Importance of Collateral Circulation in Ischemic Stroke
Revascularization encompasses all treatment-related improvements in blood flow, including recanalization of the proximal arterial occlusion and reperfusion of the downstream territory. Recanalization is required for antegrade tissue reperfusion, but recanalization may not necessarily lead to reperfusion in regions where distal emboli or established infarctions are present.1,2 On the contrary, acute reperfusion without recanalization may occur in patients who received or did not received endovascular therapies, and reperfusion ≤6 hours was consistently superior to recanalization in predicting tissue and clinical outcome.3 The cerebral collateral circulation refers to the subsidiary network of vascular channels that stabilize cerebral blood flow when principal conduits fail. Collateral status differs among patients with acute ischemic stroke. Relatively sparse attention has been devoted to the role of baseline collateral circulation in patients with acute ischemic stroke who are candidates for revascularization.
The IMS III,4 MR RESCUE,5 and SYNTHESIS Expansion trials6 were three multicenter, prospective, randomized controlled trials which failed to show a benefit from endovascular intervention for acute ischemic stroke. In addition, successful recanalization failed to improve the functional outcome in a significant proportion of patients, ranging from 26 to 49% (futile and dangerous recanalization), stimulating the need to improve the selection of patients based on individual pathophysiology.7,8
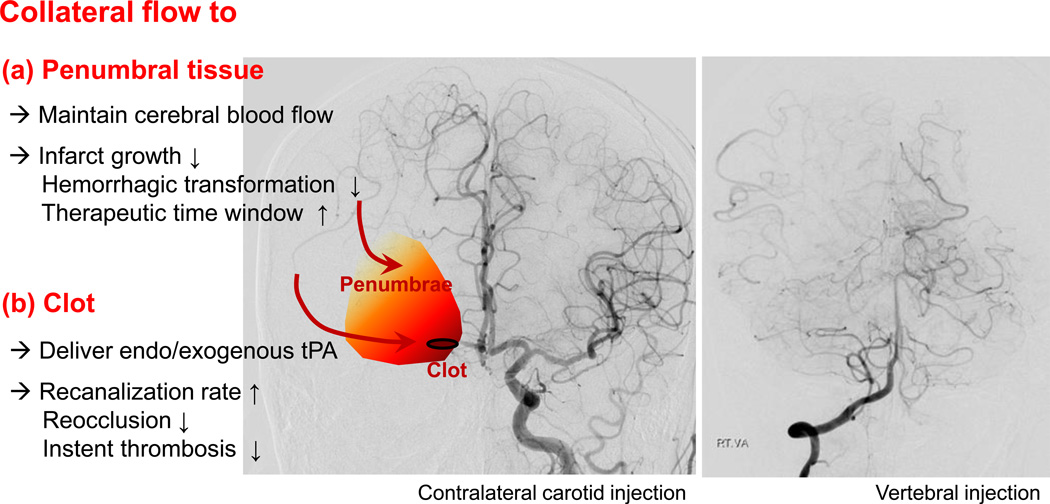
Among neuroimaging parameters, a large core and poor collaterals are demonstrated to be strong predictors of both response to endovascular therapy and functional outcome,9, 10,11,12,13 and excluding patients with large core and poor collateral circulation may improve the therapeutic benefit from endovascular therapy. In the subgroup analysis of the IMS III trial, more robust collateral grade was associated with better clinical outcomes.14 Adequate collateral circulation may contribute to the maintenance of tissue viability in the absence of recanalization. In both intravenous thrombolysis and endovascular trials, shorter time to treatment was associated with better odds for positive outcome.15,16,17 However, stroke patients presenting at later time points may still benefit from endovascular therapy,18 and the time to treatment was a predictor of outcome only when collaterals were not considered, suggesting the important role of collaterals for the determination of this time window.19 Good pial or leptomeningeal collateral circulation predicts better clinical responses to intra-arterial treatment even 5 hours after the onset of the stroke, suggesting that collateral status could extend the time window for endovascular procedures.20,21 Therefore, collateral flow to penumbral tissue beyond the clot has clinical implications in the setting of acute endovascular therapy (Figure 1).
Figure 1.
Impact of collateral flow to penumbral tissue and occluding clot.
