Abstract
Background
Ethanol (EtOH) inhibits Notch-mediated vascular smooth muscle cell (SMC) proliferation, an event that is key in vessel remodeling and atherogenesis. The object of this study was to determine if EtOH inhibits Notch signaling in SMC at the level of γ-secretase, a protease that in concert with α-secretase, catalyzes the release of the intracellular domain of the Notch receptor necessary for signaling.
Methods and Results
Human coronary artery SMC (HCASMC) were treated with a recombinant soluble Notch ligand, DLL4 (2 μg/ml), or transfected with a constitutively active Notch 1 intracellular domain (N1ICD), in the absence or presence of EtOH. Ethanol (25 mM) treatment inhibited DLL4-stimulated CBF-1/RBP-Jk dependent promoter activity (determined by luciferase assay) and downstream target gene HRT3 mRNA levels. In contrast, ethanol had no effect on N1ICD-driven CBF1/RBP-Jk dependent promoter activity or HRT-3 expression. These data suggest that EtOH inhibits Notch signaling at, or prior to, NICD generation. Gamma-secretase activity was determined in solubilized membrane preparations from HCASMC treated with/without EtOH (25 mM) or the γ-secretase inhibitor DAPT (20 μM) using (i) a fluorometric assay and (ii) western blot detection of cleavage products using a Flag-tagged Notch based substrate, N100Flag. EtOH inhibited basal and DLL4-stimulated γ-secretase activity, and SMC growth to a similar extent as DAPT, whereas it had no effect on α-secretase (TACE/ADAM 17) activity also determined by fluorometric assay. Moreover, EtOH treatment inhibited the expression of caveolin-1, a lipid raft protein implicated in regulating γ-secretase activity, and altered its cellular distribution in HCASMC.
Conclusions
Ethanol inhibits Notch signaling in vascular smooth muscle cells at the level of γ-secretase activity, possibly by affecting lipid raft function. Such a response might be expected to result in attenuation of pathologic vessel remodeling and thus may contribute to moderate alcohols’ cardioprotective effects.
Keywords: alcohol, γ-secretase, Notch, vascular smooth muscle cells, atherosclerosis
Introduction
The cell signaling mechanisms mediating the cardiovascular effects of alcohol consumption, as purported by epidemiologic studies (Thun et al., 1997) (Pearson, 1996) (Di Castelnuovo et al., 2006), warrant further investigation.
Notch signaling regulates cell-fate determination and is critical during development (Shawber & Kitajewski, 2004). It is recapitulated during various disease processes in the adult. For example, Notch regulates the function of vascular smooth muscle cells (SMC) (Proweller, Pear, & Parmacek, 2005; Sweeney et al., 2004) whose triggered growth and migration contribute to the pathophysiology of vessel disease including arteriosclerosis and atherosclerosis (Lacolley, Regnault, Nicoletti, Li, & Michel, 2012). Notch signaling controls arterial SMC phenotype in vitro and in vivo (Morrow et al., 2008b; Wang et al., 2003). The critical role of SMC Notch 1 receptor, in particular, in vascular disease has been highlighted by several studies using a variety of specific Notch 1 knockdown strategies that prevent SMC growth in vitro and injury-induced vessel remodeling in vivo (Y. Li et al., 2009) (Redmond et al., 2014).
We have previously reported that ethanol (EtOH) treatment inhibits Notch-dependent SMC proliferation (Morrow et al., 2010). EtOH inhibited Notch 1 receptor expression and downstream signaling in SMC in vitro and its anti-proliferative effect was reversed by overexpression of the constitutively active NICD (Morrow et al., 2010). Moreover, moderate alcohol consumption reduced injury-induced Notch target gene expression concomitant with inhibition of intimal medial thickening in ligated mouse vessels(Morrow et al., 2010). These data highlight the Notch pathway as a novel vascular target for EtOH, but do not illuminate the precise inhibitory mechanism involved.
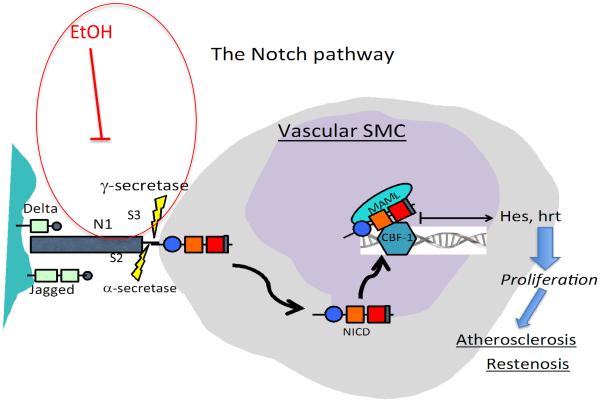
Indeed, the Notch signaling pathway involves several steps and offers numerous targets for regulatory intervention(Baron, 2003). In the canonical version, interaction of a ligand (e.g., Delta-like ligand or Jagged) with the Notch receptor initiates proteolytic cleavage at the extracellular site of the transmembrane receptor by α-secretase (a member of the ‘a disintegrin and metalloprotease domain' ADAM family, also known as ‘tumor necrosis factor-α converting enzyme’ (TACE)) followed by cleavage at the intracellular site by γ-secretase, resulting in the release of Notch-IC (also referred to as Notch intracellular domain; NICD) from the cytoplasmic side of the cell membrane. NICD is then translocated into the nucleus, mediated by nuclear translocation signals, where it interacts primarily with CSL and recruits co-activators to form a transcription–activating complex (Fig 1).
