Abstract
Background
Gestational alcohol exposure causes lifelong physical and neurocognitive deficits collectively referred to as fetal alcohol spectrum disorders (FASDs). Micronutrient deficiencies are common in pregnancies of alcohol-abusing women. Here we show the most common micronutrient deficiency of pregnancy, iron deficiency without anemia, significantly worsens neurocognitive outcomes following perinatal alcohol exposure.
Methods
Pregnant rats were fed iron-deficient (ID) or iron-sufficient diets from gestational day 13 to postnatal day (PD) 7. Pups received alcohol (0, 3.5, 5.0 g/kg) from PD 4–9, targeting the brain growth spurt. At PD 32, learning was assessed using delay or trace eyeblink classical conditioning (ECC). Cerebellar interpositus nucleus (IPN) and hippocampal CA1 cellularity was quantified using unbiased stereology.
Results
Global ANOVA revealed that ID and alcohol separately and significantly reduced ECC learning with respect to amplitude (p’s ≤0.001) and CR percentage (p’s ≤0.001). Iron and alcohol interacted to reduce CR percentage in the trace ECC task (p = 0.013). Both ID and alcohol significantly reduced IPN (p’s <0.001) and CA1 cellularity (p’s < 0.005). CR amplitude correlated with IPN cellularity (Delay 0.871, Trace 0.703, p’s <0.001) and CA1 cellularity (Delay 0.792, Trace 0.846, p’s <0.001) across both tasks. The learning impairments persisted even through the offsprings’ iron status had normalized.
Conclusion
Supporting our previous work, gestational ID exacerbates the associative learning deficits in this rat model of FASD. This is strongly associated with cellular reductions within the ECC neurocircuitry. Significant learning impairments in FASD could be the consequence, in part, of pregnancies in which the mother was also iron-inadequate.
Keywords: fetal alcohol spectrum disorders, iron deficiency, hippocampus, cerebellum, stereology, eyeblink conditioning
INTRODUCTION
Prenatal alcohol consumption (PAE) impairs fetal development to produce life-long behavioral and cognitive impairments known as fetal alcohol spectrum disorders (FASDs). FASD prevalence is as high as 2.4% to 4.8% of school children in the U.S. and Western Europe, and its more severe manifestation, fetal alcohol syndrome (FAS), may affect 6–9 per 1000 (May et al., 2014a). In both humans and animal models, PAE impairs learning performance across non-motor and motor tasks and causes disruptive neural changes, including the cerebellum and hippocampus (Mattson et al., 2013; Norman et al., 2009). FASD severity varies widely even after controlling for patterns and quantity of alcohol intake. Factors associated with greater severity include increased maternal age, low maternal education and socioeconomic status, and small maternal weight and stature (Jacobson et al., 2004; May et al., 2013). These may represent, in part, surrogate markers for nutritional factors that modify alcohol’s neurotoxicity. One nutritional candidate is gestational iron deficiency (ID). ID affects 22% of U.S. women aged 12–49 years (Stoltzfus 2001). Gestational ID causes cognitive and behavioral deficits in the offspring that persist even after iron status is normalized. These deficits involve many of the same domains affected in FASD including learning, attention, executive function, and motor skills (Beard and Connor, 2003; Congdon et al., 2012; McEchron et al., 2008), and similar physiological changes including reduced myelination and dendrite formation, and altered brain neurochemistry (Jorgenson et al., 2005; Rao et al., 2011; Yu et al., 1986).
Clinical studies identified maternal iron status as a significant modifier of outcome in alcohol-exposed pregnancies. FAS-associated growth delays and small head circumference were greatest in children who also had ID-anemia (Carter et al. 2007, 2012). We corroborated this interaction in a rat model wherein dams were fed an ID diet that met most maternal but not fetal needs, such that the offspring and not the dams had ID-anemia (Rufer et al., 2012). This condition models a common clinical manifestation of ID in which the mother’s near-normal hematological indices mask her inadequate iron stores. These neonatal pups received a repeated binge alcohol exposure during the brain growth spurt period, which in rat occurs during postnatal (P) days 4–9 and parallels neurodevelopment of the human third trimester, which is sensitive to alcohol’s damage (Goodlett et al., 1990; West et al., 1986). We found that ID interacted with alcohol to significantly impair the offsprings’ somatic growth, cerebellar myelination, and cerebellum-dependent associative learning, both delay eyeblink classical conditioning (ECC) and fear conditioning, as compared with either ID or alcohol separately (Rufer et al., 2012). These deficits persisted into adolescence (P35) even though the pups were iron-adequate from weaning onward. Maternal iron adequacy mitigated the learning and growth deficits despite equivalent alcohol exposures. Other cerebellar-dependent behaviors were normal, suggesting the ID-alcohol effects were selective and were not due to generalized malnutrition. These growth, white matter, and learning deficits also comprise key diagnostic criteria in FASD, suggesting that children who experienced gestational ID and PAE may represent individuals within the severe end of the FASD spectrum.
Here, we investigate whether the ID-PAE interaction extends to hippocampal-dependent learning and the neuronal populations that contribute to this neurocircuitry.
METHODS
Generation of IS and ID Animals
Animals were generated at East Carolina University as described (Rufer et al., 2012). Pregnant Long-Evans rats (Harlan, Richmond IN) were fed an iron-sufficient (IS) (100 ppm Fe; TD.06016) or iron-deficient (2–6 ppm Fe; TD.80369; both from Harlan-Teklad, Madison WI) diet from gestational day (G) 13 to P7. The ID iron intake increased to 20 ppm (TD.06013) from P7 to P20 to keep the dams in Stage I ID and the growing pups in a moderate Stage II/III ID, as per established protocol. Pups had free access to the maternal diet. Starting at P20, all offspring were fed commercial lab chow (Harlan, Indianapolis IN), which satisfied all nutritional needs. Liver mineral content was quantified at P40 using inductively coupled plasma atomic emission spectroscopy (Rufer et al., 2012). This study was approved by the respective Institutional Animal Care and Use Committees.
