Abstract
Combining natural α-amino acid residues and unnatural β-amino acid residues in a single chain leads to heterogeneous-backbone oligomers called α/β-peptides. Despite their unnatural backbones, α/β-peptides can manifest a variety of folding patterns and biological functions reminiscent of natural peptides and proteins. Moreover, incorporation of β-residues can impart useful properties to the oligomer such as improved stability to degradation by protease enzymes. α/β-Peptides have been developed that engage diverse biological targets, including proteins involved in apoptotic signaling, HIV-cell fusion, hormone signaling, and angiogenesis. For some systems, promising results obtained in vitro have paved the way for demonstrated activity in vivo, where α/β-peptides show equal potency and improved duration of effect compared to α-peptide counterparts.
Introduction
Protein–protein binding interactions are involved in myriad cellular functions, including those associated with many diseases. Agents that modulate binding interactions between proteins have tremendous therapeutic potential; however, the structural properties of protein–protein interfaces can make the development of inhibitors challenging.[1] Peptides and proteins have proven a promising alternative to small-molecule scaffolds for some targets. While the number of peptide-based drugs is large and growing, they suffer from drawbacks to clinical use such as rapid degradation by proteases in serum.[2] A broad field of research has demonstrated that α-peptides are just one of many oligomeric backbones capable of complex folding behavior and that their unnatural-backbone analogues can adopt similar folds while resisting enzymatic degradation. Efforts to develop unnatural oligomers with folding patterns reminiscent of peptides and proteins (“foldamers”) have recently expanded into the realm of biological function.[3-6] Among the many backbones explored in this context, chains that blend α-amino acid residues with β-amino acid residues (α/β-peptides) have emerged as an important class of heterogeneous-backbone foldamers.[7-10] The present review surveys published research from the last ~5 years on α/β-peptides of moderate length that show biological function resulting from binding to a natural protein receptor (Table 1).
Table 1.
Bioactive α/β-peptides with therapeutic potential.
| α-Peptide Prototype |
Peptide Length |
β-Residue Content |
Secondary Structure |
Binding Partner |
Function (Implication) | Ref |
|---|---|---|---|---|---|---|
| BH3 domains | 26 | 27% | α-helix | Bcl-xL, Mcl-1 | Apoptosis regulation (cancer) | [15,16] |
| gp41 (CHR domain) |
38 | 29% | α-helix | gp41 (NHR domain) |
Viral cell entry (HIV) | [23] |
| Neuropeptide Y25-36 |
12 | 17% | non-regular | Y1R, Y4R | Gastrointestinal regulation, vasoconstriction (various) |
[31,32] |
| PTH1-34 | 34 | 18% | α-helix | PTH1R | Calcium regulation (osteoporosis) |
[35] |
| GLP-1 | 31 | 16% | α-helix | GLP1R | Glucose regulation (diabetes) | [37] |
| Phage-derived VEGF ligand |
19 | 32% | non- regular1 |
VEGF | Angiogenesis (cancer, diseases of the eye) |
[40] |
| Anginex | 34 | 9% | β-sheet | Gal-1 | Angiogenesis, (cancer, diseases of the eye) |
[46] |
| Phage-derived VEGF ligand |
39 | 15%1 | helix-turn- helix1 |
VEGF | Angiogenesis, (cancer, diseases of the eye) |
[50] |
Cyclized via a disulfide bond.
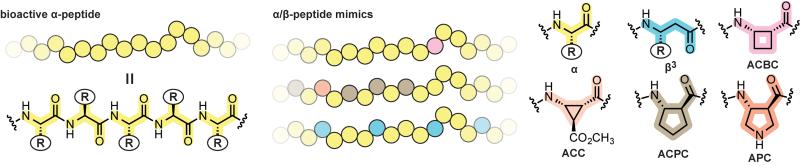
β-Residues are among the most common building blocks in foldamer research, and a number of interesting biological activities have been reported for appropriately designed β-peptides.[3-6] Although β-residues are structurally diverse, those found in the bioactive α/β-peptides covered in this review fall into one of two categories (Figure 1): β3 or βcyc. β3-Residues are backbone-homologated variants of α-residues. In α/β-peptides, β3-residues offer the advantage of retaining protein-like side chain functionality at the potential cost of increased backbone flexibility due to an additional rotatable bond. In βcyc-residues (e.g., ACBC, ACC, ACPC, APC), the central backbone torsion is constrained by a carbocyclic or heterocyclic ring. This offers the advantage of exerting control over folding behavior of the β-residue based on selection of ring size and stereochemistry.
Figure 1.
An α-peptide, example α/β-peptides, and their constituent residue structures. Replacing one or more α-amino acid residues in a biologically active α-peptide sequence with β-residues can lead to heterogeneous-backbone α/β-peptides with native-like function and improved biostability. Chemical structures and abbreviations are shown for a natural α-residue alongside those of the β-residues appearing in α/β-peptides covered in this review.
