Abstract
Background
Many alcoholics display moderate to severe cognitive dysfunction accompanied by brain pathology. A factor confounded with prolonged heavy alcohol consumption is poor nutrition and many alcoholics are thiamine deficient. Thus, thiamine deficiency (TD) has emerged as a key factor underlying alcohol–related brain damage (ARBD). TD in humans can lead to Wernicke Encephalitis that can progress into Wernicke–Korsakoff Syndrome and these disorders have a high prevalence among alcoholics. Animal models are critical for determining the exact contributions of ethanol- and TD-induced neurotoxicity, as well as the interactions of those factors to brain and cognitive dysfunction.
Methods
Adult rats were randomly assigned to one of six treatment conditions: Chronic ethanol treatment (CET) where rats consumed a 20% v/v solution of ethanol over 6 months; Severe pyrithiamine-induced TD (PTD-MAS); Moderate PTD (PTD-EAS); Moderate PTD followed by CET (PTD-CET); Moderate PTD during CET (CET-PTD); Pair-fed control (PF). After recovery from treatment, all rats were tested on spontaneous alternation and attentional set-shifting. After behavioral testing, brains were harvested for determination of mature brain-derived neurotrophic factor (BDNF) and thalamic pathology.
Results
Moderate TD combined with CET, regardless of treatment order, produced significant impairments in spatial memory, cognitive flexibility and reductions in brain plasticity as measured by BDNF levels in the frontal cortex and hippocampus. These alterations are greater than those seen in moderate TD alone and the synergistic effects of moderate TD with CET leads to a unique cognitive profile. However, CET did not exacerbate thalamic pathology seen after moderate TD.
Conclusions
These data support the emerging theory that subclinical TD during chronic heavy alcohol consumption is critical for the development of significant cognitive impairment associated with ARBD.
Keywords: Alcoholism, thiamine deficiency, hippocampus, prefrontal cortex, thalamus, brain-derived neurotropic factor, Wernicke-Korsakoff Syndrome, alcohol-related brain damage, attentional set-shifting, spontaneous alternation
Background
Over 50% of detoxified alcoholics display some degree of cognitive impairment and have significant brain damage (Dufour, 1993, Zahr et al., 2011). However, the relative contributions of different etiological factors to the development and production of alcohol-related brain damage (ARBD) and its accompanying cognitive impairment, remain elusive. Chronic alcoholism is commonly associated with malnourishment due to poor diet and decreased absorption/utilization of nutrients, in particular thiamine (vitamin B1). It has been estimated that approximately 80% of alcoholics show some degree of thiamine deficiency (TD(Galvin et al., 2010), which if left untreated can lead to Wernicke Encephalitis and ultimately progress into Wernicke–Korsakoff syndrome (WKS). Over 75% of cases of TD go undiagnosed, particularly in alcoholics (Zahr et al., 2011). Furthermore, it has been estimated that approximately 13% of alcoholics have brain abnormalities that reflect TD (Harper et al., 1986).
When uncomplicated alcoholics (patients with no comorbid diagnosis of liver disease, brain trauma or TD) were sorted using the more expansive Caine et al (1997) criteria for TD, alcoholics with one or two signs of TD displayed significant cognitive, memory and motor impairment (Pitel et al., 2011). In contrast, alcoholic patients that had no signs of TD displayed no cognitive impairment. Furthermore, the percent (16%) of the alcoholic sample that displayed 1 or 2 signs of TD closely matched the prevalence rates of WKS described post mortem (Harper et al., 1986). Thus, undetected TD in alcoholics contributes significantly to ARBD and cognitive/memory dysfunction.
Animal models of ARBD display similar brain and cognitive abnormalities to what is seen in human alcoholics. It is well known that models of TD, in particular the pyrithiamine-induced TD model (PTD), produce severe, persistent impairments in spatial and non-spatial working memory (Savage et al., 2012). These cognitive deficits have been attributed to the extensive cell loss that occurs within the diencephalon, in particular the lesions that occur within the thalamus and mammillary bodies (Mair, 1994). This diencephalic damage leads to circuit level dysfunction within the hippocampus as well as the frontal cortex (Anzalone et al., 2010). These structural and functional lesions induced by PTD are coupled with a loss of cholinergic neurons in the medial septum/diagonal band (≈30%) that together produce a severe amnestic state (Roland and Savage, 2009).
Although chronic ethanol treatment (CET) in animals has been noted to produce learning and memory dysfunction, the degree and persistence of the impairment varies (see Vetreno et al., 2011). Factors that influence both neuropathology and cognitive impairment depend on dose and duration of ethanol exposure. In rodents and monkeys with prolonged ethanol consumption there is a reduction of cholinergic forebrain neurons (Aloe and Tirassa, 1992, Arendt et al., 1989, Cadete-Leite et al., 2003, Lukoyanov et al., 2003, Savage et al., 2000) as well as morphological changes and neuronal loss within the hippocampus and cortex (Kroenke et al., 2014, Richardson et al., 2009, Walker et al., 1980). Altered neurotrophin levels (brain-derived neurotrophic factor [BDNF]; nerve growth factor [NGF]) have been detected after CET, but the outcomes have been varied: increase, decrease or no change (Davis, 2008). The diverse results in neurotrophin measures are likely due to homeostatic changes that include windows of compensatory synaptic plasticity, which occur during different time points of ethanol toxicity (during intoxication, withdrawal, extended recovery; see Crews and Nixon, 2009).
Although many studies have used animal models to separately determine the neurological consequences of CET and TD, few have examined their synergistic interactions and an array of neural and cognitive effects have been observed that are dependent on treatment parameters (Ba et al., 1999, Ciccia and Langlais, 2000, Homewood et al., 1997, Pires et al., 2001). The most common finding is that combined TD with CET does lead to neural dysfunction, but that it is not more destructive than TD alone on behavioral, biochemical or structural measures. However, there are data suggesting there may be an interactive effect of combining TD and CET. Rats from the Indiana alcohol-preferring line exposed to a full year of heavy alcohol consumption followed by a mild/moderate PTD displayed synergistic white matter pathology (He et al., 2007). Specifically, it was found that rats exposed to CET with mild/moderate TD had a higher percentage of smaller fibers and greater myelin thinning within the corpus callosum relative to rats exposed to CET or TD alone. Second, using a binge model of ethanol toxicity, a subset of rats were also injected with pyrithiamine for 5 or 10 days to produce mild PTD (Qin and Crews, 2014). After 5 days of PTD treatment, the combined ethanol and PTD rats displayed greater microglial and inflammatory gene induction in the thalamus, but as PTD treatment progressed into day 10 there was an equal induction of proinflammatory markers in both the PTD and the combined ethanol and PTD groups. Thus, there may indeed be synergistic neuropathological interactions between ethanol exposure and TD.
However, there are several critical questions about the synergistic interplay between CET and TD that remain unanswered. To date, no studies have reported whether behaviors that are dependent on specific neural circuits are more sensitive to the interactions between TD and ethanol toxicity. Second, the timing of the TD episode in relation to the chronic ethanol epoch has not been investigated. Furthermore, human data suggest that shrinkage of the thalamus is a consequence of chronic heavy alcohol consumption as well as TD (Pitel et al, 2014), but it is unknown what causes this pathology, ethanol toxicity, TD or an interaction between subclinical TD and CET. To shed light on this issue, we assessed lesion size within the thalamus as a function of TD, CET as well as the interaction. We also looked in regions beyond the thalamus that are common sites of ARBD, the hippocampus and frontal cortex, to determine if these regions display altered plasticity, as assessed by mature BDNF, after TD and CET. To summarize, the current study was designed to answer essential questions about the interactions between TD and CET on behavioral assays as well as thalamic integrity and hippocampal and frontal cortical plasticity.