Alcohol Exposure Model
Alcohol exposure was as described (Rufer et al., 2012). IS and ID pups received either 3.5 or 5.0 g/kg ethyl alcohol (AAPER, USP) as an oral bolus in milk vehicle (Carnation non-fat dry milk), split into two half-doses 2 hr apart (0 hr, 2 hr), daily from P4 to P9. These doses produce peak BACs of 250 and 450 mg/dl, respectively and iron status does not affect alcohol clearance (Rufer et al., 2012). Littermates received calorie-matched milk vehicle (0 g/kg alcohol). The IS pups are designated as IS-0, IS-3.5, or IS-5.0, and the ID pups are designated as ID-0, ID-3.5, or ID-5.0 to reflect their iron and alcohol treatment. This model of episodic binge drinking targets alcohol to the brain growth spurt period, which occurs in rats at P2–P10 and in humans during the third trimester. Alcohol exposure during this developmental period is well-established to affect the cerebellar and hippocampal neurocircuitry that mediates ECC (Goodlett et al., 2000; Green et al., 2002; Tran et al., 2005). The dam was removed during the dosing period and the pups were group-housed in the heated home cage to standardize their nutriture whilst mitigating the potential stress of maternal separation (Melo et al., 2006). Dams were returned to the home cage when the alcohol-exposed pups recovered; this was ~6hr on P4 and ~4hr on P5–9. All pups received additional milk-only vehicle at 4 hr (P4–9) and 6 hr (P4 only) to sustain their nutrition during the separation. Our prior studies in this model (Green et al., 2002; Tran et al. 2005, 2007; Thomas and Tran, 2012; Rufer et al. 2012) do not detect statistically meaningful differences in learning measures for sham-intubated and unintubated controls, and thus this control was omitted.
Eyeblink Classical Conditioning (ECC)
ECC Behavioral Testing
This was performed as described (Rufer et al., 2012; Thomas & Tran, 2012). On approximately P30 (periadolescence), rats were implanted with two stainless steel recording electrodes (Medwire, 3T) in the left orbicularis oculi muscle and a bipolar stimulating electrode adjacent to the left eye. Animals recovered for 48 hr prior to testing. For both delay and trace ECC, animals were placed in a modified operant box containing a house light and a fan (55 dB), and housed inside a larger chamber to attenuate outside noise. An animal’s EMG wires were plugged into a commutator (Plastics One), which reliably conducted electrical signals while the rat moved freely. The test boxes in turn, were connected to a computer equipped with proprietary ECC software (JSA Designs, Raleigh, NC) that recorded EMG activity and delivered the training stimuli (CS and US). Sixty-four rats underwent short-delay ECC. Data from 3 rats were excluded because of two or more sessions in which EMG signals were unreliable (i.e., baseline EMG exceeded trial EMG’s in at least 36 out of 90 paired CS-US trials per session) or because animals did not exhibit reliable unconditioned responses to the shock US (i.e., exhibit EMG’s above baseline threshold in at least 60% of the paired CS-US trials per session; final n = 61). During each trial, a tone CS (80 dB, 2.8 kHz) was presented first and following a delay of 280 ms, a shock US (60 Hz, 2.0 mA) was delivered. The shock US remained on for 100 ms, co-terminating with the tone CS. There were 90 total CS-US trials in one session; the average inter-trial interval was 30 sec (18 – 42 sec). On every 10th trial, the tone CS was presented by itself to test for learning of the CR. In total, there were 100 trials per session. Rats received two sessions per day (each session 4 hr apart) over three consecutive days and six sessions total. Each training session lasted approximately 50 min.
A separate set of rats from each litter (sixty-seven total) instead received trace ECC within 48 hr after eyelid surgery; two rats were excluded using the previous omission criteria (final n = 65). The stimulus parameters, number of sessions, and number of training days were the same as those for delay ECC, except for the CS-US contingency. The tone CS was presented first and remained on by itself for 380 ms. The tone then terminated and after a 500-ms delay, the shock US was delivered and remained on for 100 ms. This 500-ms time window represented the trace interval in which the animal is required to bridge the association between the offset of the tone CS and the onset of the shock US.
ECC Data Collection
Data were pre-screened for acceptable and unacceptable trials within each session using established criteria in rodent ECC (Skelton, 1988; Tran et al., 2007). All relevant measures (below) associated with “acceptable” trials were averaged within session. Analysis was carried out using proprietary software (JSA Designs). Each trial epoch was divided into four discrete EMG sampling periods: (1) a 280-ms pre-CS baseline before tone CS onset, (2) a startle response (SR) period during the first 80 ms after CS onset (EMG activity related exclusively to a non-associative reaction), (3) a 200-ms adaptive CR period that allowed for measuring well-timed CRs prior to shock US onset; for trace ECC, it is the 200-ms period prior to delivery of the US, and (4) a UR period which measured EMG activity that occurred from the onset of the US to the end of the trial (140 ms). Any EMG activity that exceeded the pre-CS baseline mean by at least 0.4 arbitrary units (2 standard deviations) was registered as an SR, CR, and/or UR during their respective sampling periods. The reason for analyzing the adaptive CR (matched for delay and trace ECC) is that it represents a well-timed eyeblink response just prior to (i.e., 200 ms before) US onset, which is mediated by the cerebellar cortex (in delay ECC) (Perrett et al., 1993) or hippocampus (in trace ECC) (Ivkovich & Stanton, 2001). The session means for each measure were obtained over 90 possible paired CS-US trials or 10 possible CS-alone trials (results not reported as they were found to be redundant with the paired data). Measures included percentage and amplitude of SRs, adaptive CRs, and URs.
Stereology
Tissue Preparation and Sectioning
Following completion of ECC testing, brains were perfusion-fixed using 3.0% (w/v) paraformaldehyde and 1.25% (v/v) glutaraldehyde in 0.2 M phosphate buffer. Cerebella were halved at the midline vermis and the left cerebellar hemisphere (ipsilateral to the trained eye) was processed. Hippocampi were halved and the left hippocampus processed. Tissues were embedded in GMA resin (Technovit 7100, Kulzer, Germany) and sectioned (25 μm) in the sagittal (cerebella) or horizontal plane (hippocampi) using a motorized rotary microtome (Shandon Finesse) and glass knives (Ralph glass knifemaker, Energy Beam Sciences, MA). A systematic random sampling procedure was applied, where a starting section within a pre-defined sampling interval was first saved, with every nth section saved thereafter until regions containing cerebellar lobules or hippocampal CA1 terminated (Table 2). Mounted sections were stained using cresyl violet (Sigma) modified for use with GMA-embedded tissue (see Tran and Kelly, 2003; Tran et al., 2005).
Table 2.
