Abstract
Background
Both HIV and depression are associated with increased heart failure (HF) risk. Depression, a common comorbidity, may further increase the risk of HF among HIV+ adults. We assessed the association between HIV, depression and incident HF.
Methods and Results
Veterans Aging Cohort Study (VACS) participants free from cardiovascular disease at baseline (N = 81,427; 26,908 HIV+, 54,519 HIV-) were categorized into four groups: HIV- without major depressive disorder (MDD) [reference]; HIV- with MDD; HIV+ without MDD; and HIV+ with MDD. ICD-9 codes from medical records were used to determine MDD and the primary outcome, HF. After 5.8 follow-up years, HF rates per 1000 person-years were highest among HIV+ participants with MDD (9.32; 95% CI, 8.20–10.6). In Cox proportional hazards models, HIV+ participants with MDD had significantly higher risk of HF [adjusted hazard ratio (aHR) = 1.68; 95% CI, 1.45–1.95] compared to HIV- participants without MDD. MDD was associated with HF in separate fully adjusted models for HIV- and HIV+ participants (aHR = 1.21; 1.06–1.37 and 1.29; 1.11–1.51, respectively). Among those with MDD, baseline antidepressant use was associated with lower risk of incident HF events (aHR = 0.76; 0.58–0.99).
Conclusions
Our study is the first to suggest MDD is an independent risk factor for HF in HIV+ adults. These results reinforce the importance of identifying and managing MDD among HIV+ patients. Future studies must clarify mechanisms linking HIV, MDD, antidepressants, and HF; and identify interventions to reduce HF morbidity and mortality in those with both HIV and MDD.
Keywords: HIV infection, depression, psychiatric comorbidity, heart failure, epidemiology
Introduction
Antiretroviral therapy (ART) and effective clinical management have resulted in improved life expectancy for adults with HIV infection (HIV+).1, 2 Yet, the risk for cardiovascular disease (CVD) is higher for HIV+ adults compared to those without HIV (HIV-).3 The risk of heart failure (HF) is significantly higher in HIV+ adults compared to HIV- adults who are similar in age, gender, and race/ethnicity.4–6
Poor mental health is an additional concern for HIV+ adults. Depression is common in the US with the general population facing an estimated 6.6% 12-month risk of developing major depressive disorder (MDD).7 Estimates for 12-month MDD prevalence among HIV+ adults range from 5% to 10%.8 A meta-analysis reported that the frequency of MDD diagnoses among HIV+ participants was nearly two times higher than the frequency among HIV- participants.9 Like HIV infection, MDD may also increase the risk for HF.10–13
Possible mechanisms for the association between HIV and HF include chronic inflammation and platelet activation.5 Similarly, depression is associated with autonomic nervous system dysregulation, inflammation, and platelet activation.14–16 It stands to reason that HIV+ adults with MDD may be at heightened risk for HF compared to the remaining population; however, to date, no studies have examined the risk of HF in individuals burdened with co-occurring HIV and MDD.
Our objective, therefore, was to determine the association between HIV, MDD, and incident HF among a subset of HIV+ and HIV- veterans from the Veterans Aging Cohort Study (VACS) and to explore the effects of antidepressant use, HIV severity, and ART use on this association.
Methods
Participants
Details of the prospective, longitudinal Veterans Aging Cohort Study (VACS) have been published previously.17 Since 1998, the VACS continually enrolled HIV+ and age-, sex-, race/ethnicity-, and geographic region-matched HIV- veterans in the same calendar year from the US Department of Veterans Affairs (VA) system. For this analysis, data from VACS participants alive and enrolled as of April 1, 2003 were extracted from the VA national electronic medical record system. The institutional review boards from the University of Pittsburgh, Yale University, and West Haven VA Medical Center approved this study.
Participants were included in this analysis if they were free of clinical CVD at baseline (April 1, 2003). Prevalent CVD at baseline was defined using International Classification of Diseases, Ninth Revision (ICD-9) codes for HF, acute myocardial infarction, unstable angina, stroke or transient ischemic attack, peripheral vascular disease, or cardiovascular revascularization on or before their baseline date. The final sample included 81,427 Veterans (26,908 HIV+, 54,519 HIV-).
Independent Variables
Participants were considered HIV+ if they had at least one inpatient and/or two or more outpatient ICD-9 codes for HIV at baseline. The algorithm used to identify HIV+ veterans has high sensitivity (90%), specificity (99.9%), and positive predictive value (88%).17
Participants were considered to have MDD if at least one inpatient or two outpatient ICD-9 codes for MDD (296.2x and 296.3x) identified in their electronic medical record beginning in 1998 (the earliest time point of available ICD-9 data in VACS) and up to the participant’s enrollment date in the VACS. Since 2000, the VA has promoted a strong screening, diagnosis and treatment program within the VA. Screening programs include the use of PHQ-2 instruments followed by clinical interviews in primary care and or referrals to primary care mental health clinics or specialty care (e.g., psychiatry) for confirmation of diagnosis and treatment. In a small study that compared MDD diagnoses made by general practitioners in primary care settings to Diagnostic and Statistical Manual of mental disorders, 4th edition (DSM-IV) criteria ranges for MDD, specificity was 89% while sensitivity was 79%.18 These findings were supported by a larger study that investigated the validity of billing diagnoses for clinical depression in electronic medical records.19
We categorized participants into four groups: HIV- without MDD (reference); HIV- with MDD; HIV+ without MDD; and HIV+ with MDD.