Figure 1.
The Notch signaling pathway. Interaction of a ligand (e.g., Delta or Jagged) with the Notch receptor (e.g., N1) initiates cleavage events at site 2 (S2) and site 3 (S3) by α- and γ-secretase, respectively, resulting in release of the intracellular domain of the receptor (NICD) from the cell membrane. NICD is then translocated into the nucleus where it forms a transcription activating complex triggering target gene (Hes, Hrt) transcription. Our data indicate that EtOH inhibits γ-secretase activity and thus, Notch signaling, in vascular SMC.
The γ-secretase involved in Notch signal transduction is a large protease complex and is composed of a catalytic subunit presenilin (PSN-1, PSN-2) and accessory subunits, presenilin enhancer-2 (Pen-2), anterior pharynx defective-1 (Aph1) and Nicastrin (Shih & Wang, 2007). In addition to Notch and amyloid precursor protein, there are numerous other substrates of γ-secretase(Zhang et al., 2014).
We report here that EtOH regulates Notch signaling in HCASMC by specifically inhibiting γ-secretase activity, in the absence of any effect on α-secretase. This effect of ethanol may be important in mediating the vascular protective effects, and other biological effects, of alcohol consumption.
Materials and Methods
Cell culture
Human coronary artery smooth muscle cells (HCASMC, Clonetics) were cultured in optimized SMC medium (Clonetics), supplemented with epidermal growth factor, insulin, fibroblast growth factor and 5% FCS. Cells were characterized by staining positively for SMC α-actin and used in experiments between passages 3-12.
Ethanol treatment
EtOH (200 proof, ACS/USP grade, Ultrapure LLC, Darien, CT) was diluted in media to achieve the desired concentration before being added to cultures. HCASMC were treated with a recombinant soluble Notch ligand, Delta-like ligand 4 (DLL4, R&D systems, 2 μg/ml), or transfected with constitutively active Notch 1ICD (Notch intracellular domain, N1ICD) (Morrow et al., 2010), in the absence or presence of alcohol (EtOH, 25 mM). N1IC plasmid #17623 (EF.hICN1.CMV.GFP) was obtained from Addgene (nonprofit plasmid respository).
Plasmid Preparation, Transient Transfection, Luciferase and β-Galactosidase Assays
Plasmids were prepared for transfection using a Qiagen plasmid midi kit. Cells were plated onto 6-well plates 2 days prior to transfection, at a density of 1×105 cells/well, and were transfected at 70% confluency. Plasmid transfection was performed using the Gene Pulser XcellTM system (Bio-Rad, Hercules, CA, USA). The cells were transfected with the indicated expression constructs, with the addition of a total of 5.0μg DNA/well. Transfection efficiency was confirmed and normalized to β-galactosidase (β-gal) activity following co-transfection with CMV-LacZ, a plasmid-encoding β-gal activity. Western blot analysis was performed to confirm overexpression of effector proteins. In luciferase reporter studies, cells were harvested 16–24 h post transfection, using 1 X Reporter Lysis Buffer (Promega, Madison WI). Transactivation of reporter genes was evaluated by the luciferase assay (Promega) and normalized to the β-gal activity. The latter was performed according to the manufacturer’s instructions (High Sensitivity β-galactosidase Assay; Stratagene, La Jolla, CA).
Western Blot Analysis
Proteins from cell lysates (12-15 μg) were resolved on precast SDS-PAGE gels (12% resolving, 5% stacking) prior to transfer onto nitrocellulose membrane (BIORAD, Carlsbad, CA). Membranes were stained with Ponceau S and probed for β-actin or GAPDH to ensure equal protein loading and transfer, and rinsed in wash buffer (PBS containing 0.05% Tween-20) before being probed using commercially available antibodies from Abcam (Cambridge, MA) essentially as described previously (Morrow et al., 2014).
Quantitative real-time RT-PCR
Total RNA (1-2 μg), isolated from cells using Qiagen RNeasy kit (Valencia, CA) was reverse-transcribed using iscriptTM cDNA Synthesis kit from BIO-RAD (Carlsbad, CA). The gene-specific (Hrt3, Hrt2) oligonucleotide sequences were as previously described(Morrow et al., 2008a). Real-Time RT-PCR was performed using the Stratagene MX3005 machine and the SYBER green jumpstart PCR kit (Sigma, St. Louis, MO) as described by the manufacturer.
Gamma-secretase assay
γ-secretase activity was measured using an intramolecularly quenched fluorogenic peptide substrate from Calbiochem (cat# 565764) essentially as described by Farmery et al. (2003). This substrate contains the C-terminal β-APP (amyloid β-peptide precursor protein) amino acid sequence that is cleaved by γ-secretase. The ReadyPrep (membrane II) protein extraction kit (BioRad, cat#163-2084) was used to isolate SMC cellular membranes and then to extract associated integral membrane proteins into a solution. Protein concentration in membrane preparations was determined by Bradford assay. Activity was measured by pre-incubating solubilized membranes (10 μg of total protein in assay buffer with 0.25% CHAPSO w/v), +/− DLL4, in the absence or presence of ethanol (5-25 mM) or the γ-secretase inhibitor (GSI) DAPT (20 μM, Tocris Bioscience) for 1 hr. The fluorescent peptide probe (8 μM) was then added, and tubes incubated at 37°C, overnight. Fluorescence was measured using a plate reader (POLARstar OPTIMA) with excitation wavelength at 355nm and emission wavelength at 440nm. Background fluorescence of the peptide probe was subtracted from all readings.
