Abstract
Background
Since limited in vitro tools exist for evaluating the pathophysiology of extrahepatic bile ducts, we aim to develop an extrahepatic cholangiocyte culture system from normal rats.
Methods
Extrahepatic ducts were dissected from rats, cut in half length-wise and cultured on collagen-I coated plates. Transepithelial electrical resistance was measured. At ~85% confluence, in extrahepatic cholangiocytes we measured: (i) cell size and distribution, and expression for cytokeratin-19, secretin, secretin and somatostatin receptor type II (SSTR2), cystic fibrosis transmembrane conductance regulator (CFTR), chloride bicarbonate anion exchanger 2 (AE2), vascular endothelial growth factor-A (VEGF-A) and nerve growth factor (NGF); and (ii) the effect of secretin and/or somatostatin on 3’-5’-cyclic adenosine monophosphate (cAMP) levels and proliferation.
Results
Cytokeratin-positive extrahepatic cholangiocytes were cultured for 6 passages to form a cell monolayer. Cholangiocytes proliferated to confluence over a 2-wk period. The size of extrahepatic cholangiocytes averaged ~16 µm. Extrahepatic ducts and cholangiocytes were positive for secretin, secretin and SSTR2, CFTR, AE2, VEGF-A and NGF. In extrahepatic cholangiocyte cultures, secretin increased cAMP (prevented by somatostatin), chloride efflux and proliferation.
Conclusions
Extrahepatic cholangiocyte cultures may be important for studying diseases targeting extrahepatic cholangiocytes such as biliary atresia.
Keywords: epithelia, extrahepatic bile duct, gastrointestinal hormones, proliferation
INTRODUCTION
The biliary system is formed by intrahepatic and extrahepatic bile ducts, which are lined by cholangiocytes [1, 2]. Intrahepatic cholangiocytes modify bile secreted from hepatocytes as it travels through the biliary system before reaching the duodenum [3–6]. During its transit, ductal bile is modified by gastrointestinal hormones including secretin and somatostatin [3–5, 7]. Secretin regulates ductal secretion and biliary proliferation in normal and cholestatic states [1, 3, 8–10]. Secretin stimulates ductal secretion by interacting with secretin receptors (SR) expressed only by large cholangiocytes [8]. The interaction of secretin with its receptor induces an increase in 3’-5’-cyclic adenosine monophosphate (cAMP) levels, phosphorylation of protein kinase A (PKA), opening of the cystic fibrosis transmembrane conductance regulator (CFTR) channel, which in turn activates the Cl−/HCO3− anion exchanger 2 (AE2) with subsequent secretion of HCO3− into bile [1, 3, 9, 10]. Conversely, somatostatin inhibits secretin-stimulated cAMP levels and bile secretion by interacting with SSTR2 receptor [5].
Secretin stimulates biliary proliferation by activation of cAMP signaling [11, 12]. Knockout of SR reduces cholangiocyte hyperplasia in cholestatic bile duct ligated mice. Intrahepatic cholangiocytes regulate their own function by autocrine mechanisms, as they express/secrete trophic autocrine factors such as secretin, vascular endothelial growth factor (VEGF) and nerve growth factor (NGF) [12–14].
Several studies demonstrated that extrahepatic bile ducts secrete water and electrolytes; however, limited information exists regarding the effect of secretin on the function of extrahepatic cholangiocytes [15–19]. Secretin stimulates water and electrolyte movement across extrahepatic bile duct segments [17]. Secretin-induced choleresis is associated with increased cAMP levels in extrahepatic bile duct tissue of humans and baboons [15]. While several in vitro culture models are available for evaluating the function of rodent and human intrahepatic cholangiocytes [20–26], limited in vitro tools exist for evaluating the pathophysiology of the extrahepatic common bile duct [27–29]. Therefore, the aim of the study was to develop a cell line (ECCs) from normal extrahepatic bile ducts.
MATERIALS AND METHODS
Materials
Reagents and antibodies were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. Porcine secretin was purchased from Peninsula Laboratories Inc. (Belton, CA). Somatostatin was purchased from Bachem (Torrance, CA). The polyclonal antibody against secretin receptor (SR, C-20) was purchased from Santa Cruz Biotechnology, Santa Cruz, CA. The SSTR2 (A-20) antibody was purchased from Santa Cruz Biotechnology. The rabbit polyclonal CFTR antibody (cat. 2269) was purchased from Cell Signaling Technology (Danvers, MA). The rabbit polyclonal Cl−/HCO3− anion exchanger 2 (AE2, cat. N12) antibody was purchased from Santa Cruz Biotechnology. The mouse antibody against cytokeratin-19 (CK-19, ab52625) was purchased from Abcam (Cambridge, MA).
Tri Reagent for the isolation of total RNA was purchased from Sigma Life Science. The EIA secretin kit was purchased from Phoenix Pharmaceuticals, Inc., (Burlingame, CA). Commercially available ELISA kits for the measurement of VEGF-A levels were obtained from RayBiotech, Inc., (Norcross, GA). A commercially available kit for the measurement of NGF levels was obtained from EMD Millipore (Billerica, MA).