Apoptotic Signaling in the Bcl-2 Protein Family
A network of binding interactions among B-cell lymphoma-2 (Bcl-2) family proteins regulates the life and death of cells.[11] Anti-apoptotic Bcl-2 proteins, commonly overexpressed in tumor cells, exert their biological effect by binding to an amphiphilic α-helical BH3 domain on pro-apoptotic effectors, and the inhibition of this interaction has been an active target for development of cancer therapies.[12] The BH3 domain is an important early example where α/β-peptide foldamer mimicry of a bioactive α-helix led to inhibition of a biologically important protein–protein binding interaction. While the oldest of the systems covered in this review, work on the BH3 domain helps define two paradigms for the development of bioactive α/β-peptides.
Pioneering structural studies elucidated several helical folding patterns available to α/β-peptides with an alternating 1:1 backbone repeat,[13,14] and one of these scaffolds was used to develop an α/β-peptide BH3 domain mimic.[15] Structure-guided introduction of side chains on the foldamer scaffold based on the known structure of the Bcl-2 family effector protein Bak bound to anti-apoptotic Bcl-xL resulted in an α/β-peptide that bound Bcl-xL, albeit with affinity much weaker than the prototype α-peptide BH3 domain. Replacement of the C-terminal half of the alternating α/β-peptide with a pure α-peptide backbone resulted in a chimeric oligomer with tight binding affinity for Bcl-xL as well as the ability to induce release of mitochondrial cytochrome C in cancer cell lysates. Unfortunately, the α-residue rich portion of the chimera proved highly susceptible to proteolytic degradation.
Shortly after the work on chimeric α/β-peptide BH3 mimetics, an alternate design approach was developed. Starting from the BH3 domain of Bcl-2 family member Puma, a series of α/β-peptide variants were examined in which the side chain sequence of the natural ligand was displayed on all possible α/β3-peptide backbones with an ααβαααβ repeat.[16] This “sequence-based” residue replacement strategy was inspired by findings that such modification can generate α/β-peptides with complex helix-bundle folding patterns.[17] Screening seven oligomers based on the Puma BH3 domain yielded an α/β-peptide with Bcl-xL binding affinity indistinguishable from the natural α-peptide and significantly improved proteolytic stability. Later structural studies confirmed the binding mode of the Puma-based α/β-peptide with Bcl-xL was identical to that of natural BH3 domains.[18] An important difference between the Puma α-peptide and its α/β-peptide mimic was that the unnatural backbone lost the capacity to bind tightly to the anti-poptotic Bcl-2 family protein Mcl-1; however, structure-guided side-chain modification was able to restore this function.[19]
A key finding related to the α/β-peptide Puma BH3 domain mimics was the importance of the pattern of backbone alteration. Two α/β-peptides with identical side-chain sequences but different patterns of β3-residue incorporation bound to Bcl-xL with 105-fold different association affinities.[16] This observation was explored though a comprehensive screen of all possible α/β-peptides resulting from α→β3 residue replacement in the Bim BH3 domain with an αααβ, ααβαααβ, or ααβ repeat.[20] The results underscored the idea of backbone modification as a means for controlling binding selectivity. The Bim BH3 domain binds promiscuously to anti-apoptotic Bcl-2 family members, including Bcl-xL and Mcl-1. Effective α/β-Peptide Bim BH3 mimics were found for both Bcl-xL and Mcl-1; however, the optimal pattern of α→β3 residue replacement for high affinity binding differed for the two targets.
HIV Cell Entry via the Viral Protein gp41
The rapid success of sequence-based design as a strategy to generate α/β-peptide mimics of BH3 domains suggested it might be applicable to other prototype α-peptide sequences. Another system in which the concept was explored was a protein–protein interaction involved in cell entry by human immunodeficiency virus (HIV). As an enveloped virus, HIV must deliver its genetic material through the fusion of the viral and host cell membranes.[21] This process is mediated by gp41, a trimeric viral protein anchored to the HIV membrane via its C-terminus. In the course of host cell recognition by the virus, the N-terminus of gp41 inserts into the plasma membrane, anchoring the virion to the cell. A structural rearrangement ensues in which three helical gp41 C-terminal heptad repeat (CHR) domains pack against a trimer of helices in the gp41 N-terminal heptad repeat (NHR) region. The resulting antiparallel six-helix bundle fuses the viral and host plasma membranes by inducing close physical proximity. The CHR/NHR interaction has been successfully targeted clinically for HIV treatment with the 36-residue α-peptide entry inhibitor enfuvirtude, a fragment of the gp41 CHR domain.[22]