γ-secretase activity was also determined by incubating solubilized SMC membranes with a Flag-tagged Notch-based substrate, N100Flag (a kind gift from Dr. Dennis Selkoe, Harvard Medical School)(Kimberly et al., 2003), in the presence or absence of ethanol (25 mM). N100Flag contains 99 residues of the Notch 1 sequence beginning from the ligand-dependent S2 cleavage site and includes an N-terminal methionine and a C-terminal FLAG sequence. Western blot analysis using anti-Flag M2 antibody (Sigma Aldrich, St. Louis, MO) was used to detect both the flag-tagged substrate and the C-terminal cleavage product that retains the Flag epitope.
Alpha-secretase (TACE/ADAM17) assay
Alpha-secretase activity in SMC lysates stimulated +/− DLL4, in the absence or presence of EtOH, was measured using a SensoLyte 520 TACE (α-secretase) activity kit (fluorometric assay) (AnaSpec, Freemont, CA) according to manufacturer instructions. This kit uses a 5-FAM (fluorophore) and QXL® 520 (quencher) labeled FRET peptide substrate for continuous measurement of enzyme activity. In the intact FRET peptide, the fluorescence of 5-FAM is quenched by QXL® 520. Upon cleavage of the FRET peptide by the active enzyme, the fluorescence of 5-FAM is recovered, and can be continuously monitored at excitation/emission = 490 nm/520 nm. TAPI, a broad-spectrum metalloprotease inhibitor, was used as a control α-secretase inhibitor.
Data analysis
Results are expressed as means ± SEM. Experimental points were performed in duplicate with a minimum of 3 independent experiments. A value of p<0.05 was considered significant.
Results
Ethanol inhibits ligand-stimulated, but not N1IC-stimulated, Notch signaling in HCASMC
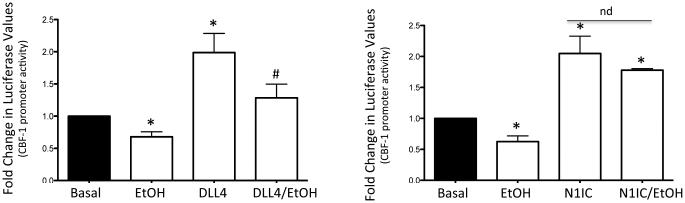
Treatment of HCASMC with recombinant soluble Delta like ligand 4 (DLL4), or transfection with the constitutively active Notch 1 intracellular domain (N1ICD), stimulated Notch signaling-associated CBF-1 promoter activity in these cells as determined by luciferase assay (Fig 2). Co-treatment with Ethanol (EtOH, 25 mM) significantly inhibited DLL4-driven promoter activity, but had no effect on N1ICD-driven promoter activity (Fig 2). Similarly, Ethanol inhibited DLL4-driven, but not N1ICD-driven Notch target gene Hrt3 expression in these cells (Fig 3). These data suggest that ethanol inhibits Notch signaling in HCASMC at, or prior to, NICD generation. Similar results were obtained using human aortic smooth muscle cells (HAoSMC, Lonza) (data not shown). As two distinct cleavage events are necessary for NICD generation and propagation of Notch signaling, we determined the effect of ethanol on the 2 proteases involved, i.e., α- and γ-secretase.
Figure 2.
Ethanol inhibits ligand-driven but not N1IC-driven CBF-1 promoter activity in HCASMC. CBF-1 promoter activity was determined by luciferase assay in SMC treated with or without recombinant Notch ligand Delta-like ligand 4 (DLL4, 2 μg/ml), or transfected with constitutively active Notch 1 IC (N1IC), in the absence or presence of EtOH (25 mM, 24h). *p<0.05 vs basal, # p<0.05 vs DLL4 alone. Data are mean± SEM (n=3).
Figure 3.
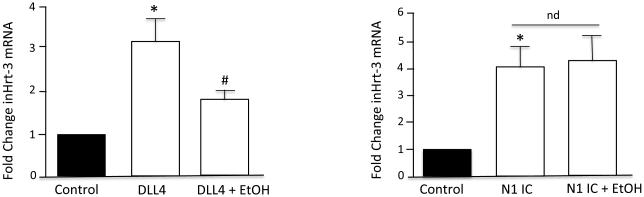
Ethanol inhibits ligand-driven but not N1IC-driven target gene HRT-3 mRNA expression. Real-time RT-PCR of HRT-3 mRNA in SMC treated with +/− recombinant (DLL4, 2 μg/ml), or transfected with constitutively active Notch 1 IC (N1IC), in the absence or presence of EtOH (25 mM, 24h). *p<0.05 vs control, # p<0.05 vs DLL4 alone. Data are mean± SEM (n=3).