Isolation of extrahepatic bile ducts (EBDs) and establishment of extrahepatic cholangiocyte cultures (ECCs)
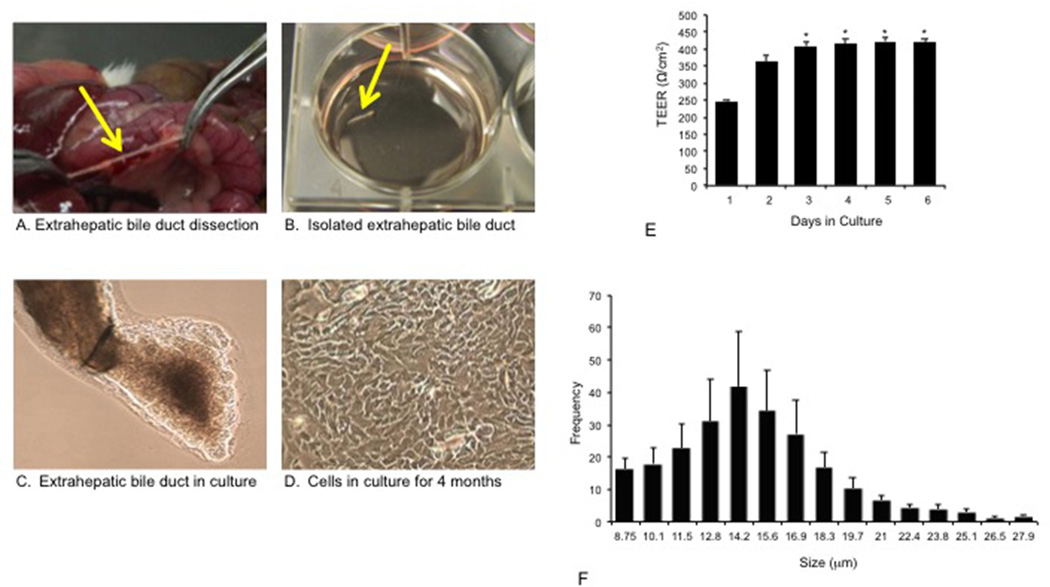
Before collection of tissue or liver perfusion, animals were injected with euthasol following the regulations of the panel of euthanasia of the American Veterinarian Medical Association with the approval of the Baylor Scott & White IACUC committee. Isolated extrahepatic bile duct fragments were obtained from male 344 Fisher rats (n = 8; 170–175 gm, Charles River Laboratories, Wilmington, PA) as follows. Following sedation, a midline incision was made in the abdomen of each animal and each extrahepatic bile duct (EBD) was located and dissected from the surrounding tissue (Figure 1A). The EBD was scraped with forceps to remove excess fat and connective tissue. The ducts were cut lengthwise and placed with the lumen side down in a well on collagen type I-coated 6 well plates (Figure 1B). Thereafter, EBDs were cultured at 37°C with 5% CO2 with 2 ml of media per well (Figure 1C). The media consists of DMEM-F12 containing 5% FBS, 1% L-glutamine, 1% Pen-Strep, 1% MEM nonessential amino acids, 1% Insulin-Transferrin-Selinum, 1% Chemically Defined Lipid Concentrate, 1% MEM Vitamin Solutions, 1% Bovine Pituitary Extract, 0.1% Epidermal Growth Factor, 0.1% 3,3’5-triiodo-L-thyromine, 0.1% Dexamethasone, 0.1% Forskolin, 0.2% Gentamicin. The media was changed after the EBDs were allowed to adhere to the plate for 48 hr. Subsequently, the media was changed every 3–4 days. After approximately 7 days, cells began to migrate from the EBD. Between 7 and 10 days, the duct floated off and cells at this stage were approximately 20–30% confluent. Following trypsinization with 1X TrypLE Express Enzyme (Invitrogen, Carlsbad, CA) for 5 minutes, cells were cultured on collagen coated 6 well plates and the media was changed twice weekly. After 4 months (approximately passage 6), the cells were 100% confluent and formed a monolayer (Figure 1D). EBDs were seeded on six-well, 3.0 mm cell culture inserts (BD Bioscience) and measurements of transepithelial electrical resistance (TEER, indicator of cell confluency) [20] were taken using a Millicell-ERS ohm-voltmeter (EVOM2, World Precision Instruments, Sarasota, Florida) every 24-h afterward until TEER readings stabilized. TEER values were calculated and expressed as Ω/cm2.
Figure 1.
Description of the isolation of extrahepatic bile ducts and development of extrahepatic cholangiocyte cultures from normal rats. [A] After a midline incision of each rat, the extrahepatic bile duct was located and dissected from the surrounding tissue. [B] The extrahepatic bile duct was carefully scraped with forceps to remove excess fat and any connective tissue. [C] The extrahepatic bile duct was cut lengthwise and placed with lumen side down in a well on collagen I coated 6 well plates. After 4 days, the cultured extrahepatic bile duct developed into cystic structures. Two weeks later, extrahepatic bile ducts were passed onto collagen cell culture plates. [D] After 3–4 months (around passage 6), the extrahepatic cholangiocytes began to proliferate to form a complete monolayer. At this stage, the cells were morphologically homogeneous under phase contrast microscopy with absence of contaminating fibroblasts. Orig. magn., x25. [E] Transepithelial electrical resistance was measured in extrahepatic bile ducts to determine formation and integrity of tight junctions between cells. There was increased transepithelial electrical resistance during time in culture, which stabilized after 3 days. *p<0.05 vs. the value of day 1 (6 evaluations). [F] Extrahepatic cholangiocyte cultures range in size from approximately 9 to 28 µm showing a similar pattern to that of intrahepatic cholangiocytes that range is size from 7 to 18 µm. TEER, transepithelial electrical resistance.
Transmission electron microscopy of EBDs and ECCs
For ultrastructural analysis [30, 31], EBDs were fixed in 2% paraformaldehyde/2.5% glutaraldehyde in 100 mM phosphate buffer, pH 7.2 for 1 hr at room temperature. Samples were washed in phosphate buffer and post-fixed in 1% osmium tetroxide (Polysciences Inc.) for 1 hr. Samples were rinsed in DI water prior to staining with 1% aqueous uranyl acetate (Ted Pella Inc., Redding, CA) for 1 hr. Following several rinses in DI water, samples were dehydrated in a graded series of ethanol and embedded in Eponate 12 resin (Ted Pella Inc.). Sections of 95 nm were cut with a Leica Ultracut UCT ultra-microtome (Leica Microsystems Inc., Bannockburn, IL), stained with uranyl acetate and lead citrate, and viewed on a JEOL 1200 EX transmission electron microscope (JEOL USA Inc., Peabody, MA) equipped with an AMT 8 megapixel digital camera (Advanced Microscopy Techniques, Woburn, MA).
Phenotypical and functional characterization of EBDs and ECCs
Cell size and distribution of ECCs were evaluated by a Cellometer Auto T4 Cell Counter (Nexcelom Bioscience LLC, Lawrence, MA). We evaluated the expression of CK-19 (biliary marker) [32], secretin, SR, CFTR and AE2 (that are expressed in the liver only by cholangiocytes) [1, 3, 7, 8, 12, 33], SSTR2 (the receptor by which somatostatin inhibits secretin-stimulated ductal secretion) [5] in: (i) cross sections of paraffin-embedded sections (4–5 µm) of EBDs by immunohistochemistry; (ii) cell smears of ECCs by immunofluorescence [21]; and (iii) real-time-PCR [21] in RNA (1 µg), respectively, from EBDs and ECCs. We used as positive control total RNA from rat portal tracts, which express the mRNA for secretin, SR, SSTR2, CFTR, AE2, VEGF-A and NGF; we used as negative control yeast-transfer RNA.
We evaluated the expression of α-fetoprotein (α-FP, marker of transformed hepatocytes) [34], albumin (marker of mature hepatocytes) [34] and vimentin (marker of mesenchymal lineage) [34] in paraffin-embedded sections (4–5 µm) of EBDs by immunohistochemistry. Immunohistochemical observations were taken in a coded fashion by a BX-40 light microscope (Olympus, Tokyo, Japan). Negative controls were included using pre-immune serum in the place of primary antibody. Following incubation with the fluorophore-conjugated secondary antibody [21], ECCs were mounted onto microscope slides with Prolong Antifade Gold containing 4’,6-diamidino-2-phenylindole (DAPI) as a nuclear counterstain (Molecular Probes, Eugene, OR).
