Abstract
An electron paramagnetic resonance spin-labeling method has been developed that allows quantitative evaluation of the amounts of phospholipids and cholesterol in lipid domains of intact fiber-cell plasma membranes isolated from cortical and nuclear regions of eye lenses. The long term goal of this research is the assessment of organizational changes in human lens fiber cell membranes that occur with age and during cataract development. The measurements needed to be performed on lens membranes prepared from eyes of single donors and from single eyes. For these types of studies it is necessary to separate the age/cataract related changes from preparation/technique related changes. Human lenses differ not only because of age, but also because of the varying health histories of the donors. To solve these problems the sample-to-sample preparation/technique related changes were evaluated for cortical and nuclear lens membranes prepared from single porcine eyes. It was assumed that the differences due to the age (animals were two year old) and environmental conditions for raising these animals were minimal. Mean values and standard deviations from preparation/technique changes for measured amounts of lipids in membrane domains were calculated. Statistical analysis (Student's t test) of the data also allowed determining the differences of mean values which were statistically significant with P ≤ 0.05. These differences defined for porcine lenses will be used for comparison of amounts of lipids in domains in human lens membranes prepared from eyes of single donors and from single eyes. Greater separations will indicate that differences were statistically significant with (P ≤ 0.05) and that they came from different than preparation/technique sources. Results confirmed that in nuclear porcine membranes the amounts of lipids in domains created due to the presence of membrane proteins were greater than those in cortical membranes and the differences were larger than the differences observed for human intact fiber cell membranes [Raguz, M. Mainali, L., O'Brien, W.J., and Subczynski, W.K. (2015) Exp. Eye Res.]. Lipids in porcine nuclear fiber cell plasma membranes were more rigid and less permeable to oxygen than in human nuclear membranes. Most likely the significant differences in the lipid composition were responsible for the observed differences.
Keywords: cholesterol, membrane domain, fluidity, hydrophobic barrier, oxygen permeation, EPR, spin labeling
1. Introduction
Lens fiber cells lose their intracellular organelles soon after they are formed (Bassnett et al., 2011; Rafferty, 1985; Wride, 2011), and the plasma membrane together with the cytoskeleton is the only supramolecular structure of the matured fiber cell. The plasma membrane accounts for essentially all lipids of the matured fiber cell. Thus, the fiber cell plasma membrane and its lipid bilayer component likely play significant roles in the maintaining fiber cell viability as well as maintaining lens homeostasis thus preventing lens opacification and development of cataract. To understand these functions of fiber cell plasma membrane it is necessary to understand functions of membrane components at the molecular level.
Using EPR spin-labeling approaches we investigated in detail the organization and properties of lens lipid membranes made of the total lipid extracts from cortical and nuclear regions of lenses from different species of animals at different ages (Mainali et al., 2011a; Mainali et al., 2013, 2015; Raguz et al., 2009). We were able to discriminate membrane domains, including the cholesterol bilayer domain (CBD), and to show that for old human donors (from age group from 61 to 70 years) nuclear membranes contain cholesterol crystals (Mainali et al., 2015). We were also able to identify cholesterol and CBD functions specific to fiber cell plasma membranes (Subczynski et al., 2012). The saturating Chol content keeps the bulk physical properties of lens lipid membranes consistent and independent of changes in phospholipid (PL) composition. Thus, CBD helps to maintain lens membrane homeostasis by providing the buffering capacity for Chol concentration in the surrounding PL bilayer, keeping it at a constant saturating level. Maintaining lens membrane homeostasis during aging is not an easy task because the lens membrane phospholipid composition changes dramatically with age (Borchman and Yappert, 2010; Li et al., 1987). Another significant conclusion from our work with lens lipid membranes is that the high Chol content, formation of CBDs, and formation of Chol crystals should not be considered as major predetermining factors for the development of age-related cataracts (Mainali et al., 2015). Investigation of lens lipid membranes (membranes without intrinsic proteins) is a necessary step in the investigation of the properties and organization of the lipid-bilayer portion of fiber-cell plasma membranes. Without this research, it is not possible to understand clearly the mechanisms by which intrinsic and extrinsic proteins affect the properties of the lipid bilayer (see (Subczynski et al., 2012) for more discussion).
All the studies cited above were completed using lens lipid membranes, thus, membranes without membrane integral proteins which should significantly alter the organization, structure, and dynamics of the lipid bilayer portion of the membrane. Aged fiber-cell membranes are loaded with integral proteins (Bassnett et al., 2011; Gonen et al., 2004; Kistler and Bullivant, 1980), the organization of which, including the formation of domains, arrays, and other structures, also change with age (Buzhynskyy et al., 2007; Costello et al., 1989; Dunia et al., 2006; Zampighi et al., 2002). Regardless of these changes, the lens normally remains transparent. Due to a lack of turnover (Lynnerup et al., 2008; Peterson and Delamere, 1992), cells in the center of the nucleus of an adult human lens are as old as the individual, and membrane proteins that perform several functions in young human lenses likely perform the same functions in older lenses with altered lipid compositions. Thus, homeostasis of the fiber-cell plasma membrane and fiber cell itself should be maintained throughout the entire human life. We believe that the fiber-cell plasma membrane, with its unique structure and properties, helps to maintain cell homeostasis.