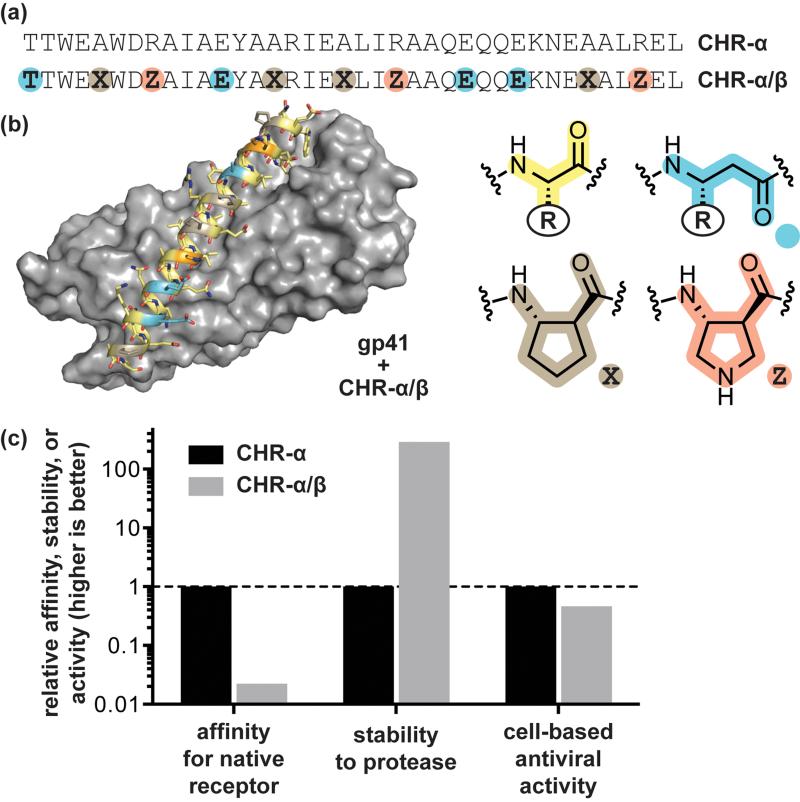
The first attempts at developing α/β-peptide inhibitors of gp41-mediated HIV cell entry were guided by success in the Bcl-2 family proteins described above; sequence-guided α→β3 residue replacement was carried out in a known CHR α-peptide ligand. These efforts were not fruitful, however, leading to a best-case α/β-peptide affinity for the gp41 CHR binding cleft 104-fold weaker than the prototype α-peptide.[23] A solution to the problem of low affinity was found through the strategic rigidification of the α/β-peptide backbone by replacing a subset of β3-residues with cyclically constrained analogues. This approach was inspired by earlier work on α/β-peptide helix bundles, in which it was found that βcyc-residues based on a trans-substituted five-membered ring (ACPC, APC) supported similar helical folds as β3 residues while considerably stabilizing the quaternary structure.[24] β3→βcyc substitution in the CHR α/β-peptide mimics had a similar stabilizing effect.[23] The best rigidified variant showed excellent structural homology to the native CHR domain, comparable antiviral activity in cell-based infectivity assays, and improved stability to degradation by proteases (Figure 2). While it is tempting to suggest an entropic effect resulting from a rigidified backbone, later work revealed that gp41 CHR mimetic α/β-peptides lacking βcyc-residues but capable of forming an array of salt bridges involving solvent-exposed β3-residues were equally potent.[25] Recent insights into the folding thermodynamics of protein-like tertiary structures with α/β-peptide backbones suggest the relationship between folding energetics among α-, β3-, and βcyc-residues is more complex than previously appreciated.[26]
Figure 2.
Design of α/β-peptide mimics of an α-peptide inhibitor of HIV-cell fusion. (a) Sequences of the prototype α-peptide inhibitor CHR-α, derived from the HIV protein gp41, and its α/β-peptide analogue CHR-α/β. (b) Crystal structure of CHR-α/β bound to an engineered variant of the HIV protein gp41 (PDB: 3O43). (c) Comparison of in vitro bioactivity data for CHR-α and CHR-α/β: relative association affinity for the engineered gp41 receptor, relative half-life to proteolytic degradation by the enzyme proteinase K, and relative activity in a cell-based HIV-1 infectivity assay. Source data are from reference [23].
G-Protein Coupled Receptors
G-protein coupled receptors (GPCRs) are responsible for transducing signals from extracellular stimuli across the membrane and into the cell. GPCRs are a diverse protein class (~800 exist in humans); this diversity is reflected in the structures of their ligands, which include small molecules, peptides (as small as 3 residues), and small proteins (<60 residues).[27] GPCRs are important in medicinal chemistry, representing the target of action of the majority of FDA-approved drugs.[28] The difficulty identifying small molecules that modulate the function of GPCRs that natively bind large peptide ligands has motivated efforts to create peptide-based therapeutics for these targets.[29] Replacement of α-residues in short peptide GPCR ligands with unnatural building blocks has a rich history in peptidomimetics research, and β-residues have found use in several such studies.[10] A body of more recent work, summarized below, highlights the potential of α/β-peptides to mimic larger GPCR peptide ligands.
The 36-residue hormone neuropeptide Y (NPY) is among the most abundant peptides in the brain and plays diverse biological roles, including regulation of food intake and mood.[30] NPY acts through binding interactions with four Y-family GPCRs: Y1R, Y2R, Y4R, and Y5R. Because of the varying functions of these different receptor subtypes, synthetic ligands with high affinity and specificity are valuable as both tools to elucidate biological roles of the receptors as well as potential therapeutic leads. Recent efforts have shown α/β-peptide analogues of NPY address an important and previously unmet need for potent ligands that selectively activate Y4R.[31] Pioneering research in the area demonstrated that incorporation of the βcyc-residue ACC into a 12-residue fragment from NPY generated an α/β-peptide a with an altered profile of binding affinity among the receptor subtypes.[32] Building on this work, a recent study reported a thorough survey of βcyc residues differing in ring size and stereochemistry incorporated at two positions in the NPY sequence.[31] Remarkably, a single α→βcyc substitution (ACBC) resulted in an α/β-peptide with potent and selective Y4R agonist activity in cell-based assays.