RNA was isolated from the samples by using the TRI Reagent from Sigma Life Science and reverse transcribed using the Reaction Ready™ First Strand cDNA synthesis kit (SABiosciences, Frederick, MD). PCR reactions were used as templates for the PCR assays using a SYBR Green PCR master mix and specific primers designed against the selected gene analyzed in the real-time thermal cycler (Agilent MX3005P thermocycler, Santa Clara, CA). A ΔΔCT (delta delta of the threshold cycle) analysis was performed using normal intrahepatic cholangiocyte cultures as control samples [35]. Data were expressed as relative mRNA levels ± SEM of the selected gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratio. The primers for rat CK-19, secretin, SR, SSTR2, CFTR, Cl−/HCO3− AE2, and GAPDH were purchased from SABiosciences and were designed according to the NCBI GenBank Accession numbers: NM_199498 (CK-19); NM_022670 (secretin); NM_031115 (SR); NM_019348 (SSTR2); NM_017048 (Cl−/HCO3− AE2); XM_001059206 (CFTR); and NM_017008 (GAPDH). An agarose gel was run to visualize the bands of the PCR products.
Effect of secretin on cAMP levels and chloride efflux assay of ECCs
We measured cAMP levels, a key messenger system regulating biliary secretory and proliferative activities [1, 3, 20, 25, 33, 36]. Following trypsinization, ECCs were treated with 0.2% BSA (basal), forskolin (10−4 M for 5 min), secretin (100 nM for 5 min), somatostatin (100 nM for 5 min) or somatostatin (pre-incubation for 5 min at 100 nM) + secretin (100 nM for 5 min) [5, 37–39] before measuring cAMP levels by EIA kits (Cayman Chemical Company, Ann Arbor, MI).
Chloride channel efflux was evaluated by commercially available kits (Abcam; ab176767). This kit provides a sensitive and robust colorimetric method for studying chloride channels. The assay takes advantage of a proprietary iodide indicator (iodide sensor blue dye). Briefly, 40,000 cholangiocytes were plated per well in a 96-well plate and allowed to grow over night. The growth media was aspirated and 100 µL/well of pre-warmed iodide-loading buffer was added and the plate was incubated for 2 to 4 hours. The iodide-loading buffer was aspirated and the wells were washed with PBS at least three times. The cells were then treated with vehicle or secretin (100 nM) [33] in 1x PBS for 5 minutes. The media was aspirated and the cells were lysed by adding 50 µL/well of cell lysis buffer provided in the kit. Iodide Sensor Blue dye (50 µL/well) and Iodide Sensor Enhancer (50 µL/well) were added to each well. The plate was incubated for 4 minutes and read at 405 nm. Data was calculated as fold-change compared to vehicle treatment.
Effect of secretin on the proliferation of ECCs
ECCs were treated with 0.2% BSA or secretin (10−7 M) for 24, 48, 72 hr and 7 days before evaluating cell proliferation by MTS assays [40]. In separate experiments, ECCs were treated with 0.2% BSA or secretin (10−7 M for 24 hr) in the absence/presence of H-89 (a PKA inhibitor, 30 µM, 1 hr pre-incubation) [41] or PD98059 (a MEK inhibitor, 10 nM, 1 hr pre-incubation) [37] before evaluating cell proliferation by MTS assays.
Expression and Secretion of Secretin, VEGF-A and NGF in ECCs
By real-time PCR, we evaluated the mRNA expression of VEGF-A and NGF, which are trophic autocrine factors secreted by intrahepatic cholangiocytes [13, 14]. The primers for rat VEGF-A (Qiagen) were designed according to the NCBI GenBank Accession numbers: NM_031836 (VEGF-A); and NM_001277055 (NGF). An agarose gel was run to visualize the bands of the PCR products. The levels of secretin, VEGF-A and NGF were evaluated in the supernatant of ECCs by commercially available EIA kits. The levels of secretin, VEGF-A and NGF secreted from the basolateral and apical domains of ECCs were measured by plating the cell lines for 72 hr on collagen-coated filters of tissue culture inserts to produce a confluent monolayer [22]. We measured the levels of VEGF-A and NGF since only the expression of these two growth factors are regulated by secretin by modulation of MicroRNA 125 and MicroRNA let7a, respectively [12].
Statistical Analysis
All data are expressed as mean ± SEM (standard error). Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Development of Extrahepatic Cholangiocyte Cultures (ECCs) from EBDs
After 4 days of culture, the extrahepatic bile ducts (EBDs) developed into cystic structures. Two weeks later, ECCs were passaged onto collagen cell culture plates. After 5–10 passages, the extrahepatic cholangiocytes began to proliferate to form a complete monolayer. At this stage, the cells were morphologically homogeneous under phase contrast microscopy. Extrahepatic cholangiocytes, when seeded on collagen-coated cell inserts, proliferated to confluency over a 1-wk period (Figure 1D). We next assessed morphological characteristics of isolated EBD epithelial junctions. The TEER increased during the days in culture and stabilized indicating the formation of tight epithelial junctions (Figure 1E). Using a cellometer, we demonstrated that ECCs range in size from approximately 9 to 28 µm (higher frequency up to 14.2 µm) (Figure 1F) showing a similar pattern to that of intrahepatic cholangiocytes that range in size from 7 to 18 µm [1].
