Introduction
Acute psychological stress can lead to atrial and ventricular arrhythmias, but the physiological pathways have not been fully elucidated. Signal processing techniques can provide insight into electrophysiological mechanisms of stress-induced arrhythmia. T-wave alternans, as well as other ECG measures of heterogeneity of repolarization, increases with emotional and cognitive stress in the laboratory setting, and may also increase with stress in “real life” settings. In the atrium, stress impacts components of the signal-averaged ECG. These changes suggest mechanisms by which everyday stressors can lead to arrhythmia.
I. ECG Signatures of Psychological Stress in the Ventricle
Epidemiological and clinical studies demonstrate that psychological distress, defined as a consciously experienced mismatch between expectations and the perceived environment associated with aversiveness, [1] can trigger both ventricular and atrial arrhythmias. The first epidemiological evidence linking acute stress and ventricular arrhythmias comes from data showing increases in sudden cardiac death (SCD) during stress-inducing population disasters such as earthquake or war. For example, increases in cardiovascular and sudden death were reported during the Iraqui missile war in Israel in 1981, [2] as well during air raids in Zagreb. [3] On the day of the earthquake in Northridge, CA, in 1994, there was a 6-fold increase in SCD compared to days prior to and following the disaster. [4] These reported sudden deaths during each of these population disasters were not related to physical injury or other direct physical involvement, implying a role of psychological rather than physical stress. SCD can be precipitated by ischemic or arrhythmic events, and the effects of stress on ischemia have been long understood. However, data from ICD patients showing an increase in ventricular arrhythmia after the World Trade Center attacks of 9/11/2001, suggest that autonomic changes related to stress may directly modulate arrhythmogenesis.[5]
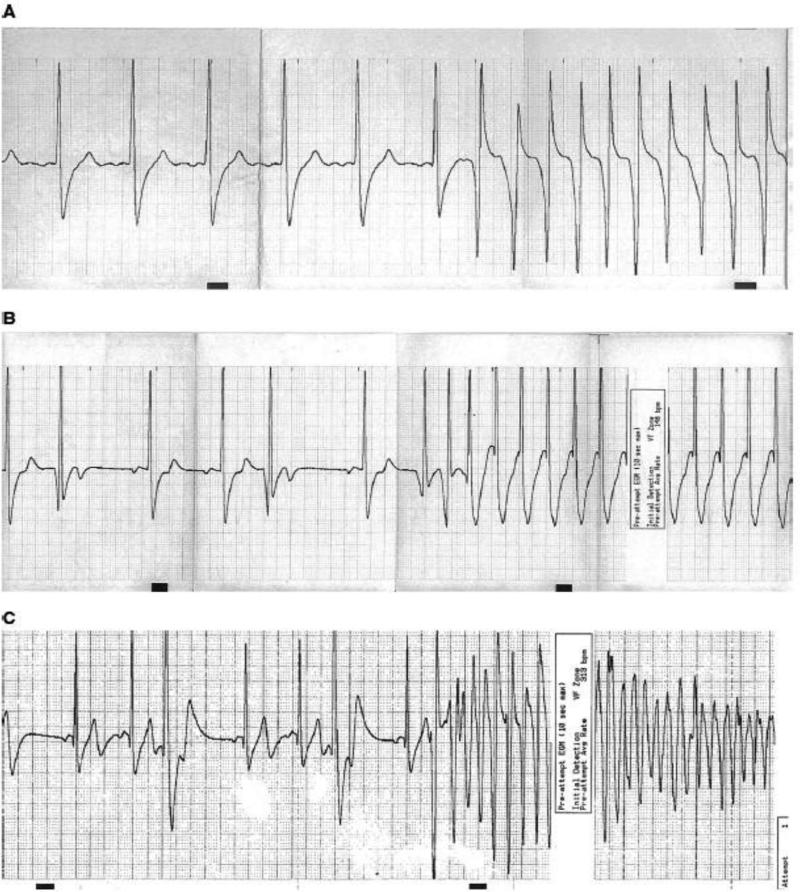
Clinical studies also show a link between stress and arrhythmia. We looked more directly at the question of whether anger or other emotions can trigger ventricular arrhythmias using a case-control study of ICD patients. In this study, patients were asked, whenever they received a shock from the ICD, to record in a diary their activities and emotions in the 15 minutes and 2 hours prior to ICD shock. They were then asked to fill out a similar diary one week later at the same time of day to serve as controls. Anger levels were greater prior to appropriate shock for ventricular arrhythmia than during control periods, demonstrating that anger can trigger ventricular arrhythmias. [6] Overall, anger-triggered arrhythmias were more likely to be polymorphic, PVC-initiated, and pause-dependent, characteristics associated with lethality (see Figure 1). [7] Because this diary study relied on self-report of anger, and because many individuals (one-third in some studies) [8] suppress the expression of anger, which is associated with physiological changes, [8] it is possible that the impact of anger on arrhythmia is even greater than seen here.
