Abstract
Background
Excessive gestational weight gain (GWG) increases the risk of childhood obesity, but little is known about its association with infant growth patterns.
Aim
To examine the GWG-infant growth association.
Methods
Pregnant women (n=743) self-reported GWG at delivery, which we classified as inadequate, adequate, or excessive based on current guidelines. Offspring weight-for-age z-scores (WAZ), length-for-age z-scores (LAZ (with height-for-age (HAZ) in place of length at 36 months)), and body mass index z-scores (BMIZ) were calculated at birth, 8, 18, and 36 months using the 2006 WHO growth standards. Linear mixed models estimated the change in z-scores from birth to 36 months by GWG.
Results
The mean (SD) WAZ was −0.22 (1.20) at birth. Overall, WAZ and BMIZ increased from birth to approximately 24 months and decreased from 24 to 36 months, while LAZ/HAZ decreased from birth through 36 months. Excessive GWG was associated with higher offspring WAZ and BMIZ at birth, 8, and 36 months, and higher HAZ at 36 months, compared with adequate GWG. Compared with the same referent, inadequate GWG was associated with smaller WAZ and BMIZ at birth and 8 months.
Conclusion
Excessive GWG may predispose infants to obesogenic growth patterns while inadequate GWG may not have a lasting impact on infant growth.
Keywords: child growth, rapid infant weight gain, gestational weight gain, prenatal factors
Introduction
Childhood obesity affects one in ten infants and toddlers aged 6 to 23 months and one in six children and adolescents aged 2 to 19 years in the U.S. [1]. A path to obesity may be established in early life [2, 3]. Importantly, infants who track along a path of faster growth may have increased risk for subsequent overweight and obesity [4–6]. Attained size is the primary outcome measure in many studies of child weight; consequently, we rely on a preponderance of cross-sectional assessments to understand a complex process beginning at conception [7]. Growth velocity, in contrast, precedes the attained size at a given assessment and includes multiple measurements [7]. Rapid infant growth is related to an increased likelihood for obesity in later childhood [4] regardless of size at birth [6] and may increase metabolic and cardiovascular risk by adolescence or early adulthood [8–10].
Maternal weight gain in pregnancy may influence growth patterns in early childhood through the mechanism of fetal programming [11]. Maternal overnutrition may irreversibly influence fetal organ and tissue development [12]. Animal models suggest that these developmental changes may induce a persistent sensitivity to fat accrual [13–15]. Gestational weight gain (GWG) has been positively associated with offspring body size at birth [11] as well as growth [16, 17] and BMI in early childhood in some studies [11], but not in others [18–21]. The majority of this literature is based on predominantly white, well-educated, mid- to upper-income samples. Studies performed in populations at high-risk for offspring obesity are needed.
The aim of our study was to evaluate the association between GWG and growth predictive of subsequent overweight and obesity risk in early childhood.
Methods
We conducted a secondary analysis using data from a prospective birth cohort study designed to evaluate the long-term effects of prenatal substance use on child development [22, 23]. Pregnant women were recruited from 1982 to 1985 at a low-income prenatal clinic at Magee-Women’s Hospital in Pittsburgh, PA. Women ≥18 years of age and in the fourth or fifth prenatal month were approached and 1,360 women agreed to initial screening interview (85% response rate). The study sample was selected based on first-trimester alcohol and marijuana use. All women who reported drinking three or more drinks per week in the first trimester of pregnancy and a random sample of those reporting less than this amount were selected for a study of the effects of prenatal alcohol exposure. Similarly, all women who reported using two or more joints per month in the first trimester of pregnancy and a random sample of those reporting less than this amount were selected for a study of prenatal marijuana exposure. These cohorts (n=829) have been merged and studied extensively [22, 24, 25].
Women were interviewed regarding lifestyle, sociodemographic characteristics, and substance use at the first study visit [mean: 18.8 (standard deviation (SD), 2.7) weeks gestation] and were followed to delivery. A total of 743 women had live, singleton births and had complete maternal weight and height data, and were therefore eligible for infant follow-up evaluations. Our Institutional Review Boards approved the original study and written, informed consent was obtained at each phase.
Maternal prepregnancy BMI (weight (kg)/height(m)2) was calculated based on self-reported prepregnancy weight and height recorded at the first prenatal visit. At delivery, women self-reported the total amount of weight that they gained since becoming pregnant. We classified women using a ratio of observed GWG to expected GWG at the gestational age of delivery based on the 2009 Institute of Medicine (IOM) guidelines [11], as described in detail previously [26]. We classified GWG as adequate (within the recommended ranges), inadequate (below the recommendations), or excessive (greater than the recommendations) [26]. Because this measure of GWG may remain correlated with length of gestation [27], we performed a sensitivity analysis using gestational-age-standardized-GWG z-scores [28].
Within 48 hours of delivery, trained study nurses measured infant crown to heel length. Gestational age at delivery and infant weight at birth were abstracted from medical records. At 8, 18, and 36 months, study nurses measured the children’s weight and length using standardized protocols. Children were measured on the same calibrated scale while wearing street clothing. Weight-for-age z-scores (WAZ), length-for-age z-scores (LAZ), and body-mass-index-for-age z-scores (BMIZ) at 0, 8, 18, and 36 months were calculated using the 2006 World Health Organization (WHO) sex- and age-specific growth standards [29, 30]. We used LAZ assessments for children <24 months and height-for-age z-scores (HAZ) at the 36 month assessment in accordance with standard methods. Z-score measurements <−5 or >5 were considered implausible and were excluded (WAZ, n=6; LAZ, n=3; BMIZ, n=9). The 743 children contributed 2,552 WAZ measurements, 2,545 LAZ/HAZ measurements, and 2,510 BMIZ measurements. We classified rapid infant weight gain as a change in WAZ from birth to 18 months >0.67 SD units, corresponding to the crossing of one centile line on growth charts [31–33].
Self-reported race, marital status, employment, monthly household income, education, and parity were available. We categorized the frequency of first trimester substance use for tobacco, alcohol, and marijuana as well as the pattern of alcohol or marijuana use over the course of pregnancy and postpartum. Elevated maternal depressive symptoms and trait anxiety were defined as scores ≥75th percentile on the Center for Epidemiologic Studies Depression Scale [34] and on the State-Trait Personality Inventory [35], respectively, and low social support was a score <25th percentile of a factor score measuring social support [36]. The mother was asked to recall at 18 months if she ever breastfed her child and the age she introduced solid foods (<6 months; ≥6 months).