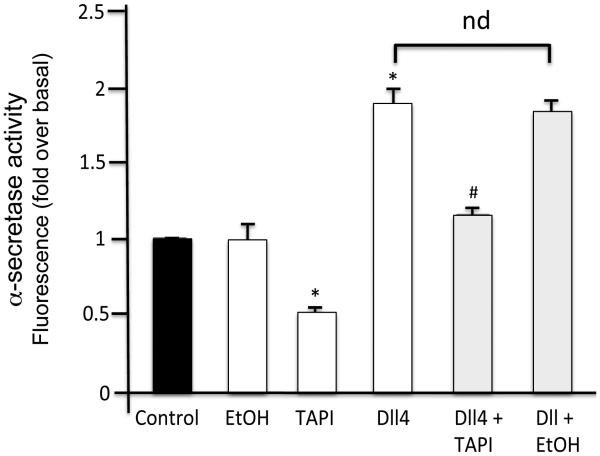
No effect of ethanol on α-secretase (TACE/ADAM17) activity
As determined using the SensoLyte® 520 TACE (α-secretase) Activity Assay Kit, DLL4 treatment stimulated α-secretase activity above basal levels (Fig 4). Basal and DLL4-stimulated α-secretase activity was inhibited by the metalloprotease inhibitor TAPI (Fig 4). In contrast, ethanol treatment (25 mM) had no significant effect on basal or DLL4-stimulated α-secretase activity in HCASMC membranes (Fig 4).
Figure 4.
Ethanol has no effect on α-secretase (TACE/ADAM17) activity. Alpha-secretase activity in HCASMC lysates stimulated +/− (DLL4, 2 μg/ml), in the absence or presence of ethanol (EtOH, 25 mM), was determined using a Sensolyte 520 TACE (α-secretase) fluorometric assay. TAPI was used as a control α-secretase inhibitor. Data are mean ±SEM, n=3. *p<0.05 vs control, # p<0.05 vs DLL4 alone.
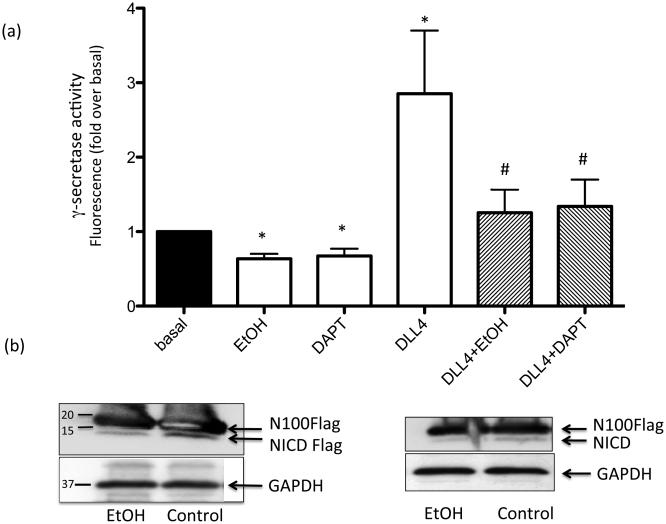
EtOH inhibits SMC γ-secretase activity
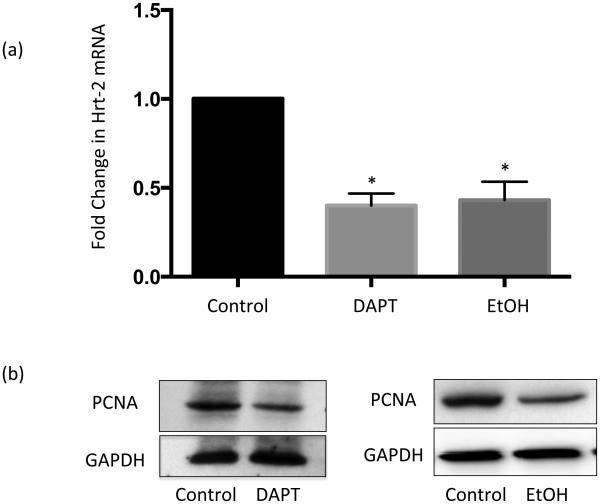
The effect of ethanol on γ-secretase activity in HCASMC membranes was determined using a fluorogenic peptide substrate. In this assay, increased fluorescence is indicative of γ-secretase cleavage activity. Treatment with DLL4 stimulated γ-secretase activity 2-4 fold above basal (Fig 5a). This ligand-stimulated γ-secretase activity was completely inhibited by the specific γ-secretase inhibitor (GSI) DAPT (20 μM) (Fig 5a). DAPT also significantly inhibited basal γ-secretase activity (Fig 5a). Treatment with Ethanol (EtOH 25 mM) significantly inhibited basal γ-secretase activity and completely inhibited DLL4-stimulated γ-secretase activity similar to the effect of DAPT (Fig 5a). Similar effects were observed with 5 and 10 mM EtOH, and in HAoSMC (data not shown). Western blot analysis of HCASMC membranes demonstrated less cleavage product of N100Flag substrate (i.e., NICD) following ethanol treatment, compared with control, indicating inhibition of γ-secretase proteolysis of Notch (Fig 5b). Of note, both EtOH and GSI treatment alone inhibited Notch target gene expression and cell proliferation, determined by proliferating cell nuclear antigen (PCNA) expression, to a similar extent in HCASMC (Fig 6).
Figure 5.