Dependent Variables
The primary outcome for this report, HF, was determined using VA and Medicare ICD-9 codes (428.xx, 429.3, 402.11, 402.91, 425.x), a method shown to have a positive predictive value of 94.3%.20 The follow-up time for participants began from their first clinical encounter on or after 4/1/2003 and continued until a HF event, death, or the last date of follow-up (12/31/2009).
Covariates
Our covariates were selected apriori based on our prior work and studies from others examining risk factors for heart failure. The Framingham Heart Study and others have identified risk factors for heart failure including age, hypertension, diabetes, smoking, cholesterol, body mass index, substance use, atrial fibrillation and flutter, and renal disease.4, 21–24 Administrative data were used to obtain age, sex, and race/ethnicity. Outpatient and laboratory reports from baseline visits stored in the VA medical record provided data regarding hypertension, diabetes mellitus, lipid concentrations, hemoglobin concentrations, renal function, atrial fibrillation, atrial flutter, alcohol abuse or dependence, cocaine abuse or dependence, and hepatitis C virus (HCV) infection. Baseline body mass index (BMI; kg/m2) and smoking status were acquired from health factor data collected by the VA and recorded in the VA medical record. Baseline antidepressant and HMG CoA reductase inhibitor (statin) data were obtained from VA pharmacy records.17
Three hypertension categories were used: none (blood pressure < 140/90 mmHg and no antihypertensive medication use); controlled (blood pressure < 140/90 mmHg and antihypertensive medication use); and uncontrolled (blood pressure ≥ 140/90 mmHg).25 Diabetes mellitus diagnosis was determined using a combination of glucose levels, anti-diabetes medication, and ICD-9 codes; an algorithm previously validated in the VACS.26 Dyslipidemia was assessed using levels of low density lipoprotein cholesterol (LDL-c), high density lipoprotein cholesterol (HDL-c), and triglycerides measured in mg/dL. Statin use was determined within six months from enrollment. Anemia was based on hemoglobin concentrations (g/dL), and renal function was based on estimated glomerular filtration rate (eGFR; mL/min/1.73 m2). Alcohol abuse or dependence and cocaine abuse or dependence were measured based on ICD-9 codes.27 Hepatitis C virus infection was defined by one or more inpatient and/or two or more outpatient ICD-9 codes for a positive HCV antibody test result.28 Body mass index and smoking status (current, past, and never) were included in the VA electronic medical health record following prompts to the clinicians during patient visits. Obesity was defined as BMI ≥ 30 kg/m2. The smoking status reported in this health factor dataset has shown high agreement with self-reported smoking status on VACS-8 surveys.29
Antidepressant use was defined as documentation of a filled prescription for selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressant (TCA), or other antidepressant use from the VA pharmacy records during the baseline period (1998–2003). Medications were classified as “other” if they were in the following classes: tetracyclic antidepressant, monoamine oxidase inhibitor, serotonin-norepinephrine reuptake inhibitor, serotonin antagonist and reuptake inhibitor, norepinephrine reuptake inhibitor, norepinephrine-dopamine reuptake inhibitor, and miscellaneous (Supplemental Table 1). Generic and brand names for antidepressants were used to search outpatient pharmacy records.
We included HIV-specific variables (CD4+ T-cell (CD4) counts, HIV-1 RNA, and ART use) for HIV+ participants from two time periods: baseline and recent.30 Baseline variables were obtained during participant visits within 180 days of the baseline enrollment date (4/1/2003). Recent variables were obtained during the visit closest to HF, death, or the last follow-up date (12/31/2009). Antiretroviral medications were based on pharmacy data and categorized by drug class [i.e., nucleoside reverse-transcriptase inhibitors (NRTI), nonnucleoside reverse-transcriptase inhibitors (NNRTI), or protease inhibitors (PI)]. Additionally, four types of ART regimens were defined: NRTI with PI; NRTI with NNRTI; other regimen; and no ART use (reference). A previous study reported that 96% of HIV+ veterans obtain their ART medications through the VA system.17
Statistical analysis
Baseline descriptive statistics were calculated for each HIV/MDD group. T-tests (or non-parametric counterpart) and chi-square tests were used to determine significant differences between the groups as appropriate. Incident HF diagnosis rates per 1000 person-years were calculated for each HIV/MDD group. Cox proportional hazards regression was used to model the association between HIV/MDD group and incident HF. We constructed three models: (1) unadjusted; (2) adjusted for demographics; and (3) adjusted for age, sex, race/ethnicity, BMI, hypertension, diabetes mellitus, LDL-c, HDL-c, triglycerides, statin use, hemoglobin, renal function, atrial fibrillation, atrial flutter, smoking status, alcohol abuse or dependence, cocaine abuse or dependence, and HCV infection. The percent variance associated with each model is presented in Supplemental Table 2. We tested assumptions for Cox proportional hazard models using Schoenfeld residual plots. These plots did not show deviation from proportionality for our main exposure variable. Additional analyses examined this association among whites and African Americans separately. A Kaplan-Meier curve was created to display time to incident HF by the four HIV/MDD groups. Among those with MDD, HF incidence rates per 1000 person-years and adjusted hazard ratios were calculated by HIV status and antidepressant use. In a second analysis, we used Cox regression to model the association of MDD and incident HF among the HIV+ participants. This analysis included additional adjustments for baseline and recent CD4 counts, HIV-1 RNA, and ART use. We included only participants with a diagnosis of MDD in our third analysis, which explored the association between antidepressant use and incident HF among HIV+ and HIV- participants.