Recently, we have extended our work in order to study the properties of the lipid bilayer portion of intact fiber-cell plasma membranes isolated from human (Raguz et al., 2014, 2015) and pig (Mainali et al., 2012) lenses. We were able to confirm the existence of three distinct lipid domains: bulk lipid domain, which appears minimally affected by membrane proteins, and two domains that appear due to the presence of membrane proteins, namely boundary lipid and trapped lipid domains. We also have made efforts to quantitatively evaluate the relative amounts of PLs and Chol in lipid domains of intact human eye lens membranes (Raguz et al., 2014, 2015). Based on our work (Raguz et al., 2014, 2015), the PL analogue spin label 12-SASL is the best to show the distribution of PLs between membrane domains discriminated in intact membranes. This spin label can be inserted into intact membranes from its dry film without the use of damaging solvents (Ligeza et al., 1998) and, thus, with a minimal disturbance to the membrane structure. Among examined spin-labeled cholesterol analogues only androstane spin label (ASL) can be inserted into intact membranes from its dry film (Mainali et al., 2012). Structures and approximate locations of these two spin labels in the lipid bilayer are shown in Fig. 1. The unique distributions of these spin labels between domains in lens lipid and intact membranes (Fig. 2) allow us to obtain unique quantitative information about the relative amounts of PLs and Chol in lipid domains. Figure 2 also provides the guideline for our experiments and interpretation of data. Quantitative results obtained for cortical and nuclear intact membranes isolated from clear human lenses of different age groups have allowed us to assess changes in the organization of lipids that occur with age. The data indicate that the amount of lipids in domains uniquely formed due to the presence of integral membrane proteins is greater in nuclear membranes than in cortical membranes and in nuclear membranes increases significantly with age. However, all of these measurements were obtained using samples pooled from about twenty clear lenses.
Fig. 1.
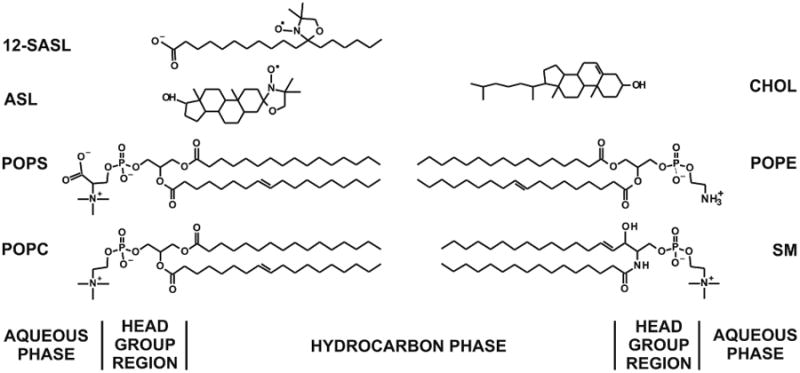
Chemical structures of 12-SASL and ASL together with the structure of Chol and the most abundant phospholipids in porcine lens membranes, namely: PC (35%), SM (26%), PS (21%, and PE (12%) (Deeley et al., 2008). Most abundant acyl chains of these PLs are palmitate (33%) and oleate (33%). Approximate locations of these molecules across the lipid bilayer membranes are illustrated.
Fig. 2.
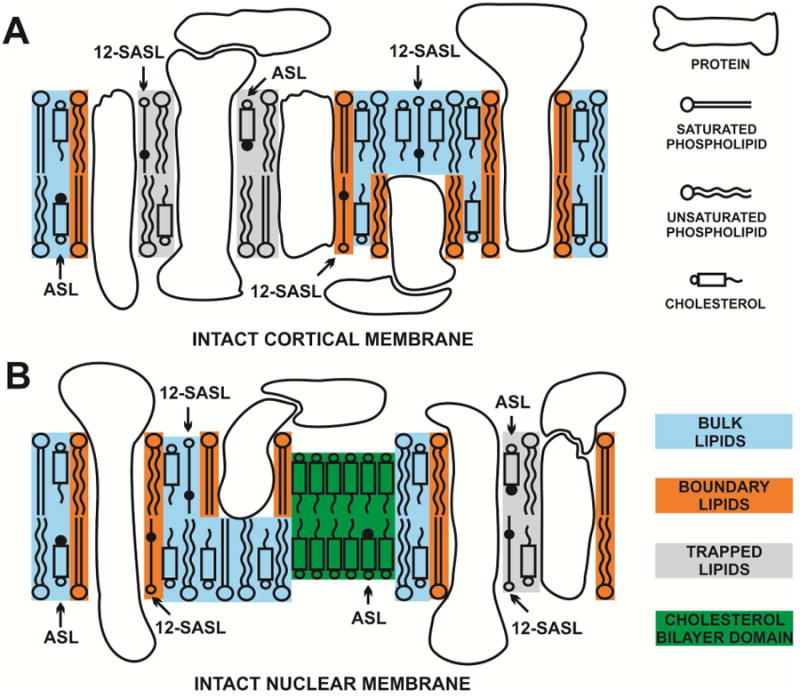
Schematic drawing of the intact porcine cortical (A) and nuclear (B) membranes. Purported lipid domains induced by the high Chol content and the presence of integral membrane proteins are indicated. Phospholipid spin label 12-SASL is distributed between bulk lipids, boundary lipids and trapped lipids; while Chol analog ASL is distributed between bulk and trapped lipids (it can be also located in the CBD). Note that Chol as well as ASL are excluded from boundary lipids. The nitroxide moieties of spin labels are indicated by black dots.
Data from figure 6 of (Raguz et al., 2015) also contains preliminary data for the quantitative evaluation of the relative amounts of PLs and Chol in lipid domains in cortical and nuclear human intact membranes from three pairs of clear lenses from three different donors and for cortical and nuclear intact membranes from left and right eye clear lenses from four different donors. These preliminary data allowed us to conclude that the differences observed between the left and the right eye of the same donor were much smaller that the scattering of data from different donors of a similar age. Thus, the significant methodological problem need to be solved before this quantitative method can be widely applied to assess organizational changes in fiber cell membranes occurring with age and during cataract development, namely: what is the contribution of the preparation/technique related experimental error? The separation of the age/cataract related changes from preparation/technique related changes cannot be performed based on measurements on single human lenses because the human lenses are different not only because of age, but also because of varying health history of the donors. Here we evaluated the preparation/technique related changes from sample-to-sample (changes due to the sample preparations and measurements) for cortical and nuclear lens membranes prepared from single porcine eyes assuming that the differences due to the age (animals were two-year-old) and environmental conditions for raising these animals (obtained from the same meat factory) are minimal. Statistical analysis of the data allowed us to evaluate preparation/technique related changes. Greater changes can be assumed to come from factors connected with health history of the donors, like age or cataract development.
