Abstract
Background
Adult studies suggest conscious sedation increases gastroesophageal reflux (GER), but the role of anesthesia on GER in children is unclear. Our aim was to study the anesthesia effects on GER and pH study interpretation in children.
Methods
Children undergoing BRAVO wireless pH capsule placement under anesthesia and study duration >36 hours were included. We evaluated the pH parameters (number of reflux episodes >5 minutes, duration of longest reflux episode, time pH <4 and fraction time pH <4) at 1, 2, 6-hour and total study duration blocks using 2 cut-off values (5.3% and 6%) for the worst day, average of both days and 1st day alone. We compared time blocks to evaluate the effect of anesthesia on GER and the proportions of studies changing interpretation after excluding the 1st hour and 1st 2-hour blocks to evaluate anesthesia effect on study interpretation.
Results
A total of 150 children were included. We found a significant increase on the pH parameters in the 1st hour compared to subsequent block times suggesting an effect of anesthesia on GER. We found no significant change in the proportion of studies interpreted as normal vs. abnormal, however, excluding the initial 2 hours of the study would change the study interpretation from abnormal to normal in up to 5% of patients.
Conclusions
We found an effect of anesthesia increasing the GER parameters mainly in the 1st hour and up to the first 6 hours of the study that may result in a change in the study interpretation.
Keywords: BRAVO wireless pH, Anesthesia, Gastroesophageal Reflux, Children
BACKGROUND
The ability to measure pH in the esophagus and send the information wirelessly to an external receiver makes the BRAVO wireless pH an attractive tool in children. The safety and tolerability of the device has been established in adults (1) and children (2–5) and has also proved to be better tolerated than the conventional pH catheter in children.(3) Little information is available about the potential effect of anesthesia on pH monitoring in children. The data on effect of intravenous conscious sedation on GER in adults is controversial, while large retrospective studies have demonstrated low day to day variability and high reproducibility of the test with no significant difference in reflux parameters and proportions of abnormal studies between studies performed with and without sedation(6–8) other smaller series have reported a potential effect from intravenous conscious sedation resulting on more GER on day 1 compared to day 2.(9) A retrospective study in children reported similar day to day reproducibility but conflicting GER results between days 1 and 2 in 10/44 patients also suggesting a potential effect from anesthesia.(2) Data on the effect of anesthesia on acid reflux is lacking. We aimed to evaluate the effect of anesthesia on GER and the potential implications of such effect on the interpretation of the pH study in children.
METHODS
Institutional IRB approval was obtained to conduct a retrospective review of records of pediatric patients undergoing BRAVO wireless pH study from June 2004 to June 2010 at 2 pediatric tertiary care centers.
Patient population
Children undergoing a BRAVO wireless pH study to evaluate for presence of prolonged esophageal acid exposure as the potential cause for their symptoms. All subjects stopped acid blockers at least 48 hours before the study (48 hours for H2 blockers and 4–5 days for proton pump inhibitors). Patients were identified by searching a hospital-based procedure database and physician records. Only patients with at least 36 hours of monitoring were included.
BRAVO pH capsule study
After an overnight fast an endoscopy under general anesthesia (propofol) was performed in all patients to evaluate for presence of esophagitis and to locate the gastroesophageal junction (GEJ) to aid in the proper location of the BRAVO capsule (3–5cm above the GEJ depending on the age). Capsule was attached as per BRAVO wireless capsule attachment protocol previously published.(1) Successful capsule attachment was documented endoscopically in all patients. All studies were planned for 48 hours and a diary recording meals, position (supine or upright) and symptoms was obtained from all patients. All patients were discharged after the capsule placement and returned the recording device and diary 2 days later.