Figure 1. Electrograms of anger-triggered and non-anger-triggered ventricular arrhythmias.
Legend: A: monomorphic sudden-onset arrhythmia (non-anger-triggered); B: Monomorphic PVC-initiated pause-dependent arrhythmia (anger-triggered); C: Polymorphic PVC-initiated pause-dependent arrhythmia (anger-triggered). From Stopper, et al, Heart Rhythm 2007 (ref 6) with permission
Signal processing techniques can provide insight into electrophysiological mechanisms of stress-induced arrhythmia. Toivonen et al [9] used a human model of stress—the on-call medical house officer—to look at QT changes during stress. QT intervals during periods of arousal due to a page were compared to periods of rest with identical heart rates. They found hysteresis of the QT interval during periods of stress, with longer QT during stress than rest, similar to the QT hysteresis found during exercise. [10] Exaggerations of QT hysteresis with exercise are thought to be one mechanism underlying sudden death in exercise, and could similarly be a mechanism of stress-related SCD.
We looked directly at effects of stress on heterogeneity of repolarization, long-recognized to be an important factor in arrhythmogenesis, by measuring T-wave alternans (TWA) during a laboratory mental stress protocol. Similar to a creating physical stress on a treadmill exercise test, we can create mental stress in the laboratory through a variety of methods. These include asking the patient to do arithmetic in his head, such as serial subtraction of 7 from a 3-digit number, or can involve a speaking task with emotional content. In our lab, we do a stressor called “anger recall”. We ask subjects to tell us about a recent incident in which they were irritated or angry, as if they were telling a friend later in the day.
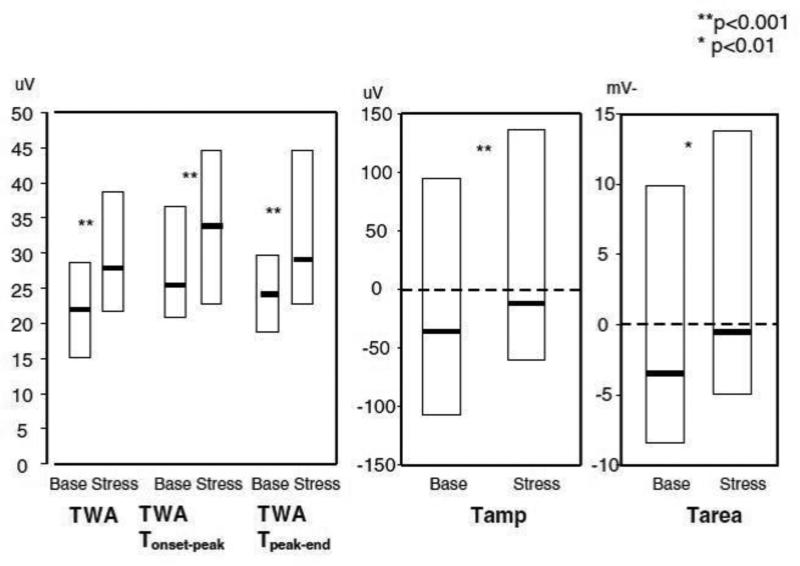
We evaluated effects of mental stress on three surface measures of heterogeneity which can be determined from Holter monitoring, T-wave alternans (TWA,) T-wave amplitude (Tamp,) and T-wave area (Tarea,) calculating TWA in the time domain using the Interbeat Average technique. [11] In this study, 33 patients with ICDs and a history of ventricular arrhythmias underwent a mental stress protocol including mental arithmetic and anger recall. TWA increased from 22 at baseline to 29 uV during mental stress (p<0.001.) All other measures of heterogeneity also increased with stress. [12] (See Figure 2) Broad-range repolarization instability, which includes non-alternans frequencies, also increased during stress. [13] In a similar study by Kop et al, mental stress was also seen to increase TWA. [14]
Figure 2. Changes in repolarization heterogeneity with mental stress.