tPA, tissue plasminogen activator
With the lessons from the aforementioned randomized clinical trials published 2013, the recent phase III randomized control trials have been conducted; the MR CLEAN,22 ESCAPE,23 EXTEND-IA,24 SWIFT PRIME,25 and REVASCAT trials.26 Most studies addressed the large core (as measured by the ASPECT score <5–7 points) and one study (the ESCAPE trial) poor collaterals in their exclusion criteria. In the ESCAPE trial, collateral status was measured in most cases by multiphasic computed tomography (CT) angiography, a dedicated CT technique to exclude patients with absent collateral.23,27 New evidence from these new randomized trials has demonstrated an overwhelming benefit from endovascular intervention, preferably with stent retriever-mediated mechanical thrombectomy, for the treatment of acute ischemic stroke secondary to large arterial occlusion. Beside the endovascular therapy field, the results of recent stroke prevention trials (WASID and SAMMPRIS) and thrombolysis trials (DIAS-2) have also emphasized the importance of collateral circulation.28,29
Images for Assessment of Collateral Status using CT or MRI
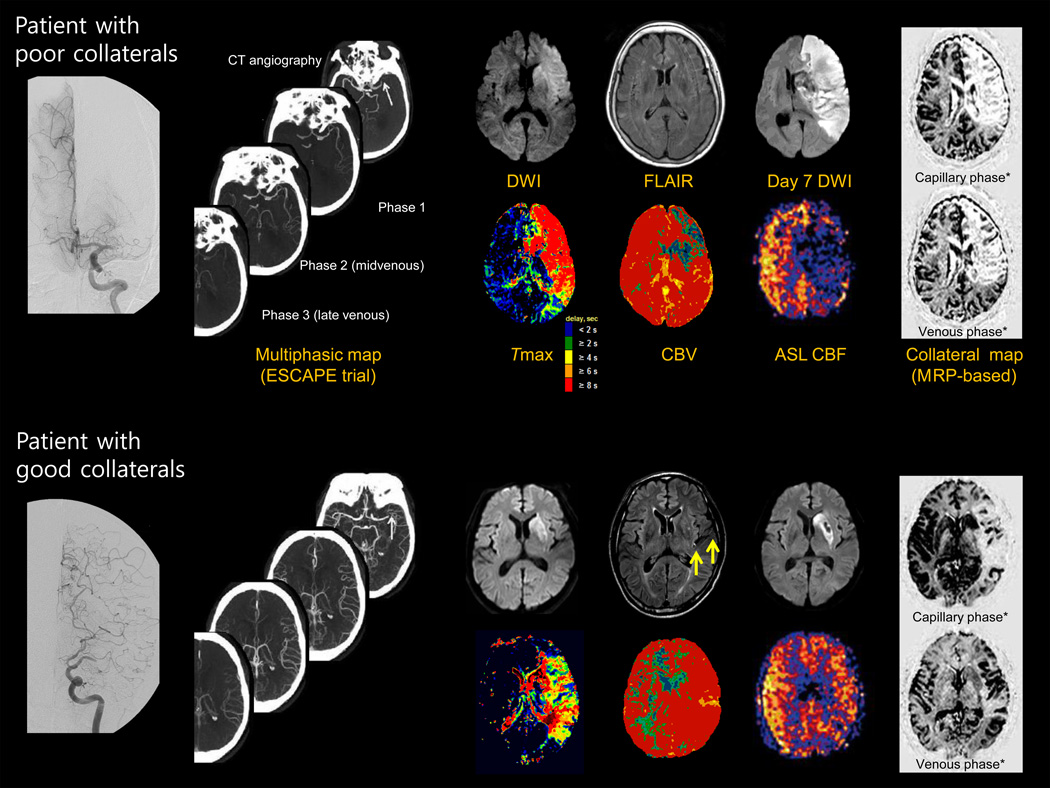
Conventional angiographic evaluation has advantages, including its reliable demonstration of occlusion vs. subtotal occlusion, well standardized recanalization grading, and high resolution visualization of leptomeningeal collaterals.1,30 However, it has several limitations. First, because conventional angiography is invasive, it requires more expertise and time to perform and carries a small risk of thrombotic events. Second, the results of angiographic collateral studies would mostly be incomplete (e.g., not including the venous phase, no contralateral or vertebrobasilar view), especially in acute setting. In addition, it is not possible to simultaneously examine both anterior and poster circulation-derived collaterals (Figure 1). Last, the information obtained regarding the effect of collateral status on the anterior and lateral views in conventional angiography cannot easily be correlated with axial images typically used to depict CT or MRI of ischemic injury. Various efforts have been made to visualize collateral flow using CT or MRI (Figure 2).
Figure 2.
Various neuroimaging parameters representing the infarcted area and collateral status.
Arrow indicate the distal hyperintense vessels
* based on the contralateral side
The Alberta Stroke Program Early CT Score (ASPECTS)
The ASPECTS on baseline imaging is an established predictor of acute ischemic stroke outcome. Both non-enhanced CT and contrast-enhanced CT ASPECTS showed a good correlation with leptomeningeal collateral grade on conventional angiography.31 Hypoattenuation on non-contrast CT is caused by a shift in brain tissue water content secondary to ischemia, and this process is dependent on the time as well as the degree of ischemia. To increase the interrater reliability of non-enhanced CT ASPECTS, especially in early phase of infarction, ASEPCTS on CT angiography source image has been developed that is less time dependent.32 The ASPECTS on contrast-enhanced CT or CT angiography source image showed better correlation with baseline stroke severity and infarct growth than non-enhanced CT ASPECTS.33
Multiphasic CT and CT angiography
Multiphasic contrast-enhanced CT collateral grades showed a good correlation with leptomeningeal collateral grade on conventional angiography in acute ischemic stroke,34,35 and has been used as a prediction tool for final infarct volume, infarct growth, subsequent brain edema, and clinical response after thrombolysis.36,37,38 More recently, the ESCAPE trialists developed a six-point score system for pial arterial filling using multiphasic CT angiography, which showed a good interrater reliability and ability to help determine clinical outcome.27 This technique is a quick and easy-to-use and needs minimal additional radiation and no additional contrast material, and no post-processing.
CT perfusion
CT perfusion may provide information of collateral status as well as core and penumbra. CT perfusion has the advantage of rapidity and wide accessibility in emergency room, and can be combined with non-enhanced CT and CT angiographic data, especially in patients with anterior circulation stroke.39,40 One retrospective study showed that the most accurate assessment of the site of occlusion, infarct core, salvageable brain tissue, and collateral circulation in patients suspected of acute stroke is afforded by a combination of CT perfusion and CT angiography.39 CT perfusion and CT angiography provide differential assessment of collateral circulation, functional and anatomic aspects, respectively.41 Cerebral blood volume may be elevated or within the normal range depending upon effectiveness of collateral supply. Moreover, virtual CT perfusion can be obtained utilizing CT angiographic information.42 CT perfusion-based selection was used in two recent randomized control trials of revascularization therapy (tenecteplase and thrombectomy).24,43 However, more specific CT perfusion criteria for collateral assessment are yet to be determined.
