Abstract
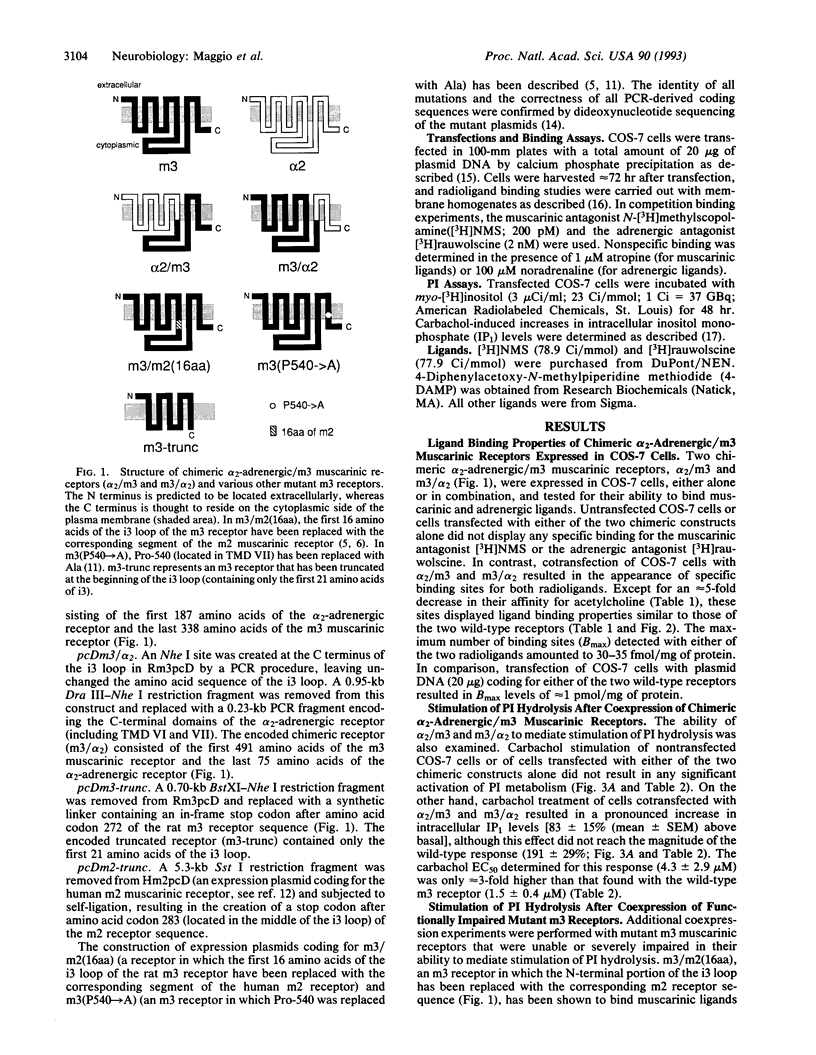
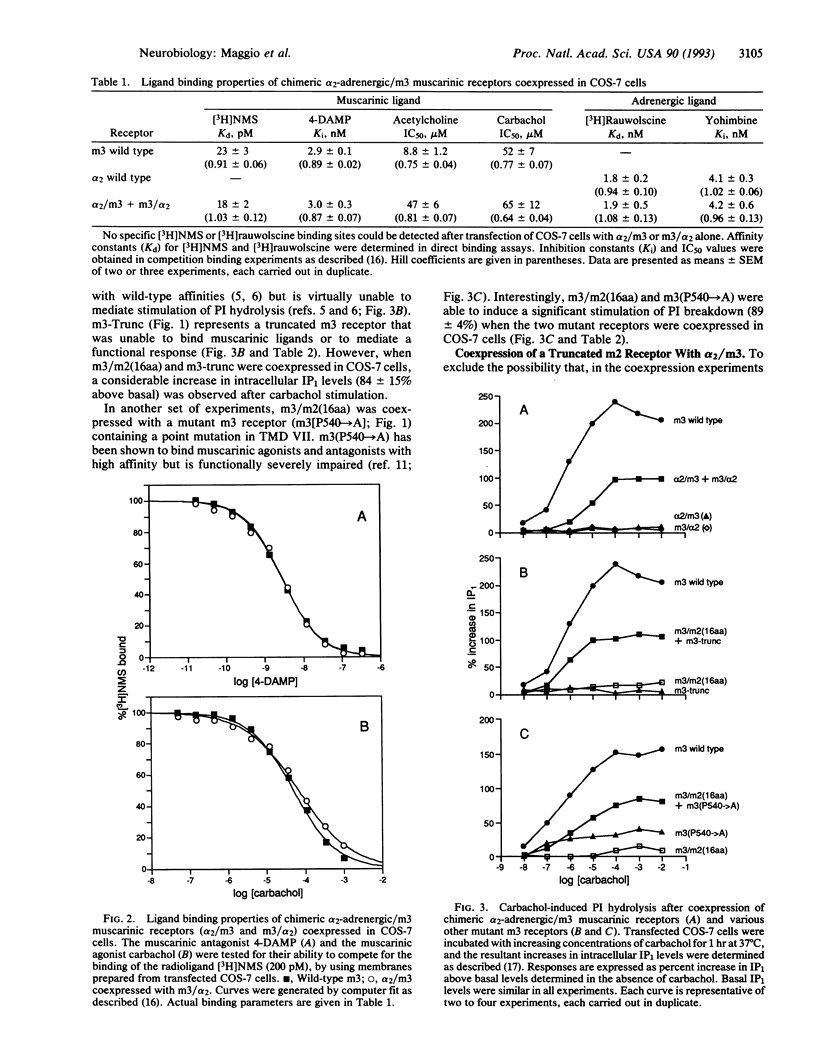
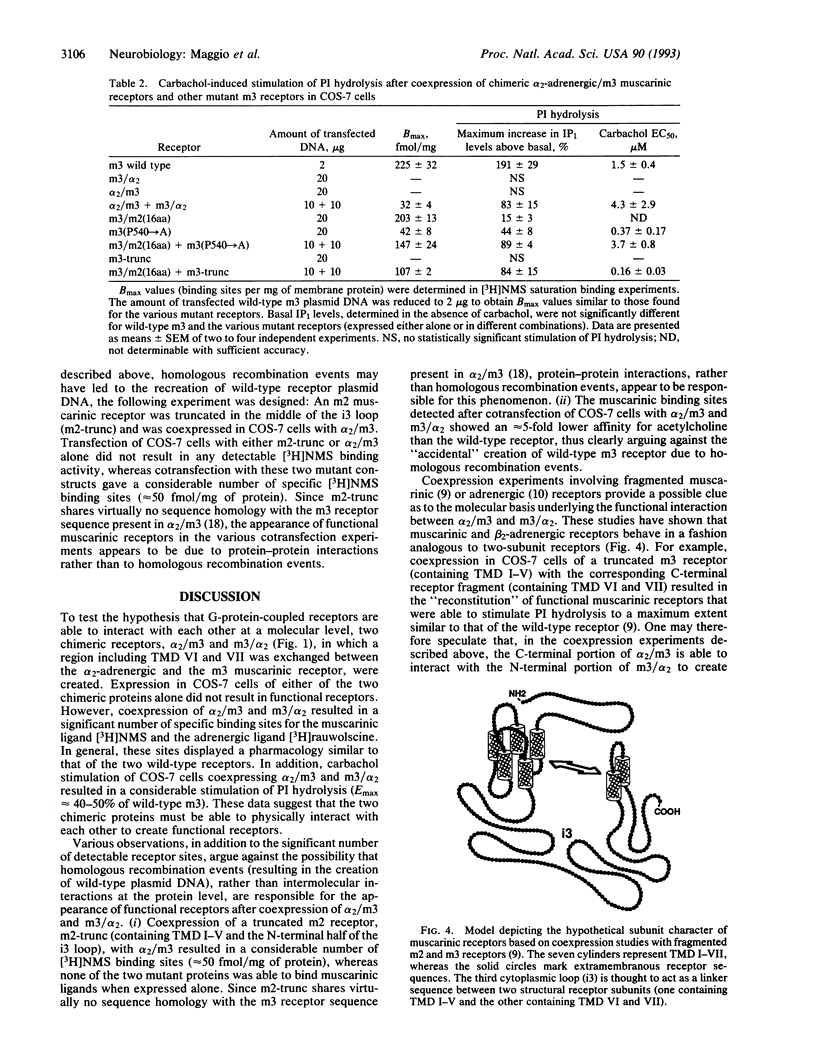
We have tested the hypothesis that guanine-nucleotide-binding-protein-coupled receptors may be able to interact with each other at a molecular level. To address this question, we have initially created two chimeric receptors, alpha 2/m3 and m3/alpha 2, in which the C-terminal receptor portions (containing transmembrane domains VI and VII) were exchanged between the alpha 2C-adrenergic and the m3 muscarinic receptor. Transfection of COS-7 cells with either of the two chimeric constructs alone did not result in any detectable binding activity for the muscarinic ligand N-[3H]methylscopolamine or the adrenergic ligand [3H]rauwolscine. However, cotransfection with alpha 2/m3 and m3/alpha 2 resulted in the appearance of specific binding sites (30-35 fmol/mg of membrane protein) for both radioligands. These sites displayed ligand binding properties similar to those of the two wild-type receptors. Furthermore, COS-7 cells cotransfected with alpha 2/m3 and m3/alpha 2 were able to mediate a pronounced stimulation of phosphatidylinositol hydrolysis upon stimulation with the muscarinic agonist carbachol (Emax approximately 40-50% of wild-type m3). A mutant m3 receptor (containing 16 amino acids of m2 receptor sequence at the N terminus of the third cytoplasmic loop) that was capable of binding muscarinic ligands but was virtually unable to stimulate phosphatidylinositol hydrolysis was also used in various cotransfection experiments. Coexpression of this chimeric receptor with other functionally impaired mutant muscarinic receptors (e.g., with an m3 receptor containing a Pro-->Ala point mutation in transmembrane region VII) resulted in a considerable stimulation of phosphatidylinositol breakdown after carbachol treatment (Emax approximately 40-50% of wild-type m3). Thus, these data suggest that guanine-nucleotide-binding-protein-coupled receptors can interact with each other at a molecular level. One may speculate that the formation of receptor dimers involving the intermolecular exchange of N- and C-terminal receptor domains (containing transmembrane domains I-V and VI and VII, respectively) may underlie this phenomenon.