Statistical analysis
Bivariate associations between mother-child dyad characteristics and GWG were tested using Pearson chi-squared tests, and their association with WAZ and BMIZ were tested using the nonparametric Kruskal-Wallis test. We used generalized linear latent and mixed models to estimate associations between GWG and WAZ, LAZ/HAZ, and BMIZ from birth to 36 months [37]. These models account for within-child correlations across study visits and variation in the number of time intervals between repeated measurements within children [38]. The underlying time variable was the child’s age, which was specified as linear and quadratic terms to reflect the nonlinear relationship with WAZ, LAZ/HAZ, and BMIZ. We calculated predicted WAZ, LAZ/HAZ, and BMIZ by GWG adequacy and tested for differences in child growth at each age using linear contrast statements at each growth measurement point. Next, we used multivariable log-binomial regression models to estimate the relative risks (RR) for the association between GWG and rapid infant weight gain.
Theory-based causal diagrams [39] were used to identify potential confounders (maternal sociodemographic variables, prepregnancy BMI, substance use, mental health, and breastfeeding) of the relationship between GWG and infant growth. Then, to achieve parsimonious regression models, we retained only those potential confounders that changed the main-effect estimate ≥10% [40]. Prepregnancy BMI and breastfeeding met our definition of confounding in all models. We additionally included maternal education, pattern of prenatal substance use, and maternal smoking status out of convention. Since our goal was to estimate the total effect of GWG on infant growth, we did not adjust for gestational age at delivery or birth weight for gestational age because they may be mediators on the causal path from GWG to child weight [41]. We used a Wald test (α=0.05) in linear regression models and the synergy index [42] in log-binomial models to test for effect modification by prepregnancy overweight BMI ≥25.0 kg/m2 vs. BMI<25.0 kg/m2), race, maternal depression, anxiety, prenatal substance use and child’s sex [11, 43, 44]..
We ran models excluding heavy substance users and we applied inverse probability sample weights [45] to reweight the study sample to resemble the original prenatal clinic sample from which the cohort was selected [46]. Finally, we evaluated whether our observed results were explained by regression to the mean [47]. Stata Software, version 11 (College Station, TX) was used for analysis.
Results
Overall, the 743 women included in this sample were normal weight, young, high-school educated, unmarried, and low income (Table 1). Prenatal substance use reflected sampling for the cohort. Nearly half of the women were African-American. On average, infants were born small (mean (SD) birth weight for gestational age z-score: −0.38 (0.95)), and 10% were born at <37 weeks. GWG varied by pregravid BMI, substance use, gestational age at delivery, and birth weight z-score. There were no significant differences in GWG by maternal education, income, prenatal marijuana use, race, child sex, or mode of infant feeding.
Table 1.
Characteristics of the study sample overall and stratified by GWG adequacy, (n=743)
| Overall, N (%) |
Inadequate GWG, n (%)* |
Adequate GWG, n (%)* |
Excessive GWG, n (%)* |
|
|---|---|---|---|---|
| Prepregnancy body mass index (kg/m2) | ||||
| Underweight (<18.5) | 91 (12) | 17 (11) | 49 (19) | 25 (7) |
| Normal weight (18.5–24.9) | 456 (61) | 110 (71) | 169 (67) | 177 (53) |
| Overweight (25.9–29.9) | 127 (17) | 16 (10) | 27 (11) | 84 (25) |
| Obese (≥30.0) | 69 (69) | 11 (7) | 8 (3) | 50 (15) |
| Maternal race/ethnicity, n (%) | ||||
| Caucasian | 363 (49) | 69 (45) | 135 (53) | 159 (47) |
| African-American | 380 (51) | 85 (55) | 118 (47) | 177 (53) |
| Maternal age (years), n (%) * | ||||
| <20 | 142 (19) | 24 (16) | 50 (20) | 68 (20) |