Methods
Subjects
Subjects were male Sprague Dawley rats (12–14 weeks-old; Harlan, Frederick, MD) pair or triad housed. After 2-weeks of acclimation to the Binghamton University vivarium, rats were randomly assigned to treatment groups. For the cohort of rats (n=48) that were behaviorally tested and had brain analyses there were six treatment conditions (group n=8; see Figure 1). During treatment, 1 rat died (unknown origin) in the PTD-CET group, reducing the number of rats in that group to 7. Behavioral and neurotrophin assays had a total of 8 or 7 rats per group. A second cohort of rats (n=24) was used to determine the blood level of active thiamine during either CET (n=6) or PTD treatment (n=9), relative to controls (n=9). All experiments were conducted according to the National Institute of Health: Guide for the Care and Use of Laboratory Animals (9th ed., National Academies Press, 2014). All experimental protocols were approved by the Institutional Animal Care and Use Committee at the State University of New York at Binghamton.
Figure 1.
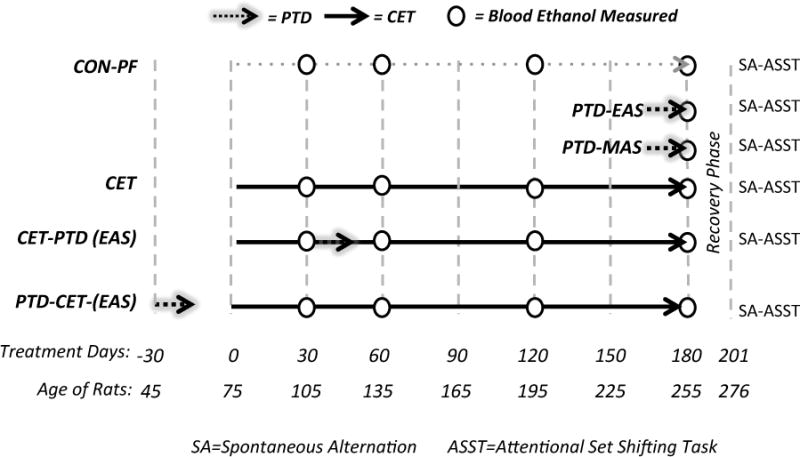
Schematic of the experimental treatment phases and timeline. CON-PF= Control pair fed; PTD-EAS= Moderate pyrithiamine–induced TD; PTD-MAS= Severe pyrithiamine–induced TD; CET = Chronic Ethanol Treatment; CET-PTD= Started on CET and after one-month exposed to moderate PTD-EAS; PTD-CET = treated with the moderate PTD-EAS and after recovery exposed to CET.
Treatments
A. Chronic Ethanol Treatment (CET)
A fading-on procedure was used to gradually expose rats to 20% v/v ethanol with 5 days of exposure to 6, 9 and 12% v/v ethanol in drinking water. After fading-on, rats were given 20% v/v ethanol for 6 months and then faded off ethanol by decreasing the concentration of ethanol in drinking water every five days. Pair-fed (PF) controls were given free access to water.
B. PTD treatment
For TD, PTD-treated rats were allowed free access to thiamine-deficient chow (Harlan Laboratories, Inc. Indianapolis, IN) for the duration of treatment that lasted 13–16 days, dependent on individual progression in TD symptoms (no group difference were observed). In addition, rats were injected daily with pyrithiamine hydrobromide (0.25 mg/kg, i.p., Sigma-Aldrich, St. Louis, MO). PTD rats displayed neurological symptoms of anorexia, ataxia, loss of righting reflexes, and ultimately opisthotonus. Rats were treated to different severity levels of TD, consisting of: (1) an early acute stage (PTD-EAS) in which rats were reversed with a bolus of 0.5 cc of thiamine hydrochloride (100 mg/mL, ip, Sigma-Aldrich) within 1 hour after the appearance of opisthotonus, and (2) a moderate acute stage (PTD-MAS) when rats were reversed 4.25-hrs after the appearance of opisthotonus. All PTD rats were returned to normal rat chow and given a second thiamine reversal injection within 24-hrs. PF rats received the thiamine-deficient chow equivalent to the consumption by PTD-treated rats to mimic the anorexic effects of treatment in conjunction with daily injections of thiamine (0.40 mg/kg, ip.).
C. Combined treatments of CET and PTD
To study the interactive effects of CET and PTD, rats were exposed to the EAS stage of TD and also CET. One group (PTD-CET) was put through TD 4-weeks prior to CET, while the other group (CET-PTD) was put through TD 4-weeks after rats were exposed to 20% v/v ethanol (Figure 1).
D. Measurements of Ethanol Consumption
Ethanol consumption was quantified through biweekly measurements of drinking bottle weights. Blood ethanol concentrations (BEC; Analox Instruments USA, Lunenburg, MA) were determined on months 1, 2, 4 and 6 at 23:00 hr (4-hrs into the dark cycle).
Behavior
A. Spontaneous Alternation
Rats were handled daily (5 min/day) for 5 days prior to behavioral testing with concurrent slight food restriction to reach 90% free-feeding weight. Spontaneous alternation was conducted using a standard plus maze (105 cm × 105 cm) consisting of 4 equidistant arms (55 cm) with clear glass walls (14 cm high) elevated 80 cm above the floor on a table as previously performed in our laboratory. The room contained various spatial cues surrounding the maze. An alternation consisted of the rat entering each of four different arms consecutively in a 4-arm sequence and the percentage of alternation was calculated from the number of alternations divided by the total possible number of alternations.
B. Attentional Set-Shifting Task (ASST)
Rats were maintained at 85% of free-feeding body weight prior to testing on this task. The ASST paradigm consisted of two dimensions, adapted from Birrell and Brown (Birrell and Brown, 2000).
Habituation. After 3 days of being trained to dig for Cheerios (Frosted Cheerios, General Mills, Dresher, PA) in white ceramic bowls (9 cm diameter, 4.5 cm height) in the home cage, each rat was habituated to the training apparatus, which was constructed from white plastic (72 cm × 40 cm × 36 cm) with a divider separating a third of the end portion of the arena as an inter-trial holding location for the rat. After 5 min, the divider was lifted allowing the rat to access the baited bowls. When a rat approached the bowls reliably within 30 sec and ate the rewards on 6-consecutive trials within 2 min, the rat would advance to discrimination training.
Discrimination Training
All rats were trained in the same sequence of discriminations. Rats were trained in discriminations of odor and media to a criterion of 6 consecutive rewarded trials.
Testing
The simple discrimination (SD) used unique exemplars (See Table 1) for either odor or media that were randomly determined and counterbalanced across each treatment condition. In compound discrimination (CD), the other dimension was introduced as a distractor, while the rewarded choice was the same as during SD. Next, the rewarded choice was reversed while all of the exemplars remained constant (R1). The next phase was an intra-dimensional shift (IDS) in which the rewarded dimension was not changed, but all new exemplars were introduced. After IDS, there was a reversal of the newly learned discrimination (R2). Lastly, an extra-dimensional shift (EDS) followed, during which the relevant dimension was reversed and all new exemplars were used. The final reversal (R3) used the same dimension as that used in EDS, but the previous non-rewarded stimulus was now correct.
Table 1.