Transmission Electron Microscopy of EBDs and ECCs
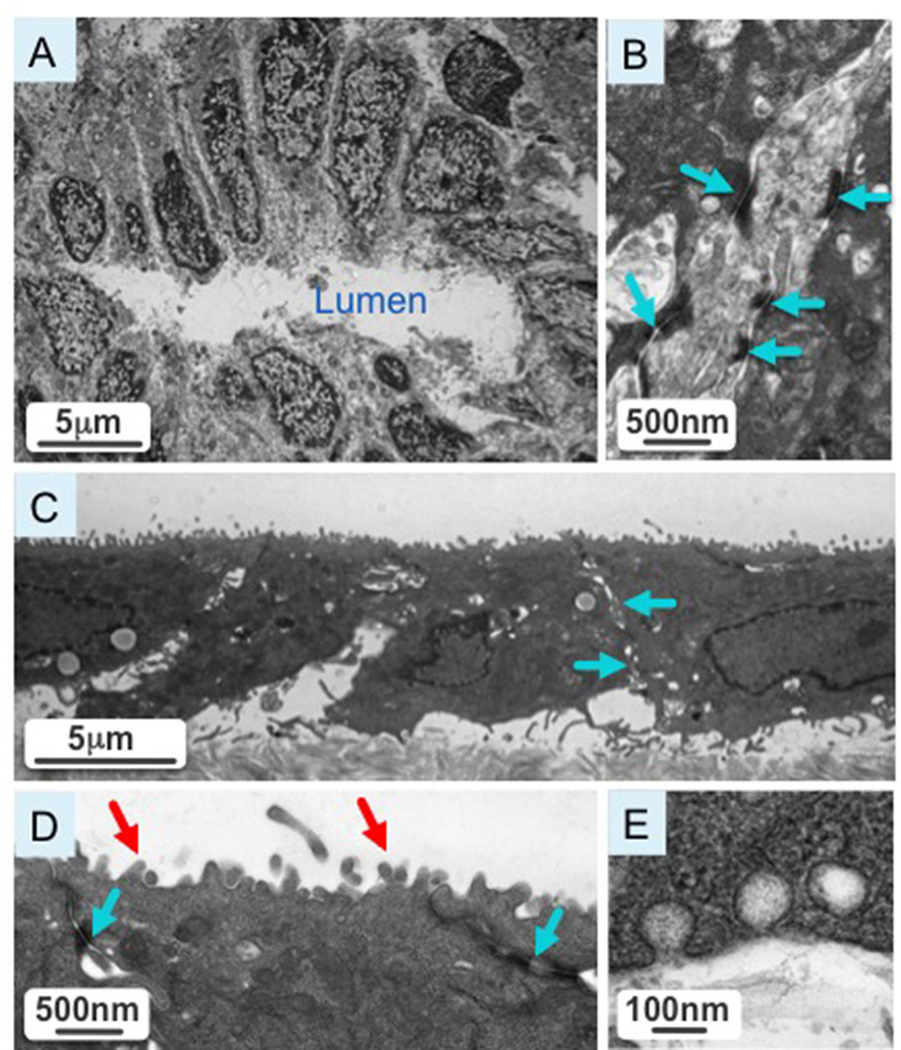
Figure 2A shows a cross-section of a normal rat extrahepatic bile duct with cholangiocytes polarized around the bile duct lumen (L). A higher magnification image of the cross-section shows intact tight junctions (depicted by blue arrows) characteristic of biliary epithelia (Figure 2B). Isolated extrahepatic cholangiocytes were cultured on transwells (Figure 2C). At higher magnification, cultured cholangiocytes show intact tight junctions (indicated by blue arrows) and an abundance of microvilli (red arrow) at the apical surface (Figure 2D). In cultured cholangiocytes we observed vesicular invaginations at both the apical and basolateral side, suggestive of active secretory and absorptive processes characteristic of bile duct epithelia (Figure 2E).
Figure 2.
[A] Cross-section of a normal extrahepatic bile duct with cholangiocytes polarized around the bile duct lumen. [B] A higher magnification image of the cross-section shows intact tight junction (blue arrows) characteristic of biliary epithelia. [C] A representative picture shows isolated extrahepatic cholangiocytes cultured on transwells. [D] A higher magnification image of cultured cholangiocytes reveals intact tight junctions (blue arrows) and an abundance of microvilli (red arrow) at the apical surface. [E] Cultured cholangiocytes have vesicular invaginations at the apical and basolateral poles, suggestive of active secretory and absorptive processes characteristic of bile duct epithelia.
Phenotypical characterization of EBDs
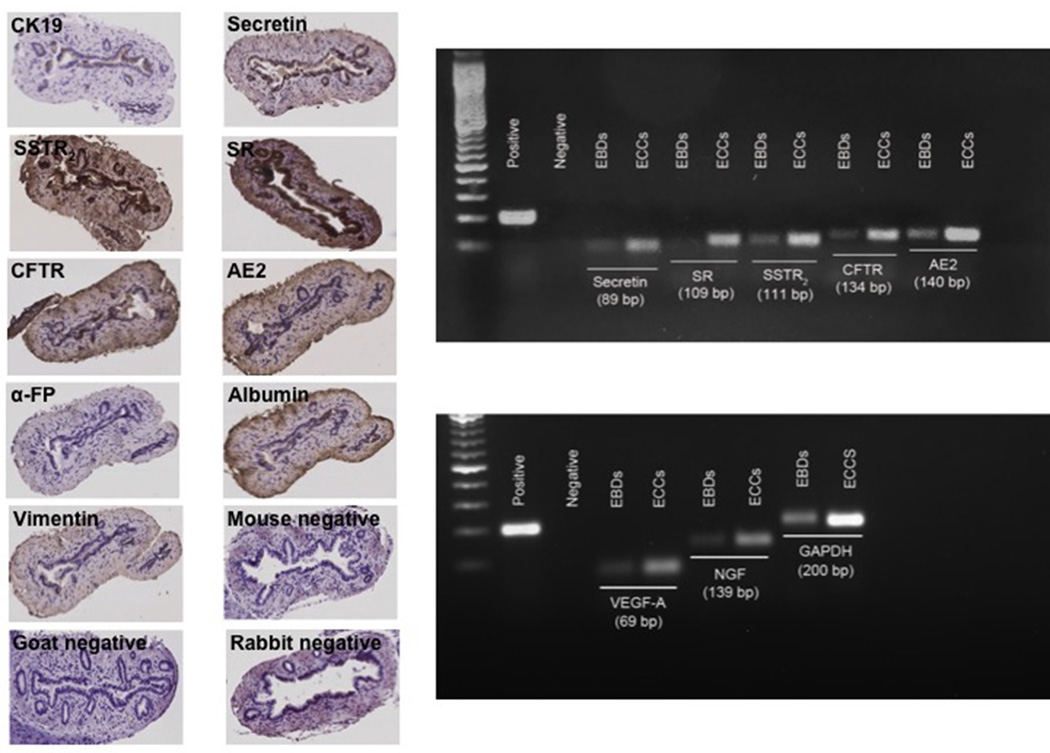
By immunohistochemistry, EBDs express CK-19, secretin, SR, SSTR2, CFTR and AE2, proteins that are key in the regulation of ductal secretory activity (Figure 3, left panel). EBDs were negative for α-FP, albumin and vimentin (Figure 3, left panel). We demonstrated that EBDs express the message for CK-19, secretin, SR, SSTR2, CFTR and AE2 (Figure 3, right panel).
Figure 3.
[Left panel] Extrahepatic bile ducts were positive for cytokeratin-19, secretin, secretin and somatostatin receptor type II, cystic fibrosis transmembrane conductance regulator and chloride bicarbonate anion exchanger 2. Extrahepatic bile ducts were negative for α-fetoprotein, albumin and vimentin. Orig. magn., x125. [Right panel] Both extrahepatic bile ducts and extrahepatic cholangiocyte cultures express the mRNA for cytokeratin, secretin, secretin and somatostatin type II receptor, cystic fibrosis transmembrane conductance regulator and chloride bicarbonate anion exchanger 2, vascular endothelial growth factor-A and nerve growth factor. AE2, chloride bicarbonate anion exchanger 2; α-FP, α-fetoprotein; CK19, cytokeratin-19; CFTR, cystic fibrosis transmembrane conductance regulator; EBDs, extrahepatic bile ducts; ECCs, extrahepatic cholangiocyte cultures; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NGF, nerve growth factor; SR, secretin receptor; SSTR2, somatostatin receptor type II; VEGF-A, vascular endothelial growth factor-A.