Body Weight at P35†
| Exposure | Female Body Weight (n=31) | Male Body Weight (n=35) | ||
|---|---|---|---|---|
| IS | ID | IS | ID | |
| 0 g/kg | 125.7 ± 5.7 g | 119.4 ± 7.0 g | 154.8 ± 6.5 g | 143.5 ± 8.6 g |
| 3.5 g/kg | 129.9 ± 8.6 g | 124.0 ± 8.2 g | 160.1 ± 7.0 g | 121.5 ± 7.0 g |
| 5.0 g/kg | 130.4 ± 7.0 g | 114.1 ± 8.6 g | 140.1 ± 7.0 g | 136.5 ± 7.7 g |
Mixed ANOVA results:
Influence with sex: (F(1, 14.013), p <0.001)
Influence of iron treatment: (F(1, 6.508), p = 0.014);
No influence of alcohol dose: (F(2, 1.036), p = 0.362)
No interaction between iron status and alcohol dose: (F(2, 0.155), p = 0.856)
Interaction between iron status x alcohol dose x sex: (F(2, 4.112), p = 0.022)
Optical Fractionator Method
We used standard criteria to identify the cerebellar lobules, those that contain the cerebellar interpositus nucleus (IPN) (Voogd, 2004) and hippocampal CA1 (Blackstad, 1956; Bonthius and West, 1991). Neurons of the IPN and hippocampal CA1 neurons were quantified in an unbiased manner using the optical fractionator method, with sampling, counting, and computational rules according to Bonthius and West (1991). Cell counting was carried out using StereoInvestigator software (MicroBrightfield); region of interest was determined under a 4x objective and cell nuclei identified with a 100x oil immersion lens. Estimates of neuron number represent unilateral values (ipsilateral to the eye used for ECC training). Investigators were blinded to treatment group.
Statistical Analysis
Dams were randomly assigned to dietary iron treatment, therefore litter was treated as the experimental unit. For ECC, the dependent variables were SR, CR, and UR measures. Dependent variables were analyzed using a full factorial model with all interactions, which included the fixed effects of Iron status (sufficient, deficient), ECC task (delay, trace), Alcohol (0, 3.5 or 5 g/kg) and Session (1–6). The model also included a random effect of litter nested within the 3-way factorial effect and the Session 1 value of the dependent variable as covariate. In order to account for repeated measures on the experimental unit across sessions, an AR(1) error structure was utilized with SAS Procedure MIXED (SAS Institute, Cary, NC).
For the cellularity analysis, data were collapsed across task because task does not affect cellularity. Dependent variables, IPN and CA1 estimated cell numbers, were analyzed using a full factorial model with all interactions which included the fixed effects of Iron status (2) and Alcohol (3).
Each dependent variable was tested with two models utilizing either equal or unequal variance among the treatment groups. A likelihood ratio test was employed to determine which model was statistically appropriate. When the effect of iron status, alcohol, and/or their interaction was significant (P < 0.05), an ANOVA analysis using a planned Tukey’s mean separation test was conducted to identify individual treatment differences. Because alcohol and ID were previously known to separately affect ECC learning and cellularity, we conducted one-tailed ANOVA to test specific hypothesis based on a priori knowledge. We also conducted specific ANOVAs based on ECC task to highlight pertinent relationships. Differences were considered significant at P < 0.05. Values are presented as Mean ± S.E.M.
To determine the relationship between cellularity and CR amplitude, linear regression was completed for Delay and Trace CR amplitude with respect to IPN and CA1 cellularity. Pearson correlation coefficients were calculated, and an analysis of covariance was utilized to determine whether slopes were significantly different across groups.
RESULTS
Body Weight at P35
Body weight of these periadolescent animals was dependent upon sex (F(1, 14.013), p <0.001) and P35 females were smaller than males across all treatments (Table 2). Iron deficiency (ID) significantly influenced body weight (F(1, 6.508), p = 0.014) and both male and female ID animals were smaller than their IS counterparts at equivalent alcohol exposures. There was no influence of alcohol dose upon body weight (p = 0.362) and no interaction between iron treatment and alcohol dose (p = 0.856).
Iron Status
Although all animals received the same iron adequate diet from weaning onward, alcohol had a lasting effect on iron status. Regardless of the preweaning diet, both the IS-5.0 and ID-5.0 offspring had significantly higher liver iron content (IS-5.0, 181.6 ± 13.7 ppm; ID-5.0, 187.0 ± 9.0 ppm; F(1, 54), p = 0.0033) as compared with their no-alcohol littermates. ID itself did not have a lasting effect on liver iron and was the same for IS-0 and ID-0 (IS-0, 141.5 ± 11.6 ppm; ID-0, 157.4 ± 11.4 ppm; p = 0.3422). Neither alcohol nor ID affected the liver content of other minerals including copper, zinc and manganese. Adequate or greater iron stores in all animals endorsed that all groups were iron-replete during the behavioral testing.
Neurobehavioral Outcomes
We first examined whether differences in learning could be explained by differences in ECC procedure, regardless of alcohol and iron status. Because the trace and delay studies were performed within the same time window and cohort of litters, we first collapsed the delay and trace results together to examine the effect of alcohol and iron-deficiency. With respect to CR percentage, the global ANOVA revealed strong separate effects of alcohol (F(2,53) = 34.55, p<0.001), iron status (F(1,53) = 10.54, p=0.001), and an alcohol-iron interaction (F(2, 53) = 3.42, p=0.02). There was also an interaction between iron status and task (F(1, 53) = 3.02, p=0.044). There was a significant effect of session (time) consistent with learning (p<0.001), and task interacted with session (p<0.001). As expected, trace ECC was more difficult to acquire than delay ECC (CR percentage: F(1, 53) = 76.82, p < 0.0001; CR amplitude: F(1, 53) = 201.78, p < 0.0001), confirming that ECC procedure strongly affected learning outcome (CR percentage: 28.69% vs. 60.24%; CR amplitude: 1.36 vs. 4.09). With respect to amplitude, the global ANOVA revealed a strong effect of alcohol (F(2, 53) = 32.79, p<0.001) and alcohol interacted with task (F(2, 53) = 5.19, p=0.003). There was a strong effect of iron status (F(1, 53) = 9.96, p=0.001) and iron status also interacted with task (F(1, 53) = 4.72, p=0.017). There was no interaction between alcohol and diet (F(2, 53) = 1.22, p=0.151), and a strong trend to an interaction between alcohol, diet, and task (F(2, 53) = 2.37, p=0.052). Amplitude increased over session (p<0.001) showing that learning occurred. Session interacted with both alcohol (p<0.001) and task (p<0.001). There was a trend for an interaction between alcohol, diet, session, and task (F(8, 216) = 1.67, p=0.053). There were no significant differences in sensory (SRs) and motor performance (URs) measures due to iron status (SR%, p = 0.087; SRAmp, p = 0.891; UR% p = 0.236; URAmp, p = 0.752), alcohol (SR%, p = 0.553; SRAmp, p = 0.171; UR% p = 0.898; URAmp, p = 0.736), or their interaction (SR%, p = 0.335; SRAmp, p = 0.245; UR% p = 0.376; URAmp, p = 0.564), suggesting that alcohol-ID challenge specifically targeted higher-order cognitive function during early brain development. Given these results, and previous results that both iron status and alcohol reduce learning in these paradigms (McEchron et al. 2008; Rufer et al., 2012; Tran et al., 2005, 2008), we performed specific ANOVAs to identify where alcohol and iron status affected learning outcomes.