Results
Within this cohort of 81,427 veterans, MDD prevalence was 17.0% overall and not significantly different between HIV+ and HIV- participants (18.8% vs. 16.1%, respectively). The four HIV/MDD groups made up the following proportions of the total sample: HIV- without MDD, 56.2%; HIV- with MDD, 10.8%; HIV+ without MDD, 26.8%; and HIV+ with MDD, 6.2%.
Baseline characteristics differed between the four HIV/MDD groups (Table 1). Regardless of MDD diagnosis, HIV+ participants were more likely than HIV- participants to have low HDL-c, high triglycerides, HCV infection, renal disease, anemia, and cocaine abuse or dependence and to be current smokers. However, HIV+ participants were less likely to have hypertension, diabetes, and high LDL-c and to be obese. Regardless of HIV status, participants with MDD were more likely than participants without MDD to have diabetes, high triglycerides, HCV infection, cocaine abuse or dependence, and alcohol abuse or dependence; to be current smokers; and to use antidepressant medications (all with p < 0.05).
Table 1.
Baseline participant characteristics by major depressive disorder (MDD) diagnosis and HIV status (N = 81,427)*
| HIV-uninfected | HIV-infected | |||
|---|---|---|---|---|
| No MDD | MDD | No MDD | MDD | |
| Characteristic | (n= 45,728) | (n= 8,791) | (n= 21,850) | (n= 5,058) |
| Age, years, mean (SD) | 48.8 (9.5) | 48.1 (7.4) | 48.3 (9.7) | 47.4 (7.9) |
| Male, % | 97.5 | 95.6 | 97.7 | 95.3 |
| Race / Ethnicity, % | ||||
| White | 37.0 | 41.6 | 37.0 | 42.5 |
| African American | 48.0 | 46.8 | 48.5 | 45.9 |
| Hispanic | 7.5 | 9.2 | 6.9 | 8.2 |
| Other | 7.4 | 2.4 | 7.7 | 3.4 |
| Body Mass Index ≥ 30 kg/m2, % | 38.7 | 38.6 | 13.7 | 16.6 |
| Hypertension, % | ||||
| None | 58.3 | 60.8 | 67.3 | 68.6 |
| Controlled | 9.4 | 10.1 | 7.0 | 7.9 |
| Uncontrolled | 32.4 | 29.0 | 25.7 | 23.6 |
| Diabetes mellitus, % | 20.0 | 23.1 | 13.3 | 16.1 |
| Lipids, mg/dL, % | ||||
| LDL cholesterol < 100 | 31.2 | 33.5 | 46.2 | 46.5 |
| LDL cholesterol 100 – 129 | 33.3 | 33.4 | 29.7 | 29.6 |
| LDL cholesterol 130 – 159 | 23.2 | 21.1 | 15.9 | 15.7 |
| LDL cholesterol ≥ 160 | 12.3 | 12.0 | 8.2 | 8.1 |
| HDL cholesterol ≥ 60 | 15.0 | 13.4 | 11.1 | 10.2 |
| HDL cholesterol 40 – 59 | 47.7 | 45.5 | 38.0 | 37.2 |
| HDL cholesterol < 40 | 37.3 | 41.1 | 50.9 | 52.6 |
| Triglycerides ≥ 150 | 37.4 | 42.2 | 46.9 | 49.1 |
| Statin use within 6 months of enrollment, % |
9.4 | 11.6 | 6.4 | 6.8 |
| Hemoglobin, g/dL, % | ||||
| ≥ 14 | 73.1 | 70.9 | 55.1 | 57.1 |
| 12 – 13.9 | 22.9 | 24.9 | 31.9 | 32.0 |
| 10 – 11.9 | 3.2 | 3.5 | 9.5 | 8.5 |
| < 10 | 0.8 | 0.8 | 3.5 | 2.5 |
| Renal function, mL/min/1.73 m2, % | ||||
| eGFR ≥ 60 | 95.3 | 95.9 | 93.6 | 94.6 |
| eGFR 30 – 59 | 4.2 | 3.7 | 5.2 | 4.4 |
| eGFR < 30 | 0.5 | 0.3 | 1.2 | 1.1 |
| Atrial fibrillation, % | 1.0 | 0.8 | 0.8 | 0.7 |
| Atrial flutter, % | 0.3 | 0.4 | 0.3 | 0.2 |
| Smoking, % | ||||
| Never | 31.4 | 22.8 | 28.4 | 19.3 |
| Past | 16.5 | 13.4 | 13.7 | 11.0 |
| Current | 52.1 | 63.8 | 57.8 | 69.7 |
| Alcohol abuse or dependence, % | 10.5 | 27.5 | 10.8 | 28.1 |
| Cocaine abuse or dependence, % | 5.5 | 16.3 | 8.5 | 23.3 |
| HCV infection, % | 13.9 | 24.0 | 32.7 | 43.2 |
| Antidepressant use, %‡ | ||||
| Any SSRI use | 20.4 | 73.7 | 22.8 | 73.6 |
| Any TCA use | 11.3 | 25.3 | 14.7 | 29.2 |
| Any other antidepressants use† | 20.6 | 70.4 | 22.2 | 68.2 |
| SSRI only use | 8.0 | 14.1 | 9.8 | 14.8 |
| TCA only use | 5.4 | 2.0 | 7.3 | 2.7 |
| Other antidepressants only use | 8.4 | 10.9 | 9.2 | 10.6 |
| No antidepressant medications | 64.1 | 10.0 | 58.4 | 9.4 |