Ethanol inhibits SMC γ-secretase activity. (a) γ-secretase activity in solubilized HCASMC membranes was measured using a fluorogenic peptide substrate (C-terminal fragment of β-APP) as described in Methods. DAPT was used as a control γ-secretase inhibitor (GSI). Data are mean ±SEM, n=3. *p<0.05 vs control, # p<0.05 vs DLL4 alone. (b) HCASMC membranes from cells treated with or without EtOH were incubated with flag-tagged Notch based substrate (N100Flag). Representative Western blots show detection of N100Flagsubstrate and its cleavage product under control and EtOH conditions. Blots were reprobed with GAPDH as a loading control.
Figure 6.
The γ-secretase inhibitor, DAPT, and Ethanol alone inhibit Notch signaling and proliferation in HCASMC to a similar extent. (a) Notch target gene HRT2 mRNA levels, determined by RT-PCR, in HCASMC treated (24 h) with or without or DAPT (20 μM) or EtOH (25 mM) (n=3, * p<0.05 vs control). (b) Representative Western blots for proliferating cell nuclear antigen (PCNA) in HCASMC treated +/− DAPT or EtOH. Blots reprobed with GAPDH as a loading control.
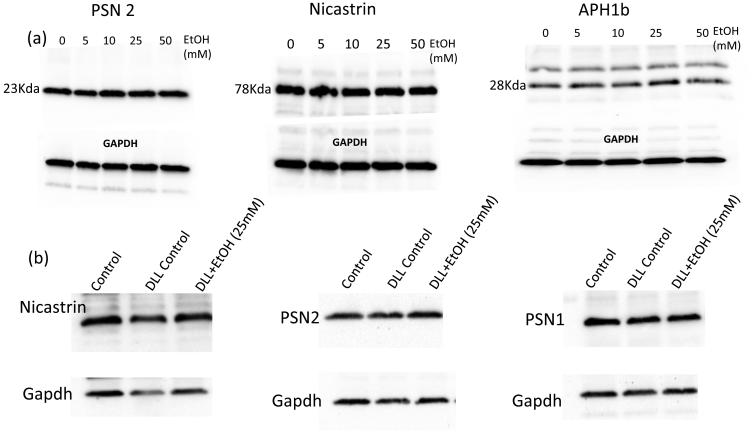
No effect of EtOH (24h) on γ-secretase subunit expression
Gamma-secretase is a complex composed of at least 4 subunits; catalytic presenilin-1 (PSN1, PSN2), and accessory subunits Pen-2, Aph1α or 1β and Nicastrin. As determined by Western Blot, Ethanol treatment (5-50 mM, 24 hr) had no effect on the expression levels of PSN1, PSN2, Aph1α or Nicastrin in HCASMC, either in control HCASMC, or those stimulated with Dll4 (Fig 7, and data not shown). Pen-2 was not successfully detected in our samples.
Figure 7.
No effect of EtOH on γ-secretase subunit protein expression. (a) HCASMC were treated with/without EtOH (0-50 mM, 24hr). Representative western blots for Presenilin2, Nicastrin and Aph1b shown. (b) HCASMC were treated with/without DLL4, in the absence or presence of EtOH (25 mM, 24h). Representative western blots shown. Blots were reprobed with GAPDH as a loading control.
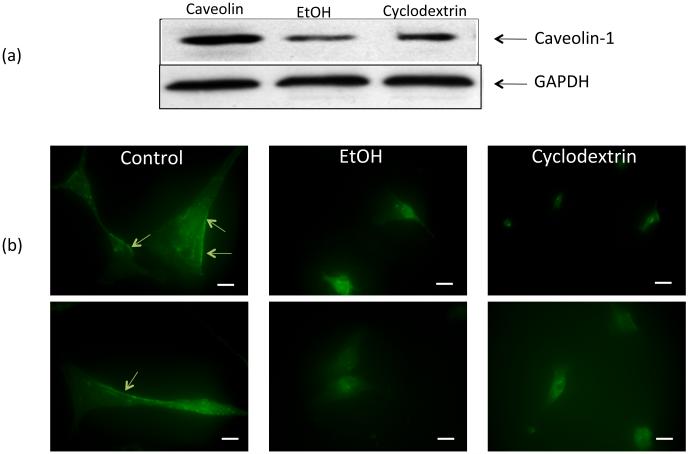
Ethanol affects SMC Caveolin expression
EtOH treatment of HCASMC affected the expression and localization of the lipid raft marker Caveolin-1 (the isoform most abundantly expressed in SMC) in these cells. Specifically, Caveolin-1 protein expression as determined by Western Blot was decreased in EtOH-treated cells (Fig 8a). Moreover, the pattern of immunofluorescent staining for Caveolin-1 in these cells was altered by ethanol, with less staining along the plasma membrane and more localized to intracellular sites (Fig 8b). The lipid raft disrupting agent methyl-β-cyclodextrin (10 mM) had similar effects on Caveolin-1 protein levels and staining pattern (Fig 8).
Figure 8.