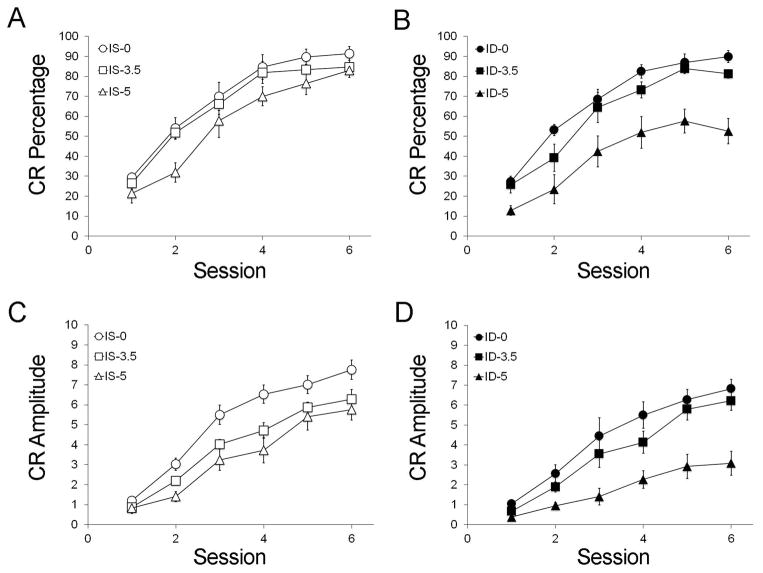
Delay ECC Results (Figure 1)
Figure 1.
Delay eyeblink classical conditioning between rats in the iron-sufficient (IS, open symbols, A, C) and iron-deficient (ID, closed symbols, B, D) groups as a function of alcohol dose (○● 0 g/kg, □■3.5 g/kg, △▲5 g/kg). Separately, alcohol and ID significantly affected learning acquisition in terms of conditioned response (CR) percentage (A, B) and amplitude (C, D) during paired training with the CS and US. Animals exposed to the combination of 5 g/kg alcohol and ID were more significantly impaired in acquiring CRs than ID animals exposed to the two lower alcohol doses (n = 9–11 animals per treatment group; error bars represent S.E.M.). Conversely, the impact of 5 g/kg alcohol on learning was drastically mitigated by an iron-sufficient diet.
Preliminary analysis found no significant sex effect, and thus analysis was pooled across this factor. For CR percentage, there were significant main effects of Iron (CR percentage, 64.12% vs. 56.37%; F(1, 26) = 6.54, p = 0.008) and Alcohol (F(2, 26) = 8.37, p < 0.001), outcomes consistent with the literature (Green et al., 2002; McEchron et al., 2008; Tran et al., 2005), but no interaction between them (p = 0.226). Rats treated with the 5 g/kg alcohol dose did not emit CRs as frequently as those receiving 3.5 g/kg or 0 g/kg (p’s < 0.032), and 3.5 and 0 trended toward differing from each other (p = 0.055). When analyzed by diet, ID5 was significantly worse than ID0 and ID3.5 (p’s < 0.042), whereas IS groups did not differ from each other. We obtained a similar result for CR amplitude with significant main effects of Iron (F(1, 26) = 6.50, p = 0.009) and Alcohol (F(2, 26) = 19.59, p < 0.001) and no diet-alcohol interaction (p = 0.194). Alcohol interacted with session (p=0.020), and diet-alcohol-session interaction approached significance (p=0.073). Impairments were again greatest at the highest alcohol dose such that 5.0 was worse than 3.5 and 0 (p’s ≤ 0.002), and 3.5 was worse than 0 (p=0.008).
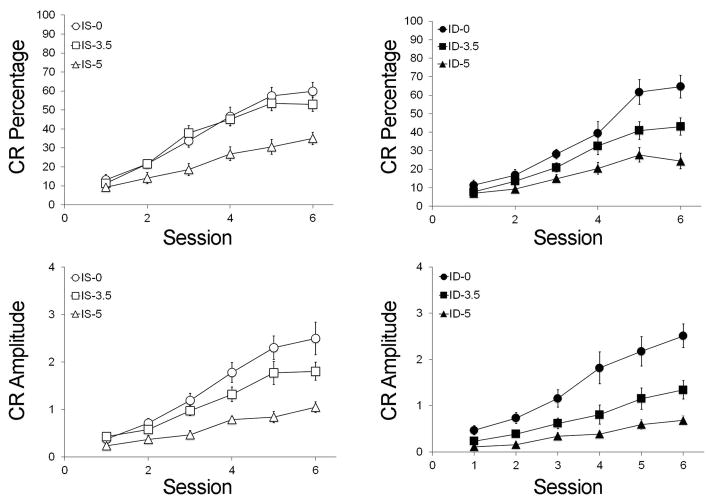
Trace ECC Results (Figure 2)
Figure 2.
Trace eyeblink classical conditioning between rats in the iron-sufficient (IS, open symbols, A, C) and iron-deficient (ID, closed symbols, B, D) groups as a function of alcohol dose (○● 0 g/kg, □■ 3.5 g/kg, △▲5 g/kg; n = 10–12 animals per treatment group; error bars represent S.E.M.). Separately, alcohol and ID significantly affected learning acquisition in terms of conditioned response (CR) percentage (A, B) and amplitude (C, D) during paired training with the CS and US. Due to the inherent difficulty with acquiring CRs in trace ECC compared to delay ECC, the overall percentages and amplitudes are lower for all groups. ID-3.5 and ID-5.0 animals were more significantly impaired in acquiring CRs than their same-dose counterparts. Differences in learning were not detected between ID-0 and IS-0 animals. Conversely, the impact of moderate or heavy alcohol exposure (3.5 g/kg and 5 g/kg) on learning was mitigated by an iron-sufficient diet.
Preliminary analysis indicated no significant sex effect and thus data were pooled across this factor. For CR percentage, there were significant main effects of Iron (F(1, 26) = 3.29, p = 0.041), Alcohol (F(2, 26) = 37.23, p < 0.001), and a significant interaction between them (F(2, 26) = 4.18, p = 0.013). For CR amplitude there was a significant main effect of Alcohol (F(2, 26) = 33.15, p < 0.001), a trend to an effect of Iron (F(1, 26) = 2.84, p = 0.052) and no interaction (p = 0.124). As with delay ECC, alcohol and ID separately affected trace ECC acquisition, and rats receiving 5 g/kg were more impaired in acquiring (p’s < 0.001) and producing CRs (p’s < 0.017) as compared to the two low dose groups, as were the 3.5 g/kg animals compared with the 0 g/kg animals (CR% p’s < 0.007; CR amplitude p’s <0.001). CR amplitude was significantly worse for ID-5 than IS-5 animals (p=0.03).
