Abstract
Objective
Medial meniscal extrusion is known to be related to structural progression of knee OA. However, it is unclear whether medial meniscal extrusion is more strongly associated with cartilage loss in certain medial femorotibial subregions than to others.
Methods
Segmentation of the medial tibial and femoral cartilage (baseline; 1-year follow-up) and the medial meniscus (baseline) was performed in 60 participants with frequent knee pain (age 61.3±9.2y, BMI 31.3±3.9 kg/m2) and with unilateral medial radiographic joint space narrowing (JSN) grade 1–3, using double echo steady state MR-images. Medial meniscal extrusion distance and extrusion area (%) between the external meniscal and tibial margin at baseline, and longitudinal medial cartilage loss in eight anatomical subregions were determined.
Results
A significant association (Pearson correlation coefficient) was seen between medial meniscus extrusion area in JSN knees and cartilage loss over one year throughout the entire medial femorotibial compartment. The strongest correlation was with cartilage loss in the external medial tibia (r=−0.34 [p<0.01] in JSN, and r=−0.30 [p=0.02] in noJSN knees).
Conclusion
Medial meniscus extrusion was associated with subsequent medial cartilage loss. The external medial tibial cartilage may be particularly vulnerable to thinning once the meniscus extrudes and its surface is “exposed” to direct, non-physiological, cartilage-cartilage contact.
Keywords: Meniscus, cartilage loss, subregions, MR imaging, knee osteoarthritis
Introduction
The menisci play an important role in the mechanical protection of knee cartilage, enabling consistent force transmission across the incongruous femorotibial joint (1–3), and keeping the mechanical stress on cartilage and subchondral bone within reasonable limits (2–5). Meniscal damage is known to be associated with meniscal extrusion (6–8,8,9), because loss of matrix integrity may reduce radial stiffness, cause an external shift of the meniscus (extrusion), and prevent the meniscus from taking up hoop stresses during load transmission. Extrusion reduces mechanical protection of the knee cartilage by the meniscus, and hence is a risk factor for incident knee osteoarthritis (OA) (5), structural progression of OA (5,10–12) and knee symptoms (13,14).
Previous studies have reported semi-quantitative measures of meniscal extrusion to be associated with subsequent cartilage loss in the medial femorotibial compartment (15,16), and quantitative (two-dimensional) measures of extrusion (in one image slice) and meniscal lesions to be related to semi-quantitative estimates of cartilage loss (i.e. longitudinal increase in cartilage lesions) (17). However, it is currently unclear whether medial meniscal extrusion is more strongly associated to cartilage loss in certain femorotibial subregions than to others. We hence used technology for MRI-based measurement of subregional femorotibial cartilage thickness loss (18) and for quantitatively measuring meniscal position (relative to the tibial surface) and size three-dimensionally (19). To study the relationship between meniscus extrusion and the subregional pattern of femorotibial cartilage loss, we relied on a sample of overweight patients with frequent knee pain, one knee with radiographic joint space narrowing (JSN) and the other knee without (20,21). Such patients would typically be seen by a doctor in context of knee pain, and are typically included in clinical trials studying the effect of disease modifying OA drugs (DMOADs) (20,21). Specifically, we hypothesized that medial meniscal extrusion is more strongly associated with cartilage loss in medial femorotibial subregions physiologically covered by the meniscus, i.e. the external aspect of the medial tibia, rather than in those that are not covered and in which cartilage-cartilage contact occurs under normal conditions, i.e. central medial tibia.
Methods
Study participants
The subsample studied here has been described previously (20–22) and was drawn from the first half (n=2678) of the OA Initiative (OAI) cohort (baseline clinical data 0.2.1; http://www.oai.ucsf.edu/datarelease/) (23). The OAI is a longitudinal multi-center, cohort study, and participants were between 45 and 79 years old (24). General exclusion criteria of the OAI were rheumatoid or other inflammatory arthritis, bilateral end stage knee OA, inability to walk without aids, and MRI contraindications. Informed consent was obtained from all participants and the study approved by the local ethics committees (24). MR images were acquired annually in the participants over 4 years, using 3 Tesla Magnetom Trio magnets (Siemens Erlangen, Germany) and quadrature transmit-receive knee coils (USA Instruments, Aurora, OH) (24–26).
The sample studied here was originally selected to compare cartilage thickness loss and loss of radiographic joint space width (JSW) between knees of persons with unilateral medial radiographic joint space narrowing (mJSN) (20,21). Specific inclusion criteria for this subsample were a body mass index (BMI) >25 kg/m2, frequent pain in both knees (most days in at least one month of the past 12 months), mJSN OARSI grades 1–3 in one knee, and no medial or lateral JSN in the contra-lateral knee (20–22). Subsequently we showed that medial tibial coverage by the medial meniscus was substantially less in knees with mJSN than in contra-lateral noJSN knees in the same person (22).