2. Materials and Methods
2.1. Materials
Doxylstearic acid spin label (12-SASL) and spin-labeled cholesterol analog (androstane spin label [ASL]) were purchased from Molecular Probes (Eugene, OR). Other chemicals of at least reagent grade were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Isolation of intact membranes from cortical and nuclear fiber cell membranes
Porcine eyes from two-year-old animals were obtained on the day of slaughter from Johnsonville Sausage, LLC (Watertown, WI). The eyes were dissected, and the lenses were collected. The cortical and nuclear regions of each lens were separated based on differences in tissue consistency (Estrada and Yappert, 2004; Rujoi et al., 2003). Cortical and nuclear intact membranes were isolated separately from tissue of each lens based on minor modifications of the method developed by Bloemendal et al. (Bloemendal et al., 1972), as reported earlier (Cenedella and Fleschner, 1992; Chandrasekher and Cenedella, 1995; Lim et al., 2005). The cortical and nuclear tissues were homogenized separately, each in 25 mL of buffer A (5 mM Tris HCL, 5 mM EDTA, 5 mM EGTA, pH 8.0). The homogenate was centrifuged (29000 g, 20 min, 4°C). The pellet was washed five times with buffer B (5 mM Tris HCL, 2 mM EDTA, 2 mM EGTA, pH 8.0) and recovered by centrifugation (29000 g, 20 min, 4°C). Finally, the pellet was washed and resuspended with buffer C (10 mM PIPES, pH 7.0) and stored at −20°C.
2.3. Spin-labeling of intact membranes
Films of 12-SASL or ASL were prepared on the bottom of a test tube by drying the appropriate amount of spin label in chloroform (usually ∼20 μL of 1 mM solution). Only one type of spin label was present in each sample. Intact membrane suspensions (∼0.2 mL) were added to the test tubes and shaken for about two hours at room temperature. Control measurements demonstrated that this incubation time was sufficient to incorporate nearly all of the spin-label molecules into the membranes. Spin-label concentration was always lower than 1 mol% of the total lipids (Mainali et al., 2012). Finally, membrane suspensions were centrifuged for a short time, and the loose pellet was transferred to a 0.6 mm i.d. capillary made of gas-permeable methylpentene polymer (TPX) and used for EPR measurements (Subczynski et al., 2005).
2.4. Isolation and spin-labeling of lens lipid membranes
Conventional EPR spectra of 12-SASL and ASL in cortical and nuclear intact membranes show a good separation of strongly and weakly immobilized components (see Sect. 3.1). These spectra can be deconvoluted, giving the contribution of each component to the superimposed spectra. These components can be approximated by the EPR spectrum of 12-SASL (see Sect. 3.2) and ASL (see Sect. 3.3) in lens lipid membranes made of the total lipid extracts from cortical and nuclear regions of porcine lenses. Because of that the total lipids from the cortical and nuclear tissue materials (polled from five lenses) were extracted separately based on minor modifications of the Folch procedure (Folch et al., 1957) which was describe earlier (Raguz et al., 2009). The resultant lipid samples were soft, white solids. They were stored at −20°C.
The lipid bilayer membranes used in this work were multilamellar dispersions (multilamellar liposomes) made from the lipid extract from porcine eye-lens cortex or nucleus containing ∼1 mol% spin label (12-SASL or ASL). The membranes were prepared using the film deposition method described earlier (Kusumi et al., 1986; Mainali et al., 2011b). Finally, membrane suspensions were centrifuged for a short time, and the loose pellet was transferred to a 0.6 mm i.d. capillary made of gas-permeable methylpentene polymer (TPX) and used for EPR measurements (Subczynski et al., 2005).
2.5. EPR measurements
To further increase the signal-to-noise ratio, samples in TPX capillaries were centrifuged as described in (Subczynski et al., 2005). Conventional EPR spectra were recorded with a Bruker EMX spectrometer equipped with temperature-control accessories. All spectra with intact membranes were obtained at 37°C with modulation amplitude of 1.0 G and an incident microwave power of 5.0 mW. Spectra from lens lipid membranes were obtained at appropriate temperatures to better match spectral characteristics of the components in the spectrum of intact membranes (see Sect. 2.6). All samples were thoroughly deoxygenated, yielding correct EPR line shapes.
2.6. Distribution of PLs (% of total PLs) and Chol (% of total Chol) between domains in intact membranes
Based on the EPR spectrum of the PL analog 12-SASL, which is a superposition of spectra coming from bulk PLs and from boundary plus trapped PLs, relative amounts of PLs in these domains were evaluated. The relative distribution of Chol was investigated based on the distribution of a Chol analog spin label, ASL. Similar evaluations, as for 12-SASL, were performed based on conventional EPR spectra of ASL in intact membranes. The precision of these evaluations was better than 5%, because a 5% change in the contribution of each component decreased the goodness of the fit. These procedures were successfully applied to intact human membranes (Raguz et al., 2014, 2015). Here, we applied these procedures for cortical and nuclear intact membranes isolated from single porcine eyes.
2.7. Statistical analysis
The Student's t test was used for determining the significance of experimental differences. P ≤ 0.05 was considered to be statistically significant.
3. Results and Discussion
In these studies we used two sets of samples isolated from single porcine eyes of two-year-old animals. Eight eyes were obtained in the summer of 2014. Intact membranes were isolated and investigated in the same time period. A second group of twelve eyes were obtained in the winter of 2015 and intact membranes prepared. All investigations were performed within one week of preparation. Information obtained from the meat company indicated that there are some differences in the maintaining of animals in the summer and in the winter. Because of that we presented the data separately for these two groups of animals.