Data analysis
The pH data was uploaded to a computer and analyzed to obtain the following pH parameters: number of reflux episodes, number of long (> 5 minutes) reflux episodes, duration of longest reflux episode (minutes), time pH was <4 (minutes) and fraction time pH was <4 (%). These parameters were measured for day 1, day 2, the average of both days and also for the following blocks of the study: 1st hour, 1st 2 hours, and 6 hour blocks. We also evaluated those measurements in both supine and upright positions. Prolonged esophageal acid exposure (abnormal BRAVO pH study) was defined as a fraction of time pH <4 higher than 6% (FT6%).(10) We also did a secondary analysis using a cut-off of 5.3% as it has been reported as the cut-off value used in BRAVO pH studies in adults(1) and children.(11) Our aims were to evaluate the potential effect of anesthesia on gastroesophageal acid reflux (GER) and on the interpretation of the BRAVO wireless pH study. To evaluate the effect of anesthesia on GER we compared the pH parameters among the different time blocks. For the effect of anesthesia on the overall interpretation of the study we compared the proportion of patients with normal and abnormal pH studies using both cut-off values for the total duration of the study, using the first day alone (FDA), the average of both days (ABD) and the worst day (WS) and we also performed the same analysis excluding the first hour of recording and the first 2 hours. We performed the analysis using the data on the first day to compare the advantage of the 48h BRAVO pH study compared to the conventional 24h pH probe study.
Statistical analysis
Continuous variables are expressed as median and range and were compared using non-parametric testing. Proportions were compared using chi square or Fisher’s exact test.
RESULTS
A total of 190 children underwent a BRAVO wireless pH monitoring and 150 studies were included in this study based on study duration >36 hours. The median age was 11y (range 4–18y) and 77 (51.3%) were female. Only one patient complained of short-lived chest pain after BRAVO capsule attachment, no other complications were reported and no patient required BRAVO capsule removal. The median fraction of time pH<4 (reflux index) of all patients for both days was 3.25 with a range of 0–78.7, for day 1 was 3.20 with a range of 0–78 and for day 2 was 2.7 with a range of 0–79.5.
Effect of anesthesia on gastroesophageal reflux
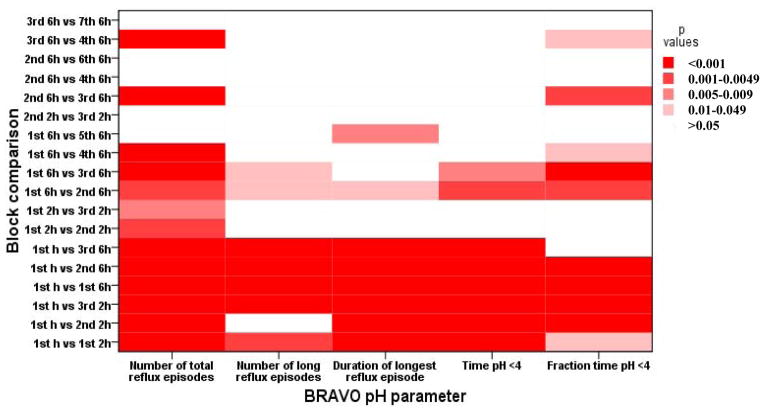
We found an increased number of acid reflux episodes during the first hour compared to subsequent hours and also during the first 6 hours compared to the subsequent 6 h blocks. There was also a significant difference in the number of prolonged reflux episodes only between the 1st 6h block compared to the subsequent 6h blocks on the first day. We also found a significant difference on the fraction of time pH was <4 in the first 6h compared to the subsequent 6h blocks on the first day. (See Figure 1)
Figure 1.
Effect of anesthesia on gastroesophageal reflux evaluated by BRAVO wireless pH study. Comparison between different blocks.
Effect of anesthesia on BRAVO pH study interpretation
Table 1 shows the effect on removing the initial hours after anesthesia on BRAVO interpretation. Taking the population as a whole we found no significant difference on the proportion of normal interpretation between those with the total duration of the study compared to those after excluding the 1st hour or the 1st 2 hours for both cut-off values for the 3 group comparisons (WD, ABD and FDA). However as can be noted below there was a small proportion of individual patients in which the final interpretation changed.
Table 1.
Changes in BRAVO interpretation if the 1st and 2nd hours of the study are removed
| Worst day | Average of both days | First day alone | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6% | 5.3% | 6% | 5.3% | 6% | 5.3% | |||||||||||||
| Study interpretation | T | T-1 | T-2 | T | T-1 | T-2 | T | T-1 | T-2 | T | T-1 | T-2 | T | T-1 | T-2 | T | T-1 | T-2 |
| Normal (n) | 94 | 96 | 97 | 90 | 90 | 90 | 111 | 112 | 112 | 108 | 107 | 107 | 107 | 109 | 110 | 104 | 104 | 105 |
| Abnormal (n) | 56 | 54 | 53 | 60 | 60 | 60 | 39 | 38 | 38 | 42 | 43 | 48 | 43 | 41 | 41 | 46 | 46 | 45 |
| Change (n) | 2 | 3 | 0 | 0 | 1 | 1 | 3 | 5 | 2 | 3 | 0 | 1 | ||||||
| p value | 0.6 | 0.6 | 0.8 | 0.9 | 0.8 | 0.8 | 0.9 | 0.5 | 0.7 | 0.9 | 0.6 | 0.7 | ||||||
T= total time, T-1= total time minus 1st hour, T-2= total time minus 1st 2 hours.
