620 | (1.6) | 316 | (1.6) | ||
| 2003 | 47,644 | (13.1) | 847 | (4.4) | 1751 | (4.5) | 847 | (4.4) | ||
| 2004 | 44,970 | (12.4) | 1398 | (7.2) | 2791 | (7.2) | 1398 | (7.2) | ||
| 2005 | 44,791 | (12.3) | 1844 | (9.6) | 3796 | (9.8) | 1844 | (9.6) | ||
| 2006 | 48,095 | (13.3) | 2618 | (13.6) | 5152 | (13.3) | 2618 | (13.6) | ||
| 2007 | 43,642 | (12.0) | 3158 | (16.4) | 6322 | (16.4) | 3158 | (16.4) | ||
| 2008 | 39,144 | (10.8) | 3883 | (20.1) | 7534 | (19.5) | 3883 | (20.1) | ||
| 2009 | 36,600 | (10.1) | 4153 | (21.5) | 8315 | (21.5) | 4153 | (21.5) | ||
| 2010 | 8078 | (2.2) | 877 | (4.5) | 1881 | (4.9) | 877 | (4.5) | ||
| Insurance | <0.0001 | 0.29 | ||||||||
| Commercial | 270,890 | (74.6) | 13,311 | (69.0) | 26,668 | (69.1) | 13,311 | (69.0) | ||
| Medicare | 39,415 | (10.9) | 3499 | (18.1) | 7158 | (18.5) | 3499 | (18.1) | ||
| Medicaid | 27,857 | (7.7) | 1267 | (6.6) | 2482 | (6.4) | 1267 | (6.6) | ||
| Uninsured | 13,253 | (3.7) | 827 | (4.3) | 1546 | (4.0) | 827 | (4.3) | ||
| Unknown | 11,636 | (3.2) | 400 | (2.1) | 754 | (2.0) | 400 | (2.1) | ||
| Marital status | <0.0001 | 0.46 | ||||||||
| Married | 204,460 | (56.3) | 9875 | (51.2) | 19,912 | (51.6) | 9875 | (51.2) | ||
| Single | 65,451 | (18.0) | 3746 | (19.4) | 7521 | (19.5) | 3746 | (19.4) | ||
| Unknown | 93,140 | (25.7) | 5683 | (29.4) | 11,175 | (28.9) | 5683 | (29.4) | ||
| Area of residence | <0.0001 | 0.36 | ||||||||
| Metropolitan | 327,639 | (90.3) | 18,278 | (94.7) | 36,485 | (94.5) | 18,278 | (94.7) | ||
| Non-metropolitan | 35,412 | (9.8) | 1026 | (5.3) | 2123 | (5.5) | 1026 | (5.3) | ||
| Region | ||||||||||
| Eastern | 42,550 | (11.7) | 2616 | (13.6) | 5233 | (13.6) | 2616 | (13.6) | ||
| Midwest | 71,617 | (19.7) | 3733 | (19.3) | 7244 | (18.8) | 3733 | (19.3) | ||
| Southern | 203,152 | (56.0) | 11,075 | (57.4) | 22,712 | (58.8) | 11,075 | (57.4) | ||
| Western | 45,732 | (12.6) | 1880 | (9.7) | 3419 | (8.9) | 1880 | (9.7) | ||
| Cancer | <0.0001 | 0.01 | ||||||||
| No | 318,583 | (87.8) | 13,187 | (68.3) | 25,973 | (67.3) | 13,187 | (68.3) | ||
| Yes | 44,468 | (12.3) | 6117 | (31.7) | 12,635 | (32.7) | 6117 | (31.7) | ||
| Comorbidity | 0.16 | |||||||||
| 0 | 254,097 | (70.0) | 9872 | (51.1) | 19,516 | (50.6) | 9872 | (51.1) | ||
| 1 | 61,796 | (17.0) | 4471 | (23.2) | 9215 | (23.9) | 4471 | (23.2) | ||
| ≥2 | 47,158 | (13.0) | 4961 | (25.7) | 9877 | (25.6) | 4961 | (25.7) | ||
| Hospital type | <0.0001 | <0.0001 | ||||||||
| Non-teaching | 209,872 | (57.8) | 10,111 | (52.4) | 19,508 | (50.5) | 10,111 | (52.4) | ||
| Teaching | 153,179 | (42.2) | 9193 | (47.6) | 19,100 | (49.5) | 9193 | (47.6) | ||
| Hospital size | 0.002 | |||||||||
| <400 beds | 173,907 | (47.9) | 8461 | (43.8) | 17,490 | (45.3) | 8461 | (43.8) | ||
| 400–600 beds | 109,211 | (30.1) | 5060 | (26.2) | 10,000 | (25.9) | 5060 | (26.2) | ||
| >600 beds | 79.933 | (22.0) | 5783 | (30.0) | 11,118 | (28.8) | 5783 | (30.0) | ||
| Hospital volume | <0.0001 | 0.25 | ||||||||
| Low | 113,736 | (31.3) | 4142 | (21.5) | 8498 | (22.0) | 4142 | (21.5) | ||