3.1. Conventional EPR
Figure 3 shows representative EPR spectra of 12-SASL coming from cortical (A) and nuclear (A′) intact membranes isolated from one porcine lens. Similar spectra were obtained for other investigated lenses. Both spectra show the presence of the strongly (component 1) and the weakly (component 2) immobilized component. The small, sharp component 3 comes from the very small amount of free 12-SASL remaining in the buffer. This component does not interfere with our evaluations, and can be subtracted from the superimposed EPR spectrum if necessary. From the qualitative analysis of these spectra it is clearly seen that the amount of the strongly immobilized component (component 1) was greater in nuclear membranes than in cortical membranes. As indicated in (Mainali et al., 2012) and (Raguz et al., 2014, 2015), the weakly immobilized component comes from bulk lipids and the strongly immobilized component from boundary plus trapped lipids. The results from the quantitative evaluation of the amount of PLs in the identified membrane domains are presented in Sect. 3.2.
Fig. 3.
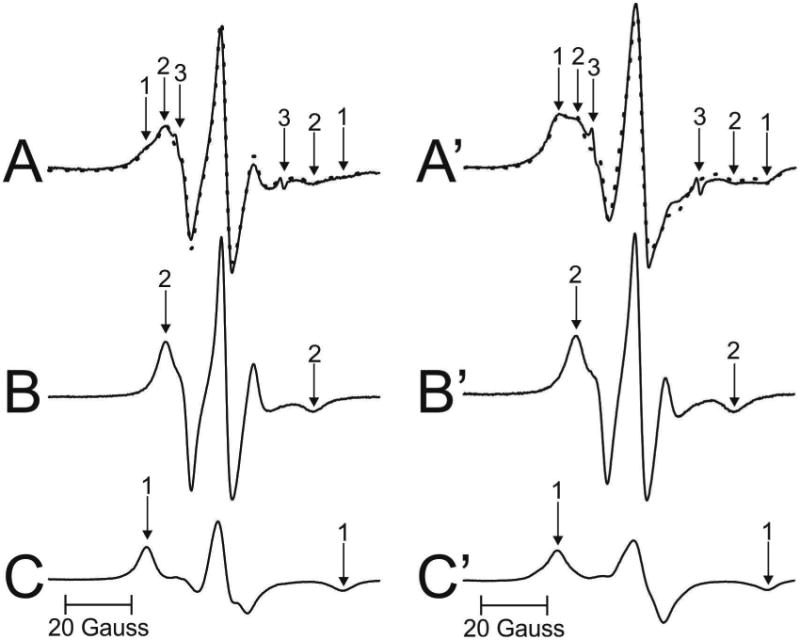
Representative EPR spectra of 12-SASL from cortical (A – solid line) and nuclear (A′ - solid line) porcine intact membranes Spectra were recorded at 37 °C. Arrows 1–3 represent spectra from strongly immobilized, weakly immobilized, and water components, respectively. See also Fig. 2 for the distribution of 12-SASL between lipid domains in intact membranes. The procedures for the evaluation of the relative amounts of PLs in domains in the lipid bilayer portions of cortical and nuclear intact membrane are also illustrated. The EPR spectra of 12-SASL in cortical (B) and nuclear (B′) lens lipid membranes obtained at 34 °C and 28 °C simulate, res pectively, the weekly immobilized components in spectra A and A′ (coming from 12-SASL located in bulk lipids). The spectra of 12-SASL in cortical (C) and nuclear (C′) lens lipid membranes obtained at −13 °C and −58 °C simulate, respectively, the strong ly immobilized component in spectra A and A′ (coming from 12-SASL located in boundary and trapped lipids). These spectra have the same maximum splitting as the weakly and the strongly immobilized component in spectra A and A′. The dotted spectrum in A (and A′) was obtained by adding 64% of spectrum B (31% of spectrum B′) and 36% of spectrum C (69% of spectrum C′), thus giving contributions of weakly and strongly immobilized components into the experimental spectrum of 12-SASL from intact cortical and nuclear membranes.
EPR spectra coming from the Chol analog spin label ASL are shown in Fig. 4. Spectra from cortical (A) and nuclear (A′) intact membranes isolated from one porcine lens are presented. EPR spectrum of ASL coming from cortical membranes (Fig. 4A) shows only the weakly immobilized component (component 2). This phenomenon was repeatedly observed in all porcine lenses investigated in this study as well as in cortical human intact membranes from donors younger than 60 years (Raguz et al., 2014, 2015). As shown in Fig. 4A′, spectrum from nuclear membranes is superimposed spectrum from both weakly (component 2) and strongly (component 1) immobilized components. Similar superimposed spectra were observed for nuclear membranes of all porcine lenses investigated in this study. As indicated in (Raguz et al., 2014), the weakly immobilized component came from ASL located in bulk lipids (and in the CBD), while the strongly immobilized component came from ASL located in trapped lipids. We were unable to identify the CBD in intact membranes (Mainali et al., 2012; Raguz et al., 2014) because the conventional EPR spectra coming from Chol analog spin labels in the CBD and in the surrounding PL bilayer saturated with Chol were practically identical (Raguz et al., 2011a, b). Additionally, we should note that Chol molecules, as well as ASL, were substantially excluded from boundary lipids (Bieri and Wallach, 1975; Warren et al., 1975). Qualitative analysis of these spectra allows us to speculate that in cortical membranes the amount of Chol in trapped lipids was negligible (undetectable using conventional EPR approach). Chol in trapped lipids in nuclear membranes was clearly detected (see also Sect. 3.3).
Fig. 4.