Example of exemplars used in the attentional set-shifting discrimination problems.
| Phase | Cup A (Odor/Media) | Cup B (Odor/Media) |
|---|---|---|
| Simple | Clove* / Cage Bedding | Nutmeg/ Cage Bedding |
| Compound | Clove*/ Confetti | Nutmeg /Cedar |
| Compound Reversal | Clove/ Confetti | Nutmeg* /Cedar |
| Intra-dimensional Shift | Rosemary* / Colored Beads | Cinnamon / Easter grass |
| Intra-dimensional Shift Reversal | Rosemary / Colored Beads | Cinnamon* / Easter grass |
| Extra-dimensional shift | Thyme / Aqua Gravel* | Citrus / Shredded Paper |
| Extra-dimensional Shift Reversal | Thyme / Aqua Gravel | Citrus / Shredded Paper* |
indicates the rewarded cue.
Note: Half of the rats in each treatment condition (randomly determined) had odor as the first relevant dimension, whereas the other half of subjects had digging media as the first relevant dimension. Original training dimension did not interact with any treatment variables.
BDNF Protein Measurement
Half of the brain was rapidly dissected at 4°C for BDNF ELISA and stored at −80°C. Tissue was weighed and diluted at 10μl/mg of tissue with a lysis buffer containing in mL: 46.5 RIPA buffer, 1.0 phosphatase inhibiter cocktail 1, 1.0 phosphatase inhibiter cocktail 2, 0.5 protease inhibiter cocktail and 1.0 phenylmethanesulfonyl fluoride (all from Sigma). Concentrations of mature BDNF were measured using the BDNF Emax Immuno assay kit (Promega, Madison, WI) according to the manufacturer instructions. Lysates were diluted 1:4 for prefrontal cortex and 1:5 for hippocampus prior to loading on a 96 well plate. A seven point BDNF standard curve between 7.9–500 pg/mL was used to measure BDNF concentrations. Concentrations were then normalized using total protein levels from a bicinchoninic acid assay and data was presented as a percent change from within plate pair-fed controls.
Histology
Half of the brain was immediately placed in 4% paraformaldehyde followed by sucrose cyroprotection and sectioned sagittally at 50 μM using a sliding microtome (SM 2000R; Leica Instruments, Wetzlar, Germany). To visualize thalamic lesions, neuronal nuclei were labeled using a primary antibody for NeuN. Washes were performed between each step (3X, 5-min each in Tris Buffered Saline, TBS). Slices were incubated for 30 min in 3% hydrogen peroxide for 30-min in a solution of 10% normal horse serum and 1% Triton X-100 in TBS and 48-hrs in monoclonal mouse anti-NeuN (1:500, Millipore, Temecula, CA) at 4°C. After rinses, slices were incubated for 2-hrs in biotinylated horse anti-mouse IgG (Vector Lab Inc, Burlington, CA) and then 2-hrs in an avidin-biotin conjugate solution (Vector ABC Elite kit; Vector Laboratories, Inc.). Finally, sections were exposed to 3,3′-diaminobenzidine for 2-min for sufficient chromogenic signal. Sections approximately 0.05, 1.0, and 2.0 mm lateral from the midline (Paxinos and Watson, 2013) were imaged at 4X on a Nikon microscope (Eclipse E400, Japan) using Image-Pro Insight software (MediaCybernetics, Rockville, MD). Extent of thalamic neuronal was assessed using Image J (NIH, freeware).
Blood Thiamine Measurement
Blood was collect via decapitation and/or cardiac puncture at two key points during treatment: (1) During active PTD treatment after loss of righting reflex; (2) During intoxication in the 28th week of CET. A group of PF subjects were used as controls. The level of the active form of thiamine, thiamine diphosphate (TDP), in whole blood was determined using HPLC (Anilytics Inc, Gaithersburg, MD).
Statistical Analyses
Analyses were performed in SPSS (IBM Anaysis, version 22). A repeated measures analysis of variance (ANOVA) was used for ethanol consumption and BECs. For spontaneous alternation, lesion size and TDP levels a one-way ANOVA was used. For ASST, a two-way repeated measures ANOVA was used with testing phase a within subjects factor and treatment group a between subjects factor. A Greenhouse-Geisser correction was used when sphericity was found to be violated by a Mauchly’s test. Relationships between behavioral and biological measures were assessed using simple bivariate Pearson correlations.
Results
1. Ethanol consumption, blood ethanol levels
Rats that had an ethanol solution as their only source of liquid (CET, CET-PTD and PTD-CET) increased their consumption during fading-on as expected (F [1, 96, 33.35] = 20.77, p<0.0001; corrected with Greenhouse-Geisser). However, there was a significant interaction between ethanol concentration and treatment group (F [3, 92, 33.35] = 5.74, p<0.001). The PTD-CET treated rats displayed a delayed increase in ethanol consumption, relative to the other groups during the fade-on period (Figure 2). Once rats reached the maximum 20% v/v ethanol concentration there was a significant interaction between weekly ethanol consumption and treatment (F [54, 540] = 4.27, p<0.0001). This effect was driven by reduced ethanol consumption in the CET-PTD group during PTD treatment followed by an increased consumption immediately following PTD treatment. It is important to note that two weeks after PTD treatment, all groups stabilized ethanol consumption at a level of 11 g/kg per day. Furthermore, BEC levels in CET groups measured at months 1, 2, 4 and 6, were not different as a function of group (F [2,18] = 0.80, p>0.46). As expected, BECs from these groups were significantly increased compared to PF rats given water (F [3,22]=7.72, p<0.001). The BECs of rats exposed to ethanol increased across treatment, but the change across months was not significant (F [2.2, 40.3] = 2.45, p>0.09). An overall BEC of 98.8 mg/dL was achieved at month 6 and this is consistent with binge drinking as defined by the NIAAA.
Figure 2.
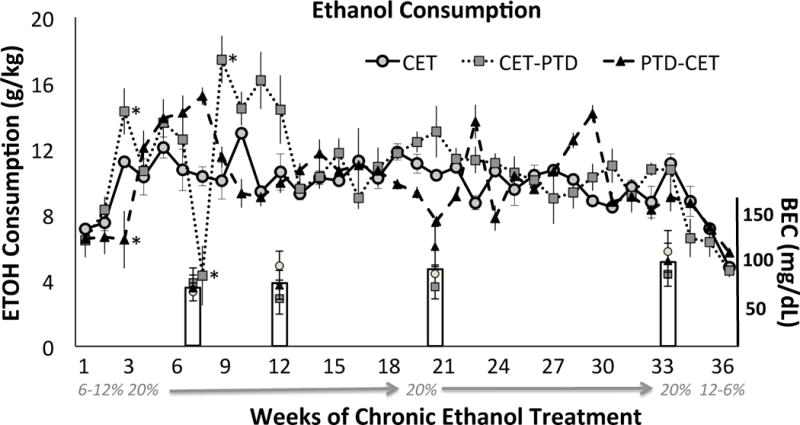
Ethanol consumption (g/kg) across months. Differences were seen during the fading on period as well as during PTD treatment in the CET-TD group, but then consumption stabilized. Inset bar charts reflect Blood Ethanol Concentrations (BECs: mean ± SEM) at months 1, 2, 4 and 6. *p<0.05 relative to other CET treatment conditions.
2. Active Thiamine Levels (TDP)
The treatment groups displayed different levels of TDP in whole blood (Figure 3, F[2,17]=14.56, p<0.001). Both CET and PTD-treated rats showed significantly lower TDP levels than PF controls (CET p<0.005, PTD, p<0.001). PTD treatment further decreased levels of TDP compared to CET (p<0.05).
Figure 3.