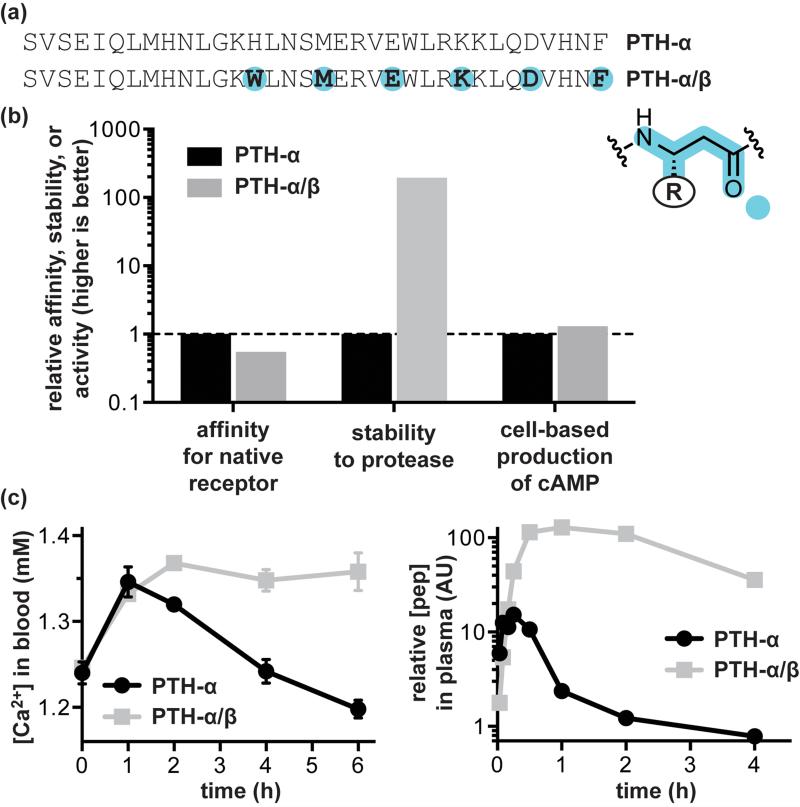
Parathyroid hormone (PTH) is a protein GPCR ligand that helps to regulate blood calcium levels. The 34-residue N-terminal peptide from PTH (PTH1-34) has the same activity as the full length protein and is a clinically used drug for the treatment of osteoporosis.[33] Structural studies on the interaction of PTH with its receptor (PTH1R) suggest that residues 15-34 fold to form an α-helix that binds with high affinity to the GPCR extracellular domain, while the N-terminal region contacts the transmembrane domain and is responsible for signaling.[34] Sequence-guided α→β3 residue replacement in an αααβ pattern generated an α/β-peptide PTH1-34 analogue with comparable receptor binding affinity and agonist activity in cell-based assays (Figure 3).[35] A particularly significant result of this work was the demonstration that α/β-peptide mimicry of the α-peptide prototype translated from in vitro experiments to an in vivo context. Mice treated with PTH1-34 or an α/β-peptide analogue showed identical initial spikes in blood calcium levels; however, a dramatic difference was observed in the duration of the effect. The α/β-peptide was able to sustain increased blood calcium several hours after levels in mice treated with the α-peptide had returned to baseline. Quantification of peptide in serum suggested that this effect results from enhanced stability of the α/β-peptide to proteolytic degradation.
Figure 3.
Design of α/β-peptide mimics of the α-peptide GPCR ligand parathyroid hormone (PTH). (a) Sequences of the prototype α-peptide PTH-α, residues 1-34 of the native hormone, and its α/β-peptide mimic PTH-α/β. (b) Comparison of in vitro bioactivity data for PTH-α and PTH-α/β: relative association affinity for parathyroid hormone receptor-1, relative half-life to proteolytic degradation by the enzyme trypsin, and relative activity in a cell-based assay of cyclic adenosine monophosphate (cAMP) production. (c) Summary of key in vivo data obtained for PTH-α and PTH-α/β in mice: blood calcium levels after treatment with the indicated peptide at a dose of 20 nmol/kg and relative concentration of peptide remaining in serum as a function of time after treatment. Source data are from reference [35].
The 30-residue hormone glucagon-like peptide 1 (GLP-1) stimulates glucose-dependent insulin release through interaction with GPCRs, and several peptide-based GLP-1 mimics are clinically approved drugs for the treatment of diabetes.[36] A drawback to GLP-1 in clinical applications is its rapid degradation in serum, motivating a recent study examining the ability of α/β-peptide analogues to combine efficacy and improved biostability.[37] Sequence-based α→β3 substitutions, quite successful in the PTH analogues described above, were not tolerated in GLP-1; incorporation of just three β3-residues in the 30mer abolished agonist activity entirely. The use of βcyc-residues proved much more effective; receptor binding affinity was retained in an analogue with five α→βcyc replacements in an αααβ pattern. Interestingly, the 12-residue N-terminal segment of GLP-1 proved quite sensitive to backbone alteration; however, this problem was solved by the judicious use of two α-aminoisobutyric acid residues to block known proteolytic cleavage sites near the N-terminus. The optimized α/β-peptide GLP-1 mimic showed agonist activity identical to the α-peptide hormone in cell-based assays and significantly improved in vitro proteolytic stability. These results were maintained in vivo, where mice treated with the more proteolytically stable α/β-peptide showed improved glucose tolerance when challenged several hours after dosing compared to mice treated with the α-peptide. Paralleling observations for the PTH mimics, improved stability to proteolytic degradation translated into a more sustained effect in vivo.