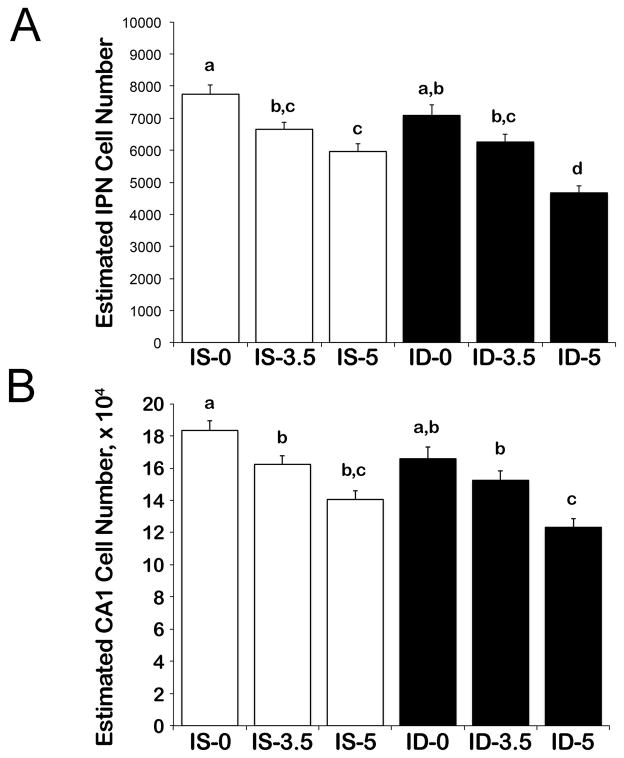
Cerebellar and Hippocampal Neuronal Cellularity
Initial ANOVAs showed no significant differences due to sex as a factor in either learning paradigm therefore it was removed from subsequent analyses. Because there is no reason to believe that task will affect cellularity, and because task itself did not affect cellularity (p = 0.881), we pooled the cell counts across ECC task and conducted 2 (Iron Status) x 3 (Alcohol) ANOVAs for the different cell types.
For the IPN (Figure 3A), we found significant main effects of Iron (F(1, 61) = 14.60, p < 0.001) and Alcohol (F(2, 61) = 36.95, p < 0.001), and their potential interaction approached significance (p = 0.083). Alcohol exposure significantly reduced cellularity within the IPN in a dose-dependent manner, and IPN neuronal numbers were lowest at 5 g/kg and highest at 0 g/kg alcohol (p’s < 0.001). Cellularity reductions were significantly greater for ID-5 than IS-5 animals (p = 0.004). With respect to hippocampal CA1 neurons (Figure 3B), we found significant main effects of Iron (F(1, 61) = 7.08, p = 0.005) and Alcohol (F(2, 61) = 23.51, p < 0.001), and no interaction (p = 0.269). Alcohol exposure significantly reduced cellularity within the CA1, and 5 g/kg was worse than for the 3.5 and 0 (p’s <0.001) and 3.5 was worse than 0 (p= 0.005).
Figure 3.
Cellularity of the (A) Interpositus Nucleus (IPN) and (B) hippocampal CA1 regions of animals that underwent ECC testing. Data from delay and trace subjects were pooled as task did not affect cellularity. Cell count estimates represent unilateral values for each brain region ipsilateral to the trained eye. Values are means ± S.E.M., n = 20–24 animals per treatment group. Means without a common letter differ, p <0.05.
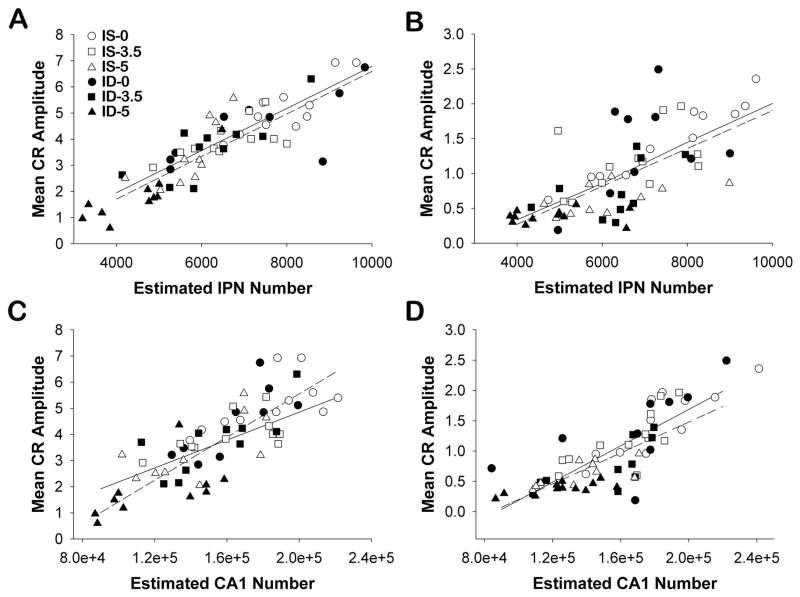
Relationship between Associative Learning and Brain Cellularity
To elucidate potential relationships between learning outcomes and their neurocircuitry, we selected mean CR amplitude across all 6 sessions as the dependent measure for learning, since it is a more continuous measure than CR percentage, reflects cellular activity between IPN neurons and neurons in the cranial motor pathway that produces blink responses, and may provide a more accurate index of learning. Using the mean CR amplitude for each rat across all training sessions was also necessary to limit the number of correlations calculated. For delay ECC, the IPN is the site of associative neuroplasticity (Clark et al., 1992; Woodruff-Pak et al., 1985). For trace ECC, the hippocampus, particularly hippocampal CA1, supports conditioned eyeblink responding (Ivkovich & Stanton, 2001; Moyer et al., 1996). Within each ECC training regimen, we found that learning was highly correlated with cell number. Neuron number in the IPN significantly correlated with CR amplitude in both the delay ECC (Pearson’s r = + 0.871, p < 0.001; Figure 4A) and the trace ECC tasks (Pearson’s r = + 0.703, p < 0.001; Figure 4B). Similarly, CA1 neuron number and CR amplitude significantly correlated in both the delay ECC (Pearson’s r = + 0.792, p < 0.001; Figure 4C) and trace ECC tasks (Pearson’s r = + 0.846, p < 0.001; Figure 4D). We also calculated separate correlations for each IS- and ID-alcohol treatment group (Table 3) and found that learning performance and cellularity were significantly correlated for the delay procedure and the IPN, and for the trace procedure and the CA1, at most alcohol exposures at a minimum p value of 0.05.
Figure 4.
