Abstract
Background and purpose
The term “metabolic syndrome” (MetS) describes the clustering of risk factors found in many individuals with obesity. Due to their pathophysiology, we hypothesized that two features of MetS, central obesity and insulin resistance (IR), would be associated with cerebrovascular changes on MRI, and specifically with incident lacunar disease and not white matter hyperintensity progression (WMH).
Methods
Risk factors were defined at study baseline in 934 participants in the Atherosclerosis Risk in Communities (ARIC) study who completed two brain MRIs approximately ten years apart. WMH progression and incident lacunes between the two MRIs were determined. An IR score for each participant was created using principal component analysis of 11 risk factors, including (among others): insulin, HOMA-IR, body mass index (BMI) and waist circumference. MetS (presence/absence), using standard clinical definitions, and IR score at the first MRI, were independent variables, evaluated in multivariate logistic regression to determine odds of WMH progression (Q5 vs. Q1–4) and incident lacunes.
Results
MetS (adjusted OR 1.98; 95% confidence interval (CI) 1.28, 3.05) and IR score (adjusted OR per 1-standard deviation increase: 1.33, 95% CI 1.05, 1.68) were associated with incident lacunes but not with WMH progression. Insulin, HOMA-IR and BMI were not associated with incident lacunes or WMH progression in separate models.
Conclusions
The IR score and central obesity are associated with incident lacunar disease but not WMH progression in individuals. Central obesity and IR may be important risk factors to target to prevent lacunar disease.
Keywords: metabolic syndrome, white matter hyperintensities, lacunes, obesity, insulin resistance, leukoaraiosis
Introduction
Brain small vessel disease, including white matter hyperintensities (WMH) and lacunes, often leads to cognitive impairment and dementia.1 As dementia and obesity are increasing in prevalence, it is important to understand their association. In obese patients, fat metabolism is dependent on insulin, which suppresses lipolysis in fat cells (adipocytes). In insulin resistance (IR), adipocytes fail to respond to the actions of insulin, resulting in lipolysis, which explains why patients with IR often have dyslipidemia.2 Although IR is associated with diabetes, not all people with IR develop diabetes. Metabolic syndrome (MetS) is a commonly used term used to describe the clustering of central obesity, hypertension, hyperglycemia and dyslipidemia and attempts to capture associations with cardiovascular risk. This may be a hypothetical construct less relevant than its individual components, in part because the individual risk factors may adequately explain disease endpoints.3, 4
The mechanisms of small vessel disease may provide insight into risk factor associations. WMHs are thought to arise from chronic ischemia and blood-brain barrier breakdown,5–7 and abnormal venous pathology, known as venous collagenosis, may also be present in these lesions.6 Two distinct pathologies are described in lacunes:8, 9 1) plaque-like accumulations with the vessel that may represent microatheromas and 2) fibrinoid necrosis of the blood vessel wall (lipohyalinosis). A prior ARIC study determined that smaller lacunes (<7mm) and larger lacunes (8–20mm) have different risk factor profiles, possibly due to unique pathologic mechanisms.10 We hypothesize that WMH are more similar to smaller lacune subtypes. Given the association with IR and dyslipidemia, we hypothesized that midlife central obesity and IR would be associated with incident lacunes, especially larger lesions (microatheromatous disease), but not with WMH progression.
Methods
Study Population
The ARIC study was conducted at four sites, with 15,792 participants aged 45 to 64 years upon initial recruitment in 1987–1989.11 Five main study visits occurred: visit 1–4 (each three years apart) and visit 5 in 2011–13. At visit 3, participants from Forsyth County, North Carolina and Jackson, Mississippi (black subjects only) were invited for the first brain MRI (1993–5).12 Ten years after, these participants were invited to participate in the Brain MRI ancillary study in 2004–06.12 Of the 1,134 participants who underwent both scans, 999 had interpretable MRI’s at both visits. Other exclusions were as follows: 9 with a prior history of stroke, 3 for non-physiologic values of MRI measurements and 53 missing MetS variables. The analytic population included 934 participants. All participants signed informed consent, and each institution’s institutional review board approved the study.