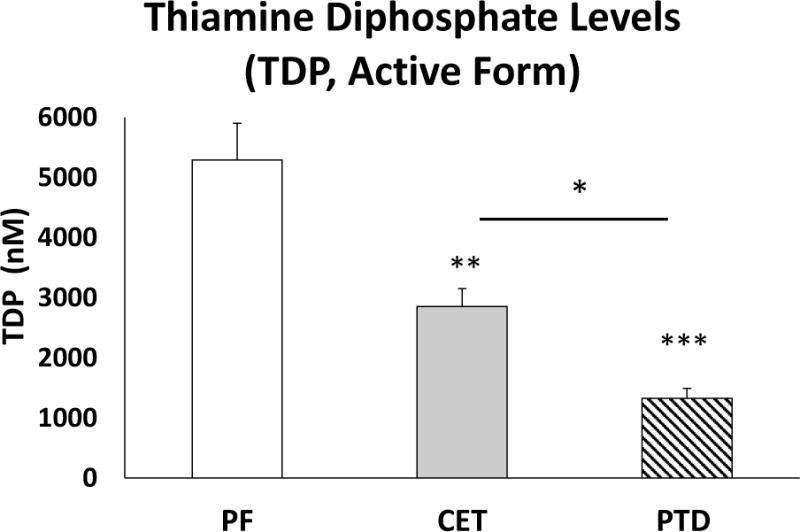
Levels of thiamine diphosphate (TDP) in red blood cells (mean ± SEM). Levels of TDP are decreased in PTD and CET treated rats, compared to PF controls. Furthermore, PTD causes a greater reduction in TDP levels than CET.. * p<0.05, **p<0.005, ***p<0.001
3. Cognitive Dysfunction
A. TD combined with chronic ethanol treatment synergistically enhances deficits in spatial memory
For spontaneous alternation behavior there was a significant main effect of treatment (Figure 4A, F [5, 41] = 7.72; p<0.0001). Rats treated with severe TD (PTD-MAS, p<0.01) and CET alone (CET, p<0.05) alternated significantly less than PF control rats. In contrast, rats treated with mild TD (PTD-EAS) performed just as well as PF treated controls (p>0.50). Interestingly, rats treated with a combination of mild TD and CET (CET-PTD, PTD-CET) alternated significantly less than PF controls and PTD–EAS rats (for all comparisons, p<0.001) and less than CET rats (CET-PTD: p<0.05; PTD-CET: p=0.056). Thus, combined CET and mild TD exacerbate spatial memory impairment to levels observed in PTD-MAS rats. No differences were found in the number of arms entered during testing demonstrating similar activity levels (F [5,41] = 1.21, p > 0.32, data not shown).
Figure 4.
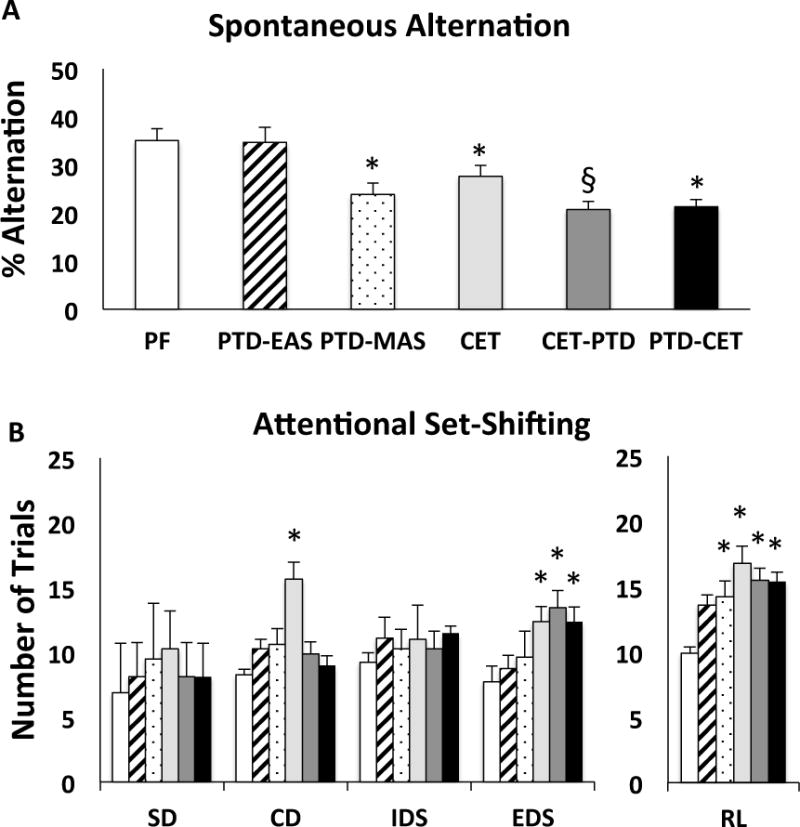
Behavioral data as a function of treatment condition. A. Mean percent alternation (mean ± SEM) as a function of treatment condition. All treatment conditions except moderate TD (PTD-EAS) where impaired. There was also a synergistic deficit in the CET-TD group. B. Trials to criterion (mean ± SEM) during the different phases in the attentional set-shifting task. All rats were able to perform the simple discrimination (SD). Rats exposed to CET were impaired, relative to PF controls, on compound discrimination (CD), the extra-dimensional shift (EDS) and in reversal learning (RL). Rats exposed to CET & TD where impaired on the EDS and RL. Rats treated with moderate TD were not impaired on any discriminations and those extended to the severe stage (PTD-MAS) where impaired on RL. *p<0.05 compared to control. § p<0.05 compared to CET as well as PTD-EAS.
B. Chronic ethanol treatment with and without TD decreases cognitive flexibility
All treatment groups performed similarly to controls during the SD (Figure 4B, F [5, 41] = 1.60, p= 0.18). In CD, when a second distractor dimension was introduced, there was an effect of treatment (F [5, 41] = 2.81, p<0.05), where CET rats required more trials to master the task (p<0.01). No effect of group was seen during IDS (F [5,41] = 1.83, p > 0.12). In contrast, during EDS there was an effect of treatment (F [5, 41] =2.39, p < 0.05). Although TD alone (PTD-MAS; PTD-EAS) had no effect on EDS (p’s > 0.30), rats exposed to CET (CET, CET-PTD, PTD-CET) took more trials than PF control rats to master the rule (all p’s<0.05). An overall effect of group on reversal learning (R1, R2, R3) was observed (F [5, 41] = 2.94, p<0.05). Ethanol treated groups (CET, CET-PTD, PTD-CET) and severe thiamine deficient rats (PTD-MAS) required more trials to reach criterion during reversal learning than PF controls (all p’s<0.05). Moderate TD was not significantly different from PF (p>0.07).
4. Neuropathology and BDNF levels
A. Thalamic lesion
As expected, the PTD MAS treatment resulted in the greatest percent of rats with a gross thalamic lesion (100%). Fewer rats displayed gross thalamic lesions in the other treatment conditions (PTD-EAS= 75%; PTD-CET=87.5%; CET-PTD=0%; CET=0%). We measured lesion size in the thalamus as a quantitative indicator of thalamic integrity. PTD-MAS rats had larger areas of thalamic damage compared to other groups (Figure 5A, p<0.05). Unexpectedly, PTD-CET and CET-PTD rats did not have an increase in lesion area relative to PTD-EAS treated rats. PTD-CET rats had the same lesion size as PTD-EAS rats (p > 0.87) and CET-PTD and CET rats did not show any lesions (Figure 5C).
Figure 5.