Conventional MRI
The diffusion-weighted image lesion volume and pattern are associated with the degree of collateral flow in acute ischemic stroke.44,45 A large lesion volume and cortical lesion pattern (regardless of the lesion volume) on diffusion-weighted image are frequently found in patients with poor collaterals. Fluid attenuation inversion recovery images can also provide information about collateral status. The presence of distal hyperintense vessels or fluid attenuation inversion recovery vascular hyperintensities and the absence of perisylvian sulcal effacement are associated with good collaterals and favorable outcome in patients with acute middle cerebral artery stroke.46,47 Similarly, conspicuous flow voids, deoxygenation seen as hypointensity, or disappearing phase mismapping on gradient echo image may also be clues about the collateral status.48
Dynamic susceptibility contrast MR perfusion
Various MR perfusion parameters have been used to measure collateral status. Compared with patients with poor collaterals, those with good collaterals show less severe delays in Tmax and relatively preserved (or even increased) cerebral blood volume within ischemic regions.10 Optimal MR perfusion parameter to predict collateral grade has seldom been reported. In our Tmax severity-weighted probabilistic model, collateral status was determined by a presence of delayed perfusion (Tmax of 16 to 22 sec) rather than by the presence of a shorter delay in perfusion (Tmax ≤10).49 In addition, collateral circulation can be easily visualized by simple post-processing using the source data of dynamic susceptibility contrast MRI. Christensen et al. reported the potential use of novel post-processing and visualization techniques (subtracting the image of the first movement map) for evaluating collaterals using bolus tracking MRI.50 Campbell et al. developed collateral vessel grading using a digitally-subtracted perfusion MRI, and showed that deterioration in collateral grade correlated with subsequent infarct growth.51 We have applied a simple semi-automatic collateral map technique (FAST-Coll program) using perfusion scan source data to assess collateral grade in acute ischemic stroke.52 A good correlation was observed between MR- and conventional angiography-based collateral assessment systems. These techniques have an advantage in that the information regarding collateral status can be directly compared with MR diffusion and perfusion image, and there is no need for additional acquisition of conventional angiography or MRI dedicated for collateral assessment.
Arterial spin labeling MRI (ASL)
ASL is a non-contrast perfusion imaging method to measure cerebral blood flow that relies on the magnetic labeling of arterial water. ASL is a promising technique for the assessment of collateral flow that can provide various types of information regarding collateral status. With ASL, late-arriving flow appears as a serpiginous high ASL signal within cortical vessels, which has been termed ‘arterial transit artifact’ (ATA). Patients with ATA had improved outcomes, suggesting that this signal may represent collateral flow.53,54 In addition, flow-direction-sensitive phase contrast MR angiography and vessel-encoded arterial arterial spin labeling could noninvasively provide information regarding the origins and distal function of collateral flow comparable to that obtained with conventional angiograms.55,56 Lastly, ASL can provide anatomic (ASL MRA) and dynamic blood flow (time-resolved) information in the circle of Willis, similar to that obtained with conventional angiography without the use of exogenous contrast agents.57,58
Imaging techniques to visualize collateral arteries
Various modalities have been used to noninvasively measure collateral flow, such as transcranial Doppler, CT angiography, or MR angiography.59 CT or MR angiography can evaluate the cerebral collateral circulation in the circle of Willis with moderate-to-good diagnostic performance, but has limitation in the evaluation of leptomeningeal collaterals. Recent advances in 7-Tesla MRI have enabled the direct visualization of pial branches in cerebral arterial disease, indicating the possibility of assessing leptomeningeal collaterals with high-resolution MR angiography.60 However, this technique is not feasible in patients with acute stroke.
Advantages and disadvantages of various neuroimaging techniques
As shown in Table 1, they differ greatly depending on the technique used in the assessment of collateral status. While there are various ways to determine collaterals using MRI, these may have limited usefulness in light of (a) recent trials where mostly CT was used; (2) further overwhelming data for the need for speed. It is unlikely that in the near future MRI images can be obtained at the same efficiency as CT based imaging. MRI techniques for collateral status deserve further investigation but are not yet ready for implementation as a valid predicting neurologic biomarker in stroke reperfusion trials for the following aspects.
Availability and scan time: CT-based collateral assessment is faster and more accessible than MRI-based techniques. However, the great potential for improving feasibility and accuracy of MR-based collateral assessment exist. For example, a 6-minute multimodal MRI protocol was proposed recently, which showed a good diagnostic quality and a significant reduction in scan time rivaling that of multimodal CT protocol.66
Visualization: The ASPECTS scoring system imperfectly accounts for brain eloquence, is limited to the middle cerebral artery territory, is dependent on attention to scan quality and technique, and takes practice to learn and use well. MRI-based or multi-modal CT-based collateral techniques may provide better quantitative and good visualization than simple non-enhanced CT techniques.
Evidences: While CT-based collateral assessments have been used in large randomized controlled trials, no studies have been focused on MRI-based collateral assessments vs. standard CT in patient selection for revascularization therapy. Randomized trials are needed to show the same kind of efficiency at CT.
Table 1.