| 20–24 | 376 (51) | 81 (53) | 141 (56) | 154 (46) |
| 25–29 | 181 (24) | 34 (22) | 53 (21) | 94 (28) |
| ≥30 | 44 (6) | 15 (10) | 9 (4) | 20 (6) |
| Maternal education (years), n (%) | ||||
| <12 | 193 (26) | 44 (289) | 74 (29) | 75 (22) |
| =12 | 449 (60) | 95 (62) | 147 (58) | 207 (62) |
| >12 | 101 (14) | 15 (10) | 32 (13) | 54 (16) |
| Marital status | ||||
| Unmarried | 498 (67) | 106 (69) | 182 (72) | 210 (63) |
| Married | 245 (33) | 48 (31) | 71 (28) | 126 (38) |
| Employment, n (%) * | ||||
| Working or in school | 196 (26) | 29 (19) | 68 (27) | 99 (29) |
| Not working or in school | 547 (75) | 125 (81) | 185 (73) | 237 (71) |
| Income Level ($/month), n (%) | ||||
| <400 | 439 (61) | 90 (61) | 160 (66) | 189 (58) |
| ≥400 | 277 (39) | 57 (39) | 83 (34) | 137 (42) |
| Parity, n (%) * | ||||
| Nulliparous | 335 (45) | 54 (35) | 117 (46) | 164 (49) |
| Multiparous | 408 (55) | 100 (65) | 136 (54) | 172 (51) |
| Prenatal smoking in the 1st trimester, n (%) * | ||||
| None | 339 (46) | 63 (41) | 100 (40) | 176 (52) |
| <0.5 packs/day | 152 (20) | 31 (20) | 52 (21) | 69 (21) |
| 0.5 to <1 packs/day | 139 (19) | 35 (23) | 60 (24) | 44 (3213 |
| ≥1 packs/day | 113 (15) | 25 (16) | 41 (16) | 47 (14) |
| Prenatal alcohol use in the 1st trimester | ||||
| None | 264 (36) | 63 (41) | 72 (29) | 129 (38) |
| >0 to <1.5 drinks/week | 158 (21) | 26 (17) | 59 (23) | 73 (22) |
| 1.5 drink/week to <1 drinks/day | 176 (24) | 35 (23) | 65 (26) | 76 (23) |
| ≥1 drinks/day | 145 (19) | 30 (19) | 57 (23) | 58 (17) |
| Prenatal marijuana use in the 1st trimester | ||||
| None | 438 (59) | 94 (61) | 154 (61) | 190 (57) |
| >0 to <0.5 joint/day | 156 (21) | 31 (20) | 45 (18) | 80 (24) |
| 0.5 to <1 joints/day | 53 (7) | 9 (6) | 23 (9) | 21 (6) |
| ≥1 joints/day | 96 (13) | 20 (13) | 31 (12) | 45 (13) |
| Pattern of prenatal cigarette use, n (%) * | ||||
| Never used in pregnancy | 314 (42) | 60 (39) | 92 (36) | 162 (48) |
| First trimester use only | 38 (5) | 6 (4) | 14 (6) | 18 (5) |
| Second and/or third trimester use | 391 (53) | 88 (57) | 147 (58) | 156 (46) |
| Pattern of prenatal alcohol use, n (%) * | ||||
| Never used in pregnancy | 226 (30) | 53 (34) | 59 (23) | 114 (34) |
| First trimester use only | 280 (38) | 49 (32) | 109 (43) | 122 (36) |
| Second and/or third trimester use | 237 (32) | 52 (34) | 85 (34) | 100 (30) |
| Pattern of prenatal marijuana use, n (%) | ||||
| Never used in pregnancy | 433 (58) | 92 (60) | 153 (61) | 188 (56) |
| First trimester use only | 178 (24) | 32 (21) | 55 (22) | 91 (27) |
| Second and/or third trimester use | 132 (18) | 30 (19) | 45 (18) | 57 (17) |
| Gestational age at delivery (weeks), n (%) * | ||||
| <37 weeks | 71 (10) | 23 (15) | 29 (11) | 19 (6) |
| ≥37 weeks | 672 (90) | 131 (85) | 224 (89) | 317 (94) |
| †Birth weight for gestational age z-score, n (%) * | ||||
| Small for age (<10th percentile) | 124 (17) | 43 (28) | 37 (15) | 44 (13) |
| Appropriate for age (10th to 90th percentile) | 591 (79) | 107 (70) | 209 (83) | 275 (82) |
| Large for age (>90th percentile) | 28 (4) | 4 (3) | 7 (3) | 17 (5) |
| Infant sex, n (%) | ||||
| Female | 368 (50) | 79 (51) | 127 (50) | 162 (48) |
| Male | 375 (50) | 75 (49) | 126 (50) | 174 (52) |
| Ever breastfed infant, n (%) | ||||
| Yes | 133 (21) | 25 (19) | 45 (21) | 63 (22) |
| No | 496 (79) | 105 (81) | 173 (79) | 218 (78) |
Birth weight for gestational age z-score reference [54]
Pearson chi-square test p<0.05
Mean WAZ at 0, 8, 18, and 36 months were lower among children born to underweight or normal weight mothers, small-for-gestational age infants, and infants born <37 weeks (Table 2). Results were similar for LAZ/HAZ and BMIZ (data not shown). Rapid infant weight gain over the first 18 months was common (45%). Infants of women who smoked during pregnancy, and infants born <37 weeks or small-for-gestational-age tended to exhibit rapid infant weight gain (Appendix Table 1).
Table 2.
Weight-for-age z-score (WAZ) by maternal and infant characteristics.