Definition of MetS and IR score
MetS was defined at visit 1 by presence of at least 3 of the following criteria: 1) waist circumference ≥102 cm in men or ≥88 cm in women; 2) HDL < 40 mg/dL in men and <50 mg/dL in women or on drug treatment; 3) elevated blood pressure ≥130 mmHg systolic or ≥85 mmHg diastolic or on drug treatment; 4) elevated triglycerides ≥150 mg/dL or on drug treatment; and 5) elevated fasting glucose ≥100 mg/dL or on treatment for diabetes.13
An IR score was defined to examine the clustering of risk factors using principal components analysis (PCA). This ad hoc construct was created to better capture the joint effects of central obesity and IR, and is specific to this population studied. Eleven metabolic factors were included: log-transformed values for insulin, HOMA-IR and triglycerides (to normalize the distributions), waist circumference, body mass index (BMI), waist-to-hip ratio, systolic blood pressure, diastolic blood pressure, fasting glucose, HDL and LDL. PCA was computed using orthogonal rotation to look for linear combinations between the exposures to establish a new set of fewer variables comprised of uncorrelated components. Components retained for the analysis had an Eigen value greater than 2.0, leaving one component explaining 36.3% of the sample variance. This component was defined by correlated metabolic factors with a loading >0.30 that included: BMI, waist-to-hip ratio, waist circumference, log insulin and log HOMA-IR (Supplemental Table I). Each participant was assigned an IR score based on these factor loadings.
Waist circumference was measured in centimeters at the level of the umbilicus. BMI was calculated in kg/m2. Fasting blood samples were collected and frozen at 70°C for storage and methods for serum triglycerides and high-density lipoprotein were described previously.14–16 Serum glucose was measured with the hexokinase method. Insulin was measured by radioimmunoassay (125-I Insulin 100 test kit; Cambridge Medical Diagnostics, Billerica, MA). The homeostatic model of IR (HOMA-IR) was calculated using the formula: (glucose mg/dL x insulin uU/mL)/ 405 with glucose and insulin measured from visit 1.17
Magnetic Resonance Imaging
Eligibility and screening protocols for the MRI visits are described in detail elsewhere.18, 19 Scans were completed on 1.5 Tesla machines, and spin-echo, spin-density/T2* weighted and T1 weighted images were collected in 5mm axial slices. A “lacune” was defined as 3–20mm in size. Locations included were the basal ganglia, thalamus, brainstem, internal capsule, deep cerebellum, and subcortical white matter. Only non-hemorrhagic lesions hyperintense on proton density and T2-weighted images and hypointense on T1-weighted images were selected. The number of lacunes at the visit 3 MRI was subtracted from the number of Brain MRI lacunes and if this value was one or greater, the individual had an “incident lacune”. Lacunes were further subdivided by size into two groups: 3–7mm and >7 to 20mm, based on maximum anterior-posterior or right-left diameter.
WMH progression was determined with two methods. Using the Cardiovascular Health Study rating scale (0–9),20, 21 periventricular and subcortical WMH were graded from visual comparison with 8 template images for both MRI visits. The change in grade between visits was calculated. At the Brain MRI visit, an automated algorithm was used to segment WMH volume on the axial fluid–attenuated inversion recovery images, with manual editing to exclude infarcts and other lesions.22 Volumetric measurements were standardized to an intracranial volume of 1500 cm3. The actual data (visual grades and measured volumes) from the brain MRI visit were used to impute the estimated volumes at visit 3, WMH progression between the two MRIs was calculated using predicted volumes for visit 3 subtracted from measured volumes at the Brain MRI.23
Statistical methods
Descriptive statistics were evaluated stratified by MetS status, using t-tests for continuous variables and chi-squared tests for categorical variables. Univariate and multivariate logistic regression was used to assess the odds of incident lacunes with each 1-SD increase in risk factors (insulin, HOMA-IR, BMI and triglycerides). MetS (presence/absence) and each 1-SD increase in the IR score were used as composite measures of these risk factors. Outcomes were WMH progression (top quintile (Q5) vs. Q1–4) and incident lacunes 3–20mm, which were further subdivided by size. Because of the possibility of non-differential misclassification of volumetric WMH progression using the prediction equation, and therefore under estimation of effect size, the analysis was also conducted using change in visual WMH grade.