| Intermediate | 124,157 | (34.2) | 6503 | (33.7) | 12,812 | (33.2) | 6503 | (33.7) | ||
| High | 125,158 | (34.5) | 8659 | (44.9) | 17,298 | (44.8) | 8659 | (44.9) | ||
| Surgeon volume | <0.0001 | 0.36 | ||||||||
| Low | 127,537 | (35.1) | 4561 | (23.6) | 9375 | (24.3) | 4561 | (23.6) | ||
| Intermediate | 119,980 | (33.1) | 4302 | (22.3) | 8571 | (22.2) | 4302 | (22.3) | ||
| High | 105,656 | (29.1) | 9943 | (51.5) | 19,692 | (51.0) | 9943 | (51.5) | ||
| Unknown | 9878 | (2.7) | 498 | (2.6) | 970 | (2.5) | 498 | (2.6) | ||
| Other procedures | ||||||||||
| Small bowel resection | 1545 | (0.4) | 273 | (1.4) | <0.0001 | 540 | (1.4) | 273 | (1.4) | 0.88 |
| Colon resection | 2461 | (0.7) | 429 | (2.2) | <0.0001 | 812 | (2.1) | 429 | (2.2) | 0.35 |
| Rectosigmoid resection | 3740 | (1.0) | 666 | (3.5) | <0.0001 | 1243 | (3.2) | 666 | (3.5) | 0.14 |
| Lysis of adhesion | 73,797 | (20.3) | 5573 | (28.9) | <0.001 | 11,319 | (29.3) | 5573 | (28.9) | 0.26 |
Table 3.
Utilization of HA-CMC in patients who underwent colectomy.
| Colectomy (unmatched) | Colectomy (propensity score matched) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||||||
|
|
||||||||||
| N | (%) | N | (%) | p-value | N | (%) | N | (%) | p-value | |
| 245,757 | (91.9) | 21,611 | (8.1) | 43,222 | 21,611 | |||||
| Age | <0.0001 | 0.52 | ||||||||
| <60 | 89,870 | (36.6) | 9046 | (41.9) | 17,979 | (41.6) | 9046 | (41.9) | ||
| ≥60 | 155,887 | (63.4) | 12,565 | (58.1) | 25,243 | (58.4) | 12,565 | (58.1) | ||
| Gender | 0.0003 | 0.89 | ||||||||
| Male | 113,479 | (46.2) | 9705 | (44.9) | 19,385 | (44.9) | 9705 | (44.9) | ||
| Female | 132,278 | (53.8) | 11,906 | (55.1) | 23,837 | (55.2) | 11,906 | (55.1) | ||
| Race | <0.0001 | <0.0001 | ||||||||
| White | 171,615 | (69.8) | 15,392 | (71.2) | 31,678 | (73.3) | 15,392 | (71.2) | ||
| Black | 25,699 | (10.5) | 2374 | (11.0) | 4432 | (10.3) | 2374 | (11.0) | ||
| Other | 48,435 | (19.7) | 3843 | (17.8) | 7112 | (16.5) | 3845 | (17.8) | ||
| Unknown | 8 | 0 | 2 | 0 | ||||||
| Year of diagnosis | <0.0001 | 0.98 | ||||||||
| 2000 | 3094 | (1.3) | 203 | (0.9) | 400 | (0.9) | 203 | (0.9) | ||
| 2001 | 5793 | (2.4) | 391 | (1.8) | 723 | (1.7) | 391 | (1.8) | ||
| 2002 | 15,197 | (6.2) | 782 | (3.6) | 1535 | (3.6) | 782 | (3.6) | ||
| 2003 | 27,834 | (11.3) | 1729 | (8.0) | 3379 | (7.8) | 1729 | (8.0) | ||
| 2004 | 29,010 | (11.8) | 2124 | (9.8) | 4255 | (9.8) | 2124 | (9.8) | ||
| 2005 | 30,241 | (12.3) | 2403 | (11.1) | 4836 | (11.2) | 2403 | (11.1) | ||
| 2006 | 34,719 | (14.1) | 3023 | (14.0) | 6068 | (14.0) | 3023 | (14.0) | ||
| 2007 | 35,081 | (14.3) | 3222 | (14.9) | 6457 | (14.9) | 3222 | (14.9) | ||
| 2008 | 33,129 | (13.5) | 3541 | (16.4) | 7192 | (16.6) | 3541 | (16.4) | ||
| 2009 | 25,737 | (10.5) | 3357 | (15.5) | 6703 | (15.5) | 3357 | (15.5) | ||
| 2010 | 5922 | (2.4) | 836 | (3.9) | 1674 | (3.9) | 836 | (3.9) | ||
| Insurance | <0.0001 | 0.02 | ||||||||
| Commercial | 94,841 | (38.6) | 9499 | (44.0) | 19,083 | (44.2) | 9499 | (44.0) | ||
| Medicare | 127,445 | (51.9) | 10,004 | (46.3) | 20,272 | (46.9) | 10,004 | (46.3) | ||