The effect of ethanol on SMC Caveolin-1 expression. HCASMC were treated with or without EtOH (25 mM) or the lipid raft disrupting agent methyl-β-cyclodextrin (10 mM) for 30 mins (immunofluorescence study) or 24 h (Western blot analysis). (a) Caveolin-1 protein levels (representative Western blot; GAPDH as a loading control); (b) Immunofluorescent staining of SMC using a polyclonal anti-Caveolin antibody and an AlexaFluor 488 labeled secondary antibody. Two representative pictures shown (top and bottom are same condition), acquired using a Nikon TE300 Diaphot microscope/Spot RT ccd digital camera). Arrows indicating Caveolin-1 in the plasma membrane. Bar = 25 μM
Discussion
Moderate alcohol consumption is a negative risk factor for atherosclerosis and its clinical sequelae heart attack and stroke (Di Castelnuovo et al., 2006; Roerecke & Rehm, 2014). We have previously described that EtOH inhibits Notch signaling and the Notch-dependent proliferation of vascular smooth muscle cells key to vessel remodeling and atherosclerotic plaque development (Morrow et al., 2010). Here we report that the mechanism whereby EtOH negatively regulates Notch signaling in these cells is by specific inhibition of the γ-secretase proteolytic activity necessary for activation of the Notch receptor. This novel effect of EtOH may be pertinent to its cardiovascular protective effects as well as to other biological actions of alcohol consumption.
Notch plays a pivotal role in the regulation of many fundamental cellular processes such as proliferation, stem cell maintenance, and differentiation during embryonic development and in the adult (Borggrefe & Liefke, 2012). It has been implicated in a variety of pathologies including vascular disease (Y. Li et al., 2009; Morrow et al., 2008b; Redmond et al., 2014), cancer and immune disorders (Li et al., 2014; Takebe et al., 2014). As a result Notch inhibitors, including γ-secretase inhibitors (GSIs) and monoclonal antibodies (mAbs) against Notch receptors or ligands, are currently in clinical development for several diseases (Takebe et al., 2014). The Notch signaling pathway belongs to a family that utilizes ‘regulated intramembrane proteolysis’ (RIP) for signal transduction. Unlike in more traditional signaling pathways, there are no G-protein coupled receptors, no ion channels activated or second messengers involved. Instead, RIP results in the release of extracellular and/or intracellular domains from transmembrane proteins; these cleaved fragments then act as biological effectors at other sites in the cell. In the case of Notch specifically, ligand (i.e., Delta-like 1,3 and 4 or Jagged 1,2) binding to the receptor (i.e., Notch 1-4) induces the proteolytic cleavage of the receptor, first at the extracellular site by α-secretase (TACE/ADAM17) followed by cleavage at the intracellular site by γ-secretase, resulting in subsequent translocation of the Notch intracellular domain (NICD) into the nucleus where it associates with the central transcription factor RBP-J and activates transcription(Baron, 2003).
While EtOH represses smooth muscle cell proliferation by inhibiting Notch signaling (Morrow et al., 2010), there are several potential regulatory points in this pathway at which it could do so. Our experiments in HCASMC demonstrated that whereas EtOH treatment inhibited Notch ligand-stimulated signaling as determined by CBF-1 promoter activity and HRT target gene expression, it had no significant effect on N1ICD-driven signaling. These data suggested that EtOH was inhibiting Notch signaling at, or prior to, NICD generation. Based on that data, we focused on determining a possible regulation of α- and/or γ-secretase proteolytic activity in HCASMC by EtOH.
Ethanol had no effect on α-secretase (TACE/ADAM17) activity. In contrast EtOH, at levels achievable by ‘moderate’ drinking, inhibited basal and DLL4-stimulated γ-secretase activity, to a degree similar to the widely used γ-secretase inhibitor DAPT. Functionally, EtOH’s inhibitory effect on smooth muscle cell proliferation was mimicked by treatment with DAPT. The peptide substrate used in the fluorogenic γ-secretase assay was the C-terminal of amyloid β-peptide precursor protein (β-APP). To date more than 90 proteins (generally type 1 transmembrane proteins) have been identified as substrates for γ-secretase, with Notch and β-APP the most characterized (Jurisch-Yaksi, Sannerud, & Annaert, 2013). Of note, we previously obtained similar results (i.e., inhibition of γ-secretase activity by EtOH) using a Notch substrate flurophore as substrate (no longer commercially available) and the Notch ligand Jag2 to stimulate activity (data not shown). Moreover, the effect of EtOH on γ-secretase activity was also determined using N100Flag, a Flag-tagged Notch 1 sequence containing the S3 cleavage site, as a substrate and Western blot analysis to detect the amount of cleaved fragment as an indicator of proteolytic activity. Taken together, our data indicate that EtOH inhibits γ-secretase cleavage of both Notch and β-APP substrates.
Gamma-secretase is a multi-subunit enzyme composed of a catalytic element presenilin (PSN)-1, PSN-2, and accessory subunits Pen-2, Aph1α or Aph1β and Nicastrin. It is likely that different subunit composition of the complex contributes to tissue and substrate specificity and to differences in susceptibility to various γ-secretase inhibitors(Shih & Wang, 2007). As determined by western blot analysis HCASMC expressed PSN1 and PSN2, Nicastrin and Aph1β. Ethanol’s inhibitory effect on γ-secretase activity in these cells occurred in the absence of any effect on the expression of these protein subunits, either under basal conditions or in cells stimulated by a Notch ligand.