Legend: Repolarization changes with mental stress. Box plots represent median and interquartile range. Tamp = T-wave amplitude; TWA = T-wave alternans; Tarea = T-wave area. Reprinted from Lampert, et al, J Cardiovasc Electrophys 2005, (ref 9) with permission
Pathways through which mental stress may increase TWA are unknown. Kop et al performed simultaneous SPECT-perfusion imaging, and so were able to confirm that the effect of anger on TWA was independent of ischemia.[14] Increasing heart rate alone (eg, through atrial pacing) can increase TWA, but in our study,(as is usual with mental stress) there was minimal increase in heart rate with mental stress. We did see that the increase in TWA correlated with increases in catecholamines, suggesting a direct sympathetic effect on cellular repolarization. Prior studies have also suggested that sympathetic activation may increase TWA beyond the effects of heart rate. Experimentally, stellectomy abolishes, while stellate ganglion stimulation increases, TWA.[15] In clinical studies, intravenous beta-blockade[16] decreased the magnitude of TWA, and TWA induced with exercise is greater than that with atrial pacing at the same heart rate.[17]
The effect of anger on TWA in the laboratory was predictive of arrhythmias in real life. In a follow-up of our study of anger and TWA,[18] we found that anger-induced TWA was a significant predictor of arrhythmia, with likelihood of ICD-treated ventricular arrhythmia for those in the top quartile of anger-induced in the lab of over 10 times that of other patients (CI 1.6-113, p<0.05.) (Figure 4) Anger-induced TWA remained predictive after controlling for standard predictors of arrhythmia such as ejection fraction, prior clinical arrhythmia, and wide QRS. Many studies, particularly those using time-domain methodology, such as the Modified Moving Average technique, to which our Interbeat Average is similar [11]have shown that TWA predicts future arrhythmia, [19] and a recent consensus statement supports its use, based on studies including over 12,000 patients: “Overall, our assessment is that it is reasonable to consider TWA evaluation whenever there is suspicion of vulnerability to lethal cardiac arrhythmias. However, there is as yet no definitive evidence from interventional trials that it can guide therapy.”[19] A particular advantage of time-domain methods is the absence of requirement for increased heart rate (as with spectral methods), which permits studies to be done on prescribed beta-blockers. Holding beta-blockers significantly decreases the predictive power of the test. [17] This finding is consistent with the impact of autonomic factors on TWA which our studies have shown: performance of TWA testing during an autonomic state similar to the patients’ usual (ie, on beta-blockers if patient is taking them) will be more predictive of future outcome. Our study was limited by size, inclusion of mainly men, and using treated arrhythmias as an outcome in an era prior to current evidence-based programming. However, the high predictive value seen here raises an intriguing possibility-- Laboratory mental stress-invoked TWA may be an even better probe of arrhythmia-risk, as this modality measures the interaction of trigger and substrate which may lead to arrhythmogenesis (rather than substrate alone).
II. ECG Signatures of Psychological Stress in the Atrium
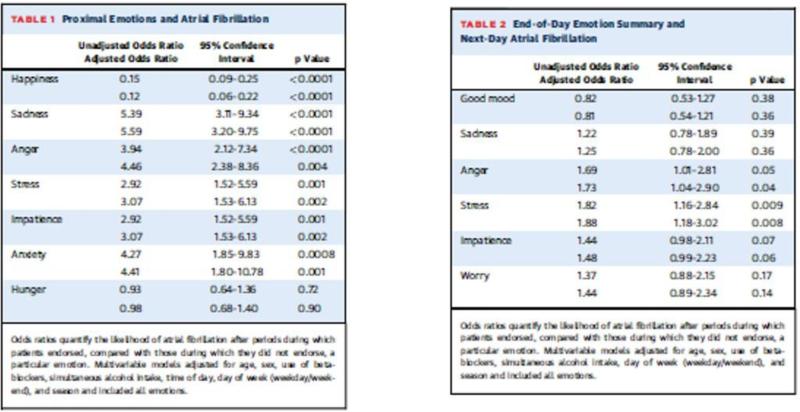
Small case-series have suggested that stressful stimuli may trigger AF. In two series reported in 1968 and 1999, [20, 21] 2-30% of AF events occurred “emotional or physical exhaustion”, with specific triggers reported of death in the family, or awakening to an alarm. [22] We have recently reported the first prospective study of emotional triggering of AF. [23] In this year-long, prospective, electronic-diary (eDiary)-based study, 95 patients with intermittent AF recorded their heart rhythm on event-monitor at the time of symptoms, and completed an eDiary query of their emotions (e.g., anger, anxiety, sadness, stress, happiness), 1) for the preceding (proximal) 30 minutes, (Figure 7) and 2) at the end of each day, summarizing their emotions for that day. Patients also underwent monthly 24-hour Holter-monitoring, completing an eDiary twice per waking hour. Emotions reported on eDiary for the 30 minutes proximal to AF were compared to those reported during 24-hr Holter monitoring during sinus rhythm. Similarly, end-of-day emotion summaries for days preceding a day with AF were compared to the end-of-day emotion summaries preceding a day without AF. Overall 228 symptomatic AF episodes were reported by 40 subjects. There were 163 episodes (34 subjects) with associated proximal emotion reports on eDiary, 11,563 emotion reports during Holter-confirmed sinus rhythm, 112 end-of-day summary emotion reports preceding days with episodes of AF (31 subjects), and 14663 end-of-day summary emotion reports preceding days without AF . Negative emotions (sadness, anxiety, anger, stress) increased the likelihood of an AF episode 2-5 fold (all p<0.01.) Happiness decreased AF likelihood by 85% (p<0.001). Anger and stress reported on end-of-day emotion summaries similarly increased the likelihood of AF the following day (HRs 1.69 and 1.82, p<0.05). (See Figure 3).