| Medicaid | 10,115 | (4.1) | 901 | (4.2) | 1637 | (3.8) | 901 | (4.2) | ||
| Uninsured | 8217 | (3.3) | 741 | (3.4) | 1363 | (3.2) | 741 | (3.4) | ||
| Unknown | 5139 | (2.1) | 466 | (2.2) | 867 | (2.0) | 466 | (2.2) | ||
| Marital status | <0.0001 | 0.01 | ||||||||
| Married | 124,545 | (50.7) | 11,190 | (51.8) | 22,859 | (52.9) | 11,190 | (51.8) | ||
| Single | 33,720 | (13.7) | 3213 | (14.9) | 6135 | (14.2) | 3213 | (14.9) | ||
| Unknown | 87,492 | (35.6) | 7208 | (33.4) | 14,228 | (32.9) | 7208 | (33.4) | ||
| Area of residence | <0.0001 | 0.22 | ||||||||
| Metropolitan | 220,996 | (89.9) | 20,055 | (92.8) | 39,995 | (92.5) | 20,055 | (92.8) | ||
| Non-metropolitan | 24,761 | (10.1) | 1556 | (7.2) | 3227 | (7.5) | 1556 | (7.2) | ||
| Region | <0.0001 | 0.002 | ||||||||
| Eastern | 37,121 | (15.1) | 4756 | (22.0) | 9975 | (23.1) | 4756 | (22.0) | ||
| Midwest | 49,948 | (20.3) | 3956 | (18.3) | 7942 | (18.4) | 3956 | (18.3) | ||
| Southern | 118,970 | (48.4) | 10,381 | (48.0) | 20,589 | (47.6) | 10,381 | (48.0) | ||
| Western | 39,718 | (16.2) | 2518 | (11.7) | 4716 | (10.9) | 2518 | (11.7) | ||
| Cancer | <0.0001 | 0.87 | ||||||||
| No | 165,694 | (67.4) | 15,473 | (71.6) | 30,920 | (71.5) | 15,473 | (71.6) | ||
| Yes | 80,063 | (32.6) | 6138 | (28.4) | 12,302 | (28.5) | 6138 | (28.4) | ||
| Comorbidity | <0.0001 | 0.34 | ||||||||
| 0 | 70,925 | (28.9) | 6685 | (30.9) | 13,505 | (31.3) | 6685 | (30.9) | ||
| 1 | 46,657 | (19.0) | 4241 | (19.6) | 8280 | (19.2) | 4241 | (19.6) | ||
| ≥2 | 128,175 | (52.2) | 10,685 | (49.4) | 21,437 | (49.6) | 10,685 | (49.4) | ||
| Hospital type | <0.0001 | 0.35 | ||||||||
| Non-teaching | 144,267 | (58.7) | 10,327 | (47.8) | 20,485 | (47.4) | 10,327 | (47.8) | ||
| Teaching | 101,490 | (41.3) | 11,284 | (52.2) | 22,737 | (52.6) | 11,284 | (52.2) | ||
| Hospital size | <0.0001 | 0.18 | ||||||||
| <400 beds | 117,538 | (47.8) | 8977 | (41.5) | 18,075 | (41.8) | 8977 | (41.5) | ||
| 400–600 beds | 74,554 | (30.3) | 7843 | (36.3) | 15,382 | (35.6) | 7843 | (36.3) | ||
| >600 beds | 53,665 | (21.8) | 4791 | (22.2) | 9765 | (22.6) | 4791 | (22.2) | ||
| Hospital volume | <0.0001 | 0.16 | ||||||||
| Low | 72,061 | (29.3) | 5828 | (27.0) | 11,837 | (27.4) | 5828 | (27.0) | ||
| Intermediate | 84,449 | (34.4) | 6796 | (31.5) | 13,278 | (30.7) | 6796 | (31.5) | ||
| High | 89,247 | (36.3) | 8987 | (41.6) | 18107 | (41.9) | 8987 | (41.6) | ||
| Surgeon volume | <0.0001 | 0.77 | ||||||||
| Low | 79,622 | (32.4) | 6515 | (30.2) | 13,177 | (30.5) | 6515 | (30.2) | ||
| Intermediate | 81,883 | (33.3) | 7431 | (34.4) | 14,843 | (34.3) | 7431 | (34.4) | ||
| High | 77,837 | (31.7) | 7175 | (33.2) | 14,207 | (32.9) | 7175 | (33.2) | ||
| Unknown | 6415 | (2.6) | 490 | (2.3) | 995 | (2.3) | 490 | (2.3) | ||
| Other procedures | ||||||||||
| Small bowel resection | 14,593 | (5.9) | 1610 | (7.5) | <0.0001 | 2887 | (6.7) | 1610 | (7.5) | 0.0003 |
| Lysis of adhesion | 35,810 | (14.6) | 4690 | (21.7) | <0.0001 | 8790 | (20.3) | 4690 | (21.7) | <0.0001 |
Figure 1.