In neural tissue, γ-secretase activity is associated with lipid rafts (Vetrivel & Thinakaran, 2010), and alterations in membrane lipid composition can affect secretase activities (Walter & van Echten-Deckert, 2013). Caveolin is a protein found in specialized lipid rafts called caveolae that are involved in cell signaling and transport (Chidlow & Sessa, 2010; Pavlides et al., 2012). Caveolin-1 is the most abundant isoform expressed in smooth muscle cells. Caveolin-1’s functional role can vary in different vascular cell types (e.g., endothelial vs smooth muscle)(Chidlow & Sessa, 2010) and it has been implicated in chronic inflammatory conditions and pathologies including atherosclerosis (Chidlow & Sessa, 2010). Lin et al., reported that a change in abundance of caveolin-1 was associated with SMC proliferation and plaque formation in hypercholesterolemic rabbits(Lin et al., 2006). Of interest, Caveolin-1 has previously been reported, albeit in non-vascular cells, to regulate γ-secretase activity (Kapoor et al., 2010), and to modulate Notch signaling (S. Wang et al., 2013). Kapoor et al (2010) demonstrated that suppression of Caveolin-1 in HEK 293 cells using small interfering RNA (siRNA) altered γ-secretase-mediated processing of Notch. In our study, we found that EtOH treatment of HCASMC inhibited ligand-stimulated γ-secretase activity and Notch signaling concomitant with inhibition of the expression of Caveolin-1, and changes in its localization in these cells. These effects of EtOH on caveolin-1 may mediate its inhibition of γ-secretase activity in HCASMC. We note that a recent report describes an opposite, stimulatory effect of a much higher concentration (i.e., ~220 mM) of EtOH on Caveolin-1 expression in HepG2 hepatocarcinoma cells (Y. Wang et al., 2015), highlighting potential cell- and/or concentration-dependent differences of EtOH on this protein.
In conclusion, ethanol represses Notch signaling in vascular smooth muscle cells by specifically targeting γ-secretase proteolytic activity, with no effect on α-secretase, thus preventing NICD generation and target gene transcription. Given the role of Notch in driving SMC proliferation, this effect of ethanol should be significant in pathologic vascular remodeling events such as atherosclerosis. Moreover, given the promiscuous nature of γ-secretase in terms of its numerous substrates, the inhibition of this protease by ethanol described here may also be important in mediating other effects of alcohol consumption in addition to its’ cardiovascular effects.
Acknowledgements
This work was supported by grants from the National Institutes of Health; 1F32AA022016 to E.H, R21AA20365 and RO1AA012610 to E.M.R, and by K99HL095650 to D.M.
Footnotes
Conflict of interest.
None to declare.
References
- Baron M. An overview of the Notch signalling pathway. Seminars in Cell & Developmental Biology. 2003;14(2):113–119. doi: 10.1016/s1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Liefke R. Fine-tuning of the intracellular canonical Notch signaling pathway. Cell Cycle (Georgetown, Tex.) 2012;11(2):264–276. doi: 10.4161/cc.11.2.18995. doi:10.4161/cc.11.2.18995. [DOI] [PubMed] [Google Scholar]
- Chidlow JH, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovascular Research. 2010;86(2):219–225. doi: 10.1093/cvr/cvq075. doi:10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Archives of Internal Medicine. 2006;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. doi:10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- Farmery MR, Tjernberg LO, Pursglove SE, Bergman A, Winblad B, Näslund J. Partial purification and characterization of gamma-secretase from post-mortem human brain. The Journal of Biological Chemistry. 2003;278(27):24277–24284. doi: 10.1074/jbc.M211992200. doi:10.1074/jbc.M211992200. [DOI] [PubMed] [Google Scholar]
- Jurisch-Yaksi N, Sannerud R, Annaert W. A fast growing spectrum of biological functions of γ-secretase in development and disease. Biochimica Et Biophysica Acta. 2013;1828(12):2815–2827. doi: 10.1016/j.bbamem.2013.04.016. doi:10.1016/j.bbamem.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Hsu W-M, Wang B-J, Wu G-H, Lin T-Y, Lee S-J, et al. Caveolin-1 regulates γ-secretase-mediated AβPP processing by modulating spatial distribution of γ-secretase in membrane. Journal of Alzheimer's Disease : JAD. 2010;22(2):423–442. doi: 10.3233/JAD-2010-100531. doi:10.3233/JAD-2010-100531. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Esler WP, Ye W, Ostaszewski BL, Gao J, Diehl T, et al. Notch and the amyloid precursor protein are cleaved by similar gamma-secretase(s) Biochemistry. 2003;42(1):137–144. doi: 10.1021/bi026888g. doi:10.1021/bi026888g. [DOI] [PubMed] [Google Scholar]
- Lacolley P, Regnault V, Nicoletti A, Li Z, Michel J-B. The vascular smooth muscle cell in arterial pathology: a cell that can take on multiple roles. Cardiovascular Research. 2012;95(2):194–204. doi: 10.1093/cvr/cvs135. doi:10.1093/cvr/cvs135. [DOI] [PubMed] [Google Scholar]