Models were built sequentially, with demographic values added first, and then history of coronary artery disease and hypertension. Hypertension was included in all models, except those in which it was a part of the variable definition (e.g. MetS). Other covariates included: age (years), sex, race (black, white), education, history of coronary artery disease, history of alcohol use, and history of tobacco use. Interaction terms were tested between MetS and sex, age and race, each, but were not included in the final model due to the lack of statistical significance. Because of the overlap between diabetes and IR, a sensitivity analysis was performed excluding all individuals with diabetes (n=73). A two-sided p-value of <0.05 was considered significant for all analyses.
Results
The mean change in WMH was 5 cm3 (standard deviation, SD 8.5) and the range of incident lacunes was 0 to 5, with 33% of those with a lacune having more than one. At baseline (ages 45–64), participants with MetS did not differ significantly from those without MetS by age, race or sex (Table 1).
Table 1.
Sample Characteristics
| Without MetS (n=609) | With MetS (n=325) | |
|---|---|---|
| Black | 282 (46.3) | 171 (52.6) |
| Women | 365 (59.9) | 210 (64.6) |
| Age (y ± SD) | 55.8 ± 4.5 | 56.0 ± 4.2 |
| Education | ||
| Less than high school | 110 (18.1) | 90 (13.8)ŧ |
| High school graduate* | 159 (26.2) | 92 (28.3) |
| More than high school | 339 (55.7) | 143 (44.0) |
| Prevalent CHD | 22 (3.6) | 12 (3.7)ŧ |
| Diabetes | 23 (3.7) | 50 (15.4)ŧ |
| Elevated waist circumference | 223 (36.6) | 263 (80.9)ț |
| Low high-density lipoprotein | 82 (13.5) | 194 (59.7)ț |
| Elevated blood pressure | 197 (32.4) | 243 (74.8)ț |
| Elevated triglycerides | 48 (7.9) | 175 (53.9)ț |
| Elevated fasting glucose | 167 (27.4) | 256 (78.8)ț |
| Current or former smoking | 297(48.8) | 156 (48.1) |
| Current or former alcohol | 385 (63.6) | 185 (57.1) |
| Mean WMH progression (cm3, mean± SD) | 4.8 ± 8.7 | 5.3 ± 8.3 |
| Participants with incident lesions | ||
| Lacunes 3–7mm | 32 (5.3) | 29 (8.9)ț |
| Lacunes >7–20mm | 20 (3.3) | 28 (8.6)ț |
| Lacunes 3–20mm | 52 (8.5) | 48 (14.8)ț |
Values are N (%) unless otherwise specified;
also includes GED or vocational school
= ten participants had incident infarcts in both groups;
WMH = white matter hyperintensity; SD = standard deviation; MetS = metabolic syndrome; CHD = coronary heart disease.
= p<0.05
MetS and its components, incident infarcts, and WMH progression
Lipid markers were associated with all lacunes (triglycerides and inverse association with HDL) but not WMHs (Supplemental Table II, Table 2) in unadjusted and adjusted models. Systolic blood pressure was associated with WMH and incident lacunes in unadjusted models (Supplemental Table II and III), but did not reach statistical significance for lacunes in adjusted models (Table 2 and 3).
Table 2.