Advantages and disadvantages of various neuroimaging techniques for collateral assessment.
| Images | A good correlation with conventional angiographic collateral grade |
Clinical Trials | Advantages | Disadvantages |
|---|---|---|---|---|
| Conventional angiography | None | Reference standard | Invasive | |
| ASPECTS score | ASPECTS on both contrast-enhanced than non-enhanced CT31 | ESCAPE23 SWIFT PRIME,25 REVASCAT26 |
Easy-to-use Routine study |
Relatively low interrater reliability61 Collaterals not visualized |
| Multiphasic CT | Multiphasic perfusion CT collateral grades34,35 | ESCAPE23 | Easy-to-use | Radiation Needs contrast dye |
| Diffusion-weighted image | Infarct volume44 Infarct pattern45 |
BRASIL62 DEFUSE63 |
Routine MRI study | Many confounders Collaterals not visualized |
| FLAIR and gradient echo image | Distal hyperintense vessel on FLAIR46 | Observational studies | Routine MRI study | Poor visualization |
| MR or CT perfusion | Tmax severity10,49 | DEFUSE63 EPITHET64 MR RESCUE5 EXTEND-IA24 SWIFT PRIME25 |
Quantitative and good visualization | Needs post-processing Needs contrast dye More specific criteria for collateral assessment are needed |
| MR-based collateral image | Collateral map using DSC PWI source data65 | Observational studies | Good visualization Simple post-processing performed at workstations; Covers the entire vascular phase |
Needs validation |
| Arterial spin labeling | Vessel-encoded ASL56,55 Arterial transit artifact53 |
iCAS Other ongoing studies* |
Vessel selective information No contrast dye |
Contamination from the partial labeling of the nearby vessels or antegrade flow T1 decay of the label may limit the extent to which slow inflows can be imaged |
ASPECTS, Alberta Stroke Program Early CT Score; ESCAPE, Endovascular treatment for small core and anterior circulation proximal occlusion with emphasis on minimizing CT to recanalization times; SWIFT PRIME, Solitaire with the intention for thrombectomy as primary endovascular treatment for acute ischemic stroke; REVASCAT, Endovascular revascularization with solitaire device versus best medical therapy in anterior circulation stroke within 8 hours; BRASIL, Bleeding risk analysis in stroke; DEFUSE, Diffusion and perfusion imaging evaluation for understanding stroke evolution imaging before thrombolysis; FLAIR, fluid attenuation inversion recovery; EPITHET, the echoplanar imaging thrombolysis evaluation trial; MR RESCUE, mechanical retrieval and Recanalization of stroke clots using embolectomy; EXTEND-IA, extending the time for thrombolysis in emergency neurological deficits-Intra-arterial; iCAS, imaging collarterals in acute stroke.
Quantifying collateral perfusion in cerebrovascular disease-Moyamoya disease and stroke patients (NCT01419275), Acute MRI in transient ischemic attack (NCT01531946), and A longitudinal study of multimodal resonance imaging in stroke patients (NCT02024503).
Strategies to enhance Collateral Circulation
Several conditions might adversely affect collateral status, including systemic illness (cardiac or pulmonary illness, dehydration, vascular risk factors, etc), medications that inhibit physiological augmentation of blood pressure (i.e., high-dose antihypertensives or wide fluctuation in blood pressure), and cerebral vascular status (widespread cerebral atherosclerosis or incomplete circle of illness).67 Optimization of these factors could help to minimize the risk of collateral failure. Beside, non-modifiable factors, such as aging and genetic factors may also influence on the collateral development and rarefaction.68,69
Collateral enhancing strategies are important ways to restore blood flow within ischemic regions, particularly in patients who are ineligible for revascularization therapy, such as those outside the therapeutic time window for intravenous thrombolysis (4.5 h) or endovascular therapy (6–8 h), or those having poor collaterals in whom unfavorable response to revascularization therapy is expected. Collateral enhancing strategies for hemodynamic manipulations include induced hypertension, lying flat head position, volume expansion, external counterpulsation, partial aortic obstruction diverting splanchnic blood flow to the upper body and brain, and sphenopalatine ganglion stimulation (Table 2).67,70 In the field of neurosurgery, emergent bypass surgery has been applied for patients with acute ischemic stroke.71,72 Application of nitric oxide,73 albumin,74 and tumor necrosis factor-α inhibitor75 has been shown to increase arteriogenesis (collateral formation from preexisting channels) in animal model of stroke. In addition, preclinical and clinical studies have showed that various pharmacological therapies may enhance angiogenesis (capillary formation from preexisting vessels) and vascuologenesis (de novo capillary formation),67,76,77 which includes sildenafil and phosphodiesterase type 5 inhibitors,78,79 erythropoietin/trophic factors,80,81,82 and statins with or without cell therapy.83,84,85,86 Large, randomized trials in acute stroke patients have seldom been performed and showed negative results.87,88,89,90 The potential reasons for these failures include inadequate patient selection and lack of assessment of the effects of such interventions on collateral blood vessels and collateral flow. Further studies are needed with optimal patient selection and rigorous assessment of the therapeutic values of collateral enhancing strategies using advanced imaging techniques for collateral flow. There are currently several ongoing clinical trials employing various strategies (Table 2).
Table 2.
Therapeutic strategies for enhancing collateral circulation via arteriogenesis and angiogenesis/vasculogenesis.
| Arteriogenesis | Clinical trials | Angiogenesis/ vasculogenesis |
Clinical trials |
|---|---|---|---|
| Induced hypertension | SETIN-Hypertension (O) | Erythropoietin | EPO stroke (C) |
| Head position | HeadPoST (O) | Trophic factors | |
| Volume expansion | Statins | ||
| Partial aortic occlusion | SENTIS (C), FASTFlo-tPA (C), Flo24 (C) |
PDE5 inhibitors | Sildenafil treatment of subacute ischemic stroke (C) |
| SPG stimulation | ImpACT-24 (O) | Stem/progenitor cells | |
| External counter pulsation | CUFFS (C), EULIPCCS (O) |
||
| Bypass surgery | |||
| Albumin | ALIAS (C) | ||
| Nitric oxide | ENOS (C) | ||
| TNF-α inhibitor |
SPG, sphenopalatine ganglion; TNF-α, tumor necrosis factor-α; PDE5, phosphodiesterase type 5 inhibitors.