Figure 3. Emotion and atrial fibrillation.
Legend: Reprinted from Lampert, et al, J Am Coll Cardiol 2014 (ref 20) with permission
Potential mechanisms of the arrhythomogenic effects of stress on AF are less well-understood. Repolarization as measured in invasive experiments by the atrial effective refractory period shortens with sympathetic stimulation in most[24] although not all[25] studies. Further, sympathetic stimulation acts synergistically when combined with vagal stimuli. [24]
Conduction in the atria can be measured noninvasively using the signal-averaged P wave, and a prolonged SA-P duration has been associated with recurrent AF.[26] In the normal atrium, sympathetic stimulation with isoproterenol shortens SA-P duration; conversely, beta-blockers slow conduction. Atropine given after beta-blockade shortened SA-P duration, suggesting that vagal activation slowed conduction.[27] In addition, P-wave duration measured from 24-hour Holter monitoring is shorter in daytime, further suggesting that changes in sympathovagal balance alter conduction through the atria. [28]
We have looked preliminarily at the effect of stress in individuals with AF, in whom atrial conduction overall is abnormal.[29] In this study, 97 AF patients and 25 control subjects underwent mental stress testing in the laboratory similar to that described above. Overall, P-wave duration was longer at both baseline and during anger than in controls, but decreased with anger similarly in both groups. However, late potentials, as indicated by RMS40 (root mean square voltage of last 40 ms) increased in the AF patients but decreased in controls. Among AF patients, there was inverse association between the change in p-wave duration and change in RMS40 (preliminary analyses, unpublished data). The electrophysiological mechanisms of this finding have not been elucidated. It is possible that anger-related autonomic changes accentuated the underlying heterogeneity of conduction in patients with known substrate for AF, for example, by shortening conduction times in normal tissue without change in abnormal tissue, leading to vulnerability to AF.
Clinical implications
Based on our and others’ findings that stress can trigger atrial and ventricular arrhythmias, it seems highly likely that interventions aimed at decreasing stress, or decreasing the physiological impact of stress on the body, can decrease arrhythmia frequency in susceptible patients. Use of ECG markers of psychological stress as described above can serve as surrogate endpoints, as well as provide mechanistic information. We are currently evaluating whether an eight-week stress reduction program aimed at reducing negative emotion can reduce arrhythmia frequency in patients with ICDs. [30] To better understand mechanisms of effect, this study is also evaluating whether stress reduction can attenuate the increases in TWA with laboratory stress described above. Complementary therapies such as yoga have been shown to decrease both atrial[31] and ventricular[32] arrhythmia symptoms. Further research is needed into both traditional psychoeducational and complimentary approaches to decreasing stress-induced arrhythmias.
Summary
Stress can trigger both atrial and ventricular arrhythmias. Evaluating ECG signatures of stress can provide mechanistic information, as well as serving as surrogate endpoints for studies investigating therapeutic approaches. While there are a number of approaches to evaluating repolarization and repolarization heterogeneity in the ventricle, options for looking noninvasively at atrial electrophysiology are fewer and this would be a valuable area of future research.