Even though the median fraction of time (%) of pH <4 during the first hour of the study was 0 (range 0–69), in 25 patients it was >5.3%, in 18 of those 25 it was >10% and in 10 it was >25%. When evaluating the first 2 hours of the study the same proportion rose to 35 patients although the median fraction pH <4 was similar at 0.95 (range 0–34) and 28 of those were >10%.
Worst day
FT6%
According to the pediatric guidelines, using the cut-off value of fraction of pH <4 higher than 6% on the worst day we noticed that 56 studies were classified as abnormal, and when the 1st hour is excluded that resulted in 2 studies changing interpretation from abnormal to normal and after excluding the 1st 2 hours a total of 3 studies changed to normal. All of those had normal esophageal histology. Of those 56 abnormal studies, the interpretation was based on day 1 being abnormal in 33, and in 23 on an abnormal day 2 only. Using a conventional 24h pH probe study we would have missed those 23 patients that had an abnormal study interpretation based solely on the abnormal fraction of pH <4 on day 2. On the other hand, of those 33 patients with abnormal study on day 1 a total of 10 also had an abnormal study on day 2, therefore only 10/33 would have been considered abnormal if we exclude day one from the analysis, independently from anesthesia; none of those 10 changed interpretation excluding the 1st hour or 1st 2 hours. From those 23 with abnormal day 1 and normal day 2, a total of 2 would have been diagnosed as having normal esophageal acid exposure if we exclude the 1st hour and 3 if we exclude the 1st 2 hours of the study. This means that in 2/150 (1.3%) and 3/150 (2%) studies the interpretation changed from abnormal to normal after excluding the 1st hour and the 1st 2 hours, respectively. Considering only those with abnormal esophageal acid exposure, those proportions would be 2/60 (3.3%) and 3/60 (5%), respectively. There is no significant difference between the proportions with normal and abnormal studies excluding the 1st hour or 1st 2 hours. (See Table 1).
FT5.3%
A total of 60 studies were interpreted as abnormal, excluding the 1st hour and 1st 2 hours does not change the study interpretation. Of those 60, the interpretation as abnormal on 35 (23%) studies was based on day 1 and on day 2 on the remainder 25 (16%). Of those 35 with abnormal study on day 1, only 13 had an abnormal day 2. Therefore only 13/35 would have been considered abnormal if we exclude day one from the analysis, independently of the effect of anesthesia. On the other hand from those 22 studies with an abnormal day 1 and normal day 2, a total of 3 patients (13 %) would have been diagnosed as having normal acid exposure if we exclude the first 2 hours of the study. This means that in 3/150 (2 %) patients the results of the BRAVO would have changed from abnormal to normal. If we take only those with abnormal acid exposure the proportion would have been 3/60 (5%).
Average of both days
FT6%
A total of 111 studies were interpreted as normal, after excluding the 1st hour one study (0.9%) changed from abnormal to normal and after excluding the 1st 2 hours the same study changed from abnormal to normal (0.9%), that patient had normal esophageal histology.
FT5.3%
A total of 108 studies were interpreted as normal and after excluding the 1st hour 2 studies (1.9%) changed from normal to abnormal (normal esophageal histology) and another changed from abnormal to normal (0.9%) (No histology obtained). After excluding the 1st 2 hours a total of 3 studies (2.8%) changed from normal to abnormal (normal esophageal histology) and 2 others (1.9%) changed from abnormal to normal (both with normal esophageal histology).
First day alone
FT6%
A total of 43 studies were interpreted as abnormal and after excluding the 1st hour 2 studies changed from abnormal to normal (4.7%) (One with normal histology and the other had no histology available) and after excluding the 1st 2 hours a total of 3 studies (7%) changed from abnormal to normal (2 with no histology available and one with normal histology).