Association of obesity and insulin resistance with small vessel disease
| WMH progression (Q5 v. Q1–4) | Incident lacunes 3–20mm | |||
|---|---|---|---|---|
| Odds ratio, 95% Confidence Interval | ||||
| Insulin* | 0.95 | 0.79, 1.14 | 1.11 | 0.91, 1.35 |
| HOMA-IR* | 1.00 | 0.84, 1.19 | 1.15 | 0.96, 1.38 |
| Body mass index* | 1.01 | 0.84, 1.21 | 1.04 | 0.83, 1.30 |
| Waist circumference* | 1.05 | 0.89, 1.25 | 1.12 | 0.91, 1.40 |
| Waist-to-hip ratio* | 1.08 | 0.90, 1.30 | 1.30 | 1.03, 1.65 |
| Triglycerides* | 0.93 | 0.76, 1.13 | 1.24 | 1.04, 1.47 |
| High density lipoprotein* | 1.12 | 0.93, 1.36 | 0.77 | 0.59, 0.99 |
| Systolic blood pressure* ț | 1.33 | 1.12, 1.58 | 1.20 | 0.97, 1.48 |
| MetS ț | 1.12 | 0.79, 1.59 | 1.98 | 1.28, 3.05 |
| IR score*ț | 1.11 | 0.92, 1.33 | 1.33 | 1.05, 1.68 |
Q= quintile; HOMA-IR = Homeostatic model assessment, insulin resistance;
1-standard deviation increase; Adjustments for age, sex, education, race, history of coronary artery disease, history of alcohol use, tobacco use and hypertension;
No adjustments for hypertension
Table 3.
Association of obesity and insulin resistance with incident lacunes, by size
| Incident lacunes3–7mm | Incident lacunes>7–20mm | |||
|---|---|---|---|---|
| Odds ratio, 95% Confidence Interval | ||||
| Insulin* | 1.13 | 0.89, 1.42 | 1.16 | 0.89, 1.51 |
| HOMA-IR* | 1.19 | 0.96, 1.46 | 1.23 | 0.97, 1.55 |
| Body mass index* | 1.00 | 0.75, 1.33 | 1.18 | 0.87, 1.58 |
| Waist circumference* | 1.07 | 0.82, 1.41 | 1.34 | 1.00, 1.81 |
| Waist-to-hip ratio* | 1.33 | 1.00, 1.80 | 1.43 | 1.02, 2.00 |
| Triglycerides* | 1.32 | 1.09, 1.60 | 1.21 | 0.97, 1.50 |
| High density lipoprotein* | 0.73 | 0.53, 1.02 | 0.67 | 0.45, 1.00 |
| Systolic blood pressure* ț | 1.22 | 0.94, 1.60 | 1.28 | 0.96, 1.71 |
| MetS ț | 1.85 | 1.09, 3.14 | 3.34 | 1.78, 6.30 |
| IR score*ț | 1.34 | 1.01, 1.79 | 1.62 | 1.16, 2.25 |
HOMA-IR = Homeostatic model assessment, insulin resistance;
1 standard deviation increase; Adjustments for age, sex, education, race, history of coronary artery disease, history of alcohol use, tobacco use and hypertension;
No adjustments for hypertension
Mean WMH progression did not differ between those with and without MetS (Table 1). When using change in WMH grade instead of quantitative WMH progression, associations with MetS components remained null (Supplemental Table IV, Table 2). Persons with MetS had more incident lacunes than those without MetS (14.8% vs. 8.5%, Table 1). This increase in lacunes in persons with MetS was predominantly due to an increase in infarcts >7–20mm (8.6% versus 3.3%, Table 1). These associations remained significant after adjustments (Tables 2 and 3). A sensitivity analysis excluding diabetics revealed similar but attenuated associations, even though diabetes (as defined by fasting glucose ≥100) is part of the MetS definition (Supplemental Tables V, VI).