| WAZ 0 mo. Mean (SD) |
WAZ 8 mo. Mean (SD) |
WAZ 18 mo. Mean (SD) |
WAZ 36 mo. Mean (SD) |
|
|---|---|---|---|---|
| Prepregnancy body mass index (kg/m2) | ||||
| Underweight (<18.5) | −0.50 (1.15)** | −0.16 (1.11) *** | 0.14 (1.07)** | −0.16 (0.93) *** |
| Normal weight (18.5–24.9) | −0.26 (1.15) | 0.13 (1.01) | 0.33 (0.96) | 0.09 (0.95) |
| Overweight (25.9–29.9) | −0.02 (1.32) | 0.35 (1.11) | 0.59 (1.09) | 0.27 (1.05) |
| Obese (≥30.0) | 0.02 (1.25) | 0.16 (1.20) | 0.46 (1.28) | 0.18 (1.00) |
| Maternal race/ethnicity | ||||
| Caucasian | 0.04 (1.08) * | 0.20 (1.02) | 0.50 (1.05)*** | 0.15 (0.95) |
| African-American | −0.47 (1.24) | 0.07 (1.10) | 0.24 (1.02) | 0.05 (0.99) |
| Pattern of prenatal smoking | ||||
| Never used in pregnancy | −0.01 (1.19) * | 0.17 (1.07) | 0.35 (1.06) | 0.10 (0.98) |
| First trimester use only | −0.06 (1.24) | 0.33 (0.96) | 0.57 (1.02) | 0.22 (1.08) |
| Second and/or third trimester use | −0.41 (1.17) | 0.09 (1.07) | 0.36 (1.03) | 0.08 (0.96) |
| Pattern of prenatal alcohol use | ||||
| Abstained entire pregnancy | −0.29 (1.22) | 0.24 (1.09) *** | 0.38 (1.09)*** | 0.18 (1.03)*** |
| Abstained after 1st trimester | −0.17 (1.24) | 0.17 (1.04) | 0.46 (0.99) | 0.14 (0.94) |
| Did not abstain after 1st trimester | −0.22 (1.11) | 0.001 (1.07) | 0.24 (1.05) | −0.03 (0.96) |
| GWG category | ||||
| Inadequate | −0.77 (1.35)* | −0.12 (1.05) * | 0.13 (1.00)** | −0.13 (1.01) * |
| Adequate | −0.29 (1.09) | 0.03 (1.03) | 0.32 (1.04) | −0.07 (0.89) |
| Excessive | 0.08 (1.10) | 0.33 (1.07) | 0.50 (1.05) | 0.33 (0.98) |
| Gestational age at delivery (weeks) | ||||
| <37 weeks | −2.28 (1.18) * | −0.45 (1.36) ** | −0.16 (1.15) * | −0.43 (1.11) * |
| ≥37 weeks | −0.02 (0.99) | 0.19 (1.01) | 0.42 (1.02) | 0.15 (0.95) |
| Birth weight for gestational age z-score | ||||
| Small for age (<10th percentile) | −1.49 (0.80)* | −0.29 (1.04) * | −0.06 (0.90)* | −0.16 (1.01) * |
| Appropriate for age (10th to 90th percentile) | −0.05 (1.05) | 0.19 (1.05) | 0.42 (1.04) | 0.12 (0.96) |
| Large for age (>90th percentile) | 1.66 (0.73) | 0.94 (0.85) | 1.04 (1.11) | 0.74 (0.77) |
| Ever breastfed infant | ||||
| Yes | −0.002 (1.07) *** | 0.15 (1.04) | 0.32 (1.00) | 0.11 (1.01) |
| No | −0.25 (1.22) | 0.15 (1.08) | 0.38 (1.05) | 0.10 (0.96) |
Statistical significance level for Kruskall-Wallis equality of populations test,
p<0.001,
p<0.01,
p<0.05
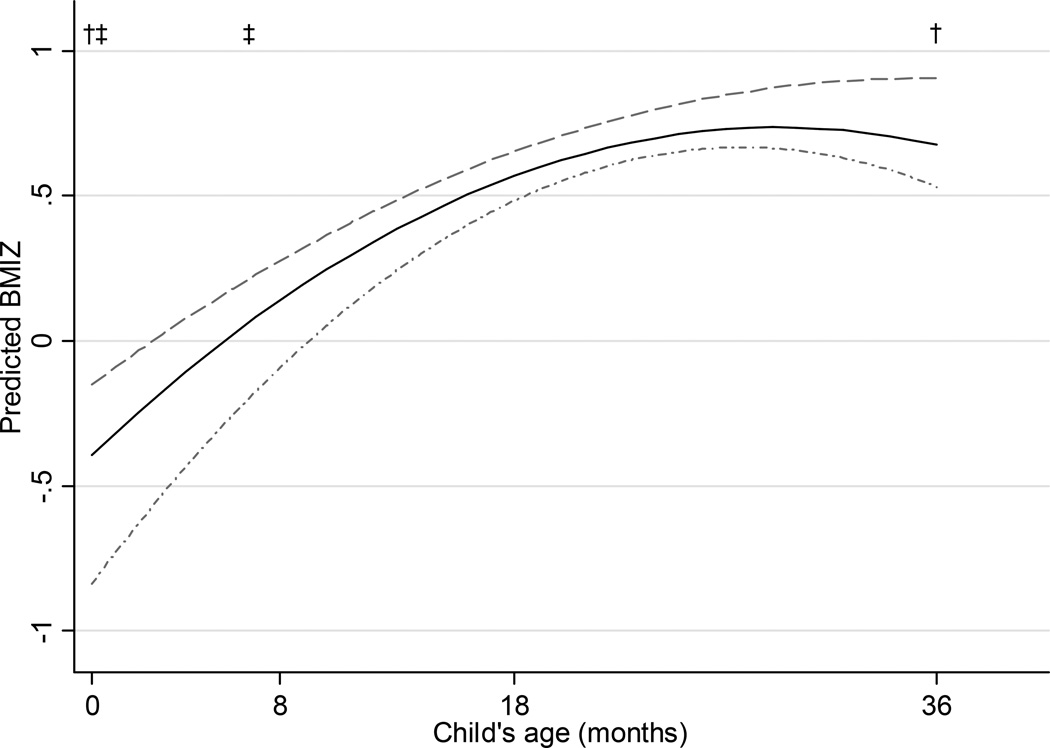
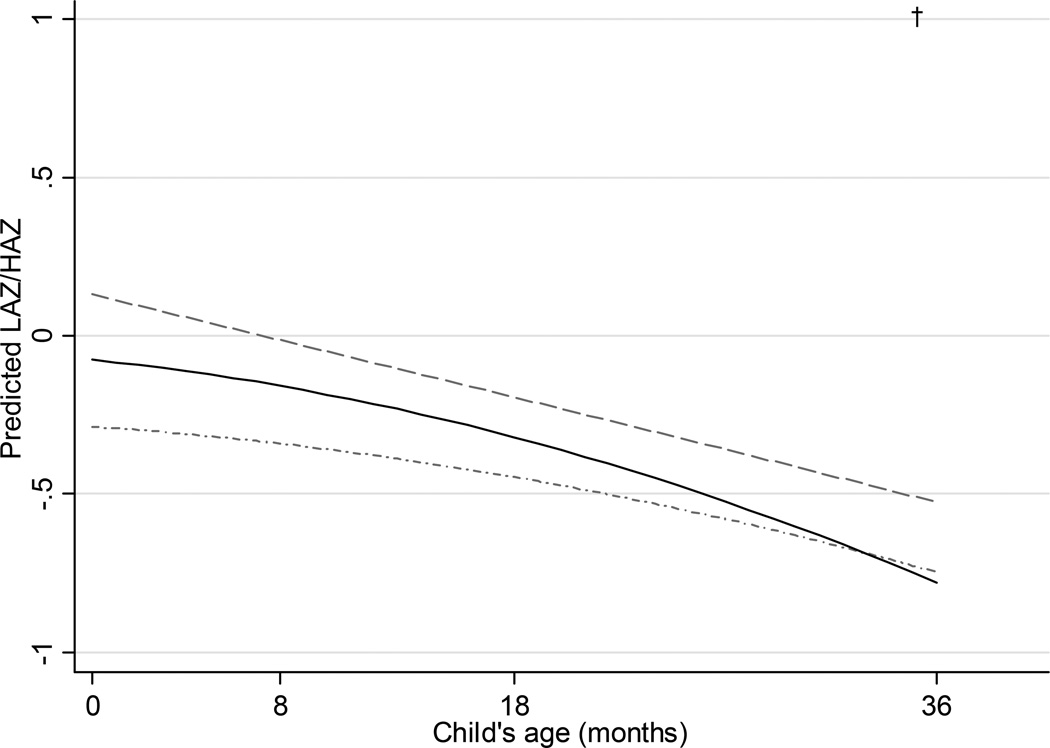
Beta coefficients from the final adjusted longitudinal multivariable models predicting WAZ, LAZ/HAZ, and BMIZ are shown within Appendix Table 2 online, and The predicted WAZ (Figure 1) and BMIZ (Figure 3) increased from birth to approximately 24 months and decreased from 24 to 36 months in all GWG groups, while there was a decrease in the predicted values for LAZ/HAZ from birth to 36 months (Figure 2). Children of women who gained inadequate weight during pregnancy had smaller WAZ [adjusted beta coefficient (95% CI): −0.38 (−0.60, −0.16)] and BMIZ [−0.44 (−0.69, −0.20)] at birth than the children of women whose GWG was within recommended ranges, but there were no statistical differences thereafter. Yet the children of women who gained inadequate weight were no different in LAZ [−0.21 (−0.46, 0.04)] at birth as compared to children of women who gained within the recommendations, and were no different in LAZ/HAZ at any age thereafter. Women with excessive GWG had children with higher WAZ [0.30 (0.12, 0.49)] and BMIZ [0.25 (0.04, 0.45)] at birth compared with children of women with adequate gain, but there was no difference in LAZ [0.21 (−0.01, 0.42)]. The children of women with excessive GWG remained heavier at 8 months and 36 months than the children of women who gained adequate weight, but there was no difference in weight at 18 months. As compared with adequate gain, children of women who gained excessive weight remained no different in length at 8 or 18 months, but were taller at 36 months.