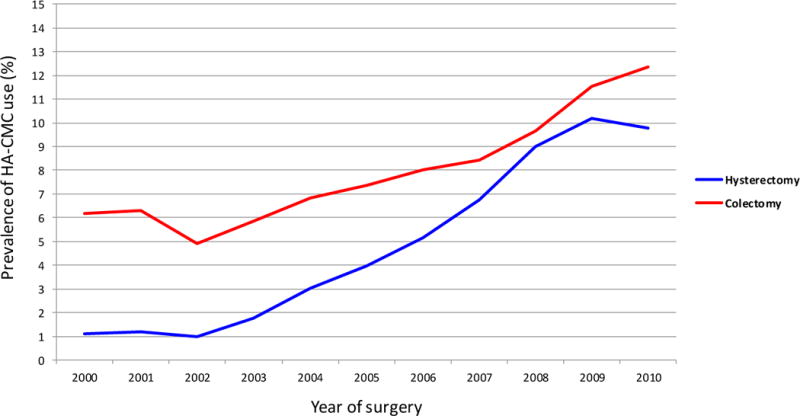
Prevalence rates of sodium hyaluronate-carboxymethylcellulose (HA-CMC) use by year of procedure for patients undergoing hysterectomy or colectomy. Prevalence rates of HA-CMC use for both hysterectomy and colectomy patients increased over time from 2000 to 2010 (P < 0.0001).
Table 4.
Multivariable models of predictors of use of HA-CMC use for patients who underwent hysterectomy and colectomy.
| Hysterectomy | Colectomy | |
|---|---|---|
| Age | ||
| <60 | Referent | Referent |
| ≥60 | 1.02 (0.97–1.07) | 0.91 (0.87–0.94)* |
| Gender | ||
| Male | – | Referent |
| Female | – | 1.05 (1.02–1.08)* |
| Race | ||
| White | Referent | Referent |
| Black | 1.04 (0.99–1.08) | 1.00 (0.95–1.05) |
| Other/unknown | 1.08 (1.03–1.13)* | 0.96 (0.91–1.01) |
| Year of diagnosis | ||
| 2000 | Referent | Referent |
| 2001 | 2.24 (1.64–3.05)* | 1.73 (1.41–2.12)* |
| 2002 | 4.48 (3.37–5.94)* | 3.07 (2.53–3.71)* |
| 2003 | 11.21 (8.56–14.68)* | 5.55 (4.61–6.67)* |
| 2004 | 19.81 (15.16–25.88)* | 6.15 (5.12–7.40)* |
| 2005 | 25.49 (19.39–32.88)* | 6.73 (5.61–8.07)* |
| 2006 | 37.21 (28.61–48.40)* | 7.35 (6.13–8.81)* |
| 2007 | 54.80 (42.13–71.27)* | 8.43 (7.03–10.11)* |
| 2008 | 71.49 (54.88–91.13)* | 9.95 (8.29–11.93)* |
| 2009 | 79.83 (61.26–104.04)* | 12.24 (10.19–14.70)* |
| 2010 | 77.18 (58.86–101.19)* | 12.63 (10.43–15.30)* |
| Insurance | ||
| Commercial | Referent | Referent |
| Medicare | 1.02 (0.97–1.08) | 0.91 (0.88–0.95)* |
| Medicaid | 0.99 (0.93–1.05) | 0.97 (0.90–1.04) |
| Uninsured | 1.00 (0.93–1.07) | 0.97 (0.90–1.05) |
| Unknown | 0.98 (0.88–1.08) | 0.94 (0.85–1.03) |
| Marital status | ||
| Married | Referent | Referent |
| Single | 1.02 (0.98–1.06) | 0.96 (0.93–1.00) |
| Unknown | 0.97 (0.93–1.00) | 0.94 (0.91–0.97)* |
| Area of residence | ||
| Metropolitan | Referent | Referent |
| Non-metropolitan | 0.48 (0.28–0.80)* | 0.62 (0.35–1.11) |
| Region | ||
| Eastern | Referent | Referent |
| Midwest | 1.17 (0.62–2.23) | 1.47 (0.72–3.01) |
| Southern | 0.94 (0.51–1.71) | 1.71 (0.88–3.35) |
| Western | 1.37 (0.67–2.80) | 1.53 (0.68–3.44) |
| Cancer | ||
| No | Referent | Referent |
| Yes | 1.38 (1.32–1.45)* | 0.86 (0.84–0.89)* |
| Comorbidity | ||
| 0 | Referent | Referent |
| 1 | 1.14 (1.09–1.19)* | 1.04 (1.00–1.08)* |
| ≥2 | 1.26 (1.20–1.33)* | 1.06 (1.02–1.10)* |
| Hospital type | ||
| Non-teaching | Referent | Referent |
| Teaching | 1.09 (0.68–1.75) | 1.90 (1.11–3.25)* |
| Hospital size | ||