Central obesity, incident infarcts and WMH progression
Increasing values of insulin, HOMA-IR and BMI were not associated with WMH progression or all incident lacunes (Table 2). Each 1-SD increase in HOMA-IR and BMI was associated with increased odds of incident large lacunes in unadjusted models (Supplemental Table III), but this result was attenuated with adjustment (Table 3). Central obesity as is measured by waist circumference, and waist-to-hip ratio was associated with incident lacunes, with a larger effect size for larger lacunes in unadjusted (Supplemental Table II and III) and adjusted models (Table 2, 3).
IR score
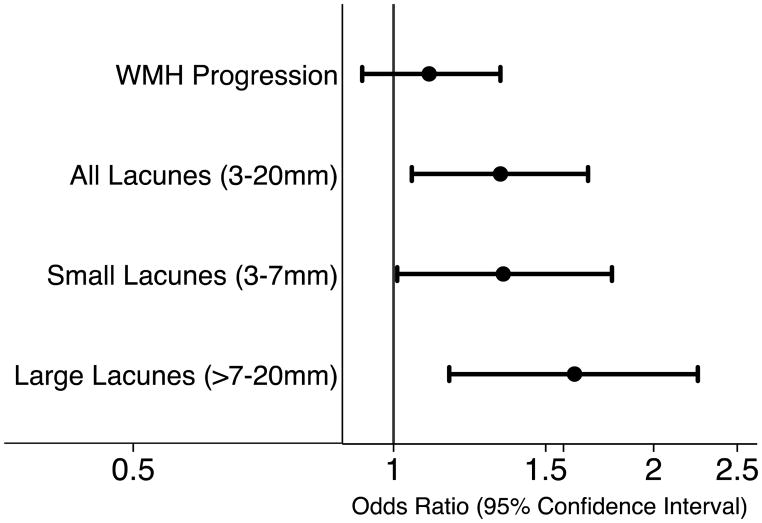
A higher IR score represents increased values of each of the weighted measures of central obesity and IR in a participant. Unlike MetS, hypertension and hyperglycemia contribute to a small part of the weighting, with higher weights given to waist circumference, waist-to-hip ratio, HOMA-IR, insulin and BMI (Supplemental Table I). Each 1-SD increase in the score was associated with increased odds of all incident lacunes, (adjusted OR 1.33, 95% CI 1.05,1.68, Table 2). Participants with a higher IR score had increased odds of having both larger and small lacunes, with increased effect size for larger lacunes (Figure 1, Table 3).
Figure 1. Insulin resistance (IR) score and incident small vessel disease.
The association of the IR score is shown in association with white matter hyperintensity (WMH) progression, incident small infarcts and incident large infarcts. The odds of being in the top quintile of WMH progression, or of having incident large or small infarcts are shown per standard deviation increase in the score.
Discussion
In this study we found unique associations with metabolic factors and brain small vessel disease in a prospective community-based cohort. Serum insulin, HOMA-IR and BMI were not independently associated with incident small vessel disease. An IR score, which may better capture their joint effects, and MetS were associated with all lacunes but not WMH progression. Although the magnitude of the association for MetS was larger than that for the IR score, we hypothesized that since MetS includes hypertension, a well-known risk factor for lacunar disease,8 its association with lacune progression may be primarily driven by hypertension. Our results instead demonstrate that this difference may be partially driven by HDL or other unmeasured covariates. These results are compelling because they demonstrate different risk factor profiles for small vessel disease, which may have important implications for dementia and cognition in later life.1
MetS was previously shown in other cohorts to be associated with WMH cross-sectionally,24, 25 and with changes in cerebral white matter microstructure, as measured by diffusion tensor imaging26, 27 These earlier studies did not, however, separate out the effect of hypertension between groups. In our study we examined WMH progression, which is a more robust measurement of microvascular disease than single measurements of WMH, as it likely partially accounts for potential confounding by social and demographic factors (which would affect WMH but not necessarily its progression). Our results suggest that WMH progression is unrelated to central obesity. Whether the difference between our study and prior work is related to unmeasured confounders is unknown and suggests that this result requires confirmation in other populations.