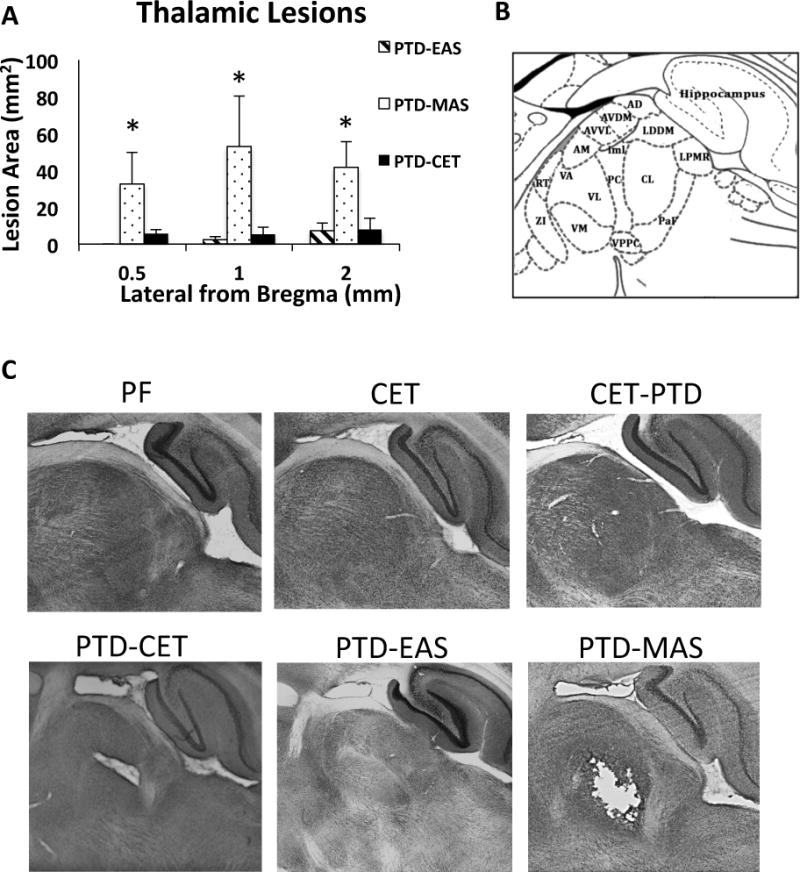
Assessment of thalamic integrity in sagittal rat brain sections stain with NeuN. A. Average lesion area for groups that had lesions in thalamus (mean ± SEM). PTD-MAS rats show the greatest area of neuronal loss in the thalamus. B. Schematic of thalamic nuclei at 1.4 mm lateral from Bregma (Paxinos and Watson, 2013). C. Images of thalamus from each treatment group. Pair fed, CET and CET-PTD rats did not have lesions, while PTD-CET, PTD-EAS and PTD-MAS treatments produced lesions with PTD-MAS causing lesions with the greatest area. AD, anterodorsal nucleus; AM, anteromedial nucleus; AVDM, anteroventral nucleus dorsoventral; AVVL, anteroventral nucleus ventrolateral; CL, centrolateral nucleus; iml, internal medullary lamina ; LDDM, laterodorsal nucleus dorsomedial ; LPMR, lateral posterior nucleous ventral; PC, paracentral nucleus; PaF, parafascicular nucleus; RT, Reticular nucleous; ZI, Zona incerta. *p<0.05.
B. Changes in mature BDNF levels
In the hippocampus, BDNF levels were altered by treatment condition (Figure 6A, F [5,39] = 4.03, p<0.01). The hippocampi of two subjects (1 CET, 1 PTD-MAS) were unable to be processed. Compared to PF controls, PTD-MAS as well as CET-PTD and PTD-CET decreased the level of BDNF in the hippocampus by approximately 70% (For all comparisons p<0.01). A significant reduction (60%) in hippocampal BDNF was also seen after CET (p<0.05). In contrast, PTD-EAS did not alter hippocampal BDNF levels (p>0.74). The levels of BDNF were also decreased in the prefrontal cortex by treatment conditions, but the reductions were not as great as those seen in the hippocampus (Figure 6B, F [5,41] = 3.31, p<0.05). Again, moderate PTD treatment (PTD-EAS) had no effect on prefrontal cortical BDNF level (p>0.27). Severe TD treatment (PTD-MAS), CET and combined moderate TD with CET (CET-PTD and PTD-CET) reduced prefrontal cortical BDNF levels in the range of 30–40% (all p’s <0.05).
Figure 6.
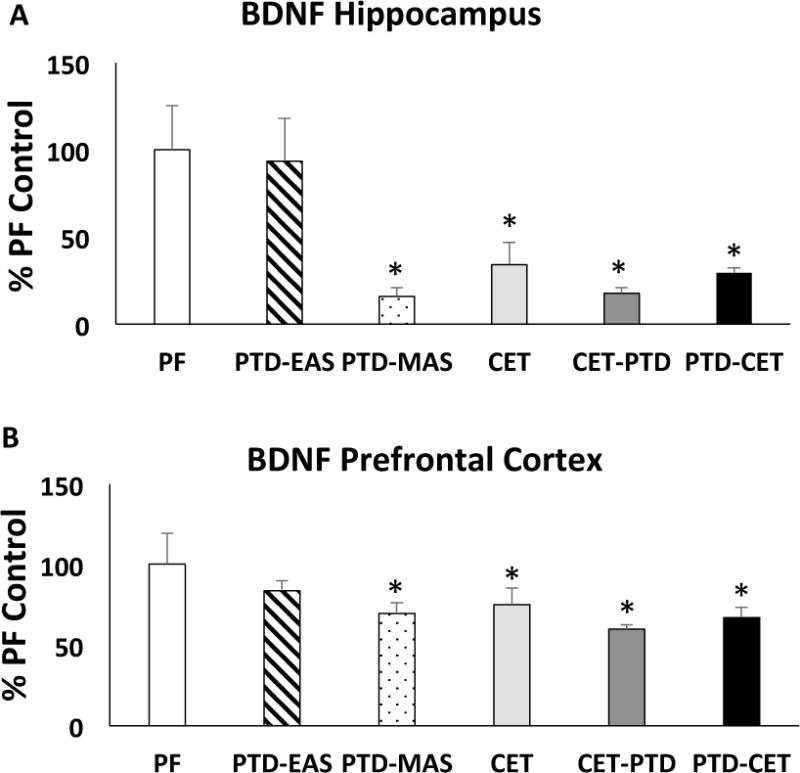
Mature BDNF levels (mean ± SEM) in the Hippocampus (A) and Prefrontal cortex (B). With the exception of moderate TD (PTD-EAS), all treatment conditions, relative to control rats, had reduced BNDF levels in both the hippocampus and frontal cortex. The reduction of BDNF level as a function of treatment condition was greater in the hippocampus than the frontal cortex. *p<0.05
5. Interactions between BDNF protein levels, BEC levels and spontaneous alternation
BDNF levels in both hippocampus and prefrontal cortex were positively correlated with spontaneous alternation performance (Figure 7A hippocampus; r[42]=0.32, p<0.05 and Figure 7B prefrontal cortex; r[44]=0.34, p < 0.05). We also found that BDNF levels in prefrontal cortex were negatively correlated with BECs (Figure 7C, r[27]=−0.57, p < 0.01), suggesting that increased exposure to ethanol is linked to decreased BDNF in prefrontal cortex. BECs were also negatively correlated with alternation performance (Figure 7D, r[27]= −0.47, p < 0.01). Correlations of BDNF and ASST were not significant (p’s > 0.12).
Figure 7.