| <400 beds | Referent | Referent |
| 400-600 beds | 1.17 (0.70–1.96) | 0.83 (0.44–1.56) |
| >600 beds | 0.95 (0.44–2.01) | 0.78 (0.31–1.95) |
| Hospital volume | ||
| Low | Referent | Referent |
| Intermediate | 1.50 (0.92–2.43) | 1.30 (0.74–2.29) |
| High | 1.41 (0.69–2.86) | 2.27 (0.99–5.20) |
| Surgeon volume | ||
| Low | Referent | Referent |
| Intermediate | 1.30 (1.24–1.36)* | 1.16 (1.12–1.20)* |
| High | 2.40 (2.29–2.50)* | 1.30 (1.25–1.35)* |
| Unknown | 2.52 (2.21–2.87)* | 1.70 (1.48–1.94)* |
Relative risk (95% confidence interval).
HA-CMC was utilized in 21,611 (8.1%) of the patients who underwent colectomy. Use of HA-CMC in patients undergoing colectomy doubled from 6.2% in 2000 to 12.4% in 2010 (p<0.0001) (Figure 1). Table 3 displays the characteristics of the unmatched and propensity matched colectomy cohorts. In a multivariable model, year of diagnosis was the strongest predictor of HA-CMC use (RR=12.63; 95% CI, 10.43–15.30 for 2010 vs. 2000) (intracluster correlaction coefficient=0.07) (Table 4). Older patients (RR=0.91; 95% CI, 0.87–0.94), patients with Medicare (RR=0.91; 95% CI, 0.88–0.95), and those with cancer (RR=0.86; 95% CI, 0.84–0.89) were less likely to receive HA-CMC. Conversely, women (RR=1.05; 95% CI, 1.02–1.08), those with ≥2 comorbidities (RR=1.06; 95% CI, 1.02–1.10), patients at teaching hospitals (RR=1.90; 95% CI, 1.11–3.25), and patients operated on by intermediate (RR=1.16; 95% CI, 1.12–1.20) and high (RR=1.30; 95% CI, 1.25–1.35) volume surgeons were more likely to receive HA-CMC. Hospital volume had no apparent association with HA-CMC use.
After propensity score matching and risk adjustment, HA-CMC use was not associated with abscess formation (RR=0.97; 95% CI, 0.84–1.12) in women who underwent hysterectomy. In the hysterectomy cohort, HA-CMC was associated with bowel obstruction (RR=1.38; 95% CI, 1.15–1.66) and ileus (RR=1.68; 95% CI, 1.59–1.78) (Table 5). Among patients who underwent colectomy, HA-CMC use was associated with a 13% increased risk of abscess formation (RR=1.13; 95% CI, 1.08–1.17). In patients who underwent colectomy, HA-CMC was also associated with wound complications (RR=1.19; 95% CI, 1.09–1.30), bowel obstruction (RR=1.13; 95% CI, 1.07–1.19), ileus (RR=1.14; 95% CI, 1.10–1.18), reoperation (RR=1.20; 95% CI, 1.10–1.30) and peritonitis (RR=1.13; 95% CI, 1.07–1.19).
Table 5.
Association between adhesion barrier use and periopeartive complications in propensity stratified cohort.