As lacunar stroke has different histopathology than WMH, it is not surprising that there are differential risk factor associations with these disease subtypes. The MetS components hypertension, diabetes, triglycerides and HDL are associated with incident lacunes in other work.28 A prior ARIC study reported that lacunes ≤7mm were associated with diabetes and hemoglobin A1C, and larger lacunes were associated with elevated LDL. This study brought to the forefront that descriptions of two lacune subtypes, by C. M. Fisher and others, seem to withstand the epidemiologic evidence.9 The current study did not focus on hyperglycemia, and we in fact demonstrated that in patients without diabetes, IR trended to increase odds of lacunes. It may be that patients who have abnormally glycosylated end products, as are present in diabetes, may have more lipohyalinosis, while abnormal central obesity, resulting in IR, predisposes to lacunes via a distinct mechanism. This theory requires further investigation. Also requiring investigation is whether targeting the “metabolic profile” of obesity and IR might be particularly important to prevent incident lacunes (especially the larger subtype). A limitation of the analysis is that we were unable to approximate how changes in metabolic factors through life affect brain small vessel disease, in that those with earlier exposures might have more disease than those who developed risk factors later. Another limitation is potential selection bias affecting subjects who received two MRIs, who may have been healthier than those who did not return for a second MRI. In addition, we did not obtain direct volumetric measurements from the visit 3 MRI, however, we also used WMH grade to compare to imputed WMH progression and found similar results. The relative health and age of the population (often before the onset of dementia) limits analysis of the extremes of disease.
In addition, although the recent STRIVE consortium recommended a definition of lacunes as 3 to 15 mm in subcortical locations; we used 3–20 mm as a size cutoff based on prior work in ARIC to allow comparability.29 Although neurologists generally attribute stroke mechanism to stroke location, there is often overlap between mechanisms at different locations (hypoperfusion, embolism and lipohyalinosis). As this is an observational study, there may be unmeasured confounders, such as socioeconomic indicators or medication adherence that contribute to the observed associations. In addition, we only evaluated whether each participant had one or more of a given type of infarct, so there is no “dose-response” effect, according to the number of lacunes. This could lead to less precision in risk factor differentiation. Finally, some participants had more than one type of incident infarct, which could lead to misclassification bias.
The strength of our study is the large prospective design rigorously measured confounders in participants with good rates of follow-up. We believe that incident imaging findings are more robust than cross-sectional data. Our exposures were measured at baseline, with outcomes measured up to 17 years later, since midlife risk factors have been shown to have greater associations with later-life disease outcomes.30
Summary
Our results lend further support that brain small vessel disease is not homogeneous, and that unique risk factors profiles exist for WMH and lacunar disease. The IR score is associated with silent lacunes, but not WMH, which may be a result of differences in pathology. This composite score focusing on central obesity and IR may better capture the effects of adiposity, as compared with BMI, which did not have the same associations. Future studies are warranted to evaluate whether interventions to treat central adiposity reduce silent MRI markers of brain disease.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding:
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Dr. Schneider was supported by NIH/NHLBI training grant T32HL007024.
Footnotes
Disclosures: Dr. Jack serves as a consultant for Eli Lilly and receives funding from the NIH and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic. Dr. Knopman serves as Deputy Editor for Neurology and serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer's Disease Cooperative Study; and receives research support from the NIH.