SETIN-Hypertension, Safety and efficacy of therapeutic induced hypertension in acute non-cardioembolic ischemic stroke; HeadPoST, Head position in stroke trial; SENTIS, Safety and efficacy of NeuroFlo technology in ischemic stroke; FASTFlot-tPA, Feasibility and safety of NeuroFlo in stroke patients receiving tissue plasminogen activator; Flo24, Safety and efficacy of NeuroFlo in 8–24 hour stroke patients; ImpACT-24, Implant for augmentation of cerebral blood flow trial, effectiveness and safety in a 24 hour window; CUFFS, Safety study of external counterpulsation as a treatment for acute ischemic stroke; EULIPCCS, Effects of upper limb ischemic postconditioning on collateral circulation after stroke; ALIAS, Albumin in acute ischemic stroke trial; ENOS, Efficacy of nitric oxide in stroke; EPO stroke, Multicenter efficacy study of recombinant human erythropoietin in acute ischemic stroke.
(C) completed trials ; (O) ongoing trials.
Conclusions and perspectives
In acute stroke patients, reperfusion after cerebral ischemia can be achieved via collaterals or through arterial revascularization. Relatively little attention has been paid to the patient’s collateral status or therapeutic strategies for collateral enhancement. Growing evidence has demonstrated that it is important to re-estimate the risk-benefits of stroke therapy, such as endovascular therapy for acute ischemic stroke, in consideration of collateral status. Neuroimaging techniques for the assessment of collaterals are rapidly developing and may provide insight on the perfusion of collaterals in patients who may not otherwise be candidates for conventional angiography. Continuous efforts are needed to develop collateral enhancing strategies, and large randomized trials are needed with monitoring their effects using imaging modalities for collateral assessment.
Acknowledgments
Funding sources: This study was supported by the Korea Health Technology R&D Project, the Ministry of Health & Welfare (HI14C1624) to O.Y.B. Dr. Liebeskind has received a research grant from the NIH-NINDS (K24NS072272 and R13NS089280), and Dr. Goyal has received a research support from Covidien.
Disclosure: M.G. has received honoria from Covidien and remuneration from GE Healthcare.
References
- 1.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke. 2013;44:2650–2663. doi: 10.1161/STROKEAHA.113.001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares BP, Chien JD, Wintermark M. Mr and ct monitoring of recanalization, reperfusion, and penumbra salvage: Everything that recanalizes does not necessarily reperfuse! Stroke. 2009;40:S24–S27. doi: 10.1161/STROKEAHA.108.526814. [DOI] [PubMed] [Google Scholar]
- 3.Cho TH, Nighoghossian N, Mikkelsen IK, Derex L, Hermier M, Pedraza S, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke. 2015;46:1582–1589. doi: 10.1161/STROKEAHA.114.007964. [DOI] [PubMed] [Google Scholar]
- 4.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Molina CA. Futile recanalization in mechanical embolectomy trials: A call to improve selection of patients for revascularization. Stroke. 2010;41:842–843. doi: 10.1161/STROKEAHA.110.580266. [DOI] [PubMed] [Google Scholar]
- 8.Shi ZS, Liebeskind DS, Xiang B, Ge SG, Feng L, Albers GW, et al. Predictors of functional dependence despite successful revascularization in large-vessel occlusion strokes. Stroke. 2014;45:1977–1984. doi: 10.1161/STROKEAHA.114.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill MD, Demchuk AM, Goyal M, Jovin TG, Foster LD, Tomsick TA, et al. Alberta stroke program early computed tomography score to select patients for endovascular treatment: Interventional management of stroke (ims)-iii trial. Stroke. 2014;45:444–449. doi: 10.1161/STROKEAHA.113.003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2008;79:625–629. doi: 10.1136/jnnp.2007.132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- 13.Kucinski T, Koch C, Eckert B, Becker V, Kromer H, Heesen C, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45:11–18. doi: 10.1007/s00234-002-0881-0. [DOI] [PubMed] [Google Scholar]
- 14.Liebeskind DS, Tomsick TA, Foster LD, Yeatts SD, Carrozzella J, Demchuk AM, et al. Collaterals at angiography and outcomes in the interventional management of stroke (ims) iii trial. Stroke. 2014;45:759–764. doi: 10.1161/STROKEAHA.113.004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 16.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: An analysis of data from the interventional management of stroke (ims iii) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prabhakaran S, Ruff I, Bernstein RA. Acute stroke intervention: A systematic review. JAMA. 2015;313:1451–1462. doi: 10.1001/jama.2015.3058. [DOI] [PubMed] [Google Scholar]
- 18.Nogueira RG, Smith WS, Sung G, Duckwiler G, Walker G, Roberts R, et al. Effect of time to reperfusion on clinical outcome of anterior circulation strokes treated with thrombectomy: Pooled analysis of the merci and multi merci trials. Stroke. 2011;42:3144–3149. doi: 10.1161/STROKEAHA.111.624163. [DOI] [PubMed] [Google Scholar]
- 19.Galimanis A, Jung S, Mono ML, Fischer U, Findling O, Weck A, et al. Endovascular therapy of 623 patients with anterior circulation stroke. Stroke. 2012;43:1052–1057. doi: 10.1161/STROKEAHA.111.639112. [DOI] [PubMed] [Google Scholar]
