Abstract
Background and Purpose
Atherosclerotic plaque vulnerability is accompanied by changes in the molecular and cellular function in the plaque shoulder, including a decrease in vascular smooth muscle cell (VSMC) proliferation. We aimed to determine if the expression of three miRNAs that regulate VSMC proliferation (miR-145, miR-221, and miR-222) are altered with plaque rupture, suggesting a role in regulating plaque stability.
Methods
miRNAs were measured in the plaque shoulder of carotid plaques obtained from patients undergoing carotid endarterectomy (CEA) for three distinct clinical scenarios: 1) patients without prior neurologic events but high-grade carotid stenosis (asymptomatic), 2) patients with an acute neurologic event within 5 days of the CEA (urgent), and 3) patients undergoing CEA > 5 days after a neurologic event (symptomatic).
Results
Mean time from plaque rupture event to CEA was 2.4 days in the urgent group. The urgent group exhibited a significant decrease in miR-221 and miR-222 expression in the plaque shoulder while no significant differences were seen in miR-145 across the three groups. Regression analysis demonstrated a significant correlation between time from the neurologic event to CEA and increasing miR-221 and miR-222, but not miR-145. mRNA encoding p27Kip1, a target of miR-221 and -222 that inhibits VSMC proliferation, was increased in the urgent group.
Conclusions
Atherosclerotic plaque rupture is accompanied by a loss of miR-221 and -222 and an increase in p27Kip1 mRNA expression in the plaque shoulder, suggesting an association between these miRNAs and atherosclerotic plaque stability.
Keywords: Atherosclerosis, Plaque Rupture, miRNA-221, miRNA-222, carotid artery endarterectomy
Carotid plaque rupture leads to acute neurologic symptoms and, similarly, coronary plaque atheroembolization leads to acute coronary syndrome. Loss of vascular smooth muscle cells (VSMCs) in the plaque shoulder region of the fibrous cap has been implicated in plaque rupture.1 MicroRNA-221 and -222 (miR-221/222) are short non-coding RNAs that inhibit the expression of the cyclin-dependent kinase inhibitor, p27Kip1, promoting VSMC proliferation and intimal thickening.2, 3 In contrast, miR-145 promotes VSMC differentiation as well as decreased intimal thickening and atherosclerotic plaque formation in animal models.4-6
The present study aimed to investigate the role of expression of these miRNAs in atherosclerotic plaque rupture. We measured miR-221/222 and miR-145 in carotid plaque shoulder segments from patients undergoing carotid endarterectomy (CEA) and stratified the data according to the time between the CEA and the neurological event. Here we report that miR-221/222 expression is significantly decreased acutely following plaque rupture and exhibits a rapid return to pre-rupture levels.
Methods
Carotid plaque specimens and pertinent medical histories were obtained from 76 patients undergoing CEA in the Department of Surgery at the Ochsner Clinic with no significant differences in age, sex, lipid profile, smoking, or presence of diabetes mellitus between the groups (Table). Similar to our previous findings7, there was a significant increase in miR-221/222 expression in diabetic versus non-diabetic patients (p < 0.01, Supplemental Figure I). Patients were stratified into three groups: without prior neurologic events (asymptomatic, n = 31), with a prior neurologic event within 5 days of the CEA (urgent, n = 25) and undergoing CEA greater than 5 days post a neurologic event (symptomatic, n = 20). miRNAs and mRNAs of interest were measured in the plaque shoulder by real-time PCR using the primers listed in Supplemental Table I. A more detailed methods section is supplied as an online supplement.
Table. Characteristics of Patients.
| Characteristics | Total (n=76) | Asymptomatic (n=31) | Urgent (n=25) | Symptomatic (n=20) | P value |
|---|---|---|---|---|---|
| Age, y | 66.8 ± 2.0 | 68.2 ± 2.9 | 66.8 ± 2.6 | 64.5 ± 5.3 | 0.28 |
| Male Sex | 50 (66) | 19 (61) | 18 (72) | 13 (65) | 0.70 |
| Body Mass Index, kg/m2 | 27.1 ± 0.8 | 28.5 ± 1.1 | 26.4 ± 1.5 | 25.9 ± 1.6 | 0.33 |
| Total Cholesterol, mg/dL | 175.9 ± 6.7 | 166.1 ± 11.4 | 184.5 ± 9.1 | 175.0 ± 17.6 | 0.42 |
| High Density Lipoprotein, mg/dL | 43.0 ± 1.6 | 44.2 ± 2.3 | 40.0 ± 2.3 | 46.8 ± 4.2 | 0.22 |
| Low Density Lipoprotein, mg/dL | 102.2 ± 5.2 | 96.7 ± 9.6 | 108.8 ± 6.5 | 98.2 ± 12.9 | 0.56 |
| Triglycerides, mg/dL | 134.2 ± 8.8 | 124.6 ± 11.6 | 153.6 ± 16.6 | 112.2 ± 11.8 | 0.14 |
| Serum Creatinine, mg/dL | 1.1 ± 0.1 | 1.0 ± 0.0 | 1.0 ± 0.1 | 1.3 ± 0.2 | 0.21 |
| Diabetes Mellitus | 13 (17) | 5 (16) | 5 (20) | 3 (15) | 0.89 |
| Smoker | 21 (28) | 9 (29) | 8 (32) | 4 (20) | 0.81 |
| Time to CEA, days | 2.4 ± 0.4 | 38 ± 8 | <0.001 |
Results
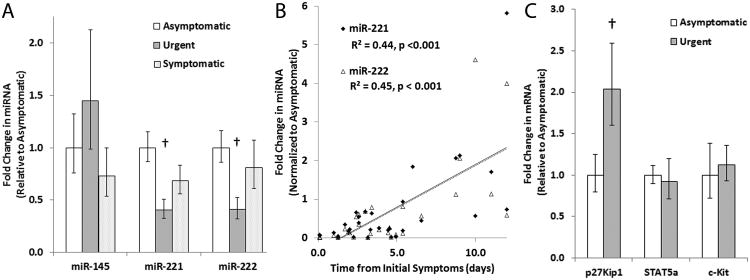
The urgent group exhibited a significant decrease in miR-221/222, but not miR-145, compared to the asymptomatic and symptomatic groups (Figure 1A). Linear regression analysis demonstrated a direct correlation between expression of miR-221/222 and increasing time between carotid plaque rupture/acute neurologic symptom onset and the time when CEA occurred (R2 = 0.44, p < 0.001 for miR-221 and R2 = 0.45, p < 0.001, Figure 1B). This correlation is not seen with miR-145 (R2 = 0.004, p = 0.85).