Effect of Secretin on cAMP levels, Cl− Efflux and Proliferation of ECCs
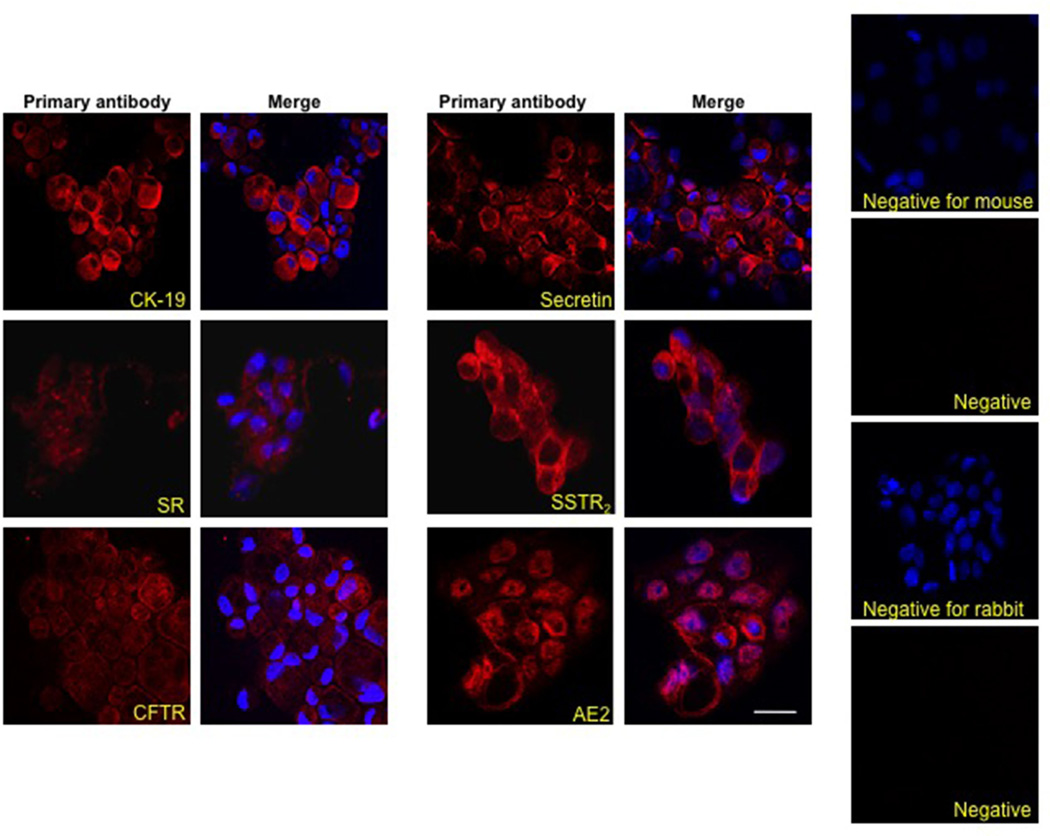
By immunofluorescence, ECCs were positive for CK-19, secretin, SR, SSTR2, CFTR and AE2 (Figure 4). Similar to intrahepatic cholangiocytes [1, 5], secretin increased cAMP levels of ECCs, increase that was prevented by pre-incubation with somatostatin (Figure 5A). Somatostatin inhibited basal cAMP levels of ECCs (Figure 5A). Similar to intrahepatic cholangiocytes [33], secretin increased chloride efflux activity in ECCs compared to the basal value (Figure 5B). Secretin induced a sustained (24 hr to 7 days) increase in the proliferation of ECCs (Figure 5C). Secretin-stimulation of ECCs proliferation was blocked by H-89 (PKA inhibitor) [41] and PD98059 (MEK inhibitor) [37] (Figure 5D).
Figure 4.
By immunofluorescence, we demonstrate that EECs express cytokeratin-19, secretin and somatostatin receptor type II, cystic fibrosis transmembrane conductance regulator and chloride bicarbonate anion exchanger 2. Nuclei are counterstained with 4’,6-diamidino-2-phenylindole. Negative controls are included. Bar = 20 µm.
AE2, chloride bicarbonate anion exchanger 2; CK19, cytokeratin-19; CFTR, cystic fibrosis transmembrane conductance regulator; DAPI, 4’,6-diamidino-2-phenylindole; SR, secretin receptor; SSTR2, somatostatin receptor type II.
Figure 5.
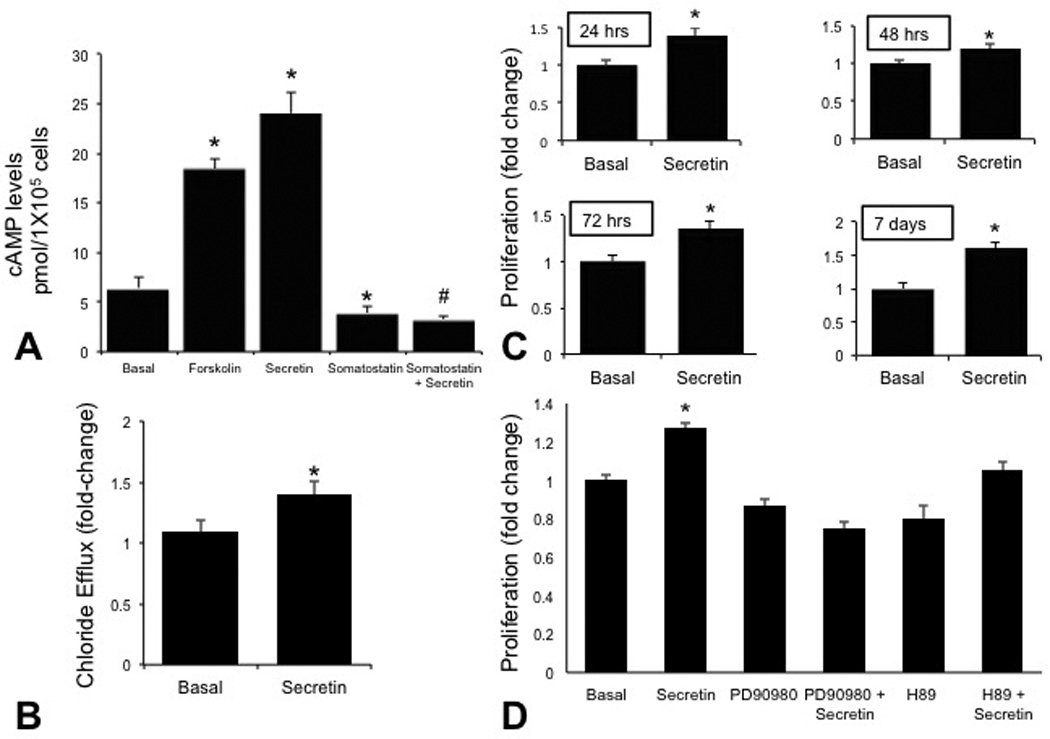
[A] Forskolin and secretin increased 3’-5’-cyclic adenosine monophosphate levels of ECCs. Secretin-stimulated 3’-5’-cyclic adenosine monophosphate levels were prevented by pre-incubation with somatostatin. Somatostatin inhibited basal 3’-5’-cyclic adenosine monophosphate levels. Data are mean ± standard error of six evaluations. *p<0.05 vs. corresponding basal values. #p<0.05 vs. secretin-stimulated 3’-5’-cyclic adenosine monophosphate levels. [B] Secretin increased chloride efflux activity in extrahepatic cholangiocyte cultures compared to the corresponding basal value. *p<0.05 vs. corresponding basal values. [C] Effect of secretin (10−7 M for 24 hr to 7 days) on the proliferation of extrahepatic cholangiocyte cultures. Secretin induced a sustained increase in the proliferation of extrahepatic cholangiocyte cultures. [D] Secretin-stimulation of the proliferation of extrahepatic cholangiocyte cultures was prevented by H-89 and PD98059. Data are expressed as mean fold change (± standard error) in absorbance (A495) compared to basal treatment at each time point (n=7). *p<0.05 vs. corresponding basal values. cAMP, 3’-5’-cyclic adenosine monophosphate; ECCs, extrahepatic cholangiocyte cultures; SEM, standard error.