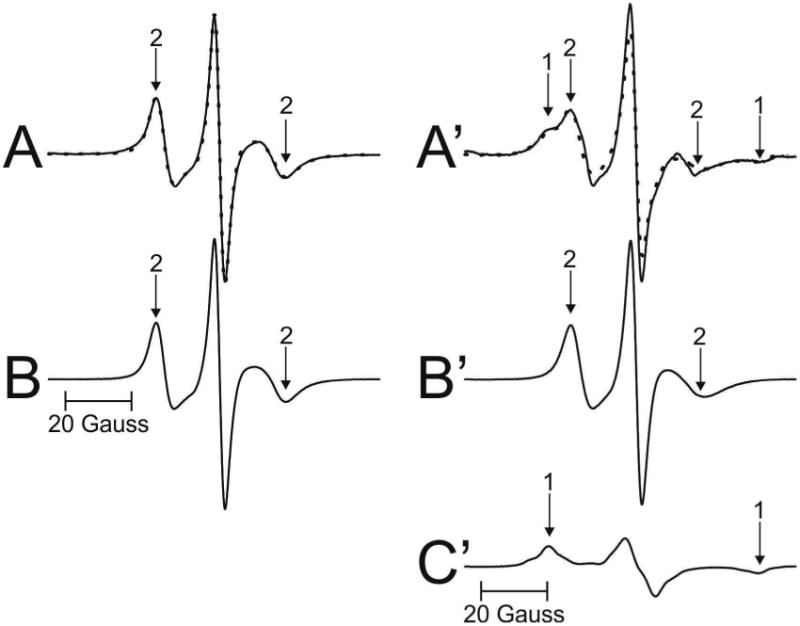
Representative EPR spectra of ASL from cortical (A – solid line) and nuclear (A′ – solid line) porcine intact membranes. Spectra were recorded at 37 °C. Arrows 1 and 2 represent spectra from strongly immobilized and weakly immobilized components, respectively. See also Fig. 2 for the distribution of ASL between lipid domains in intact membranes. The procedures for the evaluation of the relative amounts of Chol in domains in the lipid bilayer portions of cortical and nuclear intact membrane are also illustrated. The EPR spectra of ASL in cortical (B) and nuclear (B′) lens lipid membranes obtained at 34 °C simulate the weekly imm obilized components in spectra A and A′ (coming from ASL located in bulk lipids (and CBD)). The spectrum of ASL in nuclear (C′) lens lipid membranes obtained at −58 °C simulate the strongly immobilize d component in spectrum A′ (coming from ASL located in trapped lipids). This spectrum has the same maximum splitting as the strongly immobilized component in spectrum A′. The dotted spectrum in A was spectrum B, showing that the weakly immobilized component in the experimental spectrum of ASL from intact cortical membranes is coming from bulk lipids almost not affected by the presence of membrane proteins. The dotted spectrum in A′ was obtained by adding 45% of spectrum B′ and 55% of spectrum C′, thus giving contributions of weakly and strongly immobilized components into the experimental spectrum of ASL from intact nuclear membranes.
3.2. Amounts of PLs (% of total PLs) in domains uniquely formed due to the presence of membrane proteins
The PL analog spin label 12-SASL (see Fig. 1 for its structure) is the best spin label to show the distribution of PLs between membrane domains discriminated in intact membranes (Raguz et al., 2014, 15). Conventional EPR spectra of 12-SASL in intact membranes show a good separation of strongly (component 1) and weakly (component 2) immobilized components. These spectra can be deconvoluted, giving the contribution of each component to the superimposed spectra. Because the weakly immobilized component is coming from 12-SASL located in bulk lipids and the strongly immobilized component from 12-SASL located in boundary plus trapped lipids, the contribution of these component to the EPR experimental spectra reflects the relative distribution of PLs between these domains. This procedure is shown in Fig. 3A, B, and C for EPR spectrum of 12-SASL in cortical intact membranes and in Fig. 3A′, B′ and C′ for EPR spectrum of 12-SASL in nuclear intact membranes from a single porcine lens. The weakly immobilized components (components 2 in A and A′) are coming from bulk lipids which are minimally affected by membrane proteins. These components can be approximate by the EPR spectrum of 12-SASL in lens lipid membranes made of the total lipid extract from cortical (Fig. 3B) and nuclear (Fig. 3B′) intact membranes. Minimal effect of membrane proteins on these components can be adjusted by the lowering of temperature for the best fit. The best fits were obtained when these components were recorded at 34 °C. The strongly imm obilized components (components 1 in A and A′) are coming from lipids in boundary layers around integral membrane proteins and from lipids trapped within protein aggregates and arrays. EPR spectra cannot discriminate these two domains giving averaged information about boundary plus trapped lipid domain. The best approximation of these components can be obtained by EPR spectra of 12-SASL from lens lipid membranes with the motion decreased to that as in boundary plus trapped domain. This was achieved by decreasing the temperature for lens lipid membranes to −14 °C. At this tempera ture maximum splitting in spectra for lens lipid membranes were equal to maximum splitting for strongly immobilized components in spectra of intact membranes. The strongly immobilized components (components 1) obtained from cortical and nuclear lens lipid membranes are shown in Fig. 3C and C′, respectively.
The experimental EPR spectrum of 12-SASL in cortical intact membranes (A) (solid line in Fig. 3A) can be successfully reproduced by adding 64% of spectrum (B) and 36% of spectrum (C) (dotted line in Fig. 3A) where 100% of spectrum (B) and (C) indicates spectra with the same intensity (the same area under the absorption curve). Similarly the spectrum in nuclear membranes (A′) (solid line in Fig. 3A′) can be successfully reproduced by adding 31% of spectrum (B′) and 69% of spectrum (C′) (dotted line in Fig. 3A′). Similar procedures were performed for cortical and nuclear membranes isolated from other porcine lenses giving relative amounts of PLs in discriminated domains in each individual lens.