References
- 1.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MMB. Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Dearborn JL, Knopman D, Sharrett AR, Schneider AL, Jack CR, Jr, Coker LH, et al. The metabolic syndrome and cognitive decline in the atherosclerosis risk in communities study (aric) Dement Geriatr Cogn Disord. 2014;38:337–346. doi: 10.1159/000362265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: Definitions and controversies. BMC medicine. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. Journal of the neurological sciences. 2002;203–204:159–163. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 6.Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. Journal of the neurological sciences. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental mri white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 8.Lammie GA. Hypertensive cerebral small vessel disease and stroke. Brain pathology. 2002;12:358–370. doi: 10.1111/j.1750-3639.2002.tb00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher CM. Lacunes: Small, deep cerebral infarcts. Neurology. 1965;15:774–784. doi: 10.1212/wnl.15.8.774. [DOI] [PubMed] [Google Scholar]
- 10.Bezerra DC, Sharrett AR, Matsushita K, Gottesman RF, Shibata D, Mosley TH, Jr, et al. Risk factors for lacune subtypes in the atherosclerosis risk in communities (aric) study. Neurology. 2012;78:102–108. doi: 10.1212/WNL.0b013e31823efc42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: Design and objectives. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain mri: The ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; american heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 14.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--i. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thrombosis and haemostasis. 1989;61:15–19. [PubMed] [Google Scholar]
- 15.Nagele U, Hagele EO, Sauer G, Wiedemann E, Lehmann P, Wahlefeld AW, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 16.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Mosley TH, Jr, Knopman DS, Catellier DJ, Bryan N, Hutchinson RG, Grothues CA, et al. Cerebral MRI findings and cognitive functioning: The Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 19.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, et al. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The aric study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27:2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 20.Bryan RN, Manolio TA, Schertz LD, Jungreis C, Poirier VC, Elster AD, et al. A method for using mr to evaluate the effects of cardiovascular disease on the brain: The cardiovascular health study. AJNR Am J Neuroradiol. 1994;15:1625–1633. [PMC free article] [PubMed] [Google Scholar]
- 21.Manolio TA, Kronmal RA, Burke GL, Poirier V, O'Leary DH, Gardin JM, et al. Magnetic resonance abnormalities and cardiovascular disease in older adults. The cardiovascular health study. Stroke. 1994;25:318–327. doi: 10.1161/01.str.25.2.318. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR, O'Brien PC, Rettman DW, Shiung MM, Xu Y, Muthupillai R, et al. Flair histogram segmentation for measurement of leukoaraiosis volume. Journal of Magnetic Resonance Imaging. 2001;14:668–676. doi: 10.1002/jmri.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottesman RF, Coresh J, Catellier DJ, Sharrett AR, Rose KM, Coker LH, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:3–8. doi: 10.1161/STROKEAHA.109.566992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K, Yasuda N, Toyonaga S, Yamada SM, Nakabayashi H, Nakasato M, et al. Significant association between leukoaraiosis and metabolic syndrome in healthy subjects. Neurology. 2007;69:974–978. doi: 10.1212/01.wnl.0000266562.54684.bf. [DOI] [PubMed] [Google Scholar]
- 25.Bokura H, Yamaguchi S, Iijima K, Nagai A, Oguro H. Metabolic syndrome is associated with silent ischemic brain lesions. Stroke. 2008;39:1607–1609. doi: 10.1161/STROKEAHA.107.508630. [DOI] [PubMed] [Google Scholar]
- 26.Segura B, Jurado MA, Freixenet N, Falcon C, Junque C, Arboix A. Microstructural white matter changes in metabolic syndrome: A diffusion tensor imaging study. Neurology. 2009;73:438–444. doi: 10.1212/WNL.0b013e3181b163cd. [DOI] [PubMed] [Google Scholar]
- 27.Ryu SY, Coutu J-P, Rosas HD, Salat D. The relationship between insulin resistance and white matter integrity in middle-aged and older adults. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2013;9:P108. [Google Scholar]
- 28.Gouw AA, van der Flier WM, Fazekas F, van Straaten ECW, Pantoni L, Poggesi A, et al. Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period: The leukoaraiosis and disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- 29.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.