FT5.3%
A total of 46 studies were interpreted as abnormal with no change after excluding the 1st hour and one study changed from abnormal to normal (2%) (No histology available) after excluding the 1st 2 hours.
DISCUSSION
BRAVO wireless pH is a safe and well-tolerated test to evaluate GER in children as young as 4 years of age. We have demonstrated an important effect of anesthesia on the occurrence of GER in the first 2 hours with a potential effect on the diagnosis of GER (BRAVO wireless pH study interpretation), particularly in those patients with abnormal acid exposure.
The day-to-day variability of the GER on adults has been previously reported (12, 13), however data in children is controversial with some showing no significant variability on consecutive days (14, 15) and others showing important variation.(3, 16) That variability has been attributed to the potential effect of anesthesia or intravenous conscious sedation on GER but the data available is controversial. Regarding sedation, some report a minimal effect on GER(6) while others report more GER on day 1 of the study(9). However, nowadays most of the endoscopic procedures in children are being performed under anesthesia and the effect it has on GER is not known.
In a study on 66 children undergoing BRAVO wireless pH and conventional pH probes studies simultaneously placed under anesthesia, Croffie et. al. reported no difference on GER parameters on day 1 measured with conventional pH probe studies and with the wireless BRAVO pH capsule, but they found a difference between the BRAVO and the conventional l pH probe study on the day 2 and both days combined with only a single patient showing discordant results with normal GER on day 1 and abnormal on day 2, suggesting that anesthesia may play a role on increasing GER on day 1.(3) Hochman et. al. reported no significant variability on the test in 44 children but also reported conflicting results between day 1 and 2 in 10 out of 44 children(2), also suggesting a potential effect of anesthesia on GER. The mechanism of such increase in GER is unknown. A decrease in the lower esophageal sphincter pressure resulting in a hypothetical increase in GER has not been proved for conscious sedation. In children the use of sedatives like midazolam or chloral hydrate have no significant effect on the lower esophageal resting pressure.(17, 18) Also, the effect of anesthetics on the lower esophageal sphincter is controversial with some reporting a decrease on the LES resting pressure during anesthesia(19) while others reporting no significant effect(20).
Our findings demonstrated that the increase in GER occurs mainly in the first hour of the study and up to the first 6 hours after the capsule placement, as has been previously described in adult studies(21). However this increment of GER had a minimal effect on the overall interpretation of the BRAVO study. The increase in diagnostic yield of GER and symptom correlation by adding the second day has been well demonstrated in adult studies, (22, 23) however the potential effect of anesthesia on the diagnostic yield has not been thoroughly evaluated. We observed that only 1.3% of children with abnormal acid exposure using 6% cut off on the worse day would have been reclassified as being normal if we exclude the 1st hour of the study and 2% if we exclude the 1st 2 hours. However if only those patients with abnormal acid exposure are counted, excluding the first 2 hours would have changed the diagnosis from abnormal to normal acid exposure in 5%. Therefore it can be argued that in order to be more accurate on the diagnosis of GERD we may need to exclude the first hour or two of the study from the analysis. The effect of removing the 1st hour or 1st 2 hours was higher (although not statistically significant) on ABD and FDA compared to when WD is used. This can be explained because using WD the study was abnormal on the 1st day in 33/56 (59%), while in the other 23 (41%) the WD was the second day. Therefore removing the 1st hour or 1st 2 hours would not affect it the WD interpretation. Given our results we suggest that when an abnormal exposure of acid is found based on the first day of the BRAVO study, a careful analysis of the amount of reflux in the first 2 hours needs to be undertaken, or the first 2 hours need to be excluded, before a diagnosis of pathologic reflux is made. Prospective studies are needed to evaluate if removing the first or the first and second hour after anesthesia will have an impact on patient treatment and outcome. Furthermore it is also not clear what is the effect of anesthesia on the interpretation of esophageal impedance studies, as it is likely that also the number of non-acid reflux events are increased. Future studies to address this question will be needed.
This study has important limitations, mainly the fact that it is retrospective, the lack of pediatric normative data and we do not have any clinical outcome to evaluate the potential impact of our findings on clinical practice. However all pH measures are objective, were done blindly and same criteria was applied to all data analysis so they reflect changes that occurred after the anesthesia.