3.3. Amounts of Chol (% of total Chol) in domains uniquely formed due to the presence of membrane proteins
The relative distribution of Chol is indicated by the distribution of Chol analog spin label, ASL (see Fig. 1 for its structure). Similar evaluations, as illustrated in Fig. 3, can be performed based on conventional EPR spectra of ASL in intact cortical (Fig. 4A) and nuclear (Fig. 4A′) membranes. Thus the weakly immobilized component (component 2 in Fig. 4A) can be approximate by the EPR spectrum of ASL in cortical lens lipid membranes. The best fit was obtained when this spectrum was recorded at 34°C (Fig. 4B). Because ASL spectra in cortical in tact membranes were one component spectra, their fit to ASL spectra coming from lens lipid membranes was a good guide for choosing conditions (temperature) for weakly immobilized component of 12-SASL (see Sect. 3.2) and ASL in nuclear membranes. The best approximation for the weakly immobilized component in nuclear intact membranes (component 2 in Fig. 4A′) should be the EPR spectrum of ASL in nuclear lens lipid membranes. The best fit was obtained when this spectrum was recorded at 34°C (Fig. 4B′). The strongly immobilized component (component 1 in spectrum Fig. 4A′)) is coming from ASL in trapped lipid domain. The best approximation of this component can be obtained by EPR spectrum of ASL coming from nuclear lens lipid membranes with the decreased motion to match the maximum splitting of the spectrum to that of the strongly immobilized component in spectrum of intact membranes. The best match and the best fit were obtained when ASL spectrum in nuclear lens lipid membranes was recorded at −22°C (Fig. 4C′). The experimental EPR spectrum of ASL in nuclear intact membranes (A′) (solid line in Fig. 4A′) can be successfully reproduced by adding 45% of spectrum (B′) and 55% of spectrum (C′) (dotted line in Fig. 4A′). Similar procedures were performed for nuclear membranes isolated from other porcine lenses giving relative amounts of Chol in discriminated domains in each individual lens.
3.4. Sample-to-sample variability
Cumulative results for the distribution of PLs and Chol between domains in cortical and nuclear intact fiber cell porcine membranes of the porcine lens are presented in the Fig. 5A and B. This figure contains data indicating the amount of PLs (A) and the amounts of Chol (B) for all measured single lenses in the group of eight lenses and the group of twelve lenses. For two groups of lenses we calculated mean values and standard deviations (indicated as solid lines and bars, respectively). It was assumed that the differences due to the age (animals were two year old) and environmental conditions for raising these animals were minimal. Thus the sample-to-sample variations (evaluated standard deviations) were coming only from preparation/technique related changes between lenses in each group.
Fig. 5.
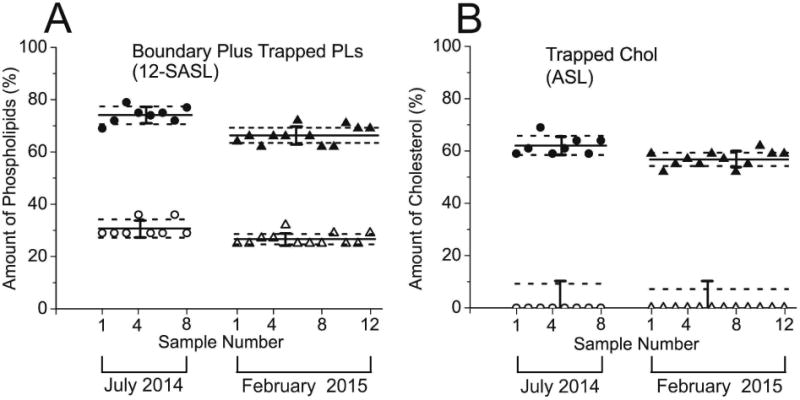
Amounts of PLs (A, % of total PLs) and Chol (B, % of total Chol) in domains uniquely formed due to the presence of membrane proteins in porcine intact cortical (open symbols) and nuclear (filled symbols) lens membranes. Presented data, represented by points, are for eight lenses obtained in July 2014, and twelve lenses obtained in February 2015. Mean values and standard deviations for each group are indicated as solid lines and bars, respectively. Broken lines indicate the interval around mean values outside of which the differences between means are statistically significant with P ≤ 0.05. Results for PLs were obtained based on conventional EPR spectra of 12-SASL (as indicated in Fig. 3) and for Chol based on conventional EPR spectra of ASL (as indicated in Fig. 4).
Using Student's t test statistical analysis we also calculated for each group the separation of mean values which will allow stating that these values are statistically different with P ≤ 0.05 and that the difference comes from different than preparation/technique related changes. Amounts of PLs in boundary plus trapped domain have to be separated by 3.45% (data from the group of eight eyes) or 1.95% (data from the group of twelve eyes) in cortical membranes and by 3.38% (data from the group of eight eyes) or 2.95% (data from the group of twelve eyes) in nuclear membranes to be considered statistically significant. These separations from the mean values are indicated as broken lines in Fig. 5A. Amounts of Chol in trapped domain of nuclear membranes have to be separated by 3.7% (data from the group of eight eyes) or 2.55% (data from the group of twelve eyes) to be considered statistically significant. These separations from the mean values are indicated as broken lines in Fig. 5B. Because in cortical membranes Chol was detected only in one domain (namely in bulk lipids), for statistical analysis we used precision of the individual measurement (5%, indicated as a bar in Fig. 5B) instead of the standard deviation. The Student's t test analysis indicated that only amounts of Chol in trapped domain greater than 4.7% (data from the group of eight eyes) or 3.6% (data from the group of twelve eyes) can be considered as statistically significant difference form zero level characteristic for cortical membranes (indicated as broken lines in Fig. 5B).
For human single lenses we are not able to separate sample-to-sample changes coming from preparation/technique factors from those connected with health history of donors. All preparation and measurement procedures are the same for porcine and human lenses and we assumed that scattering of the data coming from preparation/technical factors should be the same. Because of that the differences defined for porcine lenses will be used for comparison of amounts of lipids in domains in human lens membranes prepared from eyes of single lens. Greater separations will indicate that differences were statistically significant with (P ≤ 0.05) and that they came from different than preparation/technique sources.
3.5. Distribution of PLs and Chol between domains in intact porcine cortical and nuclear membranes
Few additional significant conclusions can be made based on results presented in the Fig. 5A and B. The amount of PLs in boundary and trapped lipid domains as well as the amount of Chol in trapped lipid domains was greater in nuclear than in cortical membranes, and these differences were larger than the differences observed for human intact fiber cell membranes (Raguz et al., 2014, 2015). In human intact membranes the differences in PL and Chol content in indicated domains between nuclear and cortical membranes were the greatest for 61- to 80-year-old donors. Surprisingly, the differences observed for two-year-old pigs were even larger than those evaluated for the oldest human donors. It is likely that the significant difference in the PL composition is responsible for such grate differences in the distribution of PLs and Chol between membrane domains in porcine and human fiber cell membranes.