Expression of VEGF-A and NGF and Secretion of Secretin, VEGF-A and NGF by ECCs
We demonstrated that ECCs express the mRNA for VEGF-A and NGF (Figure 3, right panel). The levels of secretin secreted from total ECCs (containing both apical and basolateral domains) were higher compared the basolateral and apical domains of ECCs, whereas similar levels of VEGF-A and NGF were detected between the basolateral and apical domains of ECCs and total ECCs (Figure 6).
Figure 6.
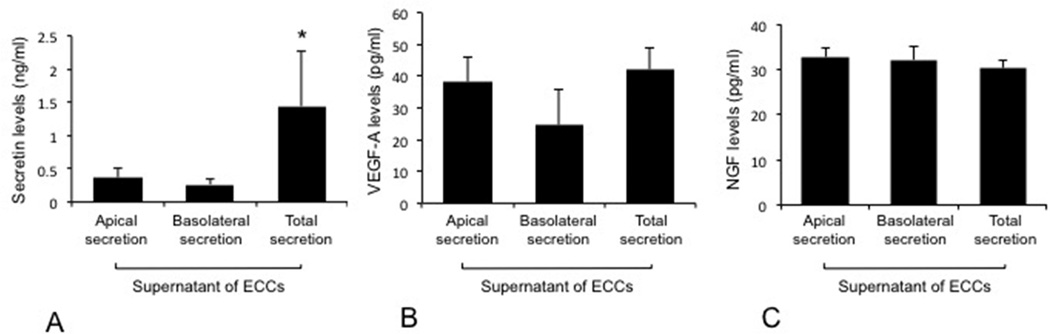
Measurement of secretin, VEGF-A and NGF levels in total (containing both apical and basolateral domains) ECCs as well as basolateral and apical domains of ECCs. The levels of secretin secreted from total ECCs (containing both apical and basolateral domains) were higher compared the basolateral and apical domains of ECCs, whereas similar levels of VEGF-A and NGF were detected between the basolateral and apical domains of ECCs and total ECCs. Data are mean ± SEM of six evaluations. *p<0.05 vs. the levels of secretin in the basolateral and apical domains of ECCs.
ECCs, extrahepatic cholangiocyte cultures; NGF, nerve growth factor; SEM, standard error; VEGF-A, vascular endothelial growth factor-A.
DISCUSSION
We isolated EBDs from normal rats and demonstrated that they are positive for CK-19 as well as secretin, SR, SSTR2, CFTR and AE2 but negative for α-FP, albumin and vimentin. Ultrastructural analysis shows that extrahepatic bile ducts are lined by polarized cholangiocytes and have morphological phenotypes similar to that of intrahepatic cholangiocytes [42]. Intact tight junctions and microvilli (characteristic of intra- and extra-hepatic biliary epithelia) [27, 43] were present in EBDs and ECCs. Similar to EBDs, ECCs express CK-19, secretin, SR, SSTR2, CFTR and AE2 and respond to secretin and somatostatin with changes in cAMP levels. Similar to intrahepatic cholangiocytes [33], secretin increased chloride efflux activity in ECCs. Furthermore, secretin increased the proliferation of ECCs that was prevented by H-89 and PD98059. Similar to intrahepatic cholangiocytes [13, 14], ECCs express and secrete the trophic autocrine factors, VEGF and NGF.
The rationale for developing lines of extrahepatic bile ducts that would respond to secretin is based on the fact that secretin is a key factor for the maintenance of biliary secretion and homeostasis by autocrine/paracrine signalling mechanisms. Secretin stimulates the secretory activity of large bile ducts by interacting with SR only expressed by cholangiocytes [1, 3], an interaction that induces the secretion of water and bicarbonate ions by activation of CFTR and AE2 [1, 3]. Secretin is a key autocrine trophic factor for cholangiocytes since it increases biliary proliferation by activation of cAMP-dependent PKA signalling mechanisms [11, 37]. Secretin receptors also modulate cholangiocyte proliferation since knockdown of SR reduces cholestasis-induced biliary hyperplasia [11]. Loss of SR and secretin response is associated with decreased bicarbonate secretion and subsequent biliary damage [44, 45]. The concept that biliary bicarbonate (stimulated by the secretin/SR axis) is a key protective mechanism for cholangiocytes in ductopenic states is supported by several studies [46, 47]. Studies performed in cultured models of rodent and human intrahepatic cholangiocytes provided important information related to the pathophysiology (especially in relationship to the secretin/SR axis) of these cells, however, limited cultured systems have been developed for evaluating the secretory functions of extrahepatic bile ducts [19, 27–29]. We have developed [20] a polarized culture system of normal rat intrahepatic cholangiocytes (NRIC) that display phenotypic markers typical of biliary epithelia and express proteins (SR, CFTR and AE2) that are key in secretin-stimulated ductal secretory activity [1, 3, 8, 38, 39]. A recent study has demonstrated activation of fluid secretion in a normal rat cholangiocyte line through a CFTR-independent mechanism [48]. A rat intrahepatic biliary cell line has been developed, which grows under hormonally defined, serum-free conditions, maintains nonmalignant and biliary phenotypes, displays morphologic and functional phenotypes of polarity and alters its proliferation rate in response to a variety of growth factors [49]. Furthermore, we have developed immortalized cell lines of small and large murine cholangiocytes originating from small and large ducts [1, 3, 23]. The large cholangiocyte lines were responsive to secretin [23].