Scatterplots of the unbiased estimated total neuron number and mean conditioned response amplitude (CR) across 6 sessions of eyeblink classical conditioning (ECC) for each rat. Interpositus nucleus (IPN) (A, B) and hippocampal CA1 (C, D) neuron cellularity for animals experiencing delay ECC (A, C) and trace ECC (B, D). Cell count estimates represent unilateral values for each brain region ipsilateral to the trained eye. Linear regressions are plotted for the IS (solid line) and ID (dotted line) animals across alcohol exposures and did not vary in slope or value.
Table 3.
Correlation between Cell Number and CR Amplitude
| ECC Task | Region | Diet | Alcohol, g/Kg | Correlation coefficient | Slope* |
|---|---|---|---|---|---|
| Delay | IPN | Pooled | Pooled | 0.871† | 8.31 x 10−4 |
| IS | Pooled | 0.835† | 8.06 x 10−4 | ||
| 0 | 0.846† | 9.13 x 10−4 | |||
| 3.5 | 0.635† | 4.70 x 10−4 | |||
| 5 | 0.762† | 1.29 x 10−3 | |||
| ID | Pooled | 0.873† | 8.15 x 10−4 | ||
| 0 | 0.713† | 5.41 x 10−4 | |||
| 3.5 | 0.806† | 8.14 x 10−4 | |||
| 5 | 0.879† | 9.16 x 10−4 | |||
| CA1 | Pooled | Pooled | 0.792† | 3.59 x 10−5 | |
| IS | Pooled | 0.741† | 2.87 x 10−5 | ||
| 0 | 0.622† | 2.30 x 10−5 | |||
| 3.5 | 0.587 | 1.62 x 10−5 | |||
| 5 | 0.673† | 2.77 x 10−5 | |||
| ID | Pooled | 0.810† | 4.13 x 10−5 | ||
| 0 | 0.783† | 4.43 x 10−5 | |||
| 3.5 | 0.757† | 3.37 x 10−5 | |||
| 5 | 0.547 | 2.02 x 10−5 | |||
| Trace | IPN | Pooled | Pooled | 0.703† | 2.79 x 10−4 |
| IS | Pooled | 0.735† | 2.78 x 10−4 | ||
| 0 | 0.968† | 3.27 x 10−4 | |||
| 3.5 | 0.447 | 1.72 x 10−4 | |||
| 5 | 0.593 | 9.79 x 10−5 | |||
| ID | Pooled | 0.622† | 2.67 x 10−4 | ||
| 0 | 0.407 | 2.43 x 10−4 | |||
| 3.5 | 0.517 | 2.04 x 10−4 | |||
| 5 | 0.118 | 1.18 x 10−5 | |||
| CA1 | Pooled | Pooled | 0.846† | 1.53 x 10−5 | |
| IS | Pooled | 0.856† | 1.50 x 10−5 | ||
| 0 | 0.861† | 1.59 x 10−5 | |||
| 3.5 | 0.736† | 1.35 x 10−5 | |||
| 5 | 0.693† | 6.72 x 10−6 | |||
| ID | Pooled | 0.821† | 1.49 x 10−5 | ||
| 0 | 0.849† | 1.11 x 10−5 | |||
| 3.5 | 0.710† | 1.02 x 10−5 | |||
| 5 | 0.722† | 3.29 x 10−6 |
Slopes did not differ as determined by analysis of covariance.
Indicates Pearson’s correlation coefficients for which p < 0.05
CONCLUSIONS
The most important finding from this study is that maternal iron status strongly influences alcohol’s neurobehavioral damage in the developing offspring. We show that gestational ID potentiates the ability of developmental alcohol exposure to impair cerebellar-dependent associative learning, confirming our previous work (Rufer et al., 2012). We extend this and show that this learning deficit was accompanied by significant neuronal losses in the circuitry that participates in this learning. We further demonstrate that ID also worsens hippocampal-dependent learning due to alcohol exposure, and this associates with cellular losses within CA1 populations that participate in that task. These data endorse the hypothesis that severe outcomes within FASD may represent, in part, alcohol-exposed pregnancies during which the mother and her offspring were iron-insufficient. The level of iron deficiency necessary for this adverse interaction is relatively modest, wherein maternal liver iron stores are depleted but maternal hematological indices are largely normal (Rufer et al., 2012). In a clinical setting, such a lack of overt anemia might preclude the ID from being diagnosed or aggressively treated. It is important to add that the learning and cellular deficits in these alcohol-exposed animals were substantially mitigated, although not fully normalized, when maternal iron intake was adequate. This suggests that normal iron nutriture protects the developing brain against alcohol’s damage and that alcohol-exposed pregnancies having a marginal iron status have heightened vulnerability to alcohol’s damage, and will likely benefit from iron supplementation.
Neurobehavioral deficits are the most important sequelae from PAE. The ECC tasks evaluated here show promise as biobehavioral markers in FASD screening and assessment (Jacobson et al., 2011; Foroud et al., 2012), hence our findings have direct relevance for FASD. Our finding that alcohol exposure reduced the cerebellar and hippocampal neuronal populations that contribute to this learning is consistent with these populations’ known vulnerability to alcohol during the brain growth spurt period (Green et al., 2002; Tran et al., 2005). Cellularity within the IPN and CA1 are important determinants of ECC learning (Clark et al., 1992; Ivkovich and Stanton, 2001; Moyer et al., 1996; Woodruff-Pak et al., 1985) and as our correlational analysis suggests, these cellular losses likely contribute to the impaired learning in these ECC tasks. With respect to iron status, gestational ID also reduces delay and trace conditional learning (McEchron et al., 2008), and our work adds to that literature by documenting that this is accompanied by cellular reductions within that neurocircuitry. The basis for these reductions is not fully understood but may involve the increased apoptosis and reduced myelination previously documented in this model (Rufer et al., 2012). It is also unknown why these neural structures are vulnerable to the ID-alcohol combination. The cerebellum is normally iron-enriched whereas the hippocampus is not (Hare et al., 2012), suggesting that cellular vulnerability to ID-alcohol cannot be explained by differential iron requirements. Gestational ID heightens hippocampal vulnerability to neurotoxic damage, such as from ischemia-induced injury (Rao et al., 2007), and could similarly act here to shape vulnerability to alcohol’s neurotoxicity. Separately, ID and alcohol disrupt numerous developmental processes in brain (Beard and Connor 2003; Miller 2006). Alcohol exposure alters iron metabolism in adult alcoholics (Harrison-Findek et al., 2006), and our preliminary data (Huebner et al., submitted) suggest that alcohol may alter how the developing fetus utilizes iron, particularly if iron is limiting. Although sex can affect the acquisition of ECC responses (i.e. Dalla & Shors, 2009), the lack of sex effects here are consistent with previous investigations of FASD (Green et al., 2002; Tran et al., 2005, 2007; Thomas & Tran, 2012). We cannot rule out that hormonal fluctuations during the estrus cycle could have masked sex-based learning differences.