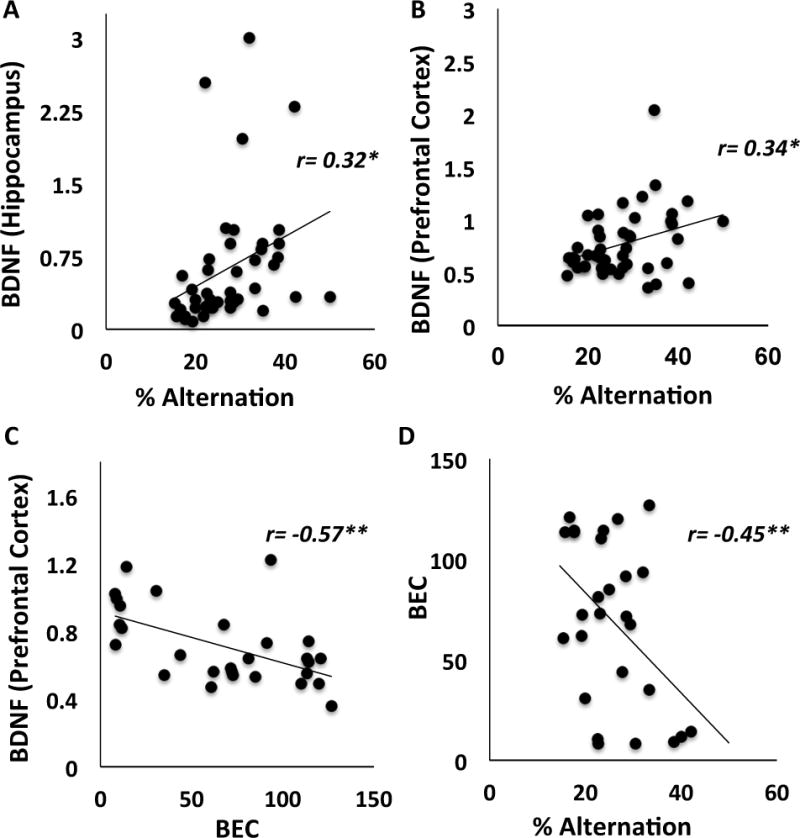
Correlations. (A) Hippocampal BDNF levels were positively correlated with spontaneous alternation scores; (B) Prefrontal cortical BDNF levels were also positively correlated with spontaneous alternation, scores; (C) BDNF levels were negatively correlated with BECs; (D) BECs were negatively correlated with spontaneous alternation scores. *p<0.05; **p<0.01
Discussion
Our findings support the hypothesis that when CET and subclinical TD interact there are additive functional deficits within neuronal circuits involved in key cognitive processes (spatial mapping, cognitive flexibility). Corresponding to the additive effects of reduced BDNF levels, CET combined with TD leads to synergistic deficits on spatial processing and cognitive flexibility. Thalamic lesion size was not increased by the combined treatment, suggesting that it is not the critical site modulating the extensive cognitive dysfunction when TD co-occurs with chronic alcohol consumption. However, BDNF levels in hippocampus and prefrontal cortex mirror the cognitive effects of combined treatment and is further correlated with behavioral performance in spontaneous alternation. Considering the role of BDNF in synaptic remodeling, decreased hippocampal-frontal cortical circuit plasticity is a likely candidate for the cognitive deficits seen when CET is combined with moderate TD.
Spatial processing relies heavily on intact hippocampal function. In spontaneous alternation, we found that CET and PTD-MAS can both independently decrease the percentage of alternation, to various degrees, supporting hippocampal dysfunction in both of these models. These results replicate our previous findings in PTD-MAS treated rats (Savage et al., 2003) and the findings of others using the CET model (Pickering et al., 2015). However, it is important to note that the effect size of severe TD (d=1.27) is larger than the effect size of CET (d=0.98). The combination of CET and PTD-EAS reduces behavioral performance to the greatest degree (CET-PTD, d=1.52; PTD-CET, d=1.58). PTD-EAS treatment alone is a subthreshold level of TD that does not impair cognitive performance. Thus, in order for extensive cognitive impairment to be observed after ARBD, severe TD is needed or moderate TD that co-occurs with alcoholism. These results replicate the types of circuit dysfunction that is seen in uncomplicated alcoholics relative to alcoholics complicated with malnourishment (see Pitel et al., 2014a).
Overall, CET leads to several types of deficits in cognitive flexibility and some of these deficits were made worse by the addition of moderate TD. CET-treatment resulted in more trials to reach criterion in the CD, the EDS, as well as reversal learning. Combined CET with moderate TD impaired the EDS and reversal learning. In our experiments, we did not see an increase in trials to criterion between the IDS and EDS, suggesting a shift in attention-set may not have occurred. Animals in this study may have been using scent cues of the medium during EDS. However, a difference in problem solving technique between treatment groups cannot be ruled out, as CET and CET-TD groups required more trials to reach criterion on the EDS. Past behavioral experience can modify both reversal and shifting behaviors, particularly in normal rats, making intact rats quicker at shifting or reversals and less dependent on certain frontal cortical regions (Young and Shapiro, 2009). This could explain why control rats, but not CET and CET-PTD groups, in this study did not show a significant increase in trials to criterion between the IDS-EDS. Thus, cognitive flexibility, as measured by reversal learning and potentially cognitive shifting is disrupted by CET with or without TD.
Various brain areas participate in ASST, depending on the phase of this task (Tait et al., 2014). Regions affected by ethanol toxicity, such the basal forebrain (Arendt et al., 1989, Cadete-Leite et al., 2003) and orbital frontal cortex (Badanich et al., 2011) play sufficient roles in reversal learning in the ASST (Tait and Brown, 2008, Chase et al., 2012). These results, along with our spontaneous alternation findings, suggest that CET and severe TD have differential effects on targeted brain areas. TD more robustly disrupted hippocampal-dependent spatial memory and CET impaired cognitive flexibility that is dependent on components of the frontal cortex. The combination of CET with TD expands the dysfunction of the individual treatments to the broader learning and memory circuit.
TD results in neuronal loss in the anterior and midline thalamic nuclei (Zhang et al., 1995) and we confirmed large lesions within these thalamic regions after severe TD and modest thalamic lesions after moderate TD. The neuropathology sequelae of CET and TD are both related to glutamate excitotoxicity (Langlais, 1995, Ward et al., 2009, Walker et al., 1980). The long-term ethanol drinking model we employed results in rats drinking significant volumes of ethanol for over 6 months and reaching BECs in the binge range, a profile that leads to cell death within the hippocampus and basal forebrain (Arendt et al., 1989, Cadete-Leite et al., 2003, Walker et al., 1980). Although there is the suggestion that both ethanol and TD have neurodegenerative effects in thalamus (Harper et al., 1986), we found no greater detection of thalamic lesions after CET and TD treatment. However, we did find that the order in which rats were treated with CET and TD influenced thalamic lesion status: some rats (87.5%) that were treated with moderate TD first followed by CET had thalamic lesions, whereas no rats treated with CET first followed by TD displayed thalamic lesions. This result suggests that CET may exhibit some early protection against the neurodegenerative processes caused by TD and such a phenomenon was recently reported (de Fatima Oliveira-Silva et al., 2015). A potential mechanism of this is an increased inhibition of excitatory responses through up regulation of GABA receptors (Zorumski et al., 2014). In addition, ethanol is a partial inhibitor at NMDA receptors (NMDARs) (Lovinger et al., 1989). Thus, four weeks of ethanol exposure prior to PTD could have altered the population of receptors, potentially rendering neurons less susceptible to excitoxicity caused by TD. Regardless of thalamic lesion size, cognitive function is the same in the combined treatments. Thus, some other common target(s) of CET and TD is allowing for the synergistic deficits in cognition. Our data point towards the circuit dysfunction between the hippocampus and prefrontal cortex.