Figure 1.
Predicted weight-for-age z-score (WAZ) from 0–36 months by gestational weight gain (GWG; excessive GWG, dashed; adequate GWG, solid; inadequate GWG, dotted; N=628).
* Predictions based on a multivariable linear model assuming prepregnancy normal weight, high school education, used alcohol in the 1st, abstained from marijuana, smoked tobacco throughout pregnancy, did not breastfeed infant, and introduced solid foods ≥6 months.
† p<0.05 for excessive compared to adequate
‡ p<0.05 for inadequate compared to adequate
Figure 3.
Predicted body mass index z-score (BMIZ) from 0–36 months by gestational weight gain (GWG; excessive GWG, dashied; adequate GWG, solid; inadequate GWG, dotted; N=628).
* Predictions based on a multivariable linear model assuming prepregnancy normal weight, high school education, used alcohol in the 1st trimester, abstained from marijuana, smoked tobacco throughout pregnancy, did not breastfeed infant, and introduced solid foods ≥6 months.
† p<0.05 for excessive compared to adequate
‡ p<0.05 for inadequate compared to adequate
Figure 2.
Predicted length-for-age/height-for-age z-score (LAZ/HAZ) from 0–36 months by gestational weight gain (GWG; excessive GWG, dashed; adequate GWG, solid; inadequate GWG, dotted; N=628).
* Predictions based on a multivariable linear model assuming prepregnancy normal weight, high school education, used alcohol in the 1st trimester, abstained from marijuana, smoked tobacco throughout pregnancy, did not breastfeed infant, and introduced solid foods ≥6 months.
† p<0.05 for excessive compared to adequate
‡ p<0.05 for inadequate compared to adequate
When we excluded women who were heavy alcohol (≥1 drink per day in the first trimester) or marijuana users (≥1 joint per day in the first trimester), excessive GWG was associated with higher predicted WAZ, LAZ/HAZ, and BMIZ over the entire study period (data not shown). For each category of GWG, the WAZ at 18 months was greater than the product of the initial WAZ at birth and the correlation between them, indicating that this change in WAZ was greater than the changes expected due to regression to the mean.
Women who gained inadequate weight were more likely to have an infant with rapid infant weight gain from birth to 18 months (Table 3). After adjustment for confounders, inadequate total GWG was associated with 28% higher risk of rapid infant weight gain compared with adequate GWG. Maternal excessive weight gain was not associated with the risk of rapid infant weight gain before or after confounder adjustment. Excluding heavy substance users did not meaningfully change these results.
Table 3.
Association between gestational weight gain (GWG) and rapid infant weight gain from 0 to 18 months (n=609).
| Rapid infant weight gain from 0 to 18 months |
Unadjusted RR (95% CI) |
Adjusted† RR (95% CI) |
||
|---|---|---|---|---|
| No | Yes | |||
| GWG category, n (%) * | ||||
| Inadequate | 55 (44) | 69 (56) | 1.27 (1.02, 1.59) | 1.28 (1.03, 1.60) |
| Adequate | 120 (56) | 93 (44) | 1.0 (ref) | 1.0 (ref) |
| Excessive | 163 (60) | 109 (40) | 0.92 (0.74, 1.13) | 0.92 (0.74, 1.14) |
Pearson chi-square test p<0.05
Adjusted for prepregnancy body mass index and infant ever breastfed.
None of our conclusions changed when we used CDC-based growth standards to calculate infant z-scores; applied inverse probability sample weights; categorized GWG based on z-scores; modeled the raw value of child’s BMI; or constrained models of rapid infant weight gain using an upper age limit (data not shown). We did not find evidence of effect modification in any of the models by prepregnancy body mass index, maternal depression, anxiety, substance use, race, or child sex.
Discussion
We found that the association between GWG and infant growth is dynamic over the first 36 months of infancy. Compared with women who gained within the recommended ranges, women with excessive GWG had children who were heavier at birth and 8 months, and both heavier and longer at 36 months, while women who had inadequate GWG had children who were lighter only at birth, but were no different in length. Among women who gained inadequate weight, there was no difference in child WAZ and LAZ/HAZ from 8 to 36 months; this is consistent with their increased risk of rapid weight gain from birth to 18 months. These associations were robust to adjustment for confounders.
Evidence suggests that excessive GWG is associated with greater adiposity [44, 48–51] and higher weight [11] in infants at birth as well as an increased risk of obesity in early childhood [52]. Yet we are aware of only two large rigorous studies of GWG in relation to infant growth. Li et al., [18] examined GWG in relation to WAZ, LAZ, and WLZ from birth to 12 months in 38,539 Chinese mothers delivering term infants, and Deierlein et al., [16] studied WAZ, LAZ, and WLZ from birth to 36 months in 476 North Carolina mothers and their term infants. Our finding that excessive GWG was related to heavier and longer infants who grew more slowly in the first year of life compared with adequate GWG were supported by Li et al. [18]. Deierlein et al. in contrast, found that these infants were heavier, longer, and gained weight and length faster from birth to 36 months. The differences in growth over time may be explained by the high prevalence of prepregnancy obesity (15%) in the Deierlein cohort compared with our cohort (9%) and that of Li et al. (6%), which may alter infant growth trajectories [53].