| Propensity Score Matched Hysterectomy Cohort | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HA-CMC No | HA-CMC Yes | ||||||
| (n=38,608) | (n=19,304) | ||||||
|
|
|||||||
| N | (%) | N | (%) | p-value | Unadjusted RR | Adjusted RR | |
| Abscess | 571 | (1.5) | 298 | (1.5) | 0.55 | 1.04 (0.91–1.20) | 0.97 (0.84–1.12) |
| Wound complication | 252 | (0.7) | 159 | (0.8) | 0.02 | 1.26 (1.03–1.54)* | 1.19 (0.97–1.45) |
| Bowel obstruction | 268 | (0.7) | 198 | (1.0) | <0.0001 | 1.48 (1.23–1.78)* | 1.38 (1.15–1.66)* |
| Ileus | 2708 | (7.0) | 2330 | (12.1) | <0.0001 | 1.72 (1.63–1.82)* | 1.68 (1.59–1.78)* |
| Reoperation | 259 | (0.7) | 137 | (0.7) | 0.59 | 1.06 (0.86–1.30) | 0.99 (0.81–1.22) |
| Peritonitis | 170 | (0.4) | 76 | (0.4) | 0.41 | 0.89 (0.68–1.17) | 0.79 (0.60–1.03) |
|
| |||||||
| Propensity Score Matched Colectomy Cohort | |||||||
|
| |||||||
| HA-CMC No | HA-CMC Yes | ||||||
| (n=43,222) | (n=21,611) | ||||||
|
|
|||||||
| N | (%) | N | (%) | p-value | Unadjusted RR | Adjusted RR | |
| Abscess | 6500 | (15.0) | 3753 | (17.4) | <0.0001 | 1.15 (1.11–1.20)* | 1.13 (1.08–1.17)* |
| Wound complication | 1276 | (3.0) | 784 | (3.6) | <0.0001 | 1.23 (1.12–1.34)* | 1.19 (1.09–1.30)* |
| Bowel obstruction | 3558 | (8.2) | 2029 | (9.4) | <0.0001 | 1.14 (1.08–1.20)* | 1.13 (1.07–1.19)* |
| Ileus | 7659 | (17.7) | 4385 | (20.3) | <0.0001 | 1.15 (1.10–1.19)* | 1.14 (1.10–1.18)* |
| Reoperation | 1551 | (3.6) | 969 | (4.5) | <0.0001 | 1.25 (1.15–1.35)* | 1.20 (1.10–1.30)* |
| Peritonitis | 3874 | (9.0) | 2259 | (10.5) | <0.0001 | 1.17 (1.11–1.23)* | 1.13 (1.07–1.19)* |
Discussion
Our findings suggest that HA-CMC use has gradually increased over the last decade for patients undergoing both hysterectomy and colectomy. Despite a number of retrospective studies and meta-analyses, use for both procedures remains <15%. We found no association between HA-CMC use and abscess formation in women who underwent hysterectomy, but did note a small but statistically significant association between HA-CMC and the development of abscesses in patients who underwent colectomy.
A large number of studies have examined the efficacy of HA-CMC for the prevention of adhesions over the last decade. In addition to a number of institutional studies, several randomized trials and meta-analyses have suggested that HA-CMC may reduce the risk of postoperative adhesions.1,6,27–30 In one of the largest studies that included over 1700 patients who underwent intestinal resection, Fazio and colleagues noted that HA-CMC reduced the risk of small bowel obstruction requiring reoperation.28 Despite the evidence supporting HA-CMC use, we noted that the utilization of HA-CMC for both hysterectomy and colectomy has been modest. In addition to more recent year of surgery, treatment by a high-volume surgeon appears to be one of the most important factors in use of HA-CMC for both procedures. While cancer patients undergoing hysterectomy were more likely to receive HA-CMC, the opposite was true for colectomy.
Safety concerns for anti-adhesion products have been raised in a number of studies.31–33 A trial of ferric hyaluronate (FeHA) of 700 patients undergoing open colorectal surgery was terminated after only 32 patients were enrolled. The investigators cited unacceptably high morbidity with cases of prolonged ileus, peritonitis, and anastamotic dehiscence in the study population.31 The constellation of signs and symptoms has been termed “possible Intergel Reaction Syndrome” (pIRS) or “Intergel Belly” and has led to legal actions and recall of the product in the U.S.32,34
The potential association between HA-CMC use and abscess formation was highlighted by a meta analysis in 2007.14 Although the study was criticized for a number of methodological issues, institutional studies, particularly for gynecologic surgery, have also identified an association between HA-CMC and postoperative fluid collections.9–11,13,35–37 In an analysis of over 400 patients who underwent laparotomy for ovarian, tubal, or peritoneal cancer, Leitao and colleagues noted intra-abdominal fluid collections in 8% of patients who received HA-CMC compared to 3% in those who did not receive HA-CMC.13 The risk appeared greatest in those who underwent a cytoreductive procedure.13 A retrospective report of 357 patients who underwent cytoreduction for ovarian cancer also noted an increased risk in pelvic abscess formation (OR=2.66; 95% CI: 1.21-5.86) in patients who received HA-CMC.11 A recent Cochrane review reported no statistically significantly increased risk in abscess formation or overall morbidity in patients who received HA-CMC for non-gynecologic surgery.1