Angiogenesis
Angiogenesis, the growth of new blood vessels, is fundamental to normal physiology but also plays a role in disease.[38] A crucial contributor to angiogenesis is the protein vascular endothelial growth factor (VEGF), which stimulates blood vessel growth through a signaling cascade that begins with binding to a receptor tyrosine kinase. Inhibitors of angiogenesis, including antibodies that block the interaction between VEGF and its receptors, have found clinical use in the treatment of cancer and diseases of the eye.[38] Efforts to develop smaller agents that block VEGF/VEGFR signaling led to the identification by phage display of a 19-residue α-peptide that blocks angiogenesis by engaging the receptor-binding region of VEGF.[39] In recent work, this sequence was used as a starting point to design an α/β-peptide mimic.[40] A combination of alanine scanning and “β3 scanning” (individual sequence-guided α→β3 substitutions) identified sites in the prototype sequence tolerant to backbone and/or side chain modification. Combining several of the most effective substitutions generated an α/β-peptide analogue with ~1/3 unnatural backbone content. The α/β-peptide bound VEGF with much lower affinity than the α-peptide prototype; however, it showed markedly improved proteolytic stability and retained some activity in cell-based assays.
While anti-VEGF therapies have shown great promise in the treatment of cancer, their utility is limited in many cases by rapid development of resistance.[41] The protein galectin-1 (Gal-1) has been identified as participating in one compensatory pathway that enables sustained angiogenesis in the face of VEGF-targeted treatment.[42] Interestingly, much earlier work identified a 33-residue α-peptide of de novo design (anginex) that inhibits tumor cell proliferation in vitro and in vivo through a mechanism involving Gal-1 as its primary target.[43,44] From the standpoint of α/β-peptide mimicry, anginex poses the challenge of having a multi-stranded β-sheet as its bioactive conformation. Every other example of an α/β-peptide engaging a biological target covered in this review involves the mimicry of helix or loop structure in the native ligand. Sheets have proven less straightforward than helices for mimicry by α/β-peptides, but some approaches for sequence-guided α→β residue replacement have been reported.[45] Scanning a stripe of α→β3 residue replacement along the sheet of anginex led to two analogues that showed dose-dependent inhibition of endothelial tumor cell proliferation comparable to the parent α-peptide.[46]
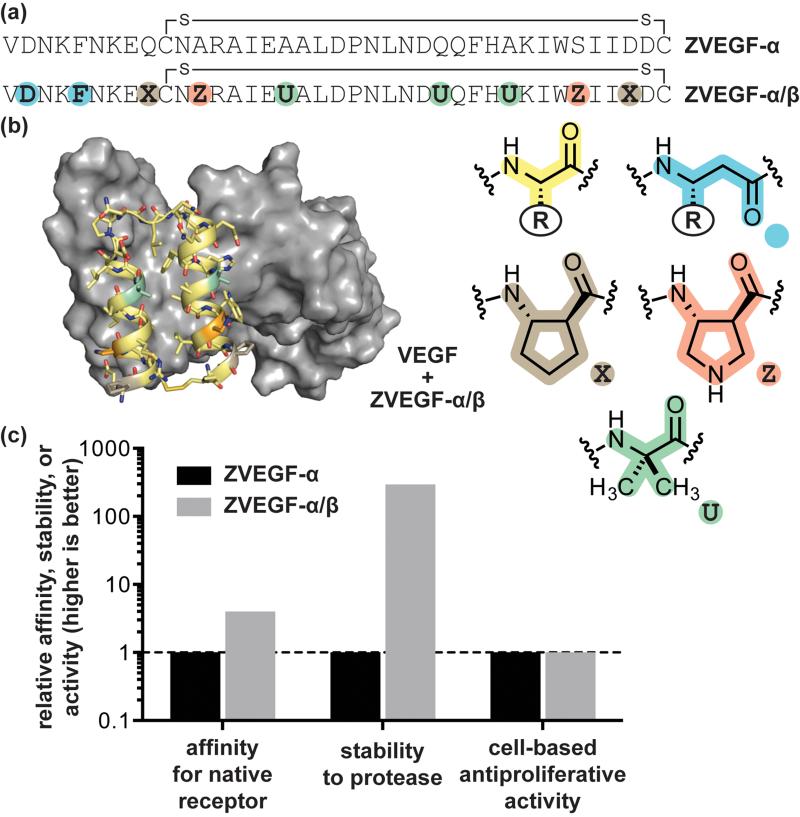
A promising class of α-peptide ligands for diverse protein targets, termed affibodies, have been developed through phage-display on a 58-residue three-helix bacterial protein scaffold.[47] Later work showed the three-helix parent protein could be truncated to a shorter 38-residue two-helix variant without compromising activity.[48] Phage-derived sequences based on both the parent scaffold and truncated version have been shown effective at targeting VEGFR in vivo for tumor imaging applications.[49] Sequence-guided α→β3 residue replacement in the two-helix VEGF-binding α-peptide, along with incorporation of βcyc-residues and Aib, generated an α/β-peptide mimic with identical affinity for VEGF (Figure 4). Comparison of the crystal structures of each ligand in complex with VEGF showed the details of molecular recognition were unchanged between the α-peptide and α/β-peptide.[50] The α/β-peptide showed improved stability to proteolytic degradation in vitro and was able to inhibit VEGF-induced proliferation in cell-based assays. The potential generality of the affibody scaffold as a source of α/β-peptide ligands for diverse targets was demonstrated through the design of α/β-peptide analogues of previously reported phage-derived sequences for immunoglobulin G and tumor necrosis factor-α.