| HIV-specific risk factors | ||||
| CD4 cell count, mm3, % | ||||
| ≥ 500 at baseline | … | … | 31.4 | 34.6 |
| 200 – 499 at baseline | … | … | 40.0 | 40.5 |
| < 200 at baseline | … | … | 28.6 | 24.9 |
| ≥ 500 at recent visit | … | … | 41.5 | 41.7 |
| 200 – 499 at recent visit | … | … | 39.2 | 38.4 |
| < 200 at recent visit | … | … | 19.3 | 20.0 |
| HIV-1 RNA, ≥ 500 copies/mL at baseline, % |
… | … | 55.1 | 56.5 |
| HIV-1 RNA, ≥ 500 copies/mL at recent visit, % |
… | … | 24.5 | 26.4 |
| ART use, % | ||||
| NRTI at baseline | … | … | 48.3 | 49.6 |
| NNRTI at baseline | … | … | 22.6 | 22.1 |
| PI at baseline | … | … | 25.3 | 27.1 |
| NRTI at recent visit | … | … | 73.7 | 68.1 |
| NNRTI at recent visit | … | … | ||
| PI at recent visit | … | … | 38.1 | 39.8 |
| Regimen, % | ||||
| NRTI + PI at baseline | … | … | 20.4 | 21.6 |
| NRTI + NNRTI at baseline | … | … | 22.1 | 21.6 |
| Other at baseline | … | … | 6.7 | 7.2 |
| No ART at baseline | … | … | 50.9 | 49.6 |
| NRTI + PI at recent visit | … | … | 32.4 | 34.1 |
| NRTI + NNRTI at recent visit | … | … | 35.9 | 28.6 |
| Other at recent visit | … | … | 8.3 | 8.6 |
| No ART at recent visit | … | … | 23.4 | 28.8 |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index (calculated as kg/m2); eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; LDL, low-density lipoprotein; MDD, major depressive disorder; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; SSRI, selective serotonin reuptake inhibitors; TCA, tricyclic antidepressants
All characteristics were significantly different between groups (p < 0.05) except atrial flutter (p = 0.201), recent CD4 cell counts (p = 0.492), baseline HIV-1 RNA concentration (p = 0.104), baseline NRTI use (p = 0.093), baseline NNRTI use (p = 0.436), and baseline ART regimens (p = 0.080)
See Supplemental Table for medications included in each antidepressant medication category
Any Antidepressant use refers to the proportion of Veterans in a category that were taking a class of antidepressant, regardless of whether they were on multiple medications. “Only use” refers to the proportion of Veterans who are only taking that one class of medication. “No medication” refers to the proportion of Veterans who were not on any of the classes of antidepressant medication.
During a median of 5.8 (25th-75th percentile: 3.3–6.6) years of follow-up, there were 2666 incident HF events. The HF rate per 1000 person-years was significantly higher among HIV+ participants with co-occurring MDD compared to rates in the other three groups, i.e. HIV- without MDD, HIV- with MDD, and HIV+ without MDD (Table 2).
Table 2.
Unadjusted rates of incident heart failure by major depressive disorder (MDD) diagnosis and HIV status (N = 81,427)
| HIV status / MDD diagnosis |
Number of participants |
Number of HF events |
Rates of HF per 1000 p-y (95% CI) |
|---|---|---|---|
| HIV -- | No MDD | 45,728 | 1,339 | 6.04 (5.73 – 6.38) |
| HIV -- | MDD | 8,791 | 319 | 6.87 (6.16 – 7.67) |
| HIV + | No MDD | 21,850 | 774 | 7.56 (7.05 – 8.11) |
| HIV + | MDD | 5,058 | 234 | 9.32 (8.20 – 10.60) |
Abbreviations: HF, heart failure; HIV, human immunodeficiency virus; MDD, major depressive disorder; p-y, person-years
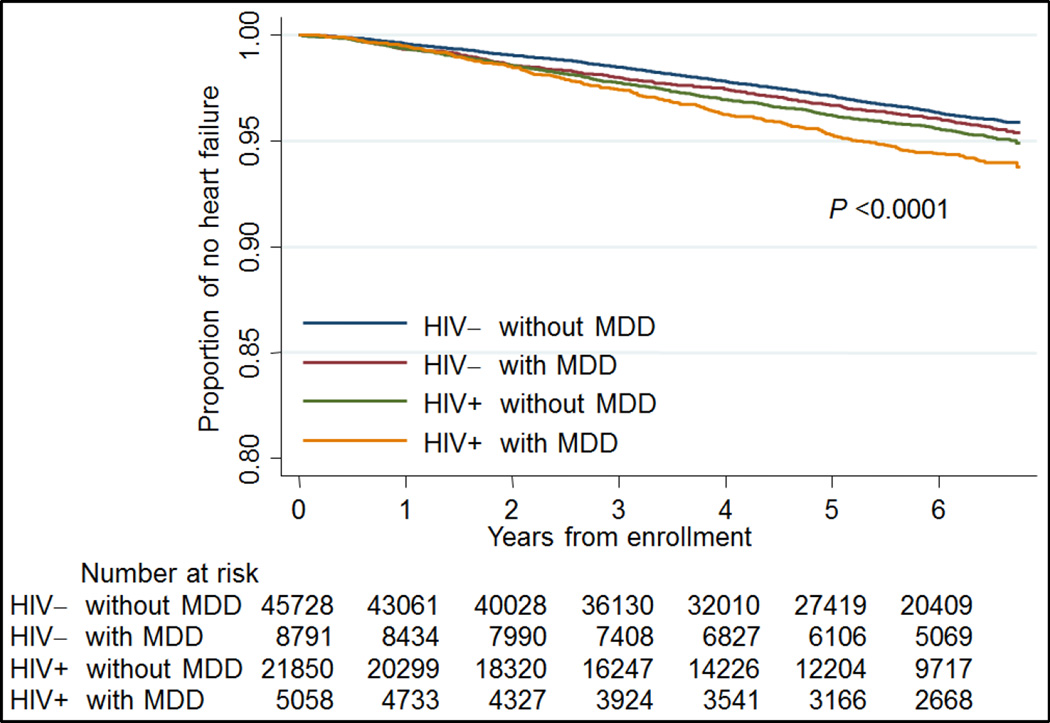