Our findings are notable in that we identified an elevated risk of abscess formation in patients who underwent colectomy, but found no association between HA-CMC use and abscess development in women who underwent hysterectomy. Further, we noted an increase in ileus and bowel obstruction after both hysterectomy and colectomy with use of HA-CMC. A major concern of any study examining complications after HA-CMC use is the issue of unmeasured confounding. In the clinical setting, those patients at greatest risk for adhesive disease and at the highest risk for perioperative complications are likely the patients who are most likely to receive HA-CMC. Although we attempted to minimize bias by using propensity score techniques and further adjusted our models for perioperative events, we cannot exclude the possibility that unmeasured factors influenced our findings. Prior work has suggested that the risk of abscess formation is highly dependent on the specific procedure and population under study.11–13
While our study benefits from the inclusion of a large number of patients, we recognize several important limitations. We cannot exclude the possibility that we did not identify all patients who received HA-CMC. HA-CMC use was captured based on billing records, as has been previously described and validated for other drugs and devices using this database.18 Further, given the cost of these products, it is unlikely that many patients would have received HA-CMC without a charge. Similarly, adverse outcomes such as abscess may have been underreported. While complications are underreported using administrative data, a priori we analyzed only major perioperative complications that would likely have generated a claim and been recorded. Additionally, underreporting of complications would likely have been balanced across the groups. Along the same lines, using administrative data it is impossible to determine the timeframe of the occurrence of the complications under study. Although we did not select patients who underwent a minimally invasive procedure, prior to the introduction of specific ICD-9 codes these patients were likely coded as an open procedure and thus included in the analysis. While our dataset samples hospitals from across the U.S., we cannot exclude the possibility that these findings are not generalizable to other hospitals. We also recognize that patients who had disease-related complications such as an abscess prior to surgery were probably less likely to receive HA-CMC which may have confounded our findings. Lastly, the goal of our analysis was only to analyze short-term perioperative complications. The long-term effects of HA-CMC on intra-abdominal complications warrant further study.
Like other studies, we noted a small but statistically significant increase in the rate of postoperative abscess formation as well as bowel complications. These data are of concern to practicing surgeons and raise the question of when HA-CMC should be used. It appears that much of the morbidity of HA-CMC use is context specific; for women undergoing gynecologic surgery the risks appear to be greater for those with ovarian cancer than for other procedures.11–13 For patients undergoing intestinal surgery, complications are particularly high when HA-CMC is applied directly to the anastomotic line.27 For patients undergoing hysterectomy and colectomy surgeons must weigh the risks and benefits of HA-CMC use carefully. Large-scale studies to examine the safety of HA-CMC and define subgroups of patients at higher risk for complications are warranted.
Footnotes
No conflicts of interest
Contribution: all authors participated in conception and design, analysis and interpretation of data, drafting and final approval of the manuscript.
References
- 1.Kumar S, Wong PF, Leaper DJ. Intra-peritoneal prophylactic agents for preventing adhesions and adhesive intestinal obstruction after non-gynaecological abdominal surgery. Cochrane Database Syst Rev. 2009:CD005080. doi: 10.1002/14651858.CD005080.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Stanciu D, Menzies D. The magnitude of adhesion-related problems. Colorectal Dis. 2007;9(Suppl 2):35–38. doi: 10.1111/j.1463-1318.2007.01346.x. [DOI] [PubMed] [Google Scholar]
- 3.Menzies D. Peritoneal adhesions. Incidence, cause, and prevention. Surg Annu. 1992;24(Pt 1):27–45. [PubMed] [Google Scholar]
- 4.Menzies D, Parker M, Hoare R, Knight A. Small bowel obstruction due to postoperative adhesions: treatment patterns and associated costs in 110 hospital admissions. Ann R Coll Surg Engl. 2001;83:40–46. [PMC free article] [PubMed] [Google Scholar]
- 5.Parker MC, Wilson MS, van Goor H, et al. Adhesions and colorectal surgery - call for action. Colorectal Dis. 2007;9(Suppl 2):66–72. doi: 10.1111/j.1463-1318.2007.01342.x. [DOI] [PubMed] [Google Scholar]
- 6.Metwally M, Watson A, Lilford R, Vandekerckhove P. Fluid and pharmacological agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2006:CD001298. doi: 10.1002/14651858.CD001298.pub3. [DOI] [PubMed] [Google Scholar]
- 7.diZerega GS. Contemporary adhesion prevention. Fertil Steril. 1994;61:219–235. doi: 10.1016/s0015-0282(16)56507-8. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad G, Duffy JM, Farquhar C, et al. Barrier agents for adhesion prevention after gynaecological surgery. Cochrane Database Syst Rev. 2008:CD000475. doi: 10.1002/14651858.CD000475.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Beck DE. Seprafilm review and meta-analysis. World J Surg. 2008;32:1883–1884. doi: 10.1007/s00268-007-9455-y. author reply 5. [DOI] [PubMed] [Google Scholar]