Figure 4.
Design of an α/β-peptide mimic of a phage-derived α-peptide that inhibits the interaction between VEGF and its receptors. (a) Sequences of the prototype VEGF-binding α-peptide ZVEGF-α and its α/β-peptide mimic ZVEGF-α/β. (b) Crystal structure of ZVEGF-α/β bound to VEGF (PDB: 4WPB). (c) Comparison of in vitro bioactivity data for ZVEGF-α and ZVEGF-α/β: relative association affinity for VEGF, relative half-life to proteolytic degradation by the enzyme proteinase K, and relative activity in a cell-based assay of VEGF-induced proliferation. Source data are from reference [50].
Conclusions
α/β-Peptides are a class of molecules with significant therapeutic potential, especially in cases where the biological target is an extended protein surface. While early studies on mixed backbones containing β-residues focused heavily on the mimicry of natural protein structure, more recent work has shifted toward the functional mimicry of bioactive α-peptide sequences. These studies have shown that α/β-peptides can be generated with biological properties identical to a prototype α-peptide, but with increased stability to degradation by proteases in serum. Examples of α/β-peptides with long in vivo lifetimes represent a significant milestone toward improving bioavailability, a major limitation of current α-peptide pharmaceuticals. Although questions of potential toxicity and immunogenicity remain areas for further investigation, α/β-peptides already show tremendous promise as therapeutic agents and appear to be on a trajectory toward potential clinical use.
Highlights.
α/β-Peptides blend natural α-amino acid residues with unnatural β-residues
α/β-Peptides are more resistant than α-peptides to degradation by protease enzymes
α/β-Peptide mimics have been reported for diverse bioactive α-peptides
Biological targets include viral-cell fusion, GPCRs, and angiogenesis
Relative to α-peptides, α/β-peptides can show potent, sustained activity in vivo
Acknowledgements
Funding for this work was provided by the National Institutes of Health (R01GM107161).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arkin Michelle R, Tang Y, Wells James A. Small-molecule inhibitors of protein-protein interactions: Progressing toward the reality. Chem Biol. 2014;21:1102–1114. doi: 10.1016/j.chembiol.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fosgerau K, Hoffmann T. Peptide therapeutics: Current status and future directions. Drug Discovery Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Bautista AD, Craig CJ, Harker EA, Schepartz A. Sophistication of foldamer form and function in vitro and in vivo. Curr Opin Chem Biol. 2007;11:685–692. doi: 10.1016/j.cbpa.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman CM, Choi S, Shandler S, DeGrado WF. Foldamers as versatile frameworks for the design and evolution of function. Nat Chem Biol. 2007;3:252–262. doi: 10.1038/nchembio876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guichard G, Huc I. Synthetic foldamers. Chem Commun. 2011;47:5933–5941. doi: 10.1039/c1cc11137j. [DOI] [PubMed] [Google Scholar]
- 6.Horne WS. Peptide and peptoid foldamers in medicinal chemistry. Expert Opin Drug Discov. 2011;6:1247–1262. doi: 10.1517/17460441.2011.632002. [DOI] [PubMed] [Google Scholar]
- 7.Horne WS, Gellman SH. Foldamers with heterogeneous backbones. Acc Chem Res. 2008;41:1399–1408. doi: 10.1021/ar800009n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilsl LKA, Reiser O. α/β-Peptide foldamers: State of the art. Amino Acids. 2011;41:709–718. doi: 10.1007/s00726-011-0894-2. [DOI] [PubMed] [Google Scholar]
- 9.Johnson LM, Gellman SH. α-Helix mimicry with α/β-peptides. Methods Enzymol. 2013;523:407–429. doi: 10.1016/B978-0-12-394292-0.00019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrele C, Martinek TA, Reiser O, Berlicki Ł . Peptides containing β-amino acid patterns: Challenges and successes in medicinal chemistry. J Med Chem. 2014;57:9718–9739. doi: 10.1021/jm5010896. [DOI] [PubMed] [Google Scholar]
- 11.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The Bcl-2 family reunion. Molecular Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the Bcl-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 13.Hayen A, Schmitt MA, Ngassa FN, Thomasson KA, Gellman SH. Two helical conformations from a single foldamer backbone: “Split personality” in short α/β-peptides. Angew Chem Int Ed. 2004;43:505–510. doi: 10.1002/anie.200352125. [DOI] [PubMed] [Google Scholar]
- 14.De Pol S, Zorn C, Klein CD, Zerbe O, Reiser O. Surprisingly stable helical conformations in α/β-peptides by incorporation of cis-β-aminocyclopropane carboxylic acids. Angew Chem Int Ed. 2004;43:511–514. doi: 10.1002/anie.200352267. [DOI] [PubMed] [Google Scholar]
- 15.Sadowsky JD, Fairlie WD, Hadley EB, Lee H-S, Umezawa N, Nikolovska-Coleska Z, Wang S, Huang DCS, Tomita Y, Gellman SH. (α/β+α)-Peptide antagonists of BH3 domain/Bcl-xL recognition: Toward general strategies for foldamer-based inhibition of protein-protein interactions. J Am Chem Soc. 2007;129:139–154. doi: 10.1021/ja0662523. [DOI] [PubMed] [Google Scholar]
- 16*.Horne WS, Boersma MD, Windsor MA, Gellman SH. Sequence-based design of α/β-peptide foldamers that mimic BH3 domains. Angew Chem Int Ed. 2008;47:2853–2856. doi: 10.1002/anie.200705315. The development of α/β-peptides capable of modulating apoptotic signaling in the Bcl-2 protein family is an important early example of sequence-based backbone modification as a strategy for generating bioactive and protease-resistant oligomers.