Results in the Fig. 5A and B also indicated that the differences in the amount of PLs and Chol in membrane domains obtained for two different eye groups were statistically significant. In nuclear membranes the amount of lipids in domains created due to the presence of membrane proteins in the eight lens group was greater than evaluated for the twelve lens group (P < 0.001 for PL and P < 0.001 for Chol). In cortical membranes the amount of PLs in domains created due to the presence of membrane proteins in eight lens group was greater than that evaluated for twelve lens group (P < 0.001). The only difference between these two groups was that porcine eyes were obtained in the summer (group of eight lenses) and in the winter (group of twelve lenses). The meat company informed us that the conditions at which animals are maintained in the summer and in the winter are somewhat different. These differences in the maintaining conditions can be considered as a “health history” of donors. Our results indicated that the investigated distribution of lipids between membrane domains is a sensitive parameter.
4. Concluding remarks
Preliminary data for the quantitative evaluation of the relative amounts of lipids in domains in cortical and nuclear human intact membranes from clear lenses of the left and the right eye (Raguz et al., 2015) indicated that the differences observed between the left and the right eye of the same donor were much smaller that the scattering of data from different donors of a similar age. These data address significant methodological problem, i.e.: what is the contribution of the preparation/technique related changes to the scattering of the data? Our evaluations were performed on two different groups of porcine eye lenses giving the guideline for our future measurements with human lenses. In the future, differences of 3.45% and 3.38% in the amounts of PLs, respectively; in cortical and nuclear membranes will be considered as statistically significant with P ≤ 0.05. For differences of the amounts of Chol in cortical and nuclear membranes these values will be, respectively, 4.7% and 3.7%. These or greater differences will be considered as coming from other than preparation/technique factors.
In previous studies to quantitatively evaluate the relative amounts of lipids in domains in intact fiber cell membranes, we prepared samples from ∼20 pooled lenses (Raguz et al., 2015). In addition to the methodological benefits (handling the sample, good signal-to-noise ratio), mixing the samples from 20 lenses enabled us to obtain averaged information about the organization of lipids in membranes from different age groups and to assess organizational changes that occur with age. However in the case of human lenses, many elements of donors' health history were lost during the pooling process. We recognized that the single lens experimental approach is critical because it will allow presentation of results from the cortex and nucleus of a single lens in relation to donor age and health history. With this approach, data can be grouped by features of the individual lenses, such as transparent or cataractous. The latter group can be further subdivided based on whether a donor had a nuclear or cortical cataract. This individual approach allows donor health history, including risk factors such as history of previous eye surgery (especially vitreous or retina surgery), statin use, diabetes, or radiation of the head, to be considered in evaluation of cataract development, opening invaluable opportunities in eye lens research. The single lens approach combined with EPR spin-labeling methods should help to elucidate the biophysical basis of lens transparency on a molecular level and help determine the causes and mechanisms of age-related changes in the lens membrane that lead to cataracts.
Amount of boundary and trapped lipids was greater in nucleus than in cortex
Lipids in porcine nuclear membranes were more rigid than in human nuclear membranes
The sample-to-sample preparation/technique related changes were evaluated
Acknowledgments
This work was supported by grants EY015526, EB002052, EB001980, and EY001931 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans Royal Soc Lond Ser B Biol Sci. 2011;366:1250–1264. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri VG, Wallach DF. Variations of lipid-protein interactions in erythrocyte ghosts as a function of temperature and pH in physiological and non-physiological ranges. A study using a paramagnetic quenching of protein fluorescence by nitroxide lipid analogues. Biochim Biophys Acta. 1975;406:415–423. doi: 10.1016/0005-2736(75)90020-6. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, Zweers A, Vermorken F, Dunia I, Benedetti EL. The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1972;1:91–106. doi: 10.1016/0045-6039(72)90032-2. [DOI] [PubMed] [Google Scholar]
- Borchman D, Yappert MC. Lipids and the ocular lens. J Lipid Res. 2010;51:2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhynskyy N, Hite RK, Walz T, Scheuring S. The supramolecular architecture of junctional microdomains in native lens membranes. EMBO Rep. 2007;8:51–55. doi: 10.1038/sj.embor.7400858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenedella RJ, Fleschner CR. Selective association of crystallins with lens ‘native’ membrane during dynamic cataractogenesis. Curr Eye Res. 1992;11:801–815. doi: 10.3109/02713689209000753. [DOI] [PubMed] [Google Scholar]
- Chandrasekher G, Cenedella RJ. Protein associated with human lens ‘native’ membrane during aging and cataract formation. Exp Eye Res. 1995;60:707–717. doi: 10.1016/s0014-4835(05)80012-0. [DOI] [PubMed] [Google Scholar]
- Costello MJ, McIntosh TJ, Robertson JD. Distribution of gap junctions and square array junctions in the mammalian lens. Invest Ophthalmol Vis Sci. 1989;30:975–989. [PubMed] [Google Scholar]