- 20.Ribo M, Flores A, Rubiera M, Pagola J, Sargento-Freitas J, Rodriguez-Luna D, et al. Extending the time window for endovascular procedures according to collateral pial circulation. Stroke. 2011;42:3465–3469. doi: 10.1161/STROKEAHA.111.623827. [DOI] [PubMed] [Google Scholar]
- 21.Hwang YH, Kang DH, Kim YW, Kim YS, Park SP, Liebeskind DS. Impact of time-to-reperfusion on outcome in patients with poor collaterals. AJNR Am J Neuroradiol. 2015;36:495–500. doi: 10.3174/ajnr.A4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 23.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 24.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 25.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 26.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 27.Menon BK, d'Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase ct angiography: A new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275:510–520. doi: 10.1148/radiol.15142256. [DOI] [PubMed] [Google Scholar]
- 28.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–974. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liebeskind DS. Reversing stroke in the 2010s: Lessons from desmoteplase in acute ischemic stroke-2 (dias-2) Stroke. 2009;40:3156–3158. doi: 10.1161/STROKEAHA.109.559682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higashida RT, Furlan AJ, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–e137. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 31.Choi JY, Kim EJ, Hong JM, Lee SE, Lee JS, Lim YC, et al. Conventional enhancement ct: A valuable tool for evaluating pial collateral flow in acute ischemic stroke. Cerebrovasc Dis. 2011;31:346–352. doi: 10.1159/000322602. [DOI] [PubMed] [Google Scholar]
- 32.Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Kulkens S, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- 33.Bhatia R, Bal SS, Shobha N, Menon BK, Tymchuk S, Puetz V, et al. Ct angiographic source images predict outcome and final infarct volume better than noncontrast ct in proximal vascular occlusions. Stroke. 2011;42:1575–1580. doi: 10.1161/STROKEAHA.110.603936. [DOI] [PubMed] [Google Scholar]
- 34.Lee KH, Cho SJ, Byun HS, Na DG, Choi NC, Lee SJ, et al. Triphasic perfusion computed tomography in acute middle cerebral artery stroke: A correlation with angiographic findings. Arch Neurol. 2000;57:990–999. doi: 10.1001/archneur.57.7.990. [DOI] [PubMed] [Google Scholar]
- 35.Kim SJ, Noh HJ, Yoon CW, Kim KH, Jeon P, Bang OY, et al. Multiphasic perfusion computed tomography as a predictor of collateral flow in acute ischemic stroke: Comparison with digital subtraction angiography. Eur Neurol. 2012;67:252–255. doi: 10.1159/000334867. [DOI] [PubMed] [Google Scholar]
- 36.Lee KH, Lee SJ, Cho SJ, Na DG, Byun HS, Kim YB, et al. Usefulness of triphasic perfusion computed tomography for intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Arch Neurol. 2000;57:1000–1008. doi: 10.1001/archneur.57.7.1000. [DOI] [PubMed] [Google Scholar]
- 37.Na DG, Ryoo JW, Lee KH, Moon CH, Yi CA, Kim EY, et al. Multiphasic perfusion computed tomography in hyperacute ischemic stroke: Comparison with diffusion and perfusion magnetic resonance imaging. Journal of computer assisted tomography. 2003;27:194–206. doi: 10.1097/00004728-200303000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Lee KH, Na DG, Byun HS, Kim YB, Shon YM, et al. Multiphasic helical computed tomography predicts subsequent development of severe brain edema in acute ischemic stroke. Arch Neurol. 2004;61:505–509. doi: 10.1001/archneur.61.4.505. [DOI] [PubMed] [Google Scholar]
- 39.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-ct and ct-angiography in acute stroke patients. Ann Neurol. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 40.Lin L, Bivard A, Parsons MW. Perfusion patterns of ischemic stroke on computed tomography perfusion. Journal of stroke. 2013;15:164–173. doi: 10.5853/jos.2013.15.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keedy AW, Fischette WS, Soares BP, Arora S, Lau BC, Magge R, et al. Contrast delay on perfusion ct as a predictor of new, incident infarct: A retrospective cohort study. Stroke. 2012;43:1295–1301. doi: 10.1161/STROKEAHA.111.639229. [DOI] [PubMed] [Google Scholar]
- 42.Tong E, Wintermark M. Cta-enhanced perfusion ct: An original method to perform ultra-low-dose cta-enhanced perfusion ct. Neuroradiology. 2014;56:955–964. doi: 10.1007/s00234-014-1416-1. [DOI] [PubMed] [Google Scholar]
- 43.Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–1107. doi: 10.1056/NEJMoa1109842. [DOI] [PubMed] [Google Scholar]
- 44.Souza LC, Yoo AJ, Chaudhry ZA, Payabvash S, Kemmling A, Schaefer PW, et al. Malignant cta collateral profile is highly specific for large admission dwi infarct core and poor outcome in acute stroke. AJNR Am J Neuroradiol. 2012;33:1331–1336. doi: 10.3174/ajnr.A2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bang OY, Saver JL, Alger JR, Starkman S, Ovbiagele B, Liebeskind DS. Determinants of the distribution and severity of hypoperfusion in patients with ischemic stroke. Neurology. 2008;71:1804–1811. doi: 10.1212/01.wnl.0000335929.06390.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SJ, Ha YS, Ryoo S, Noh HJ, Ha SY, Bang OY, et al. Sulcal effacement on fluid attenuation inversion recovery magnetic resonance imaging in hyperacute stroke: Association with collateral flow and clinical outcomes. Stroke. 2012;43:386–392. doi: 10.1161/STROKEAHA.111.638106. [DOI] [PubMed] [Google Scholar]