Acknowledgments
Research by the author discussed in this review was supported by an American Heart Association Scientist Development Grant, #0030190 and National Heart, Lung, and Blood Institute grant R01 HL073285 and National Institutes of Health/National Center for Research Resources CTSA grant UL1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no relationships with industry relevant to the content of the manuscript
References
- 1.Goldstein D. Stress, Catecholamines, and Cardiovascular disease. Oxford University Press; New York: 1995. [Google Scholar]
- 2.Meisel SR, Kutz I, Dayan KI, Pauzner H, Chetboun I, Arbel Y, et al. Effect of Iraqi missile war on incidence of acute myocardial infarction and sudden death in Israeli civilians. Lancet. 1991;338:660–661. doi: 10.1016/0140-6736(91)91234-l. [DOI] [PubMed] [Google Scholar]
- 3.Bergovec M, Mihatov S, Prpic H, Rogan S, Batarelo V, Sjerobabske V. Acute myocardial infarction among civilians in Zagreb city area. Lancet. 1992;339:303. doi: 10.1016/0140-6736(92)91370-n. [DOI] [PubMed] [Google Scholar]
- 4.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg JS, Arshad A, Kowalski M, Kukar A, Suma V, Vloka M, et al. Increased incidence of life-threatening ventricular arrhythmias in implantable defibrillator patients after the World Trade Center attack. J Am Coll Cardiol. 2004;44:1261–4. doi: 10.1016/j.jacc.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Lampert R, Joska T, Burg M, Batsford W, McPherson C, Jain D. Emotional and physical precipitants of ventricular arrhythmia. Circulation. 2002;106(4):1800–1805. doi: 10.1161/01.cir.0000031733.51374.c1. [DOI] [PubMed] [Google Scholar]
- 7.Stopper M, Joska T, Burg MM, Batsford WP, McPherson CA, Jain D, et al. Electrophysiologic characteristics of anger-triggered arrhythmias.[see comment]. Heart Rhythm. 2007;4(3):268–73. doi: 10.1016/j.hrthm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Dimsdale JEJ. Suppressed anger and blood pressure: the effects of race, sex, social class, obesity, and age. Psychosomatic medicine. 1986;48(6):430–436. doi: 10.1097/00006842-198607000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Toivonen L, Helenius K, Viitasalo M. Electrocardiographic repolarization during stress from awakening on alarm call. J Am Coll Cardiol. 1997;30:774–779. doi: 10.1016/s0735-1097(97)00222-2. [DOI] [PubMed] [Google Scholar]
- 10.Sarma JS, Venkataraman SK, Samant DR, Gadgil U. Hysteresis in the human RR-QT relationship during exercise and recovery. Pacing & Clinical Electrophysiology. 1987;10(3 Pt 1):485–91. doi: 10.1111/j.1540-8159.1987.tb04510.x. [DOI] [PubMed] [Google Scholar]
- 11.Shusterman V, Goldberg A. Tracking repolarization dynamics in real-life data. J Electrocardiol. 2004;37(Suppl):180–6. doi: 10.1016/j.jelectrocard.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 12.Lampert R, Shusterman V, Burg M, Lee FA, Earley C, Goldberg A, et al. Effects of psychological stress on repolarization and relationship to autonomic and hemodynamic factors. J Cardiovasc Electrophysiol. 2005;16:372–377. doi: 10.1046/j.1540-8167.2005.40580.x. [DOI] [PubMed] [Google Scholar]
- 13.Abisse SS, Lampert R, Burg M, Soufer R, Shusterman V. Cardiac repolarization instability during psychological stress in patients with ventricular arrhythmias. Journal of Electrocardiology. 2011;44(6):678–83. doi: 10.1016/j.jelectrocard.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kop WJ, Krantz DS, Nearing BD, Gottdiener JS, Quigley JF, O'Callahan M, et al. Effects of acute mental stress and exercise on T-wave alternans in patients with implantable cardioverter defibrillators and controls. Circulation. 2004;109:1864–1869. doi: 10.1161/01.CIR.0000124726.72615.60. [DOI] [PubMed] [Google Scholar]
- 15.Nearing BD, Huang AH, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T wave. Science. 1991;252:437–440. doi: 10.1126/science.2017682. [DOI] [PubMed] [Google Scholar]
- 16.Rashba EJ, Cooklin M, MacMurdy K, Kavesh N, Kirk M, Sarang S, et al. Effects of selective autonomic blockade on T-wave alternans in humans. Circulation. 2002;105:837–842. doi: 10.1161/hc0702.104127. [DOI] [PubMed] [Google Scholar]