In our current model, we show a detrimental effect of combined CET and TD treatment on BDNF levels in both the hippocampus and prefrontal cortex, with a larger effect in hippocampus. The down regulation of BDNF is measured after 3-wks of recovery from treatment and follows the same pattern as the change in spontaneous alternation: Higher levels of BDNF are positively correlated with higher levels of spontaneous alternation. As BDNF levels have been shown to directly modify LTP (Korte et al., 1995), it is likely that this decreased hippocampal BDNF is partially responsible for the decrease in spatial memory. As BDNF is critical for the modulation of mature neuronal dendritic and spine architecture in both the cortex and hippocampus (Kellner et al., 2014), a detailed neuroanatomical examination of these regions is needed to fully understand the effects of chronic alcoholism and moderate TD.
Another potential neuropathological target of both TD and CET is cell loss within the basal forebrain cholinergic system. TD reduces the number of cholinergic cells (30%) in the medial septum (Roland & Savage, 2009) and chronic ethanol treatment produces a similar profile in the medial septum and extends to the nucleus basalis magnocellularis (Arendt et al., 1989, Cadete-Leite et al., 2003, Floyd et al., 1997, Savage et al., 2000). At this point it is unknown whether CET combined with moderate TD exacerbates this cholinergic loss. However, innervation of cholinergic projections from the basal forebrain is critical for normal function in the hippocampus and prefrontal cortex (Hodges et al., 1991, Savage et al., 2000). It should be noted that neurotrophins, such as BDNF, play a key role in the viability of cholinergic neurons (Knusel et al., 1992), and thus examination of the cholinergic forebrain will be critical in future studies assessing the neuropathological consequences of TD and chronic alcoholism.
Summary
These data are the first to demonstrate both synergistic cognitive deficits and additive neural deficits after chronic alcohol consumption combined with moderate TD in an animal model. The cognitive deficits, but not thalamic lesion status, occur whether the treatments are in parallel (CET-PTD) or sequence (PTD-CET). Although clinical data points towards the thalamus as the critical region for severe cognitive dysfunction in human alcoholics with or without TD (Pitel et al., 2014b), our data suggest a wider network dysfunction. This network dysfunction includes the hippocampus and prefrontal cortex, and a key marker of neuroplasticity (BDNF).
Even though the thalamic pathology of the combined CET and moderate TD groups is considerably less than the severe TD conditions, the reductions in hippocampal and frontal cortical BDNF are similar, suggesting that the functional deactivation of these areas are equal across severe TD and moderate TD combined with CET. We further show that both PTD and CET treatments decrease TDP levels, with of course PTD treatment producing the lowest level of active thiamine. Thus, our data support the emerging clinical theory (Pitel et al., 2011, Zahr et al., 2011) that subclinical TD that co-occurs with chronic alcoholism can lead to a behavioral phenotype that is indistinguishable from WKS, including a severe amnestic state of impaired learning, memory and cognitive flexibility. Reduction in hippocampal and frontal cortical BDNF levels after CET combined with TD suggests that these regions are lacking in mechanisms for activity-dependent synaptic plasticity.
Acknowledgments
The research group would like to thank the Developmental Exposure Alcohol Research Center for intellectual input and technical training. In addition we thank Sian Sealy and Alex Yevtuhk for their assistance in behavioral testing.
This research was funded by an NIAAA R01 grant to LMS (RO1AA021775).
Footnotes
Conflict of Interest: No authors report any conflict of interest.
References
- ALOE L, TIRASSA P. The Effect of Long-Term Alcohol Intake on Brain Ngf-Target Cells of Aged Rats. Alcohol. 1992;9:299–304. doi: 10.1016/0741-8329(92)90070-q. [DOI] [PubMed] [Google Scholar]
- ANZALONE S, VETRENO RP, RAMOS RL, SAVAGE LM. Cortical cholinergic abnormalities contribute to the amnesic state induced by pyrithiamine-induced thiamine deficiency in the rat. Eur J Neurosci. 2010;32:847–58. doi: 10.1111/j.1460-9568.2010.07358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARENDT T, ALLEN Y, MARCHBANKS RM, SCHUGENS MM, SINDEN J, LANTOS PL, GRAY JA. Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience. 1989;33:435–62. doi: 10.1016/0306-4522(89)90397-7. [DOI] [PubMed] [Google Scholar]
- BA A, SERI BV, AKA KJ, GLIN L, TAKO A. Comparative effects of developmental thiamine deficiencies and ethanol exposure on the morphometry of the CA3 pyramidal cells. Neurotoxicol Teratol. 1999;21:579–86. doi: 10.1016/s0892-0362(99)00014-8. [DOI] [PubMed] [Google Scholar]
- BADANICH KA, BECKER HC, WOODWARD JJ. Effects of chronic intermittent ethanol exposure on orbitofrontal and medial prefrontal cortex-dependent behaviors in mice. Behav Neurosci. 2011;125:879–91. doi: 10.1037/a0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRRELL JM, BROWN VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CADETE-LEITE A, PEREIRA PA, MADEIRA MD, PAULA-BARBOSA MM. Nerve growth factor prevents cell death and induces hypertrophy of basal forebrain cholinergic neurons in rats withdrawn from prolonged ethanol intake. Neuroscience. 2003;119:1055–69. doi: 10.1016/s0306-4522(03)00205-7. [DOI] [PubMed] [Google Scholar]
- CAINE D, HALLIDAY GM, KRIL JJ, HARPER CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62:51–60. doi: 10.1136/jnnp.62.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHASE EA, TAIT DS, BROWN VJ. Lesions of the orbital prefrontal cortex impair the formation of attentional set in rats. Eur J Neurosci. 2012;36:2368–75. doi: 10.1111/j.1460-9568.2012.08141.x. [DOI] [PubMed] [Google Scholar]
- CICCIA RM, LANGLAIS PJ. An examination of the synergistic interaction of ethanol and thiamine deficiency in the development of neurological signs and long-term cognitive and memory impairments. Alcohol Clin Exp Res. 2000;24:622–34. [PubMed] [Google Scholar]
- CREWS FT, NIXON K. Mechanisms of Neurodegeneration and Regeneration in Alcoholism. Alcohol and Alcoholism. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS MI. Ethanol-BDNF interactions: still more questions than answers. Pharmacol Ther. 2008;118:36–57. doi: 10.1016/j.pharmthera.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE FATIMA OLIVEIRA-SILVA I, PEREIRA SR, FERNANDES PA, RIBEIRO AF, PIRES RG, RIBEIRO AM. Mild thiamine deficiency and chronic ethanol consumption modulate acetylcholinesterase activity change and spatial memory performance in a water maze task. J Mol Neurosci. 2015;55:217–26. doi: 10.1007/s12031-014-0306-7. [DOI] [PubMed] [Google Scholar]
- DUFOUR MC. The epidemiology of alcohol-induced brain damage. In: HUNT WA, NIXON SJ, editors. Alcohol-induced Brain Damage. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1993. [Google Scholar]
- FLOYD EA, YOUNG-SEIGLER AC, FORD BD, REASOR JD, MOORE EL, TOWNSEL JG, RUCKER HK. Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol. 1997;14:93–8. doi: 10.1016/s0741-8329(97)86147-2. [DOI] [PubMed] [Google Scholar]