Mounting evidence suggests it may not be coincidental that populations with both widespread ID and frequent alcohol consumption have some of the highest rates of FASD. In low-income populations of South Africa, where FASD rates may be highest, iron intakes of pregnant and lactating women seldom attain requirements (Hattingh et al. 2008; May et al. 2014b) and prevalence estimates range from 19%–86% for ID and 9%–20% for ID-anemia (Faber et al., 2001; Nojilana et al. 2007). Such disadvantaged populations will have a substantial percentage of alcohol-exposed pregnancies that are also iron-deficient and thus are at risk for worsened outcomes due to these ID-alcohol interactions (Carter et al., 2007, 2012; Rufer et al. 2012). Clinical studies strongly endorse that maternal ID is a risk factor that exacerbates the damage of FASD. In an urban Coloured community in South Africa, ID-anemia is more common in infants whose mothers’ binge-drink, and those infants have the smallest head circumference and the greatest delays in weight gain (Carter et al., 2007, 2012). Those growth delays are reproduced in our rat model of ID-alcohol (Rufer et al., 2012), and importantly, in those same animals ECC learning is further impaired by the ID-alcohol combination. In this model, multiple features of FASD are significantly worsened by concurrent ID including brain size, body weight gain, myelination, cerebellar and hippocampal cellularity, and associative learning (Rufer et al., 2012; this study), suggesting additional ID-alcohol effects may be identified in clinical populations. Although it is without question that PAE damages the developing embryo and fetus, the collective clinical and animal data emphasize that maternal/fetal iron status is a crucial modifier of FASD outcome. Under ID-5, the animals had difficulty in acquiring the Delay and Trace ECC tasks and performed worse than the other iron-sufficient and iron-deficient groups. Thus, iron sufficiency appears to confer protection especially against the higher alcohol exposure, and ID heightened vulnerability as alcohol dose increased. The collective data suggest that improvements in gestational and perinatal iron status should significantly mitigate alcohol’s developmental damage.
Unfortunately, gestational ID remains a significant public health problem in child-bearing age women across socioeconomic levels because of the appreciable iron losses from menstruation (14 mg/menses) and childbirth (700–800 mg/term birth). Oral iron supplements can address this, but they have low bioavailability and are poorly tolerated because they irritate the gut. Although alcohol consumption is associated with increased liver iron stores through its suppression of liver hepcidin (Harrison-Findik et al. 2006), this mechanism is irrelevant when the diet is iron deficient. Indeed, ID is not uncommon in alcohol-exposed pregnancies, and heavy-drinking pregnant woman are more likely to have low rather than high liver iron stores, as indicated by their reduced serum ferritin and transferrin saturation despite hemoglobin levels in the normal range (Streissguth et al. 1983). This situation parallels that of our ID rat dams (Rufer et al. 2012) and is in a range to interact with alcohol and further impair the child’s developmental outcome. Conversely, compared with offspring born to ID dams, outcomes were generally better in the IS offspring who experienced equivalent alcohol exposures. This was seen not only in the associative learning and brain neuronal counts here, but also in the myelination levels and growth potential (Rufer et al., 2012). Thus, although the potential impact of PAE upon maternal/fetal iron utilization is unknown and under active investigation, these studies endorse the importance of providing adequate iron to the alcohol-exposed pregnancy, because both the animal and clinical data predict that improvements of maternal iron status, especially in populations with high ID risk, will likely improve gestational outcomes despite high maternal alcohol use.
In summary, we validate our previous study and demonstrate that poor iron status heightens the offspring’s vulnerability to alcohol’s neurodevelopmental damage. These data endorse that the treatment of maternal ID through normalization of iron status should improve the child’s developmental outcome despite the gestational alcohol exposure.
Table 1.
Stereological Parameters
| IPN | CA1 | |
|---|---|---|
| a(frame), μm | 75 x 75 | 30 x 30 |
| a(X, Y) step, μm | 161 x 318 | 70 x 231 |
| Disector h, μm | 12 | 12 |
| Guard h, μm | 3 | 3 |
| Section sampling fraction | 1/3 | 1/4 |
| Nominal thickness* | 25 μm | 25 μm |
Mean section thickness as measured with a Z-axis encoder was used to compute the number estimate
Acknowledgments
This work was funded by NIH F32 AA21311 (S.M.H.), R21 AA17281 (S.M.S, T.D.T.), R01 AA22999 (S.M.S.), and R37 AA11085 (S.M.S.).
Footnotes
Conflict of Interest. The authors declare no conflict of interest.
Contributor Information
Shane M. Huebner, Email: shuebner@wisc.edu.
Tuan D. Tran, Email: trant@ecu.edu.
Echoleah S. Rufer, Email: esrufer@gmail.com.
Peter M. Crump, Email: peter.crump@wisc.edu.
Susan M. Smith, Email: suesmith@nutrisci.wisc.edu.