In our study, inadequate GWG was associated with smaller infant WAZ only at birth and rapid weight gain from 0 to 18 months compared with adequate GWG, but no differences in LAZ. In contrast, Li et al. reported that inadequate GWG was associated with consistently lower WAZ and LAZ from 0 to 12 months, faster changes in WAZ, LAZ, and WLZ from 0 to 12 months. Between GWG groups, Deierlein et al. found no difference in WAZ or LAZ from birth to 36 months [16] nor in rapid weight gain from birth to 6 months in models unadjusted for birthweight [17], similar to findings from a small British sample [31]. The use of CDC growth references to define z-scores in both studies by Deierlein et al. [16, 17] and Ong et al. [31] rather than the WHO standards used here and by Li et al.. Because the velocity of weight gain differs between the CDC and WHO growth references [54] and the varied age ranges studied, the respective classifications of rapid weight gain may contribute to the observed inconsistences among findings. We hypothesize that in our sample of children born to predominantly normal weight, low-income mothers, infants experienced catch-up growth [31] that was exhibited through weight, but not length. For growth restricted infants, a period of postnatal catch-up growth may confer a number of advantages [55], yet, catch-up growth continues to be associated with the risk of childhood obesity [4, 56]. Children in our sample with rapid weight gain were more likely than their counterparts to be obese on CDC growth charts at 6, 10, 14, 16, and 22 years of age, but not at 3 years (data available upon request). Ongoing analyses will enhance our understanding of long-term child growth in this cohort.
Our results should be considered in light of several limitations. Self-reported maternal weight and height may lead to misclassification. However women in our study recalled their prepregnancy weight early in pregnancy and reported their GWG within 48 hours after delivery, which may improve recall [57]. WAZ and BMIZ can only estimate adiposity [58], and it is thus unclear whether the differences we observed by GWG were due to fat or fat-free mass.
We cannot conclude whether GWG is causally associated with infant growth or due to unmeasured common factors related to maternal weight gain and child growth, including shared maternal-child genetic traits or shared environment. While we adjusted for the child ever having been breastfed, we lacked information on breastfeeding intensity or duration. Residual confounding, therefore, may exist. Our findings may only be generalizable to low-income, urban samples with a large proportion of substance users. While substance use was common in our study population, no women abused substances and few used substances heavily. Notably, our results were consistent or strengthened when we excluded heavy users from the analysis.
Current US data confirm that prenatal substance use is widespread [59] thus, controlling for this confounding using a detailed, validated assessment [60] was a strength of our study. Our results suggest that GWG may impact infant growth. Whether this relationship reflects causality will be clarified by the results of ongoing randomized clinical trials to optimize GWG. Trials employing rigorous longitudinal anthropometric assessments of the offspring will best elucidate the link between maternal BMI-specific GWG and growth trajectory of the offspring.
Acknowledgements
none.
The authors alone are responsible for the content and writing of this manuscript.
We received funding support from the following
NIH/NICHD grant: R01 HD072008 (L.B.)
NIH/NIAAA grant: AA06666 (N.D.)
NIH/NIDA grant: DA03874 (N.D.)
Appendix Table 1
Characteristics by rapid infant weight gain from 0 to 18 months.
| Rapid infant weight gain from 0 to 18 months (N=609) |
||
|---|---|---|
| Not Rapid | Rapid | |
| Prepregnancy body mass index (kg/m2), % | ||
| Underweight (<18.5) | 54 | 46 |
| Normal weight (18.5–24.9) | 55 | 45 |
| Overweight (25.9–29.9) | 55 | 45 |
| Obese (≥30.0) | 60 | 40 |
| Maternal race/ethnicity, % | ||
| White | 59 | 41 |
| Black | 52 | 48 |
| Income level ($/month), % | ||
| <400 | 55 | 45 |
| ≥400 | 57 | 43 |
| Parity | ||
| Nulliparous | 54 | 46 |
| Multiparous | 57 | 43 |
| Pattern of prenatal smoking, % * | ||
| Never used in pregnancy | 64 | 36 |
| First trimester use only | 51 | 49 |
| Second and/or third trimester use | 49 | 51 |
| Pattern of prenatal alcohol use, % | ||
| Never used in pregnancy | 52 | 48 |
| First trimester use only | 54 | 46 |
| Second and/or third trimester use | 61 | 39 |
| Pattern of prenatal marijuana use, % | ||
| Never used in pregnancy | 58 | 42 |
| First trimester use only | 56 | 44 |
| Second and/or third trimester use | 47 | 53 |
| Gestational age at delivery (weeks), (%) * | ||
| <37 weeks | 4 | 96 |
| ≥37 weeks | 60 | 40 |
| Birth weight for gestational age z-score, % * | ||
| Small for age (<10th percentile) | 25 | 75 |
| Appropriate for age (10th to 90th percentile) | 60 | 40 |
| Large for age (>90th percentile) | 91 | 9 |
| Infant sex, % | ||
| Female | 55 | 45 |
| Male | 56 | 44 |
| Ever breastfed infant, % | ||
| Yes | 63 | 37 |
| No | 54 | 47 |
Pearson chi-square test p<0.05
Appendix Table 2
Beta coefficients for weight-for-age z-scores (WAZ; N=628), length-for-age/height-for-age z-scores (LAZ/HAZ; N=628), and body mass index z-score (BMIZ; N=628) by gestational weight gain (GWG).