- 10.Holmdahl L. A misleading meta-analysis of seprafilm. World J Surg. 2008;32:1888–9. doi: 10.1007/s00268-008-9575-z. author reply 90–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krill LS, Ueda SM, Gerardi M, Bristow RE. Analysis of postoperative complications associated with the use of anti-adhesion sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier after cytoreductive surgery for ovarian, fallopian tube and peritoneal cancers. Gynecol Oncol. 2011;120:220–223. doi: 10.1016/j.ygyno.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Leitao MM, Jr, Byrum GV, 3rd, Abu-Rustum NR, et al. Postoperative intra-abdominal collections using a sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier at the time of laparotomy for uterine or cervical cancers. Gynecol Oncol. 2010;119:208–211. doi: 10.1016/j.ygyno.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Leitao MM, Jr, Natenzon A, Abu-Rustum NR, et al. Postoperative intra-abdominal collections using a sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier at the time of laparotomy for ovarian, fallopian tube, or primary peritoneal cancers. Gynecol Oncol. 2009;115:204–208. doi: 10.1016/j.ygyno.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Q, Yu Z, You J, Zhang Q. Efficacy and safety of Seprafilm for preventing postoperative abdominal adhesion: systematic review and meta-analysis. World J Surg. 2007;31:2125–2131. doi: 10.1007/s00268-007-9242-9. discussion 32. [DOI] [PubMed] [Google Scholar]
- 15.Lagu T, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Lindenauer PK. The relationship between hospital spending and mortality in patients with sepsis. Arch Intern Med. 2011;171:292–299. doi: 10.1001/archinternmed.2011.12. [DOI] [PubMed] [Google Scholar]
- 16.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 17.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. Jama. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 18.Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Ann Intern Med. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 21.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69:871–875. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 24.Hemmila MR, Birkmeyer NJ, Arbabi S, Osborne NH, Wahl WL, Dimick JB. Introduction to propensity scores: A case study on the comparative effectiveness of laparoscopic vs open appendectomy. Arch Surg. 2010;145:939–945. doi: 10.1001/archsurg.2010.193. [DOI] [PubMed] [Google Scholar]
- 25.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. Jama. 2007;297:278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck DE, Cohen Z, Fleshman JW, Kaufman HS, van Goor H, Wolff BG. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46:1310–1319. doi: 10.1007/s10350-004-6739-2. [DOI] [PubMed] [Google Scholar]
- 28.Fazio VW, Cohen Z, Fleshman JW, et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rectum. 2006;49:1–11. doi: 10.1007/s10350-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 29.Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double-blind multicenter study. J Am Coll Surg. 1996;183:297–306. [PubMed] [Google Scholar]
- 30.Diamond MP. Reduction of de novo postsurgical adhesions by intraoperative precoating with Sepracoat (HAL-C) solution: a prospective, randomized, blinded, placebo-controlled multicenter study. The Sepracoat Adhesion Study Group. Fertil Steril. 1998;69:1067–1074. doi: 10.1016/s0015-0282(98)00057-0. [DOI] [PubMed] [Google Scholar]
- 31.Tang CL, Jayne DG, Seow-Choen F, Ng YY, Eu KW, Mustapha N. A randomized controlled trial of 0.5% ferric hyaluronate gel (Intergel) in the prevention of adhesions following abdominal surgery. Ann Surg. 2006;243:449–455. doi: 10.1097/01.sla.0000207837.71831.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiseman DM. Possible Intergel Reaction Syndrome (pIRS) Ann Surg. 2006;244:630–632. doi: 10.1097/01.sla.0000239619.93579.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tzianabos AO, Cisneros RL, Gershkovich J, et al. Effect of surgical adhesion reduction devices on the propagation of experimental intra-abdominal infection. Arch Surg. 1999;134:1254–1259. doi: 10.1001/archsurg.134.11.1254. [DOI] [PubMed] [Google Scholar]
- 34.Wiseman DM. Registries for anti-adhesion products? Fertil Steril. 2006;86:771. doi: 10.1016/j.fertnstert.2006.07.1462. author reply -2. [DOI] [PubMed] [Google Scholar]
- 35.Tyler JA, McDermott D, Levoyer T. Sterile intra-abdominal fluid collection associated with seprafilm use. Am Surg. 2008;74:1107–1110. [PubMed] [Google Scholar]
- 36.Cohen Z, Senagore AJ, Dayton MT, et al. Prevention of postoperative abdominal adhesions by a novel, glycerol/sodium hyaluronate/carboxymethylcellulose-based bioresorbable membrane: a prospective, randomized, evaluator-blinded multicenter study. Dis Colon Rectum. 2005;48:1130–1139. doi: 10.1007/s10350-004-0954-8. [DOI] [PubMed] [Google Scholar]
- 37.Mohri Y, Kusunoki M. Efficacy and safety of seprafilm: systematic review and meta-analysis. World J Surg. 2008;32:1886–1887. doi: 10.1007/s00268-008-9524-x. author reply 90–91. [DOI] [PubMed] [Google Scholar]