The BRAVO study was safe. Only one patient complained of discomfort or increased symptoms after capsule placement but overall no side effects were reported from the study and no patient required any intervention associated to the placement of the BRAVO capsule and the performance of the study.
In conclusion we have shown that the presence of GER seems to be increased in the first 2 hours after BRAVO placement, changing the diagnosis in a small percent of patients, and in up to 5% of patients with abnormal acid exposure. We therefore suggest that before a diagnosis of pathologic reflux is made when the increased acid exposure is based on the first day of the
BRAVO study a careful analysis of the first 2 hours after the anesthesia needs to be done. Future studies will be needed to decide if excluding the first 2 hours after anesthesia has a major impact on the long-term outcome of the patients.
Key Message.
Anesthesia increases gastroesophageal reflux for up to 6 hours but it does not result in a significant change in the study interpretation but after excluding first 2 hours of pH study it does result in a change of interpretation in a small proportion of patients.
Acknowledgments
This work was supported by NIH grant K24DK082792A (SN)
Footnotes
Disclosures
Conception and design of the study: LR and SN Data collection: LR, AMF, AS and LR Analysis and data interpretation: LR and SN
Drafting of the manuscript: LR and SN
Critical Revision and approval of the Manuscript: LR, AMF, AS, LR and SN
References
- 1.Pandolfino JE, Richter JE, Ours T, Guardino JM, Chapman J, Kahrilas PJ. Ambulatory esophageal pH monitoring using a wireless system. Am J Gastroenterol. 2003;98(4):740–9. doi: 10.1111/j.1572-0241.2003.07398.x. [DOI] [PubMed] [Google Scholar]
- 2.Hochman JA, Favaloro-Sabatier J. Tolerance and reliability of wireless pH monitoring in children. J Pediatr Gastroenterol Nutr. 2005;41(4):411–5. doi: 10.1097/01.mpg.0000177312.81071.c8. [DOI] [PubMed] [Google Scholar]
- 3.Croffie JM, Fitzgerald JF, Molleston JP, Gupta SK, Corkins MR, Pfefferkorn MD, et al. Accuracy and tolerability of the Bravo catheter-free pH capsule in patients between the ages of 4 and 18 years. J Pediatr Gastroenterol Nutr. 2007;45(5):559–63. doi: 10.1097/MPG.0b013e3180dc9349. [DOI] [PubMed] [Google Scholar]
- 4.Lacy BE, Edwards S, Paquette L, Weiss J, Kelley ML, Jr, Ornvold K. Tolerability and clinical utility of the Bravo pH capsule in children. J Clin Gastroenterol. 2009;43(6):514–9. doi: 10.1097/MCG.0b013e31818fba38. [DOI] [PubMed] [Google Scholar]
- 5.Gunnarsdottir A, Stenstrom P, Arnbjornsson E. Wireless esophageal pH monitoring in children. J Laparoendosc Adv Surg Tech A. 2008;18(3):443–7. doi: 10.1089/lap.2007.0191. [DOI] [PubMed] [Google Scholar]
- 6.Belafsky PC, Godin DA, Garcia JC, Rahim N. Comparison of data obtained from sedated versus unsedated wireless telemetry capsule placement: does sedation affect the results of ambulatory 48-hour pH testing? Laryngoscope. 2005;115(6):1109–13. doi: 10.1097/01.MLG.0000163757.77580.D5. [DOI] [PubMed] [Google Scholar]
- 7.Nusrat S, Roy PM, Bielefeldt K. Wireless ambulatory pH studies: manometric or endoscopic guidance? Dis Esophagus. 2011 doi: 10.1111/j.1442-2050.2011.01218.x. [DOI] [PubMed] [Google Scholar]
- 8.Chander B, Hanley-Williams N, Deng Y, Sheth A. 24 Versus 48-hour Bravo pH Monitoring. J Clin Gastroenterol. 2011 doi: 10.1097/MCG.0b013e31822f3c4f. [DOI] [PubMed] [Google Scholar]