| WAZ Adjusted* beta coefficient (95% CI) |
LAZ/HAZ Adjusted* beta coefficient (95% CI) |
BMIZ Adjusted* beta coefficient (95% CI) |
|
|---|---|---|---|
| Intercept | −0.31 (−0.52, −0.11) | −0.08 (−0.30, 0.15) | −0.40 (−0.60, −0.19) |
| Child’s age (months) | 0.05 (0.04, 0.07) | −0.01 (−0.02, 0.01) | 0.08 (0.06, 0.09) |
| Inadequate GWG vs. adequate GWG | −0.38 (−0.60, −0.16) | −0.21 (−0.46, 0.04) | −0.44 (−0.69, −0.20) |
| Inadequate GWG * child’s age | 0.02 (−0.001, 0.04) | 0.003 (−0.02, 0.03) | 0.03 (0.01, 0.06) |
| Inadequate GWG * child’s age squared | −0.0003 (−0.001, 0.0002) | 0.0001 (−0.001, 0.001) | −0.001 (−0.001, 0.00001) |
| Excessive GWG vs. adequate GWG | 0.30 (0.12, 0.49) | 0.21 (−0.01, 0.42) | 0.25 (0.04, 0.45) |
| Excessive GWG * child’s age | −0.02 (−0.03, −0.001) | −0.01 (−0.03, 0.01) | −0.02 (−0.04, 0.004) |
| Excessive GWG * child’s age squared | 0.001 (0.0001, 0.001) | 0.0003 (−0.0002, 0.001) | 0.001 (−0.0001, 0.001) |
Multivariable linear model adjusted for prepregnancy body mass index, child’s age squared, inadequate × child’s age squared, excessive × child’s age squared, maternal education, pattern of prenatal alcohol, marijuana, cigarette smoking, ever breastfed infant, and solid foods introduced ≥6 months.
Footnotes
Declaration of Interest: The authors report no declarations of interest.
Contributor Information
Jill C. Diesel, Email: jdiesel@bcbsm.com.
Cara L. Eckhardt, Email: cara2@pdx.edu.
Nancy L. Day, Email: nday@pitt.edu.
Maria M. Brooks, Email: brooks@edc.pitt.edu.
Silva A. Arslanian, Email: Silva.Arslanian@chp.edu.
Lisa M. Bodnar, Email: bodnar@edc.pitt.edu.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA: the Journal of the American Medical Association. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slining MM, Herring AH, Popkin BM, Mayer-Davis EJ, Adair LS. Infant BMI trajectories are associated with young adult body composition. J Dev Orig Hlth Dis. 2013;4(1):56–68. doi: 10.1017/S2040174412000554. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones-Smith JC, Neufeld LM, Laraia B, Ramakrishnan U, Garcia-Guerra A, Fernald LC. Early life growth trajectories and future risk for overweight. Nutr Diabetes. 2013;3:e60. doi: 10.1038/nutd.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Bmj. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 6.Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing Growth Percentiles in Infancy and Risk of Obesity in Childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. doi: 10.1001/archpediatrics.2011.167. [DOI] [PubMed] [Google Scholar]
- 7.Cameron N, Bogin B. Human growth and development. 2nd ed. Amsterdam, Boston: Academic Press; 2012. [Google Scholar]
- 8.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SA, Micklesfield L, Hallal P, Victora CG. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones A, Charakida M, Falaschetti E, Hingorani AD, Finer N, Masi S, Donald AE, Lawlor DA, Smith GD, Deanfield JE. Adipose and height growth through childhood and blood pressure status in a large prospective cohort study. Hypertension. 2012;59(5):919–925. doi: 10.1161/HYPERTENSIONAHA.111.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menezes AM, Hallal PC, Dumith SC, Matijasevich AM, Araujo CL, Yudkin J, Osmond C, Barros FC, Victora CG. Adolescent blood pressure, body mass index and skin folds: sorting out the effects of early weight and length gains. J Epidemiol Community Health. 2012;66(2):149–154. doi: 10.1136/jech.2010.124842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IOM. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington DC: National Academy Press; 2009. [PubMed] [Google Scholar]
- 12.Alfaradhi MZ, Ozanne SE. Developmental programming in response to maternal overnutrition. Front Genet. 2011;2:27. doi: 10.3389/fgene.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Long NM, Hein SM, Ma Y, Nathanielsz PW, Ford SP. Maternal obesity in ewes results in reduced fetal pancreatic beta-cell numbers in late gestation and decreased circulating insulin concentration at term. Domest Anim Endocrinol. 2011;40(1):30–39. doi: 10.1016/j.domaniend.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.George LA, Uthlaut AB, Long NM, Zhang L, Ma Y, Smith DT, Nathanielsz PW, Ford SP. Different levels of overnutrition and weight gain during pregnancy have differential effects on fetal growth and organ development. Reprod Biol Endocrinol. 2010;8:75. doi: 10.1186/1477-7827-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long NM, George LA, Uthlaut AB, Smith DT, Nijland MJ, Nathanielsz PW, Ford SP. Maternal obesity and increased nutrient intake before and during gestation in the ewe results in altered growth, For Peer Review adiposity, and glucose tolerance in adult offspring. J Anim Sci. 2010;88(11):3546–3553. doi: 10.2527/jas.2010-3083. [DOI] [PubMed] [Google Scholar]
- 16.Deierlein AL, Siega-Riz AM, Herring AH, Adair LS, Daniels JL. Gestational weight gain and predicted changes in offspring anthropometrics between early infancy and 3 years. Pediatric Obesity. 2012;7(2):134–142. doi: 10.1111/j.2047-6310.2011.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deierlein AL, Siega-Riz AM, Adair LS, Herring AH. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011;158(2):221–226. doi: 10.1016/j.jpeds.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, Liu E, Guo J, Pan L, Li B, Wang P, Liu J, Wang Y, Liu G, Hu G. Maternal Prepregnancy Body Mass Index and Gestational Weight Gain on Offspring Overweight in Early Infancy. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rooney BL, Mathiason MA, Schauberger CW. Predictors of obesity in childhood, adolescence, and adulthood in a birth cohort. Matern Child Health J. 2011;15(8):1166–1175. doi: 10.1007/s10995-010-0689-1. [DOI] [PubMed] [Google Scholar]
- 20.Ehrenthal DB, Maiden K, Rao A, West DW, Gidding SS, Bartoshesky L, Carterette B, Ross J, Strobino D. Independent relation of maternal prenatal factors to early childhood obesity in the offspring. Obstet Gynecol. 2013;121(1):115–121. doi: 10.1097/aog.0b013e318278f56a. [DOI] [PubMed] [Google Scholar]
- 21.Whitaker RC. Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics. 2004;114(1):e29–e36. doi: 10.1542/peds.114.1.e29. [DOI] [PubMed] [Google Scholar]