- Li D, Masiero M, Banham AH, Harris AL. The notch ligand JAGGED1 as a target for anti-tumor therapy. Frontiers in Oncology. 2014;4:254. doi: 10.3389/fonc.2014.00254. doi:10.3389/fonc.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Takeshita K, Liu P-Y, Satoh M, Oyama N, Mukai Y, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119(20):2686–2692. doi: 10.1161/CIRCULATIONAHA.108.790485. doi:10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W-W, Lin Y-C, Chang T-Y, Tsai S-H, Ho H-C, Chen Y-T, Yang VC. Caveolin-1 expression is associated with plaque formation in hypercholesterolemic rabbits. The Journal of Histochemistry and Cytochemistry : Official Journal of the Histochemistry Society. 2006;54(8):897–904. doi: 10.1369/jhc.5A6869.2006. doi:10.1369/jhc.5A6869.2006. [DOI] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Cahill PA, Redmond EM. Ethanol stimulates endothelial cell angiogenic activity via a Notch- and angiopoietin-1-dependent pathway. Cardiovascular Research. 2008a;79(2):313–321. doi: 10.1093/cvr/cvn108. doi:10.1093/cvr/cvn108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM. Alcohol inhibits smooth muscle cell proliferation via regulation of the Notch signaling pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2597–2603. doi: 10.1161/ATVBAHA.110.215681. doi:10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O'Brien C, et al. Notch and vascular smooth muscle cell phenotype. Circulation Research. 2008b;103(12):1370–1382. doi: 10.1161/CIRCRESAHA.108.187534. doi:10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- Morrow D, Hatch E, Hamm K, Cahill PA, Redmond EM. Flk-1/KDR mediates ethanol-stimulated endothelial cell Notch signaling and angiogenic activity. Journal of Vascular Research. 2014;51(4):315–324. doi: 10.1159/000367807. doi:10.1159/000367807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides S, Gutierrez-Pajares JL, Danilo C, Lisanti MP, Frank PG. Atherosclerosis, caveolae and caveolin-1. Advances in Experimental Medicine and Biology. 2012;729:127–144. doi: 10.1007/978-1-4614-1222-9_9. doi:10.1007/978-1-4614-1222-9_9. [DOI] [PubMed] [Google Scholar]
- Pearson TA. Alcohol and heart disease. Circulation. 1996;94(11):3023–3025. doi: 10.1161/01.cir.94.11.3023. [DOI] [PubMed] [Google Scholar]
- Proweller A, Pear WS, Parmacek MS. Notch signaling represses myocardin-induced smooth muscle cell differentiation. The Journal of Biological Chemistry. 2005;280(10):8994–9004. doi: 10.1074/jbc.M413316200. doi:10.1074/jbc.M413316200. [DOI] [PubMed] [Google Scholar]
- Redmond EM, Liu W, Hamm K, Hatch E, Cahill PA, Morrow D. Perivascular delivery of Notch 1 siRNA inhibits injury-induced arterial remodeling. PloS One. 2014;9(1):e84122. doi: 10.1371/journal.pone.0084122. doi:10.1371/journal.pone.0084122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, Rehm J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Medicine. 2014;12(1):182. doi: 10.1186/s12916-014-0182-6. doi:10.1186/s12916-014-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber CJ, Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. BioEssays. 2004;26(3):225–234. doi: 10.1002/bies.20004. doi:10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- Shih I-M, Wang T-L. Notch signaling, gamma-secretase inhibitors, and cancer therapy. Cancer Research. 2007;67(5):1879–1882. doi: 10.1158/0008-5472.CAN-06-3958. doi:10.1158/0008-5472.CAN-06-3958. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, et al. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jk dependent pathway. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(12):1421–1423. doi: 10.1096/fj.04-1700fje. doi:10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacology & Therapeutics. 2014;141(2):140–149. doi: 10.1016/j.pharmthera.2013.09.005. doi:10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun MJ, Peto R, Lopez AD, Monaco JH, Henley SJ, Heath CW, Doll R. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. The New England Journal of Medicine. 1997;337(24):1705–1714. doi: 10.1056/NEJM199712113372401. doi:10.1056/NEJM199712113372401. [DOI] [PubMed] [Google Scholar]
- Vetrivel KS, Thinakaran G. Membrane rafts in Alzheimer's disease beta-amyloid production. Biochimica Et Biophysica Acta. 2010;1801(8):860–867. doi: 10.1016/j.bbalip.2010.03.007. doi:10.1016/j.bbalip.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, van Echten-Deckert G. Cross-talk of membrane lipids and Alzheimer-related proteins. Molecular Neurodegeneration. 2013;8:34. doi: 10.1186/1750-1326-8-34. doi:10.1186/1750-1326-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Kan Q, Sun Y, Han R, Zhang G, Peng T, Jia Y. Caveolin-1 regulates neural differentiation of rat bone mesenchymal stem cells into neurons by modulating Notch signaling. International Journal of Developmental Neuroscience : the Official Journal of the International Society for Developmental Neuroscience. 2013;31(1):30–35. doi: 10.1016/j.ijdevneu.2012.09.004. doi:10.1016/j.ijdevneu.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Wang W, Prince CZ, Hu X, Pollman MJ. HRT1 modulates vascular smooth muscle cell proliferation and apoptosis. Biochemical and Biophysical Research Communications. 2003;308(3):596–601. doi: 10.1016/s0006-291x(03)01453-0. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tong J, Chang B, Wang B-F, Zhang D, Wang B-Y. Effects of ethanol on the expression of caveolin-1 in HepG2 cells. Molecular Medicine Reports. 2015;11(6):4409–4413. doi: 10.3892/mmr.2015.3296. doi:10.3892/mmr.2015.3296. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li Y, Xu H, Zhang Y-W. The γ-secretase complex: from structure to function. Frontiers in Cellular Neuroscience. 2014;8:427. doi: 10.3389/fncel.2014.00427. doi:10.3389/fncel.2014.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]