After adjusting for traditional CVD risk factors, HIV- participants with MDD, HIV+ participants without MDD, and HIV+ participants with MDD had higher risk of incident HF than HIV- participants without MDD (Table 3). Age, hypertension, diabetes, low hemoglobin, low eGFR, atrial fibrillation, current smoking, cocaine abuse or dependence and HCV infection were independently associated with increased risk of incident HF. Hispanic race/ethnicity was associated with a lower risk of incident HF. When this model was additionally adjusted for SSRI, TCA, and other antidepressant use, the risk of HF remained elevated among HIV- participants with MDD (adjusted HF (aHR) = 1.17; 95% CI, 1.02 – 1.34), HIV+ participants without MDD (1.28; 1.16 – 1.41), and HIV+ participants with MDD (1.64; 1.41 – 1.92) compared to the referent group. Importantly this association was present for whites and African Americans (Table 4). Moreover, when participants without MDD who were taking antidepressants were removed from the analysis, the HIV infected and depressed Veterans continued to have the highest risk of HF (HR 1.73; 1.47–2.01). Participants with co-occurring HIV infection and MDD diagnosis had the poorest survival free of HF of all four groups (Figure 1). In separate analyses, we did not find a significant multiplicative interaction between HIV and MDD (p values>0.05).
Table 3.
Cox proportional hazard regression models examining the association between HIV/MDD group and incident heart failure
| Characteristic | Model 1 HR (95% CI) |
Model 2 HR (95% CI) |
Model 3 HR (95% CI) |
|---|---|---|---|
| HIV / MDD Group | |||
| HIV -- | No MDD | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| HIV -- | MDD | 1.13 (1.00 – 1.27) | 1.30 (1.15 – 1.47) | 1.19 (1.05 – 1.35) |
| HIV + | No MDD | 1.25 (1.15 – 1.37) | 1.32 (1.20 – 1.44) | 1.28 (1.16 – 1.41) |
| HIV + | MDD | 1.54 (1.34 – 1.77) | 1.87 (1.62 – 2.15) | 1.68 (1.45 – 1.95) |
| Age, 10 year intervals | 2.04 (1.96–2.12) | 1.78 (1.70 – 1.86) | |
| Female sex | 1.00 (0.77–1.31) | 1.01 (0.77 – 1.33) | |
| Race / Ethnicity | |||
| White | 1[Reference] | 1 [Reference] | |
| African American | 1.34 (1.24–1.46) | 1.05 (0.96 – 1.15) | |
| Hispanic | 0.80 (0.68–0.95) | 0.77 (0.65 – 0.92) | |
| Other | 0.88 (0.73–1.07) | 0.92 (0.76 – 1.11) | |
| Body Mass Index ≥ 30 | 1.25 (1.14 – 1.36) | ||
| Hypertension | |||
| None | 1 [Reference] | ||
| Controlled | 1.78 (1.58 – 2.01) | ||
| Uncontrolled | 1.94 (1.77 – 2.12) | ||
| Diabetes mellitus | 1.75 (1.61 – 1.91) | ||
| Lipids, mg/dL | |||
| LDL cholesterol < 100 | 1 [Reference] | ||
| LDL cholesterol 100 – 129 | 0.88 (0.79 – 0.98) | ||
| LDL cholesterol 130 – 159 | 0.88 (0.78 – 1.00) | ||
| LDL cholesterol ≥ 160 | 0.93 (0.78 – 1.12) | ||
| HDL cholesterol ≥ 60 | 1 [Reference] | ||
| HDL cholesterol 40 – 59 | 1.04 (0.90 – 1.21) | ||
| HDL cholesterol < 40 | 1.04 (0.89 – 1.21) | ||
| Triglycerides ≥ 150 | 1.00 (0.91 – 1.10) | ||
| Statin use within 6 months | 1.08 (0.97 – 1.21) | ||
| Hemoglobin, g/dL | |||
| ≥ 14 | 1 [Reference] | ||
| 12 – 13.9 | 1.32 (1.20 – 1.45) | ||
| 10 – 11.9 | 1.84 (1.58 – 2.14) | ||
| < 10 | 2.23 (1.75 – 2.85) | ||
| Renal function, mL/min/1.73 m2 | |||
| eGFR ≥ 60 | 1 [Reference] | ||
| eGFR 30 – 59 | 2.01 (1.78 – 2.28) | ||
| eGFR < 30 | 5.21 (4.31 – 6.30) | ||
| Atrial fibrillation | 2.15 (1.63 – 2.84) | ||
| Atrial flutter | 1.59 (0.91 – 2.78) | ||
| Smoking | |||
| Never | 1 [Reference] | ||
| Past | 1.09 (0.96 – 1.23) | ||
| Current | 1.42 (1.29 – 1.57) | ||
| Alcohol abuse or dependence | 0.98 (0.86 – 1.12) | ||
| Cocaine abuse or dependence | 1.27 (1.09 – 1.48) | ||
| HCV infection | 1.30 (1.19 – 1.43) |
Model 1: HIV MDD only
Model 2: Model 1+ age, sex, race/ethnicity
Model 3: Model 2+ all covariates
Abbreviations: BMI, body mass index (calculated as kg/m2); eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; HR, hazard ratio; LDL, low-density lipoprotein; MDD, major depressive disorder
Table 4.