- GALVIN R, BRATHEN G, IVASHYNKA A, HILLBOM M, TANASESCU R, LEONE MA, EFNS EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–18. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- HARPER CG, GILES M, FINLAY-JONES R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49:341–5. doi: 10.1136/jnnp.49.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE X, SULLIVAN EV, STANKOVIC RK, HARPER CG, PFEFFERBAUM A. Interaction of thiamine deficiency and voluntary alcohol consumption disrupts rat corpus callosum ultrastructure. Neuropsychopharmacology. 2007;32:2207–16. doi: 10.1038/sj.npp.1301332. [DOI] [PubMed] [Google Scholar]
- HODGES H, ALLEN Y, SINDEN J, MITCHELL SN, ARENDT T, LANTOS PL, GRAY JA. The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behav Brain Res. 1991;43:7–28. doi: 10.1016/s0166-4328(05)80048-8. [DOI] [PubMed] [Google Scholar]
- HOMEWOOD J, BOND NW, MACKENZIE A. The effects of single and repeated episodes of thiamin deficiency on memory in alcohol-consuming rats. Alcohol. 1997;14:81–91. doi: 10.1016/s0741-8329(96)00111-5. [DOI] [PubMed] [Google Scholar]
- KELLNER Y, GODECKE N, DIERKES T, THIEME N, ZAGREBELSKY M, KORTE M. The BDNF effects on dendritic spines of mature hippocampal neurons depend on neuronal activity. Front Synaptic Neurosci. 2014;6:5. doi: 10.3389/fnsyn.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNUSEL B, BECK KD, WINSLOW JW, ROSENTHAL A, BURTON LE, WIDMER HR, NIKOLICS K, HEFTI F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992;12:4391–402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORTE M, CARROLL P, WOLF E, BREM G, THOENEN H, BONHOEFFER T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROENKE CD, ROHLFING T, PARK B, SULLIVAN EV, PFEFFERBAUM A, GRANT KA. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 2014;39:823–30. doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGLAIS PJ. Pathogenesis of diencephalic lesions in an experimental model of Wernicke’s encephalopathy. Metab Brain Dis. 1995;10:31–44. doi: 10.1007/BF01991781. [DOI] [PubMed] [Google Scholar]
- LOVINGER DM, WHITE G, WEIGHT FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–4. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- LUKOYANOV NV, PEREIRA PA, PAULA-BARBOSA MM, CADETE-LEITE A. Nerve growth factor improves spatial learning and restores hippocampal cholinergic fibers in rats withdrawn from chronic treatment with ethanol. Exp Brain Res. 2003;148:88–94. doi: 10.1007/s00221-002-1290-7. [DOI] [PubMed] [Google Scholar]
- MAIR RG. On the role of thalamic pathology in diencephalic amnesia. Rev Neurosci. 1994;5:105–40. doi: 10.1515/revneuro.1994.5.2.105. [DOI] [PubMed] [Google Scholar]
- PAXINOS G, WATSON C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 2013. [DOI] [PubMed] [Google Scholar]
- PICKERING C, ALSIO J, MORUD J, ERICSON M, ROBBINS TW, SODERPALM B. Ethanol impairment of spontaneous alternation behaviour and associated changes in medial prefrontal glutamatergic gene expression precede putative markers of dependence. Pharmacol Biochem Behav. 2015;132:63–70. doi: 10.1016/j.pbb.2015.02.021. [DOI] [PubMed] [Google Scholar]
- PIRES RG, PEREIRA SR, PITTELLA JE, FRANCO GC, FERREIRA CL, FERNANDES PA, RIBEIRO AM. The contribution of mild thiamine deficiency and ethanol consumption to central cholinergic parameter dysfunction and rats’ open-field performance impairment. Pharmacol Biochem Behav. 2001;70:227–35. doi: 10.1016/s0091-3057(01)00593-7. [DOI] [PubMed] [Google Scholar]
- PITEL AL, EUSTACHE F, BEAUNIEUX H. Chapter 13 - Component processes of memory in alcoholism: pattern of compromise and neural substrates. In: EDITH VS, ADOLF P, editors. Handbook of Clinical Neurology. Elsevier; 2014a. [DOI] [PubMed] [Google Scholar]
- PITEL AL, SEGOBIN SH, RITZ L, EUSTACHE F, BEAUNIEUX H. Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci Biobehav Rev. 2014b doi: 10.1016/j.neubiorev.2014.07.023. [DOI] [PubMed] [Google Scholar]
- PITEL AL, ZAHR NM, JACKSON K, SASSOON SA, ROSENBLOOM MJ, PFEFFERBAUM A, SULLIVAN EV. Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology. 2011;36:580–8. doi: 10.1038/npp.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIN L, CREWS FT. Focal thalamic degeneration from ethanol and thiamine deficiency is associated with neuroimmune gene induction, microglial activation, and lack of monocarboxylic acid transporters. Alcohol Clin Exp Res. 2014;38:657–71. doi: 10.1111/acer.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON HN, CHAN SH, CRAWFORD EF, LEE YK, FUNK CK, KOOB GF, MANDYAM CD. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROLAND JJ, SAVAGE LM. The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience. 2009;160:32–41. doi: 10.1016/j.neuroscience.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVAGE LM, CANDON PM, HOHMANN HL. Alcohol-induced brain pathology and behavioral dysfunction: Using an animal model to examine sex differences. Alcoholism-Clinical and Experimental Research. 2000;24:465–475. [PubMed] [Google Scholar]
- SAVAGE LM, CHANG Q, GOLD PE. Diencephalic damage decreases hippocampal acetylcholine release during spontaneous alternation testing. Learn Mem. 2003;10:242–6. doi: 10.1101/lm.60003. [DOI] [PubMed] [Google Scholar]
- SAVAGE LM, HALL JM, RESENDE LS. Translational rodent models of Korsakoff syndrome reveal the critical neuroanatomical substrates of memory dysfunction and recovery. Neuropsychol Rev. 2012;22:195–209. doi: 10.1007/s11065-012-9194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAIT DS, BROWN VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–8. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- TAIT DS, CHASE EA, BROWN VJ. Attentional set-shifting in rodents: a review of behavioural methods and pharmacological results. Curr Pharm Des. 2014;20:5046–59. doi: 10.2174/1381612819666131216115802. [DOI] [PubMed] [Google Scholar]
- VETRENO RP, HALL JM, SAVAGE LM. Alcohol-related amnesia and dementia: animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011;96:596–608. doi: 10.1016/j.nlm.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER DW, BARNES DE, ZORNETZER SF, HUNTER BE, KUBANIS P. Neuronal loss in hippocampus induced by prolonged ethanol consumption in rats. Science. 1980;209:711–3. doi: 10.1126/science.7394532. [DOI] [PubMed] [Google Scholar]
- WARD RJ, LALLEMAND F, DE WITTE P. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or ‘binge drinking’ alcohol abuse. Alcohol Alcohol. 2009;44:128–35. doi: 10.1093/alcalc/agn100. [DOI] [PubMed] [Google Scholar]
- YOUNG JJ, SHAPIRO ML. Double Dissociation and Hierarchical Organization of Strategy Switches and Reversals in the Rat PFC. Behavioral neuroscience. 2009;123:1028. doi: 10.1037/a0016822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAHR NM, KAUFMAN KL, HARPER CG. Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol. 2011;7:284–94. doi: 10.1038/nrneurol.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG SX, WEILERSBACHER GS, HENDERSON SW, CORSO T, OLNEY JW, LANGLAIS PJ. Excitotoxic cytopathology, progression, and reversibility of thiamine deficiency-induced diencephalic lesions. J Neuropathol Exp Neurol. 1995;54:255–67. doi: 10.1097/00005072-199503000-00012. [DOI] [PubMed] [Google Scholar]
- ZORUMSKI CF, MENNERICK S, IZUMI Y. Acute and Chronic Effects of Ethanol on Learning-Related Synaptic Plasticity. Alcohol (Fayetteville NY) 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