- 9.Bechtold ML, Holly JS, Thaler K, Marshall JB. Bravo (wireless) ambulatory esophageal pH monitoring: how do day 1 and day 2 results compare? World J Gastroenterol. 2007;13(30):4091–5. doi: 10.3748/wjg.v13.i30.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) J Pediatr Gastroenterol Nutr. 2009;49(4):498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 11.Carroccio A, Cavataio F, Acierno E, Montalto G, Lorello D, Tumminello M, et al. Use of 24-hour oesophageal pH-metry for the detection of gastro-oesophageal reflux in infants: what is the ideal score and the optimal threshold? A receiver-operating-characteristic analysis. Ital J Gastroenterol Hepatol. 1997;29(4):297–302. [PubMed] [Google Scholar]
- 12.Ahlawat SK, Novak DJ, Williams DC, Maher KA, Barton F, Benjamin SB. Day-to-day variability in acid reflux patterns using the BRAVO pH monitoring system. J Clin Gastroenterol. 2006;40(1):20–4. doi: 10.1097/01.mcg.0000190753.25750.0e. [DOI] [PubMed] [Google Scholar]
- 13.Tseng D, Rizvi AZ, Fennerty MB, Jobe BA, Diggs BS, Sheppard BC, et al. Forty-eight-hour pH monitoring increases sensitivity in detecting abnormal esophageal acid exposure. J Gastrointest Surg. 2005;9(8):1043–51. doi: 10.1016/j.gassur.2005.07.011. discussion 51–2. [DOI] [PubMed] [Google Scholar]
- 14.Vandenplas Y, Helven R, Goyvaerts H, Sacre L. Reproducibility of continuous 24 hour oesophageal pH monitoring in infants and children. Gut. 1990;31(4):374–7. doi: 10.1136/gut.31.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunnarsdottir A, Stenstrom P, Arnbjornsson E. 48-hour wireless oesophageal pH-monitoring in children: are two days better than one? Eur J Pediatr Surg. 2007;17(6):378–81. doi: 10.1055/s-2007-989222. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan L, Wyllie R, Oliva L, Balsells F, Steffen R, Kay M. Reproducibility of 24-hour intraesophageal pH monitoring in pediatric patients. Pediatrics. 1998;101(2):260–3. doi: 10.1542/peds.101.2.260. [DOI] [PubMed] [Google Scholar]
- 17.Fung KP, Math MV, Ho CO, Yap KM. Midazolam as a sedative in esophageal manometry: a study of the effect on esophageal motility. J Pediatr Gastroenterol Nutr. 1992;15(1):85–8. doi: 10.1097/00005176-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Vanderhoof JA, Rapoport PJ, Paxson CL., Jr Manometric diagnosis of lower esophageal sphincter incompetence in infants: use of a small, single-lumen perfused catheter. Pediatrics. 1978;62(5):805–8. [PubMed] [Google Scholar]
- 19.Thorn K, Thorn SE, Wattwil M. The effects on the lower esophageal sphincter of sevoflurane induction and increased intra-abdominal pressure during laparoscopy. Acta Anaesthesiol Scand. 2006;50(8):978–81. doi: 10.1111/j.1399-6576.2006.01069.x. [DOI] [PubMed] [Google Scholar]
- 20.Thorn K, Thorn SE, Wattwil M. The effects of cricoid pressure, remifentanil, and propofol on esophageal motility and the lower esophageal sphincter. Anesth Analg. 2005;100(4):1200–3. doi: 10.1213/01.ANE.0000147508.31879.38. [DOI] [PubMed] [Google Scholar]
- 21.Bhat YM, McGrath KM, Bielefeldt K. Wireless esophageal pH monitoring: new technique means new questions. J Clin Gastroenterol. 2006;40(2):116–21. doi: 10.1097/01.mcg.0000196188.57543.75. [DOI] [PubMed] [Google Scholar]
- 22.Prakash C, Clouse RE. Value of extended recording time with wireless pH monitoring in evaluating gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2005;3(4):329–34. doi: 10.1016/s1542-3565(05)00021-2. [DOI] [PubMed] [Google Scholar]
- 23.Domingues GR, Moraes-Filho JP, Domingues AG. Impact of prolonged 48-h wireless capsule esophageal pH monitoring on diagnosis of gastroesophageal reflux disease and evaluation of the relationship between symptoms and reflux episodes. Arq Gastroenterol. 2011;48(1):24–9. doi: 10.1590/s0004-28032011000100006. [DOI] [PubMed] [Google Scholar]