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar S., Amitai G., Sokolovsky M. Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. Proc Natl Acad Sci U S A. 1983 Jan;80(1):156–159. doi: 10.1073/pnas.80.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar S., Moscona-Amir E., Sokolovsky M. Photoaffinity labeling reveals two muscarinic receptor macromolecules associated with the presence of calcium in rat adenohypophysis. FEBS Lett. 1982 Dec 27;150(2):343–346. doi: 10.1016/0014-5793(82)80765-5. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989 Apr;12(4):148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörje F., Wess J., Lambrecht G., Tacke R., Mutschler E., Brann M. R. Antagonist binding profiles of five cloned human muscarinic receptor subtypes. J Pharmacol Exp Ther. 1991 Feb;256(2):727–733. [PubMed] [Google Scholar]
- Hartig P., Kao H. T., Macchi M., Adham N., Zgombick J., Weinshank R., Branchek T. The molecular biology of serotonin receptors. An overview. Neuropsychopharmacology. 1990 Oct-Dec;3(5-6):335–347. [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Kurtenbach E., Curtis C. A. Muscarinic acetylcholine receptors: structure and function. Biochem Soc Trans. 1991 Feb;19(1):133–138. doi: 10.1042/bst0190133. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio R., Vogel Z., Wess J. Reconstitution of functional muscarinic receptors by co-expression of amino- and carboxyl-terminal receptor fragments. FEBS Lett. 1993 Mar 15;319(1-2):195–200. doi: 10.1016/0014-5793(93)80066-4. [DOI] [PubMed] [Google Scholar]
- Nathanson N. M. Molecular properties of the muscarinic acetylcholine receptor. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and alpha 2-adrenergic receptors based upon characteristics in primary structure. J Biol Chem. 1992 Apr 25;267(12):8342–8346. [PubMed] [Google Scholar]