- 47.Lee KY, Latour LL, Luby M, Hsia AW, Merino JG, Warach S. Distal hyperintense vessels on flair: An mri marker for collateral circulation in acute stroke? Neurology. 2009;72:1134–1139. doi: 10.1212/01.wnl.0000345360.80382.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liebeskind DS. Imaging the future of stroke: I. Ischemia. Ann Neurol. 2009;66:574–590. doi: 10.1002/ana.21787. [DOI] [PubMed] [Google Scholar]
- 49.Lee MJ, Son JP, Kim SJ, Ryoo S, Woo SY, Cha J, et al. Predicting collateral status with mr perfusion parameters: A probabilistic approach with a tmax-derived prediction model. Stroke; a journal of cerebral circulation. 2015 doi: 10.1161/STROKEAHA.115.009828. In-press. [DOI] [PubMed] [Google Scholar]
- 50.Christensen S, Calamante F, Hjort N, Wu O, Blankholm AD, Desmond P, et al. Inferring origin of vascular supply from tracer arrival timing patterns using bolus tracking mri. Journal of magnetic resonance imaging : JMRI. 2008;27:1371–1381. doi: 10.1002/jmri.21386. [DOI] [PubMed] [Google Scholar]
- 51.Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1168–1172. doi: 10.1038/jcbfm.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SJ, Son JP, Ryoo S, Lee MJ, Cha J, Kim KH, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol. 2014;76:356–369. doi: 10.1002/ana.24211. [DOI] [PubMed] [Google Scholar]
- 53.Zaharchuk G. Arterial spin label imaging of acute ischemic stroke and transient ischemic attack. Neuroimaging clinics of North America. 2011;21:285–301. doi: 10.1016/j.nic.2011.01.003. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaharchuk G, Do HM, Marks MP, Rosenberg J, Moseley ME, Steinberg GK. Arterial spin-labeling mri can identify the presence and intensity of collateral perfusion in patients with moyamoya disease. Stroke. 2011;42:2485–2491. doi: 10.1161/STROKEAHA.111.616466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu B, Wang X, Guo J, Xie S, Wong EC, Zhang J, et al. Collateral circulation imaging: Mr perfusion territory arterial spin-labeling at 3t. AJNR Am J Neuroradiol. 2008;29:1855–1860. doi: 10.3174/ajnr.A1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chng SM, Petersen ET, Zimine I, Sitoh YY, Lim CC, Golay X. Territorial arterial spin labeling in the assessment of collateral circulation: Comparison with digital subtraction angiography. Stroke. 2008;39:3248–3254. doi: 10.1161/STROKEAHA.108.520593. [DOI] [PubMed] [Google Scholar]
- 57.Sallustio F, Kern R, Gunther M, Szabo K, Griebe M, Meairs S, et al. Assessment of intracranial collateral flow by using dynamic arterial spin labeling mra and transcranial color-coded duplex ultrasound. Stroke; a journal of cerebral circulation. 2008;39:1894–1897. doi: 10.1161/STROKEAHA.107.503482. [DOI] [PubMed] [Google Scholar]
- 58.Robson PM, Dai W, Shankaranarayanan A, Rofsky NM, Alsop DC. Time-resolved vessel-selective digital subtraction mr angiography of the cerebral vasculature with arterial spin labeling. Radiology. 2010;257:507–515. doi: 10.1148/radiol.10092333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol. 2012;33:576–582. doi: 10.3174/ajnr.A2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song HS, Kang CK, Kim JS, Park CA, Kim YB, Lee DH, et al. Assessment of pial branches using 7-tesla mri in cerebral arterial disease. Cerebrovasc Dis. 2010;29:410. doi: 10.1159/000288056. [DOI] [PubMed] [Google Scholar]
- 61.Puetz V, Dzialowski I, Hill MD, Demchuk AM. The alberta stroke program early ct score in clinical practice: What have we learned? International journal of stroke : official journal of the International Stroke Society. 2009;4:354–364. doi: 10.1111/j.1747-4949.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- 62.Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding risk analysis in stroke imaging before thrombolysis (brasil): Pooled analysis of t2*-weighted magnetic resonance imaging data from 570 patients. Stroke. 2007;38:2738–2744. doi: 10.1161/STROKEAHA.106.480848. [DOI] [PubMed] [Google Scholar]
- 63.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 64.Nagakane Y, Christensen S, Ogata T, Churilov L, Ma H, Parsons MW, et al. Moving beyond a single perfusion threshold to define penumbra: A novel probabilistic mismatch definition. Stroke. 2012;43:1548–1555. doi: 10.1161/STROKEAHA.111.643932. [DOI] [PubMed] [Google Scholar]
- 65.Kim SJ, Son JP, Ryoo S, Lee MJ, Cha J, Kim KH, et al. A novel magnetic resonance imaging approach to collateral flow imaging in ischemic stroke. Ann Neurol. 2014;76:356–369. doi: 10.1002/ana.24211. [DOI] [PubMed] [Google Scholar]
- 66.Nael K, Khan R, Choudhary G, Meshksar A, Villablanca P, Tay J, et al. Six-minute magnetic resonance imaging protocol for evaluation of acute ischemic stroke: Pushing the boundaries. Stroke. 2014;45:1985–1991. doi: 10.1161/STROKEAHA.114.005305. [DOI] [PubMed] [Google Scholar]
- 67.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 68.Teunissen PF, Horrevoets AJ, van Royen N. The coronary collateral circulation: Genetic and environmental determinants in experimental models and humans. Journal of molecular and cellular cardiology. 2012;52:897–904. doi: 10.1016/j.yjmcc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Epstein SE, Lassance-Soares RM, Faber JE, Burnett MS. Effects of aging on the collateral circulation, and therapeutic implications. Circulation. 2012;125:3211–3219. doi: 10.1161/CIRCULATIONAHA.111.079038. [DOI] [PubMed] [Google Scholar]