We evaluated the ultrastructural characteristics of extrahepatic cholangiocytes, which resemble those already described in other studies in both intra- and extra-hepatic bile ducts [27, 42]. The size of our ECCs (ranging from approximately 9 to 28 µm, with higher frequency up to 14.2 µm) is similar to that of intrahepatic cholangiocytes ranging in size from 7 to 18 µm [1]. The possible heterogeneity of our ECCs may be due to the presence of subpopulations of stem/progenitor cells from peribiliary glands of the extrahepatic bile ducts and mature cholangiocytes lining the biliary epithelium [50, 51]. In support of the heterogeneity of our ECCs, several studies have shown [52, 53] that peribiliary glands contain a population of stem/progenitor cells with a cell diameter compatible with the low range of the cell diameters observed in our ECCs. However, further studies are necessary to determine the possible morphological and functional heterogeneity of our ECCs. Thereafter, we phenotypically characterized EBDs as well as ECCs by evaluating the presence of biliary markers (i.e., CK-19) and the absence of markers specific to other cell types such as mature and/or transformed hepatocytes and mesenchymal cells [34, 38], to support the biliary characteristics of our preparations. The absence of vimentin in our EBDs is supported by previous studies in intra- and extrahepatic cholangiocytes showing that these cells do not express this marker of mesenchymal origin [19, 54]. The absence of α-FP and albumin in our EBDs and ECCS is supported by previous studies [34, 54]. Supporting our findings, a study has demonstrated that biliary stem/progenitor cells residing in the deeper glands within the wall of large intrahepatic and extrahepatic bile ducts of normal human liver were negative for mature cell markers such as albumin [52]. Conversely, another study has demonstrated that very few hepatic progenitor cells positive for albumin were present in cultured extrahepatic cholangiocytes from bile duct ligated mice [55]. This discrepancy is likely due to the different species and model (cholestatic vs. normal) used in these studies.
At the functional level, the expression of secretin, SR, SSTR2, CFTR (expressed only by cholangiocytes in the liver) [56] and AE2 (expressed mostly by cholangiocytes and at very low levels by hepatocytes) [57] and responsiveness to secretin (with changes in secretion and cAMP levels) all support the concept that extrahepatic cholangiocytes behave functionally in a fashion identical to intrahepatic cholangiocytes.
The finding that: (i) secretin stimulates the proliferation of extrahepatic cholangiocytes by up-regulation of cAMP signaling (sensitivity to PKA and MAPK inhibitors); and (ii) somatostatin inhibits secretin-stimulated cAMP levels supports the concept that our extrahepatic preparations behave mitotically as intrahepatic large, cAMP-dependent cholangiocytes. This is supported by findings in intrahepatic cholangiocytes showing that somatostatin inhibits both basal and secretin-stimulated ductal choleresis (functional indices of biliary proliferation) [4, 38, 39] and large biliary proliferation [5, 39]. The inhibitory effect of somatostatin on the proliferation of extrahepatic cholangiocytes is supported by the fact that somatostatin reduces the growth of both intra- and extra-hepatic cholangiocarcinoma [58, 59].
In summary, we have developed a culture system of polarized EBDs displaying morphological, phenotypical and functional features of normal intrahepatic cholangiocytes. This culture system may be a good approach for a better understanding of diseases targeting extrahepatic bile ducts such as primary sclerosing cholangitis and biliary atresia. We would like to emphasize the possible utility of this cell culture system for the study of the oncogenic processes of the biliary epithelium.
Acknowledgments
Bryan Moss, Medical Illustration, BaylorScott & White and Imaging Facility, Department of Molecular Microbiology, Center for Infectious Disease Research, Washington University School of Medicine.
This research was supported in part by the Veterans Health Administration. Views are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs.
Funding
This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a VA Research Career Scientist Award, a VA Merit Award to Dr. Alpini, a VA Merit Award to Dr. Meng, a VA Merit Award to Dr. Glaser, VA CD2 Awards to Dr. Francis (IK2 BX001760), an NIH R01 award (DK082435) to Dr. DeMorrow, by University of Rome “La Sapienza,” to Drs Gaudio and Onori, FIRB Accordi di Programma 2010 #RBAP10Z7FS to Dr. Gaudio, and the NIH grant DK58411, DK062975 and DK07698 to Drs. Alpini, Meng and Glaser.
This research was supported in part by the Veterans Health Administration.
Abbreviations
- AE2
chloride bicarbonate anion exchanger 2
- cAMP
3’-5’-cyclic adenosine monophosphate
- CFTR
cystic fibrosis transmembrane conductance regulator
- ECCs
extrahepatic cholangiocyte cultures
- EBDs
extrahepatic bile ducts
- DAPI
4’,6-diamidino-2-phenylindole
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NGF
nerve growth factor
- PKA
protein kinase A
- SR
secretin receptor
- SSTR2
somatostatin receptor type 2
- TEER
transepithelial electrical resistance
- VEGF-A
vascular endothelial growth factor-A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 2.Kanno N, LeSage G, Glaser S, et al. Functional heterogeneity of the intrahepatic biliary epithelium. Hepatology. 2000;31:555–561. doi: 10.1002/hep.510310302. [DOI] [PubMed] [Google Scholar]
- 3.Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 4.Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tietz PS, Alpini G, Pham LD, et al. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1995;269:G110–G118. doi: 10.1152/ajpgi.1995.269.1.G110. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 7.Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 8.Alpini G, Ulrich CD, 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 9.Francis H, LeSage G, DeMorrow S, et al. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Gastrointest Cell Physiol. 2007;293:C1252–C1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 10.Strazzabosco M, Mennone A, Boyer JL. Intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1991;87:1503–1512. doi: 10.1172/JCI115160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser S, Lam IP, Franchitto A, et al. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser S, Meng F, Han Y, et al. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. e12. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 14.Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209. doi: 10.1053/j.gastro.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Levine RA, Hall RC. Cyclic AMP in secretin choleresis. Evidence for a regulatory role in man and baboons but not in dogs. Gastroenterology. 1976;70:537–544. [PubMed] [Google Scholar]
- 16.Chenderovitch J. Secretory function of the rabbit common bile duct. Am J Physiol Gastrointest Liver Physiol. 1972;223:695–706. doi: 10.1152/ajplegacy.1972.223.3.695. [DOI] [PubMed] [Google Scholar]
- 17.Nahrwold DL. Secretion by the common duct in response to secretin. Surg forum. 1971;22:386–387. [PubMed] [Google Scholar]