Cox proportional hazard regression models examining the association between HIV/MDD group and incident heart failure in Whites and African Americans
| Whites* | African Americans* | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | P value | HR (95% CI) | P value |
| HIV / MDD Group | ||||
| HIV -- | No MDD | 1.00 | 1.00 | ||
| HIV -- | MDD | 1.25 (1.02, 1.53) | 0.03 | 1.07 (0.90, 1.27) | 0.46 |
| HIV + | No MDD | 1.22 (1.04, 1.44) | 0.02 | 1.27 (1.11, 1.45) | <0.001 |
| HIV + | MDD | 1.63 (1.28, 2.09) | <0.001 | 1.71 (1.40, 2.09) | <0.001 |
Abbreviations: HIV, human immunodeficiency virus; HR, adjusted hazard ratio; MDD, major depressive disorder
All models for HF were adjusted for age, sex, body mass index, hypertension, diabetes mellitus, lipids, statin use, hemoglobin, renal function, atrial fibrillation, atrial flutter, smoking, alcohol abuse or dependence, cocaine abuse or dependence, and HCV infection
Figure 1.
Kaplan Meier Survival Curves for incident heart failure stratified by HIV and major depressive disorder status.
We further explored incident HF risk in HIV- and HIV+ participants separately. MDD was associated with increased risk of HF in both groups (aHR = 1.21; 95% CI, 1.06 – 1.37 and 1.29; 1.11 – 1.51, respectively). Among HIV+ participants, MDD remained a significant risk factor for HF after further adjusting for baseline traditional CVD risk factors, CD4 count, baseline HIV-1 RNA concentration, and ART regimen documented at the baseline visit (aHR = 1.30, 95% CI, 1.11 – 1.51). We found similar results when the model included; recent CD4 count, recent HIV-1 RNA concentration and recent ART regimen; or recent PI, NRTI, and NRTI use.
The majority of participants with MDD had at least one antidepressant prescription during the baseline period (90.2%). Among all participants in the sample with MDD, baseline antidepressant use was associated with fewer incident HF events, adjusting for HIV and cardiovascular disease risk factors (aHR = 0.76; 95% CI, 0.58 – 0.99). In a model that stratified the MDD participants by both HIV status and antidepressant use, the rates of incident HF were highest among HIV+ participants who did not use antidepressants (Table 5).
Table 5.
Rates and adjusted hazard ratios of incident heart failure among those with baseline major depressive disorder (MDD) (N = 13,849) stratified by HIV and baseline antidepressant use
| HIV status |
Anti- depressant use |
N | HF events |
HF rates per 1000 p- y (95% CI) |
P value | aHR* (95% CI) |
P value |
|---|---|---|---|---|---|---|---|
| HIV -- | Yes | 7916 | 283 | 6.73 (5.99 – 7.56) | 0.25† | 1.00 [Reference] | 0.28‡ |
| HIV -- | No | 875 | 36 | 8.25 (5.95 – 11.44) | 1.21 (0.85 – 1.71) | ||
| HIV + | Yes | 4582 | 206 | 8.97 (7.82 – 10.28) | 0.055§ | 1.39 (1.14 – 1.68) | 0.053∥ |
| HIV + | No | 476 | 28 | 13.20 (9.11 – 19.11) | 2.07 (1.39 – 3.08) |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HF, heart failure; HIV, human immunodeficiency virus; p-y, person-years
All models for HF were adjusted for age, sex, race/ethnicity, body mass index, hypertension, diabetes mellitus, lipids, statin use, hemoglobin, renal function, atrial fibrillation, atrial flutter, smoking, alcohol abuse or dependence, cocaine abuse or dependence, and HCV infection
P value for the comparison of HF rates; HIV- participants who did not use antidepressants at baseline compared to HIV- antidepressant users
P value for the comparison of aHRs; HIV- participants who did not use antidepressants at baseline compared to HIV- antidepressant users
P value for the comparison of HF rates; HIV+ participants who did not use antidepressants at baseline compared to HIV+ antidepressant users
P value for the comparison of aHRs; HIV+ participants who did not use antidepressants at baseline compared to HIV+ antidepressant users
Discussion
Our findings indicate that both depression and HIV are associated with an increased risk of HF in veterans, even after adjusting for traditional CVD risk factors, comorbidities, and substance use. Participants with co-occurring HIV and MDD had the highest rates and risk of HF relative to those with only one of the conditions or neither condition. Furthermore, among HIV+ veterans, depression was associated with an increased risk of HF after additional adjustment for CD4 cell count, HIV-1 RNA levels, and ART use.