- 17.Horne WS, Price JL, Keck JL, Gellman SH. Helix bundle quaternary structure from α/β-peptide foldamers. J Am Chem Soc. 2007;129:4178–4180. doi: 10.1021/ja070396f. [DOI] [PubMed] [Google Scholar]
- 18.Lee EF, Smith BJ, Horne WS, Mayer KN, Evangelista M, Colman PM, Gellman SH, Fairlie WD. Structural basis of Bcl-xL recognition by a BH3-mimetic α/β-peptide generated by sequence-based design. ChemBioChem. 2011;12:2025–2032. doi: 10.1002/cbic.201100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith BJ, Lee EF, Checco JW, Evangelista M, Gellman SH, Fairlie WD. Structure-guided rational design of α/β-peptide foldamers with high affinity for Bcl-2 family prosurvival proteins. ChemBioChem. 2013;14:1564–1572. doi: 10.1002/cbic.201300351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boersma MD, Haase HS, Peterson-Kaufman KJ, Lee EF, Clarke OB, Colman PM, Smith BJ, Horne WS, Fairlie WD, Gellman SH. Evaluation of diverse α/β-backbone patterns for functional α-helix mimicry: Analogues of the Bim BH3 domain. J Am Chem Soc. 2012;134:315–323. doi: 10.1021/ja207148m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. BBA-Biomembranes. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 22.Matthews T, Salgo M, Greenberg M, Chung J, DeMasi R, Bolognesi D. Enfuvirtide: The first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat Rev Drug Discov. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 23*.Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH. Structural and biological mimicry of protein surface recognition by α/β-peptide foldamers. Proc Natl Acad Sci USA. 2009;106:14751–14756. doi: 10.1073/pnas.0902663106. The extended surface involved in the binding interaction between CHR and NHR domains of the viral protein gp41 represents one of the largest targeted by an α/β-peptide.
- 24.Horne WS, Price JL, Gellman SH. Interplay among side chain sequence, backbone composition, and residue rigidification in polypeptide folding and assembly. Proc Natl Acad Sci USA. 2008;105:9151–9156. doi: 10.1073/pnas.0801135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson LM, Mortenson DE, Yun HG, Horne WS, Ketas TJ, Lu M, Moore JP, Gellman SH. Enhancement of α-helix mimicry by an α/β-peptide foldamer via incorporation of a dense ionic side-chain array. J Am Chem Soc. 2012;134:7317–7320. doi: 10.1021/ja302428d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinert ZE, Horne WS. Folding thermodynamics of protein-like oligomers with heterogeneous backbones. Chem Sci. 2014;5:3325–3330. doi: 10.1039/C4SC01094A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 28.Overington JP, Al-Lazikani B, Hopkins AL. How many drug targets are there? Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 29.Bellmann-Sickert K, Beck-Sickinger AG. Peptide drugs to target G protein-coupled receptors. Trends Pharmacol Sci. 2010;31:434–441. doi: 10.1016/j.tips.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Cabrele C, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J Pept Sci. 2000;6:97–122. doi: 10.1002/(SICI)1099-1387(200003)6:3<97::AID-PSC236>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 31*.Berlicki L, Kaske M, Gutiérrez-Abad R, Bernhardt G, Illa O, Ortuño RM, Cabrele C, Buschauer A, Reiser O. Replacement of Thr32 and Gln34 in the C-terminal neuropeptide Y fragment 25-36 by cis-cyclobutane and cis-cyclopentane β-amino acids shifts selectivity toward the Y4 receptor. J Med Chem. 2013;56:8422–8431. doi: 10.1021/jm4008505. Work on α/β-peptide mimics of neuropeptide Y is an important demonstration of the potential use of backbone modification as a tool to modulate binding selectivity when a ligand can engage multiple biological receptors.