- Deeley JM, Mitchell TW, Wei X, Korth J, Nealon JR, Blanksby SJ, Truscott RJ. Human lens lipids differ markedly from those of commonly used experimental animals. Biochim Biophys Acta. 2008;1781:288–298. doi: 10.1016/j.bbalip.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Dunia I, Cibert C, Gong X, Xia CH, Recouvreur M, Levy E, Kumar N, Bloemendal H, Benedetti EL. Structural and immunocytochemical alterations in eye lens fiber cells from Cx46 and Cx50 knockout mice. Eur J Cell Biol. 2006;85:729–752. doi: 10.1016/j.ejcb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Estrada R, Yappert MC. Regional phospholipid analysis of porcine lens membranes by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Mass Spectrom. 2004;39:1531–1540. doi: 10.1002/jms.759. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gonen T, Cheng Y, Kistler J, Walz T. Aquaporin-0 membrane junctions form upon proteolytic cleavage. J Mol Biol. 2004;342:1337–1345. doi: 10.1016/j.jmb.2004.07.076. [DOI] [PubMed] [Google Scholar]
- Kistler J, Bullivant S. Lens gap junctions and orthogonal arrays are unrelated. FEBS Lett. 1980;111:73–78. doi: 10.1016/0014-5793(80)80764-2. [DOI] [PubMed] [Google Scholar]
- Kusumi A, Subczynski WK, Pasenkiewicz-Gierula M, Hyde JS, Merkle H. Spin-label studies on phosphatidylcholine-cholesterol membranes: effects of alkyl chain length and unsaturation in the fluid phase. Biochim Biophys Acta. 1986;854:307–317. doi: 10.1016/0005-2736(86)90124-0. [DOI] [PubMed] [Google Scholar]
- Li LK, So L, Spector A. Age-dependent changes in the distribution and concentration of human lens cholesterol and phospholipids. Biochim Biophys Acta. 1987;917:112–120. doi: 10.1016/0005-2760(87)90291-8. [DOI] [PubMed] [Google Scholar]
- Ligeza A, Tikhonov AN, Hyde JS, Subczynski WK. Oxygen permeability of thylakoid membranes: electron paramagnetic resonance spin labeling study. Biochim Biophys Acta. 1998;1365:453–463. doi: 10.1016/s0005-2728(98)00098-x. [DOI] [PubMed] [Google Scholar]
- Lim J, Lam YC, Kistler J, Donaldson PJ. Molecular characterization of the cystine/glutamate exchanger and the excitatory amino acid transporters in the rat lens. Invest Ophthalmol Vis Sci. 2005;46:2869–2877. doi: 10.1167/iovs.05-0156. [DOI] [PubMed] [Google Scholar]
- Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 2008;3:e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali L, Raguz M, Camenisch TG, Hyde JS, Subczynski WK. Spin-label saturation-recovery EPR at W-band: applications to eye lens lipid membranes. J Magn Reson. 2011a;212:86–94. doi: 10.1016/j.jmr.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali L, Raguz M, O'Brien WJ, Subczynski WK. Properties of fiber cell plasma membranes isolated from the cortex and nucleus of the porcine eye lens. Exp Eye Res. 2012;97:117–129. doi: 10.1016/j.exer.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali L, Raguz M, O'Brien WJ, Subczynski WK. Properties of membranes derived from the total lipids extracted from the human lens cortex and nucleus. Biochim Biophys Acta. 2013;1828:1432–1440. doi: 10.1016/j.bbamem.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali L, Raguz M, O'Brien WJ, Subczynski WK. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61-70-year-old human donors. Eur Biiophys J. 2015;44:91–102. doi: 10.1007/s00249-014-1004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainali L, Raguz M, Subczynski WK. Phase-separation and domain-formation in cholesterol-sphingomyelin mixture: pulse-EPR oxygen probing. Biophys J. 2011b;101:837–846. doi: 10.1016/j.bpj.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CA, Delamere NA. The lens. In: Hart WM Jr, editor. Physiology of the eye. 9th. Mosby-Year Book; St Louis: 1992. pp. 348–390. [Google Scholar]
- Rafferty NS. Lens morphology. In: Maisel H, editor. The ocular lens:structure, function and pathology. Marcel Dekker; New York: 1985. pp. 1–60. [Google Scholar]
- Raguz M, Mainali L, O'Brien WJ, Subczynski WK. Lipid-protein interactions in plasma membranes of fiber cells isolated from the human eye lens. Exp Eye Res. 2014;120:138–151. doi: 10.1016/j.exer.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguz M, Mainali L, O'Brien WJ, Subczynski WK. Lipid domains in intact fiber-cell plasma membranes isolated from cortical and nuclear regions of human eye lenses of donors from different age groups. Exp Eye Res. 2015;132:78–90. doi: 10.1016/j.exer.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguz M, Mainali L, Widomska J, Subczynski WK. The immiscible cholesterol bilayer domain exists as an integral part of phospholipid bilayer membranes. Biochim Biophys Acta. 2011a;1808:1072–1080. doi: 10.1016/j.bbamem.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguz M, Mainali L, Widomska J, Subczynski WK. Using spin-label electron paramagnetic resonance (EPR) to discriminate and characterize the cholesterol bilayer domain. Chem Phys Lipids. 2011b;164:819–829. doi: 10.1016/j.chemphyslip.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguz M, Widomska J, Dillon J, Gaillard ER, Subczynski WK. Physical properties of the lipid bilayer membrane made of cortical and nuclear bovine lens lipids: EPR spin-labeling studies. Biochim Biophys Acta. 2009;1788:2380–2388. doi: 10.1016/j.bbamem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujoi M, Jin J, Borchman D, Tang D, Yappert MC. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci. 2003;44:1634–1642. doi: 10.1167/iovs.02-0786. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Felix CC, Klug CS, Hyde JS. Concentration by centrifugation for gas exchange EPR oximetry measurements with loop-gap resonators. J Magn Reson. 2005;176:244–248. doi: 10.1016/j.jmr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Subczynski WK, Raguz M, Widomska J, Mainali L, Konovalov A. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J Membr Biol. 2012;245:51–68. doi: 10.1007/s00232-011-9412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GB, Houslay MD, Metcalfe JC, Birdsall NJ. Cholesterol is excluded from the phospholipid annulus surrounding an active calcium transport protein. Nature. 1975;255:684–687. doi: 10.1038/255684a0. [DOI] [PubMed] [Google Scholar]
- Wride MA. Lens fibre cell differentiation and organelle loss: many paths lead to clarity. Philos Trans Royal Soc Lond Ser B Biol Sci. 2011;366:1219–1233. doi: 10.1098/rstb.2010.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampighi GA, Eskandari S, Hall JE, Zampighi L, Kreman M. Micro-domains of AQP0 in lens equatorial fibers. Exp Eye Res. 2002;75:505–519. doi: 10.1006/exer.2002.2041. [DOI] [PubMed] [Google Scholar]