Our findings are consistent with previous studies reporting associations between (a) HIV and incident HF and (b) depression and incident HF in HIV- samples.4, 5, 13, 31 However, this study is the first to simultaneously examine HIV status, MDD diagnosis, and antidepressant use as predictors of incident HF in a large national cohort of HIV infected and uninfected people. In addition, our results are the first to suggest that MDD is an independent risk factor for HF in HIV+ adults. This finding is important as rates of incident HF among the HIV+ participants with depression in this cohort (~9 per 1000 person years) were higher than in the U.S. population (~2–5 per 1000 person years).32
The physiological mechanisms underlying the associations of HIV infection and depression with future HF have yet to be elucidated. With HIV infection, the risk of HF may be explained by vascular damage associated with the virus itself, chronic inflammation, lack of vascular repair due to CD4 cell depletion, or dyslipidemia following HIV infection or ART-induced metabolic syndrome.4, 30, 33–35 With depression, possible mechanisms include alterations to the hypothalamic-pituitary-adrenal axis, dysregulation of the autonomic nervous system, chronic inflammation, and platelet activation.36, 37 In addition to these physiological changes, both HIV infection and depression are associated with unhealthy behavioral changes, such as substance use and decreased physical activity, which are risk factors for HF.38, 39
In this study, HIV+ participants with co-occurring MDD had the highest risk of HF. The Infectious Diseases Society of America has stressed the importance of recognizing depression in HIV+ patients.40 Our findings bolster this recommendation by adding another reason for doing so – i.e., depression as a potential risk factor for HF. In Table 4, we report preliminary evidence that raises the possibility that antidepressant use may decrease the excess risk for HF in HIV+ and HIV- adults. However, a prospective randomized controlled trial is needed to determine whether depression is a causal risk factor for HF and whether depression interventions reduce the risk of HF in the HIV infected and uninfected populations.41, 42
There are limitations of this study that warrant discussion. First, as our participants were predominantly men, the findings might not generalize to women. Second, HF and depression were based on ICD-9 codes. Therefore, some misclassification may have occurred (e.g., patients with true MDD or HF that was not indicated in their medical record with an ICD-9 code), however, such misclassification would have biased our results to the null. Third, baseline depressive disorder diagnosis was used as a predictor. Depressive symptom severity tends to fluctuate over time, and previous findings suggest that the mean of symptom severity assessed at multiple time points is a stronger predictor of subclinical CVD than depressive symptom severity assessed at one time point.43 However, because depressive episodes are likely to recur in those with a MDD diagnosis, a baseline measure of MDD diagnosis as we defined it should capture those at risk for chronic exposure to the unhealthy impact of depression.44, 45 Moreover, we did not assess whether “current” MDD (i.e., MDD within six months of and before baseline) was associated with HF because restricting the time window could lead to misclassification (i.e., participants who had been diagnosed with MDD but were not seen in clinic within six months before the baseline enrollment date would be classified as non-MDD). Lastly, our findings that depression treatment is associated with a reduced risk of HF among HIV+ veterans with MDD, while novel and intriguing, should be interpreted with caution. As this is an observational study and not a randomized control trial, we cannot eliminate confounding due to indication associated with the treatment of depression. Moreover, improved HF outcomes may be attributed to more frequent visits with a provider and better management of CVD risk factors and other medical conditions in addition to MDD treatment. Additionally, only 10% of participants with an MDD diagnosis did not have a prescription for an antidepressant during the baseline period. The high prevalence of treatment is likely a reflection of the fact that MDD in our study was determined by clinical diagnostic codes and not by a formal depression screening instrument administered to all participants. It is likely that some participants with unrecognized depression existed in our non MDD group. This possible misclassification, however, would lead us to underestimate the strength of the MDD-HF relationship, as veterans with unrecognized depression would be included in our referent group.
In conclusion, HIV and MDD are each associated with an increased risk of incident HF. HIV+ veterans with MDD had the highest rates of and risk for HF compared to veterans with either HIV, depression, or neither condition. These results suggest that MDD is a possible independent risk factor for HF among HIV+ patients and thus reinforce the importance of screening for and effectively managing depression in this patient population. Future studies in both HIV infected and uninfected adults should aim (a) to clarify the mechanisms underlying the depression-HF association and (b) to determine whether evidence-based depression treatment may help to reduce HF morbidity and mortality in these populations.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by grants HL095136, and T32 Cardiovascular Epidemiology Training Program HL083825, from the National Heart, Lung, and Blood Institute and grants AA013566, AA020790, and AA020794 from the National Institutes of Health on Alcohol Abuse and Alcoholism at the NIH.
Footnotes
Disclosures: None. The views expressed in this article are those of the authors and do not necessarily reflect the position or policies of the Department of Veterans Affairs.