- 32.Koglin N, Zorn C, Beumer R, Cabrele C, Bubert C, Sewald N, Reiser O, Beck-Sickinger AG. Analogues of neuropeptide Y containing β-aminocyclopropane carboxylic acids are the shortest linear peptides that are selective for the Y1 receptor. Angew Chem Int Ed. 2003;42:202–205. doi: 10.1002/anie.200390078. [DOI] [PubMed] [Google Scholar]
- 33.Gensure RC, Gardella TJ, Jüppner H. Parathyroid hormone and parathyroid hormone-related peptide, and their receptors. Biochem Biophys Res Commun. 2005;328:666–678. doi: 10.1016/j.bbrc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Vilardaga J-P, Romero G, Friedman P, Gardella T. Molecular basis of parathyroid hormone receptor signaling and trafficking: A family B GPCR paradigm. Cell Mol Life Sci. 2011;68:1–13. doi: 10.1007/s00018-010-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Cheloha RW, Maeda A, Dean T, Gardella TJ, Gellman SH. Backbone modification of a polypeptide drug alters duration of action in vivo. Nat Biotech. 2014;32:653–655. doi: 10.1038/nbt.2920. The development of α/β-peptide mimics of parathyroid hormone is the first example of sequence-guided backbone alteration leading to an agent with biological efficacy in vivo. The α/β-peptide showed longer circulation times and increased duration of effect compared to the α-peptide administered at the same dose.
- 36.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 37**.Johnson LM, Barrick S, Hager MV, McFedries A, Homan EA, Rabaglia ME, Keller MP, Attie AD, Saghatelian A, Bisello A, et al. A potent α/β-peptide analogue of GLP-1 with prolonged action in vivo. J Am Chem Soc. 2014;136:12848–12851. doi: 10.1021/ja507168t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fairbrother WJ, Christinger HW, Cochran AG, Fuh G, Keenan CJ, Quan C, Shriver SK, Tom JYK, Wells JA, Cunningham BC. Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry. 1998;37:17754–17764. doi: 10.1021/bi981931e. [DOI] [PubMed] [Google Scholar]
- 40.Haase HS, Peterson-Kaufman KJ, Lan Levengood SK, Checco JW, Murphy WL, Gellman SH. Extending foldamer design beyond α-helix mimicry: α/β-Peptide inhibitors of vascular endothelial growth factor signaling. J Am Chem Soc. 2012;134:7652–7655. doi: 10.1021/ja302469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shojaei F. Anti-angiogenesis therapy in cancer: Current challenges and future perspectives. Cancer Letters. 2012;320:130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Croci DO, Cerliani JP, Dalotto-Moreno T, Mendez-Huergo SP, Mascanfroni ID, Dergan-Dylon S, Toscano MA, Caramelo JJ, Garcia-Vallejo JJ, Ouyang J, et al. Glycosylation-dependent lectin-receptor interactions preserve angiogenesis in anti-VEGF refractory tumors. Cell. 2014;156:744–758. doi: 10.1016/j.cell.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 43.van der Schaft DWJ, Dings RPM, de Lussanet QG, van Eijk LI, Nap AW, Beets-Tan RGH, Bouma-Ter Steege JCA, Wagstaff J, Mayo KH, Griffioen AW. The designer antiangiogenic peptide anginex targets tumor endothelial cells and inhibits tumor growth in animal models. FASEB J. 2002 doi: 10.1096/fj.02-0509fje. [DOI] [PubMed] [Google Scholar]
- 44.Thijssen VLJL, Postel R, Brandwijk RJMGE, Dings RPM, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci USA. 2006;103:15975–15980. doi: 10.1073/pnas.0603883103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lengyel GA, Horne WS. Design strategies for the sequence-based mimicry of side-chain display in protein β-sheets by α/β-peptides. J Am Chem Soc. 2012;134:15906–15913. doi: 10.1021/ja306311r. [DOI] [PubMed] [Google Scholar]
- 46*.Hegedüs Z, Wéber E, Kriston-Pál É, Makra I, Czibula Á, Monostori É, Martinek TA. Foldameric α/β-peptide analogs of the β-sheet-forming antiangiogenic anginex: Structure and bioactivity. J Am Chem Soc. 2013;135:16578–16584. doi: 10.1021/ja408054f. Analogues of the anti-angiogenic α-peptide anginex are one of the few examples of α/β-peptide bioactivity not involving mimicry of helical secondary structure.
- 47.Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010;584:2670–2680. doi: 10.1016/j.febslet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Starovasnik MA, Braisted AC, Wells JA. Structural mimicry of a native protein by a minimized binding domain. Proc Natl Acad Sci USA. 1997;94:10080–10085. doi: 10.1073/pnas.94.19.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fedorova A, Zobel K, Gill Herman S, Ogasawara A, Flores Judith E, Tinianow Jeff N, Vanderbilt Alexander N, Wu P, Meng YG, Williams S-P, et al. The development of peptide-based tools for the analysis of angiogenesis. Chem Biol. 2011;18:839–845. doi: 10.1016/j.chembiol.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 50**.Checco JW, Kreitler DF, Thomas NC, Belair DG, Rettko NJ, Murphy WL, Forest KT, Gellman SH. Targeting diverse protein-protein interaction interfaces with α/β-peptides derived from the Z-domain scaffold. Proc Natl Acad Sci USA. 2015;112:4552–4557. doi: 10.1073/pnas.1420380112. The strategy reported for developing α/β-peptide mimics of phage-derived sequences based on the Z domain scaffold may prove a general source of protease-stable ligands for diverse biological targets.