Figure 1.
Expression of miRNA and their targets in the carotid plaque shoulder. A. Quantification of miR-145 and miR-221/222 in the plaque shoulder of patients with no prior neurologic event (asymptomatic), a neurologic event within 5 days prior to the CEA (urgent), and a neurologic event > 5 days prior to the CEA (symptomatic). B. X-Y scatterplot of fold changes in miR-221/222 expression versus time between neurologic event and CEA for patients undergoing CEA within 12 days of the cerebrovascular event. The solid and dashed lines represent a linear regression fit to the miR-221 and miR-222 data, respectively. C. Quantification of p27Kip1, STAT5A, and c-Kit in the plaque shoulder of asymptomatic and urgent patients. † indicates p < 0.05.
Levels of the mRNA encoding the miR-221/222 target, p27Kip1, were increased acutely post-plaque rupture (urgent group) (Figure 1C). Levels of two other targets of miR-221/222 that are involved in neovascularization 8, 9, c-Kit and the signal transducer and activator of transcription 5A (STAT5A), remained unchanged across the groups, suggesting neovascularization is not altered by the changes in expression of miR-221/222 that occur with rupture. These data suggest a role for loss of miR-221/222 inhibition of p27Kip1 in the thinning of the fibrous cap that promotes plaque instability and rupture.
Discussion
This is the first demonstration that miR-221/222 expression in the plaque shoulder is decreased acutely after plaque rupture. The loss of miR-221/222 was accompanied by an increase in the mRNA encoding its target, p27Kip1. Loss of p27Kip1 through increased miR-221/222 expression results in increased intimal thickening in animal models of vascular injury.3 VSMCs isolated from advanced atherosclerotic plaques exhibit lower proliferation rates.10, 11 Similarly, VSMCs isolated from CEA specimens obtained from asymptomatic patients exhibit higher proliferative responses and lower p27Kip1 levels than those obtained from symptomatic patients.12 Our data support a role for miR-221/222 in the intimal thickening associated with plaque development and that loss of miR-221/222 may underlie the reduced VSMC proliferation associated with fibrous cap thinning and plaque rupture. Down-regulation of miR-221/222 has also been implicated in the regulation of plaque neovascularization and monocyte differentiation to macrophages.9, 13 While we focused our studies on the effects of loss of miR-221/222 in VSMCs, further studies examining the role of miR-221/222 in regulating plaque neovascularization and macrophage accumulation are therefore warranted.
By focusing on carotid plaques obtained from patients undergoing ‘urgent’ CEAs, we were able to identify modulations in miRNA expression that occur acutely with plaque rupture but would not be detected in the standard symptomatic patient. Namely, that miR-221/222 expression is reduced in the carotid plaque shoulder at the time of rupture and then returns to pre-rupture levels within two weeks. It is not possible, however, to determine whether the decrease in miR-221/222 occurs prior to or as a result of plaque rupture. These data highlight the unique value of obtaining samples immediately following an acute neurologic event when examining the mechanism underlying plaque rupture and suggest a link between miR-221/222 and cell proliferation in this region.
Supplementary Material
Acknowledgments
Sources of Funding: Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers P30GM103337 and U54GM104940 and an American Diabetes Association Basic Research Award (1-13-BS-210).
Footnotes
Disclosures: None.
References
- 1.Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: Role of extracellular lipid, macrophage, and smooth muscle cell content. British heart journal. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of mir-221/222 in vessels: Molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–255. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of mir-221 and mir-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. Mir-145 and mir-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–710. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through mirnas. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 7.Coleman CB, Lightell DJ, Jr, Moss SC, Bates M, Parrino PE, Woods TC. Elevation of mir-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Molecular and cellular endocrinology. 2013;374:125–129. doi: 10.1016/j.mce.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. Micrornas modulate the angiogenic properties of huvecs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 9.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. Microrna-222 controls neovascularization by regulating signal transducer and activator of transcription 5a expression. Arterioscler Thromb Vasc Biol. 2010;30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 10.O'Brien ER, Alpers CE, Stewart DK, Ferguson M, Tran N, Gordon D, et al. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993;73:223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- 11.Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia G, Cheng G, Agrawal DK. Differential effects of insulin-like growth factor-1 and atheroma-associated cytokines on cell proliferation and apoptosis in plaque smooth muscle cells of symptomatic and asymptomatic patients with carotid stenosis. Immunology and cell biology. 2006;84:422–429. doi: 10.1111/j.1440-1711.2006.01449.x. [DOI] [PubMed] [Google Scholar]
- 13.Eigsti RL, Sudan B, Wilson ME, Graff JW. Regulation of activation-associated microrna accumulation rates during monocyte-to-macrophage differentiation. J Biol Chem. 2014;289:28433–28447. doi: 10.1074/jbc.M114.599316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.