- Potter L. T., Ballesteros L. A., Bichajian L. H., Ferrendelli C. A., Fisher A., Hanchett H. E., Zhang R. Evidence of paired M2 muscarinic receptors. Mol Pharmacol. 1991 Feb;39(2):211–221. [PubMed] [Google Scholar]
- Potter L. T., Ferrendelli C. A., Hanchett H. E. Two affinity states of M1 muscarine receptors. Cell Mol Neurobiol. 1988 Jun;8(2):181–191. doi: 10.1007/BF00711244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt M. M., McCune S. K., Kanterman R. Y., Felder C. C. The rat alpha 2-C4 adrenergic receptor gene encodes a novel pharmacological subtype. FEBS Lett. 1991 Jan 14;278(1):45–50. doi: 10.1016/0014-5793(91)80080-m. [DOI] [PubMed] [Google Scholar]
- Wess J., Bonner T. I., Brann M. R. Chimeric m2/m3 muscarinic receptors: role of carboxyl terminal receptor domains in selectivity of ligand binding and coupling to phosphoinositide hydrolysis. Mol Pharmacol. 1990 Dec;38(6):872–877. [PubMed] [Google Scholar]
- Wess J., Bonner T. I., Dörje F., Brann M. R. Delineation of muscarinic receptor domains conferring selectivity of coupling to guanine nucleotide-binding proteins and second messengers. Mol Pharmacol. 1990 Oct;38(4):517–523. [PubMed] [Google Scholar]
- Wess J., Brann M. R., Bonner T. I. Identification of a small intracellular region of the muscarinic m3 receptor as a determinant of selective coupling to PI turnover. FEBS Lett. 1989 Nov 20;258(1):133–136. doi: 10.1016/0014-5793(89)81633-3. [DOI] [PubMed] [Google Scholar]
- Wess J., Gdula D., Brann M. R. Site-directed mutagenesis of the m3 muscarinic receptor: identification of a series of threonine and tyrosine residues involved in agonist but not antagonist binding. EMBO J. 1991 Dec;10(12):3729–3734. doi: 10.1002/j.1460-2075.1991.tb04941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J., Nanavati S., Vogel Z., Maggio R. Functional role of proline and tryptophan residues highly conserved among G protein-coupled receptors studied by mutational analysis of the m3 muscarinic receptor. EMBO J. 1993 Jan;12(1):331–338. doi: 10.1002/j.1460-2075.1993.tb05661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]