- 70.Olavarria VV, Arima H, Anderson CS, Brunser AM, Munoz-Venturelli P, Heritier S, et al. Head position and cerebral blood flow velocity in acute ischemic stroke: A systematic review and meta-analysis. Cerebrovasc Dis. 2014;37:401–408. doi: 10.1159/000362533. [DOI] [PubMed] [Google Scholar]
- 71.Hwang G, Oh CW, Bang JS, Jung CK, Kwon OK, Kim JE, et al. Superficial temporal artery to middle cerebral artery bypass in acute ischemic stroke and stroke in progress. Neurosurgery. 2011;68:723–729. doi: 10.1227/NEU.0b013e318207a9de. discussion 729–730. [DOI] [PubMed] [Google Scholar]
- 72.Nussbaum ES, Janjua TM, Defillo A, Lowary JL, Nussbaum LA. Emergency extracranial-intracranial bypass surgery for acute ischemic stroke. Journal of neurosurgery. 2010;112:666–673. doi: 10.3171/2009.5.JNS081556. [DOI] [PubMed] [Google Scholar]
- 73.Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circulation research. 2012;110:727–738. doi: 10.1161/CIRCRESAHA.111.253419. [DOI] [PubMed] [Google Scholar]
- 74.Defazio RA, Zhao W, Deng X, Obenaus A, Ginsberg MD. Albumin therapy enhances collateral perfusion after laser-induced middle cerebral artery branch occlusion: A laser speckle contrast flow study. J Cereb Blood Flow Metab. 2012;32:2012–2022. doi: 10.1038/jcbfm.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yukami T, Yagita Y, Sugiyama Y, Oyama N, Watanabe A, Sasaki T, et al. Chronic elevation of tumor necrosis factor-alpha mediates the impairment of leptomeningeal arteriogenesis in db/db mice. Stroke. 2015;46:1657–1663. doi: 10.1161/STROKEAHA.114.008062. [DOI] [PubMed] [Google Scholar]
- 76.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23:1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 77.Tongers J, Roncalli JG, Losordo DW. Role of endothelial progenitor cells during ischemia-induced vasculogenesis and collateral formation. Microvascular research. 2010;79:200–206. doi: 10.1016/j.mvr.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance t2*wi. Stroke. 2008;39:1563–1568. doi: 10.1161/STROKEAHA.107.502146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silver B, McCarthy S, Lu M, Mitsias P, Russman AN, Katramados A, et al. Sildenafil treatment of subacute ischemic stroke: A safety study at 25-mg daily for 2 weeks. J Stroke Cerebrovasc Dis. 2009;18:381–383. doi: 10.1016/j.jstrokecerebrovasdis.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 80.Sahinarslan A, Yalcin R, Kocaman SA, Ercin U, Tanalp AC, Topal S, et al. The relationship of serum erythropoietin level with coronary collateral grade. The Canadian journal of cardiology. 2011;27:589–595. doi: 10.1016/j.cjca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 81.Yada T, Shimokawa H, Hiramatsu O, Satoh M, Kashihara N, Takaki A, et al. Erythropoietin enhances hydrogen peroxide-mediated dilatation of canine coronary collateral arterioles during myocardial ischemia in dogs in vivo. Am J Physiol Heart Circ Physiol. 2010;299:H1928–H1935. doi: 10.1152/ajpheart.00331.2010. [DOI] [PubMed] [Google Scholar]
- 82.Todo K, Kitagawa K, Sasaki T, Omura-Matsuoka E, Terasaki Y, Oyama N, et al. Granulocyte-macrophage colony-stimulating factor enhances leptomeningeal collateral growth induced by common carotid artery occlusion. Stroke. 2008;39:1875–1882. doi: 10.1161/STROKEAHA.107.503433. [DOI] [PubMed] [Google Scholar]
- 83.Ovbiagele B, Saver JL, Starkman S, Kim D, Ali LK, Jahan R, et al. Statin enhancement of collateralization in acute stroke. Neurology. 2007;68:2129–2131. doi: 10.1212/01.wnl.0000264931.34941.f0. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang R, Li Y, He G, Zhang D, Zhang F. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Molecular biology reports. 2012;39:285–293. doi: 10.1007/s11033-011-0737-y. [DOI] [PubMed] [Google Scholar]
- 85.Hibbert B, Ma X, Pourdjabbar A, Simard T, Rayner K, Sun J, et al. Pre-procedural atorvastatin mobilizes endothelial progenitor cells: Clues to the salutary effects of statins on healing of stented human arteries. PLoS One. 2011;6:e16413. doi: 10.1371/journal.pone.0016413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee MJ, Bang OY, Kim SJ, Kim GM, Chung CS, Lee KH, et al. Role of statin in atrial fibrillation-related stroke: An angiographic study for collateral flow. Cerebrovasc Dis. 2014;37:77–84. doi: 10.1159/000356114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ginsberg MD, Palesch YY, Hill MD, Martin RH, Moy CS, Barsan WG, et al. High-dose albumin treatment for acute ischaemic stroke (alias) part 2: A randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013;12:1049–1058. doi: 10.1016/S1474-4422(13)70223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shuaib A, Bornstein NM, Diener HC, Dillon W, Fisher M, Hammer MD, et al. Partial aortic occlusion for cerebral perfusion augmentation: Safety and efficacy of neuroflo in acute ischemic stroke trial. Stroke. 2011;42:1680–1690. doi: 10.1161/STROKEAHA.110.609933. [DOI] [PubMed] [Google Scholar]
- 89.Bath PM, Woodhouse L, Scutt P, Krishnan K, Wardlaw JM, Bereczki D, et al. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (enos): A partial-factorial randomised controlled trial. Lancet. 2015;385:617–628. doi: 10.1016/S0140-6736(14)61121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]