References
- 1.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, Burchell AN, Cohen M, Gebo KA, Gill MJ, Justice A, Kirk G, Klein MB, Korthuis PT, Martin J, Napravnik S, Rourke SB, Sterling TR, Silverberg MJ, Deeks S, Jacobson LP, Bosch RJ, Kitahata MM, Goedert JJ, Moore R, Gange SJ. North American ACCoR Design of Ie DEA Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K, Collaboration C. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, Gibert CL, Oursler KK, Rodriguez-Barradas MC, Lim J, Kazis LE, Gottlieb S, Justice AC, Freiberg MS. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171:737–743. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng B, MacPherson P, Haddad T, Dwivedi G. Heart failure in HIV infection: focus on the role of atherosclerosis. Curr Opin Cardiol. 2014;29:174–179. doi: 10.1097/HCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 6.Freiberg M, Chang J, Oursler KA, Gottdiener J, Gottlieb S, Warner A, Leaf D, Rodriguez-Barradas M, Felter S, Butt AA. The risk of and survival with preserved vs. reduced ejection fraction heart failure by HIV status; Boston, MA. Paper presented at: Conference on Retroviruses and Opportunistic Infections.2013. [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. National Comorbidity Survey R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 8.Rabkin JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–171. doi: 10.1007/s11904-008-0025-1. [DOI] [PubMed] [Google Scholar]
- 9.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 10.Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- 11.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001;161:1725–1730. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 12.Williams SA, Kasl SV, Heiat A, Abramson JL, Krumholz HM, Vaccarino V. Depression and risk of heart failure among the elderly: a prospective community-based study. Psychosom Med. 2002;64:6–12. doi: 10.1097/00006842-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol. 2006;48:1527–1537. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 14.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;(67 Suppl 1):S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 15.Kop WJ, Gottdiener JS. The role of immune system parameters in the relationship between depression and coronary artery disease. Psychosom Med. 2005;(67 Suppl 1):S37–S41. doi: 10.1097/01.psy.0000162256.18710.4a. [DOI] [PubMed] [Google Scholar]
- 16.Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;(67 Suppl 1):S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- 17.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, Justice AC. Development and verification of a "virtual" cohort using the National VA Health Information System. Med Care. 2006;44:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 18.van Weel-Baumgarten EM, van den Bosch WJ, van den Hoogen HJ, Zitman FG. The validity of the diagnosis of depression in general practice: is using criteria for diagnosis as a routine the answer? Br J Gen Pract. 2000;50:284–287. [PMC free article] [PubMed] [Google Scholar]
- 19.Trinh NH, Youn SJ, Sousa J, Regan S, Bedoya CA, Chang TE, Fava M, Yeung A. Using electronic medical records to determine the diagnosis of clinical depression. Int J Med Inform. 2011;80:533–540. doi: 10.1016/j.ijmedinf.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–269. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kottgen A, Russell SD, Loehr LR, Crainiceanu CM, Rosamond WD, Chang PP, Chambless LE, Coresh J. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 24.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention DE. Treatment of High Blood Pressure. National Heart L, Blood I and National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 26.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40:115–119. doi: 10.1002/hep.20289. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer KL, McGinnis KA, Skanderson M, Cook R, Gordon A, Conigliaro J, Shen Y, Fiellin DA, Justice AC. Alcohol problems and health care services use in human immunodeficiency virus (HIV)-infected and HIV-uninfected veterans. Med Care. 2006;44:S44–S51. doi: 10.1097/01.mlr.0000223703.91275.78. [DOI] [PubMed] [Google Scholar]
- 28.Goulet JL, Fultz SL, McGinnis KA, Justice AC. Relative prevalence of comorbidities and treatment contraindications in HIV-mono-infected and HIV/HCV-co-infected veterans. AIDS. 2005;(19 Suppl 3):S99–S105. doi: 10.1097/01.aids.0000192077.11067.e5. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis KA, Brandt CA, Skanderson M, Justice AC, Shahrir S, Butt AA, Brown ST, Freiberg MS, Gibert CL, Goetz MB, Kim JW, Pisani MA, Rimland D, Rodriguez-Barradas MC, Sico JJ, Tindle HA, Crothers K. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA, Rimland D, Rodriguez Barradas M, Brown S, Gibert C, McGinnis K, Crothers K, Sico J, Crane H, Warner A, Gottlieb S, Gottdiener J, Tracy RP, Budoff M, Watson C, Armah KA, Doebler D, Bryant K, Justice AC. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grinspoon SK. Metabolic syndrome and cardiovascular disease in patients with human immunodeficiency virus. Am J Med. 2005;(118 Suppl 2):23S–28S. doi: 10.1016/j.amjmed.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 35.So-Armah KA, Chang J, Alcorn C, Lo Re V, Baker JV, Tracy R, Butt AA, Agan BK, Rimland D, Gibert CL, Goetz MB, Oursler KK, Rodriguez-Barradas MC, Kuller LH, Brown ST, Stein JH, Skanderson M, Justice AC, Freiberg MS. HIV infection, antiretroviral therapy initiation and longitudinal changes in biomarkers of organ function. Curr HIV Res. 2014;12:50–59. doi: 10.2174/1570162x1201140716101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–962. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 37.Joynt KE, Whellan DJ, O'Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003;54:248–261. doi: 10.1016/s0006-3223(03)00568-7. [DOI] [PubMed] [Google Scholar]
- 38.Sher Y, Lolak S, Maldonado JR. The impact of depression in heart disease. Curr Psychiatry Rep. 2010;12:255–264. doi: 10.1007/s11920-010-0116-8. [DOI] [PubMed] [Google Scholar]
- 39.Brion JM, Rose CD, Nicholas PK, Sloane R, Corless IB, Lindgren TG, Wantland DJ, Kemppainen JK, Sefcik EF, Nokes KM, Kirksey KM, Eller L, Hamilton MJ, Holzemer WL, Portillo CJ, Mendez MR, Robinson LM, Moezzi S, Rosa M, Human S, Maryland M, Arudo J, Ros AV, Nicholas TP, Cuca Y, Huang E, Bain C, Tyer-Viola L, Zang SM, Shannon M, Peters-Lewis A, Willard S. Unhealthy substance-use behaviors as symptom-related self-care in persons with HIV/AIDS. Nurs Health Sci. 2011;13:16–26. doi: 10.1111/j.1442-2018.2010.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, Stone VE, Kaplan JE. America HIVMAotIDSo. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 41.Lang UE, Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JC, Perkins AJ, Callahan CM. Effect of collaborative care for depression on risk of cardiovascular events: data from the IMPACT randomized controlled trial. Psychosom Med. 2014;76:29–37. doi: 10.1097/PSY.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthews KA, Chang YF, Sutton-Tyrrell K, Edmundowicz D, Bromberger JT. Recurrent major depression predicts progression of coronary calcification in healthy women: Study of Women's Health Across the Nation. Psychosom Med. 2010;72:742–747. doi: 10.1097/PSY.0b013e3181eeeb17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text rev. Fourth ed. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- 45.Burcusa SL, Iacono WG. Risk for recurrence in depression. Clin Psychol Rev. 2007;27:959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.