- 17.Hohnloser SH, Klingenheben T, Zabel M, Li Y.g., Albrecht P, Cohen RJ. T wave alternans during exercise and atrial pacing in humans. J Cardiovasc Electrophysiol. 1997;8:987–993. doi: 10.1111/j.1540-8167.1997.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 18.Lampert R, Shusterman V, Burg M, Batsford W, McPherson C, Soufer R. Anger-induced T-wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J Am Coll Cardiol. 2009;53:774–8. doi: 10.1016/j.jacc.2008.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verrier RL, Klingenheben T, Malik M, El-Sherif N, Exner DV, Hohnloser SH, et al. Microvolt T-wave alternans physiological basis, methods of measurement, and clinical utility--consensus guideline by International Society for Holter and Noninvasive Electrocardiology. Journal of the American College of Cardiology. 2011;58(13):1309–24. doi: 10.1016/j.jacc.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peter RH, Gracey JG, Beach TB. A clinical profile of idiopathic atrial fibrillation. Annals of Internal Medicine. 1968;68:1288–1295. doi: 10.7326/0003-4819-68-6-1288. [DOI] [PubMed] [Google Scholar]
- 21.Levy S, Maarek M, Coumel P, Guize L, Lekieffre F, Medvedowsky J-L, et al. Characterization of different subsets of atrial fibrillation in general practice in France. Circulation. 1999;99(23):3028–3035. doi: 10.1161/01.cir.99.23.3028. [DOI] [PubMed] [Google Scholar]
- 22.Houghton JL, Devlin CW, Besson WT, III, Crawford W, Fincher RE, Flowers NC, et al. Possible triggering of paroxysmal atrial fibrillation in normal hearts by psychological stressors: a report of two cases. Am J Med Sci. 1990;300(4):234–236. doi: 10.1097/00000441-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Lampert R, Jamner L, Burg M, Dziura J, Brandt C, Liu H, et al. Triggering of Symptomatic Atrial Fibrillation by Negative Emotion. Journal of the American College of Cardiology. 2014;64(14):1533–1534. doi: 10.1016/j.jacc.2014.07.959. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractgoriness heterogeneity. Am J Physiol. 1997;273(2.2):H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 25.Zipes D, Mihalick M, Robbins G. Effects of selective vagal and stellate ganglion stimulation on atrial refractoriness. Cardiovascular Research. 1974;8(5):647–655. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]
- 26.Raitt M, Ingram K, Thurman S. Signal-averaged p wave duration predicts early recurrence of atrial fibrillation after cardioversion. PACE. 2000;23:259–265. doi: 10.1111/j.1540-8159.2000.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 27.Cheema A, Ahmed M, Kadish A, Goldberger J. Effects of autonomic stimulation and blockade on signal-averaged p wave duration. J Am Coll Cardiol. 1995;26:497–502. doi: 10.1016/0735-1097(95)80028-f. [DOI] [PubMed] [Google Scholar]
- 28.Dilaveris P, Frabom P, Batchvorov V, Ghuran A, Malik M. Circadian behavior of P-wave duration, P-wave area, and PR interval in healthy subjects. Annals of Non-invasive Electrocardiology. 2001;6:92–97. doi: 10.1111/j.1542-474X.2001.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guidera S, Steinberg J. The signal-averaged p wave duration: a rapid and noninvasive marker of risk of atrial fibrillation. J Am Coll Cardiol. 1993;21:1645–51. doi: 10.1016/0735-1097(93)90381-a. [DOI] [PubMed] [Google Scholar]
- 30.Donahue RG, Lampert R, Dornelas E, Clemow L, Burg MM, Investigators R. Rationale and design of a randomized clinical trial comparing stress reduction treatment to usual cardiac care: the Reducing Vulnerability to Implantable Cardioverter Defibrillator Shock-Treated Ventricular Arrhythmias (RISTA) trial. Psychosomatic Medicine. 2010;72(2):172–7. doi: 10.1097/PSY.0b013e3181c932d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, et al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. Journal of the American College of Cardiology. 2013;61(11):1177–82. doi: 10.1016/j.jacc.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 32.Toise SC, Sears SF, Schoenfeld MH, Blitzer ML, Marieb MA, Drury JH, et al. Psychosocial and cardiac outcomes of yoga for ICD patients: a randomized clinical control trial. Pacing & Clinical Electrophysiology. 2014;37(1):48–62. doi: 10.1111/pace.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]