- 22.Day NL, Richardson GA, Geva D, Robles N. Alcohol, marijuana, and tobacco: effects of prenatal exposure on offspring growth and morphology at age six. Alcohol Clin Exp Res. 1994;18(4):786–794. doi: 10.1111/j.1530-0277.1994.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 23.Day NL, Jasperse D, Richardson G, Robles N, Sambamoorthi U, Taylor P, Scher M, Stoffer D, Cornelius M. Prenatal exposure to alcohol: effect on infant growth and morphologic characteristics. Pediatrics. 1989;84(3):536–541. [PubMed] [Google Scholar]
- 24.Day NL, Zuo Y, Richardson GA, Goldschmidt L, Larkby CA, Cornelius MD. Prenatal alcohol use and offspring size at 10 years of age. Alcohol Clin Exp Res. 1999;23(5):863–869. [PubMed] [Google Scholar]
- 25.Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcohol Clin Exp Res. 2002;26(10):1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91(6):1642–1648. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Paediatr Perinat Epidemiol. 2012;26(2):109–116. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. American journal of clinical nutrition. 2013;97(5):1062–1067. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO MGRSG. Geneva: World Health Organization; 2006. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development; p. 312. [Google Scholar]
- 30.Grummer-Strawn LM, Reinold C, Krebs NF. Use of World Health Organization and CDC growth charts for children aged 0–59 months in the United States. MMWR Recomm Rep. 2010;59(RR-9):1–15. [PubMed] [Google Scholar]
- 31.Ong KKL, Ahmed ML, Emmett PM, Preece MA, Dunger DB Team ALSoPaCS. Association between postnatal catchup growth and obesity in childhood: prospective cohort study. Bmj. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Association of weight gain in infancy and early childhood with metabolic risk in young adults. J Clin Endocrinol Metab. 2007;92(1):98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- 33.Ekelund U, Ong K, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83(2):324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 34.CES-D. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 35.Spielberger CD. STPI. Center for Research in Behavioral Medicine and Health Psychology. Tampa: PCD 4118G, University of South Florida; 1979. Preliminary manual for the State-Trait Personality Inventory (STPI) [Google Scholar]
- 36.Goldschmidt L, Richardson GA, Stoffer DS, Geva D, Day NL. Prenatal alcohol exposure and academic achievement at age six: a nonlinear fit. Alcohol Clin Exp Res. 1996;20(4):763–770. doi: 10.1111/j.1530-0277.1996.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 37.Rabe-Hesketh S, Skrondal A, Pickles A. GLLAMM Manual (Second Edition); UC Berkeley Division of Biostatistics Working Paper Series; 2004. pp. 1–140. Working Paper 160. [Google Scholar]
- 38.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. New York: John Wiley and Sons; 2004. [Google Scholar]
- 39.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 40.Weng HY, Hsueh YH, Messam LL, Hertz-Picciotto I. Methods of covariate selection: directed acyclic graphs and the change-in-estimate procedure. Am J Epidemiol. 2009;169(10):1182–1190. doi: 10.1093/aje/kwp035. [DOI] [PubMed] [Google Scholar]
- 41.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, Poole C. Illustrating bias due to conditioning on a collider. Int J Epidemiol. 2010;39(2):417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103(5):506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 43.Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicol Teratol. 1992;14(6):407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- 44.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, Navder K, Yu A, Dorsey K, Gallagher D. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205(3):211, e1–e7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 46.Vanderwerker LC, Day NL, Baker CE, Richardson GA, Stone RA. Unpublished doctoral dissertation. University of Pittsburgh; 2003. Depression in children: A comparison of mother and child reports. [Google Scholar]
- 47.Cameron N, Preece MA, Cole TJ. Catch-up growth or regression to the mean? Recovery from stunting revisited. American journal of human biology : the official journal of the Human Biology Council. 2005;17(4):412–417. doi: 10.1002/ajhb.20408. [DOI] [PubMed] [Google Scholar]
- 48.Crozier SR, Inskip HM, Godfrey KM, Cooper C, Harvey NC, Cole ZA, Robinson SM. Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. American Journal of Clinical Nutrition. 2010;91(6):1745–1751. doi: 10.3945/ajcn.2009.29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tikellis G, Ponsonby AL, Wells JC, Pezic A, Cochrane J, Dwyer T. Maternal and infant factors associated with neonatal adiposity: results from the Tasmanian Infant Health Survey (TIHS) Int J Obes (Lond) 2012;36(4):496–504. doi: 10.1038/ijo.2011.261. [DOI] [PubMed] [Google Scholar]
- 50.Josefson JL, Hoffmann JA, Metzger BE. Excessive weight gain in women with a normal pre-pregnancy BMI is associated with increased neonatal adiposity. Pediatric Obesity. 2013;8(2):e33–e36. doi: 10.1111/j.2047-6310.2012.00132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davenport MH, Ruchat SM, Giroux I, Sopper MM, Mottola MF. Timing of Excessive Pregnancy- Related Weight Gain and Offspring Adiposity at Birth. Obstet Gynecol. 2013;122(2):255–261. doi: 10.1097/AOG.0b013e31829a3b86. [DOI] [PubMed] [Google Scholar]
- 52.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322, e1–e8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80(6):1579–1588. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 54.de Onis M, Garza C, Onyango AW, Borghi E. Comparison of the WHO child growth standards and the CDC 2000 growth charts. J Nutr. 2007;137(1):144–148. doi: 10.1093/jn/137.1.144. [DOI] [PubMed] [Google Scholar]
- 55.Lundgren EM, Cnattingius S, Jonsson B, Tuvemo T. Intellectual and psychological performance in males born small for gestational age with and without catch-up growth. Pediatr Res. 2001;50(1):91–96. doi: 10.1203/00006450-200107000-00017. [DOI] [PubMed] [Google Scholar]
- 56.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lederman SA, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Matern Child Health J. 1998;2(2):123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- 58.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75(6):978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 59.Substance Abuse and Mental Health Services Administration OoAS. 2009. The NSDUH Report: Substance Use among Women During Pregnancy and Following Childbirth. [Google Scholar]
- 60.Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33(1):129–136. doi: 10.1016/j.ntt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]