- 18.Strasberg SM, Petrunka CN, Ilson RG. The contribution of the extrahepatic bile ducts to bile formation. Can J Physiol Pharmacol. 1976;54:757–763. doi: 10.1139/y76-105. [DOI] [PubMed] [Google Scholar]
- 19.Yamada G, Sawada N, Mori M. Evidence for fluid-phase pinocytosis of extrahepatic bile duct cells isolated from normal rats in culture. Cell Struct Funct. 1992;17:67–75. doi: 10.1247/csf.17.67. [DOI] [PubMed] [Google Scholar]
- 20.Alpini G, Phinizy JL, Glaser S, et al. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066–G1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 21.Francis H, Glaser S, DeMorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Gastrointest Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y, DeMorrow S, Invernizzi P, et al. Melatonin exerts by an autocrine loop antiproliferative effects in cholangiocarcinoma: its synthesis is reduced favoring cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2011;301:G623–G633. doi: 10.1152/ajpgi.00118.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueno Y, Alpini G, Yahagi K, et al. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 24.Paradis K, Sharp HL. In vitro duct-like structure formation after isolation of bile ductular cells from a murine model. J Lab Clin Med. 1989;113:689–694. [PubMed] [Google Scholar]
- 25.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303–313. [PubMed] [Google Scholar]
- 26.Kumar U, Jordan TW. Isolation and culture of biliary epithelial cells from the biliary tract fraction of normal rats. Liver. 1986;6:369–378. doi: 10.1111/j.1600-0676.1986.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 27.Chai C, Zheng S, Feng J, et al. A novel method for establishment and characterization of extrahepatic bile duct epithelial cells from mice. In Vitro Cell Dev Biol Anim. 2010;46:820–823. doi: 10.1007/s11626-010-9346-7. [DOI] [PubMed] [Google Scholar]
- 28.Katayanagi K, Kono N, Nakanuma Y. Isolation, culture and characterization of biliary epithelial cells from different anatomical levels of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–98. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoerl BJ, Vroman BT, Kasperbauer JL, et al. Biological characteristics of primary cultures of human gallbladder epithelial cells. Lab Invest. 1992;66:243–250. [PubMed] [Google Scholar]
- 30.Bitoun JP, Liao S, Xie GG, et al. Deficiency of BrpB causes major defects in cell division, stress responses and biofilm formation by Streptococcus mutans. Microbiology. 2014;160:67–78. doi: 10.1099/mic.0.072884-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao X, Beatty WL, Fan H. Exploration of chlamydial type III secretion system reconstitution in Escherichia coli. PloS One. 2012;7:e50833. doi: 10.1371/journal.pone.0050833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 34.Alpini G, Aragona E, Dabeva M, et al. Distribution of albumin and alpha-fetoprotein mRNAs in normal, hyperplastic, and preneoplastic rat liver. Am J Pathol. 1992;141:623–632. [PMC free article] [PubMed] [Google Scholar]
- 35.Francis H, DeMorrow S, Venter J, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut. 2012;61:753–764. doi: 10.1136/gutjnl-2011-300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992;267:15523–15529. [PubMed] [Google Scholar]
- 37.Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 38.LeSage G, Glaser SS, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 39.Alpini G, Glaser S, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 40.Renzi A, DeMorrow S, Onori P, et al. Modulation of the biliary expression of arylalkylamine N-acetyltransferase alters the autocrine proliferative responses of cholangiocytes in rats. Hepatology. 2013;57:1130–1141. doi: 10.1002/hep.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerrier M, Attili F, Alpini G, et al. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg Nutr. 2014;3:118–1125. doi: 10.3978/j.issn.2304-3881.2014.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 43.Cho WK, Mennone A, Boyer JL. Isolation of functional polarized bile duct units from mouse liver. Am J Physiol Gastrointest Liver Physiol. 2001;280:G241–G246. doi: 10.1152/ajpgi.2001.280.2.G241. [DOI] [PubMed] [Google Scholar]
- 44.LeSage G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- 45.LeSage G, Glaser S, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 46.Hohenester S, Wenniger LM, Paulusma CC, et al. A biliary HCO3 umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 47.Beuers U, Hohenester S, de Buy Wenniger LJ, et al. The biliary HCO(3)(−) umbrella: a unifying hypothesis on pathogenetic and therapeutic aspects of fibrosing cholangiopathies. Hepatology. 2010;52:1489–1496. doi: 10.1002/hep.23810. [DOI] [PubMed] [Google Scholar]
- 48.Spirli C, Fiorotto R, Song L, et al. Glibenclamide stimulates fluid secretion in rodent cholangiocytes through a cystic fibrosis transmembrane conductance regulator-independent mechanism. Gastroenterology. 2005;129:220–233. doi: 10.1053/j.gastro.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 49.de Groen PC, Vroman B, Laakso K, et al. Characterization and growth regulation of a rat intrahepatic bile duct epithelial cell line under hormonally defined, serum-free conditions. In Vitro Cell Dev Biol Anim. 1998;34:704–710. doi: 10.1007/s11626-998-0066-1. [DOI] [PubMed] [Google Scholar]
- 50.Nakanuma Y, Hoso M, Sanzen T, et al. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552–570. doi: 10.1002/(SICI)1097-0029(19970915)38:6<552::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 51.Gaudio E, Onori P, Pannarale L, et al. Microcirculation of the extrahepatic biliary tree: a scanning electron microscopy study of corrosion casts. J Anat. 1993;182:37–44. [PMC free article] [PubMed] [Google Scholar]
- 52.Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carpino G, Cardinale V, Gentile R, et al. Evidence for multipotent endodermal stem/progenitor cell populations in human gallbladder. J Hepatol. 2014;60:1194–1202. doi: 10.1016/j.jhep.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Alpini G, Glaser S, Robertson W, et al. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G518–G529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- 55.Irie T, Asahina K, Shimizu-Saito K, et al. Hepatic progenitor cells in the mouse extrahepatic bile duct after a bile duct ligation. Stem Cells Dev. 2007;16:979–987. doi: 10.1089/scd.2007.0037. [DOI] [PubMed] [Google Scholar]
- 56.Shen H, Fan Y, Yang X, et al. Increased expression of cystic fibrosis transmembrane conductance regulator in rat liver after common bile duct ligation. J Cell Physiol. 2005;203:599–603. doi: 10.1002/jcp.20259. [DOI] [PubMed] [Google Scholar]
- 57.Alvaro D, Della Guardia P, Bini A, et al. Effect of glucagon on intracellular pH regulation in isolated rat hepatocyte couplets. J Clin Invest. 1995;96:665–675. doi: 10.1172/JCI118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan CK, Podila PV, Taylor JE, et al. Human cholangiocarcinomas express somatostatin receptors and respond to somatostatin with growth inhibition. Gastroenterology. 1995;108:1908–1916. doi: 10.1016/0016-5085(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 59.Zhao B, Zhao H, Zhao N, et al. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J Hepatobiliary Pancreat Surg. 2002;9:497–502. doi: 10.1007/s005340200062. [DOI] [PubMed] [Google Scholar]