References
- Beard JL, Connor JR. Iron status and neural functioning. Ann Rev Nutr. 2003;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Permanent neuronal deficits in rats exposed to alcohol during the brain growth spurt. Teratology. 1991;44:147–163. doi: 10.1002/tera.1420440203. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson SW, Molteno CD, Jacobson JL. Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics. 2007;120:559–567. doi: 10.1542/peds.2007-0151. [DOI] [PubMed] [Google Scholar]
- Carter RC, Jacobson JL, Molteno CD, Jiang H, Meintjes EM, Jacobson SW, Duggan C. Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol Clin Exp Res. 2012;36:1973–1982. doi: 10.1111/j.1530-0277.2012.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Zhang AA, Lavond GD. Reversible lesions of the cerebellar interpositius nucleus during acquisition and retention of a classically conditioned behavior. Behav Neurosci. 1992;106:879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, Nelson CA. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatrics. 2012;160:1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber M, Jogessar VB, Benade AJS. Nutritional status and dietary intakes of hcildren aged 2–5 years and their caregivers in a rural South African community. Int J Food Sci Nutr. 2001;52:401–411. doi: 10.1080/09637480120078285. [DOI] [PubMed] [Google Scholar]
- Foroud T, Wetherill L, Vinci-Booher S, Moore ES, Ward RE, Hoyme HE, Robinson LK, Rogers J, Meintjes EM, Molteno CD, Jacobson JL, Jacobson SW. Relation over time between facial measurements and cognitive outcomes in fetal alcohol-exposed children. Alcohol Clin Exp Res. 2012;36:1634–1646. doi: 10.1111/j.1530-0277.2012.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Marcussen BL, West JR. A single day of alcohol exposure during the brain growth spurt induces microencephaly and cerebellar Purkinje cell loss. Alcohol. 1990;7:107–114. doi: 10.1016/0741-8329(90)90070-s. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Stanton ME, Steinmetz JE. Alcohol-induced damage to the developing brain: Functional approaches using classical eyeblink conditioning. In: Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink Classical Conditioning: Volume 2, Animal Models. Kluwer Academic Publishers; Boston: 2000. pp. 135–154. [Google Scholar]
- Green JT, Tran TD, Steinmetz JE, Goodlett CR. Neonatal ethanol produces cerebellar deep nuclear cell loss and correlated disruption of eyeblink conditioning in adult rats. Brain Res. 2002;956:302–311. doi: 10.1016/s0006-8993(02)03561-8. [DOI] [PubMed] [Google Scholar]
- Hare DJ, Lee JK, Beavis Ad, van Gramberg A, George J, Adlard PA, Kinkelstein DI, Doble PA. Three-dimensional atlas of iron, copper, and zinc in the mouse cerebrum and brainstem. Anal Chem. 2012;84:3990–3997. doi: 10.1021/ac300374x. [DOI] [PubMed] [Google Scholar]
- Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- Hattingh Z, Walsh CM, Bester CJ, Oguntibeju OO. An analysis of dietary micronutrient intakes in two age groups of black South African women. West Indian Med J. 2008;57:431–437. [PubMed] [Google Scholar]
- Ivkovich D, Stanton ME. Effects of early hippocampal lesions on trace, delay, and long-delay eyeblink conditioning in developing rats. Neurobiol Learn Mem. 2001;76:426–446. doi: 10.1006/nlme.2001.4027. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Chiodo LM, Corobana R. Maternal age, alcohol abuse history, and quality of parenting as moderators of the effects of prenatal alcohol exposure on 7.5-year intellectual function. Alcohol Clin Exp Res. 2004;28:1732–1745. doi: 10.1097/01.alc.0000145691.81233.fa. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychol Rev. 2011;21:148–166. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson LA, Sun M, O’Connor M, Georgieff MK. Fetal iron deficiency disrupts the maturation of synaptic function and efficacy in area CA1 of the developing rat hippocampus. Hippocampus. 2005;15:1094–1102. doi: 10.1002/hipo.20128. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Roesch SC, Glass L, Deweese BN, Coles CD, Kable JA, May PA, Kalberg WO, Sowell ER, Adnams CM, Jones KL, Riley EP CIFASD. Further development of a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2013;37:517–528. doi: 10.1111/j.1530-0277.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Baete A, Russo J, Elliott AJ, Blankenship J, Kalberg WO, Buckley D, Brooks M, Hasken J, Abdul-Rahman O, Adam MP, Robinson LK, Manning M, Hoyme HE. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics. 2014a;134:855–866. doi: 10.1542/peds.2013-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais AS, Brooke LE, Blankenship J, Hoyme HE, Gossage JP. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod Toxicol. 2014b;46:31–39. doi: 10.1016/j.reprotox.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning MA, Blankenship J, Buckley D, Hoyme HE, Adnams CM. Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. J Dev Behav Pediatr. 2013;34:314–325. doi: 10.1097/DBP.0b013e3182905587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Alexander DN, Gilmartin MR, Paronish MD. Perinatal nutritional iron deficiency impairs hippocampus-dependent trace eyeblink conditioning in rats. Dev Neurosci. 2008;30:243–254. doi: 10.1159/000110502. [DOI] [PubMed] [Google Scholar]
- Melo AI, Lovic V, Gonzalez A, Madden M, Sinopoli K, Fleming AS. Maternal and littermate deprivation disrupts maternal behavior and social-learning of food preference in adulthood: tactile stimulation, nest odor, and social rearing prevent these effects. Dev Psychobiol. 2006;48:209–219. doi: 10.1002/dev.20130. [DOI] [PubMed] [Google Scholar]
- Miller MW. Brain Development. Oxford University Press; New York: 2006. [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;6:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojilana B, Norman R, Dhansay MA, Labadarios D, van Stuijvenberg ME, Bradshaw D the South African Comparative Risk Assessment Collaborating Group. Estimating the burden of disease attributable to iron deficiency anaemia in South Africa in 2000. S Afr Med J. 2007;97:741–746. [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Dev Disabil Res Rev. 2009;15:209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eyelid responses. J Neurosci. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Tkac I, Schmidt AT, Georgieff MK. Fetal and neonatal iron deficiency causes volume loss and alters the neurochemical profile of the adult rat hippocampus. Nutr Neurosci. 2011;14:59–65. doi: 10.1179/1476830511Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R, Tkac I, Townsend EL, Ennis K, Gruetter R, Georgieff MK. Perinatal iron deficiency predisposes the developing rat hippocampus to greater injury from mild to moderate hypoxia-ischemia. J Cereb Blood Flow Metab. 2007;27:729–740. doi: 10.1038/sj.jcbfm.9600376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufer ES, Tran TD, Attridge MM, Andrzejewski ME, Flentke GR, Smith SM. Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders. PLoS One. 2012;7(10):e47499. doi: 10.1371/journal.pone.0047499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav Neurosci. 1988;102:586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr. 2001;131:697S–700S. doi: 10.1093/jn/131.2.697S. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Labbe RF, Smith JR, Darby BL, Smith NF, Martin DC, Doan RN. Alcohol use and iron status in pregnant women. Alcohol Clin Exp Res. 1983;7:227–230. doi: 10.1111/j.1530-0277.1983.tb05447.x. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22:619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TD, Horn KH, Jackson HD, Goodlett CR. Vitamin E antioxidant therapy does not protect against neonatal ethanol-induced conditioned eyeblink learning or neuronal loss in the cerebellum of rats. Alcohol Clin Exp Res. 2005;29:117–129. doi: 10.1097/01.alc.0000150004.53870.e1. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicol Teratol. 2003;25:519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Tran TD, Stanton ME, Goodlett CR. Binge-like ethanol exposure during the early postnatal period impairs eyeblink conditioning at short and long CS-US intervals in rats. Dev Psychobiol. 2007;49:589–605. doi: 10.1002/dev.20226. [DOI] [PubMed] [Google Scholar]
- Voogd J. The human cerebellum. J Chem Neuroanat. 2003;26:243–252. doi: 10.1016/j.jchemneu.2003.07.005. [DOI] [PubMed] [Google Scholar]
- West JR, Hamre KM, Cassell MD. Effects of ethanol exposure during the third trimester equivalent on neuron number in rat hippocampus and dentate gyrus. Alcohol Clin Exp Res. 1986;10:190–197. doi: 10.1111/j.1530-0277.1986.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Yu GS, Steinkirchner TM, Rao GA, Larkin EC. Effect of prenatal iron deficiency on myelination in rat pups. Am J Pathol. 1986;125:620–624. [PMC free article] [PubMed] [Google Scholar]