Quantitative analysis of meniscal position
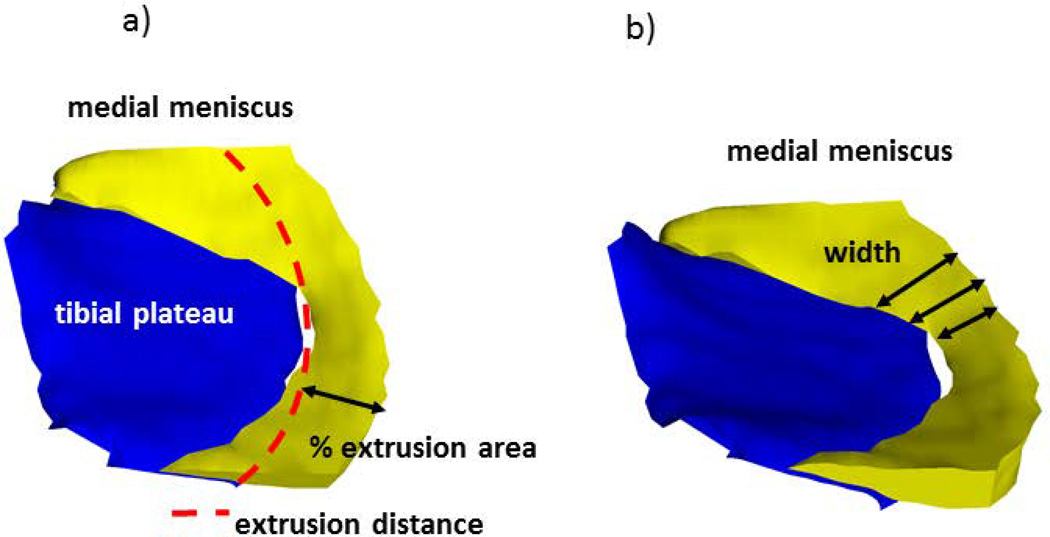
Baseline coronal multi-planar reconstructions of the sagittal double echo steady state sequence with water excitation (DESSwe) were used for manual segmentation of the meniscus (reconstructed slice thickness=1.5mm, in-plane resolution 0.37mm ×0.7mm, interpolated to 0.37mm), because the extrusion in medial/lateral direction cannot be accurately assessed in sagittal MRI. The software used (Chondrometrics GmbH, Ainring, Germany) (19) and the specific segmentation approach have been described previously (27,28). Manual segmentation of the medial tibial and weight-bearing femoral plateau area, and of the medial meniscus surfaces (Fig. 1a) were performed by a single, experienced reader (K.B.), starting anteriorly and ending posteriorly in the first/last image in which the tibial cartilage could be identified. Internally (towards the center of the tibia), the border of the meniscus was defined by the internal margin of the cartilage surface of the medial tibia, because of the continuity of the meniscus with the transverse and menisco-femoral ligaments. Meniscus segmentation and morphometry from DESS images has previously been shown to yield high intra-observer reproducibility [14, 28] and acceptable inter-observer reliability and good agreement with measurements from clinical sequences (29), and quantitative measures of meniscal extrusion derived from the DESS was strongly associated with semi-quantitative scores of meniscal extrusion at corresponding locations (30). From various measures available (19,31), we selected the two-dimensional extrusion distance of the meniscus (i.e. the distance by which it exceeds the margin of the medial tibial cartilage) in a central slice of the medial tibia, and in a slice located 6mm posterior to the center. The latter was chosen because it is the location where semi-quantitative extrusion scores (e.g. MOAKS) are commonly evaluated (32). We further determined extrusion as the relative (percent) area of the meniscus surface extruding the medial tibial plateau medially (Fig.2a). Finally, we determined the width of the entire medial meniscus (the distance between the medial and lateral margin), because this measure was previously shown to be as strongly associated with tibial plateau coverage as meniscal extrusion (30). (Fig.2b)
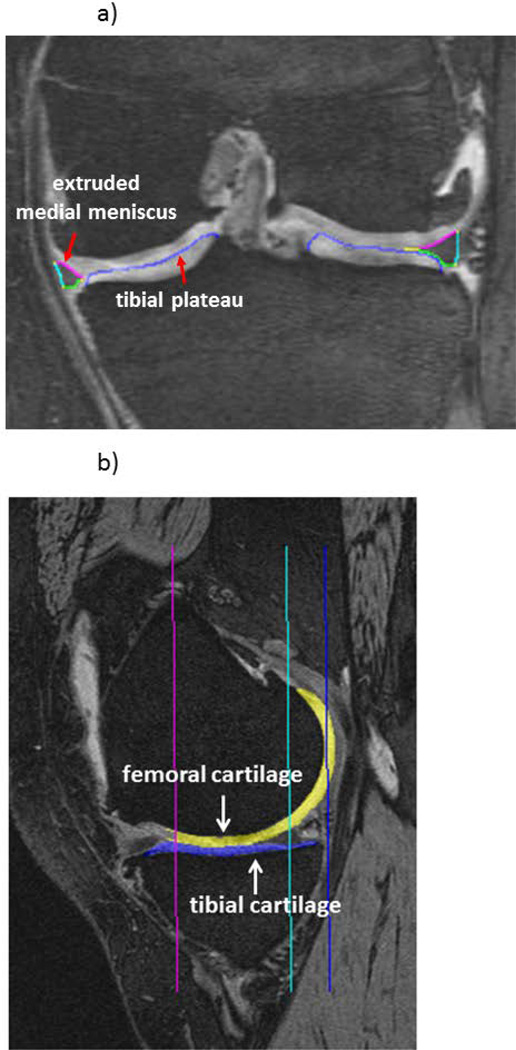
Fig. 1.
a) Coronal DESSwe MRI showing manual segmentation of the medial meniscus: FA=femoral area, TA=tibial area, EA=external area covering the tibial plateau (ACdAB); b) Sagittal DESSwe MRI showing manual segmentation of the femoral and tibial cartilage thickness.
Fig. 2.
3D reconstruction of the medial meniscus; a) extrusion distance and that % area of the meniscus surface extruding the tibial plateau margin and b) meniscus width are indicated schematically.
Quantitative analysis of subregional femorotibial cartilage loss
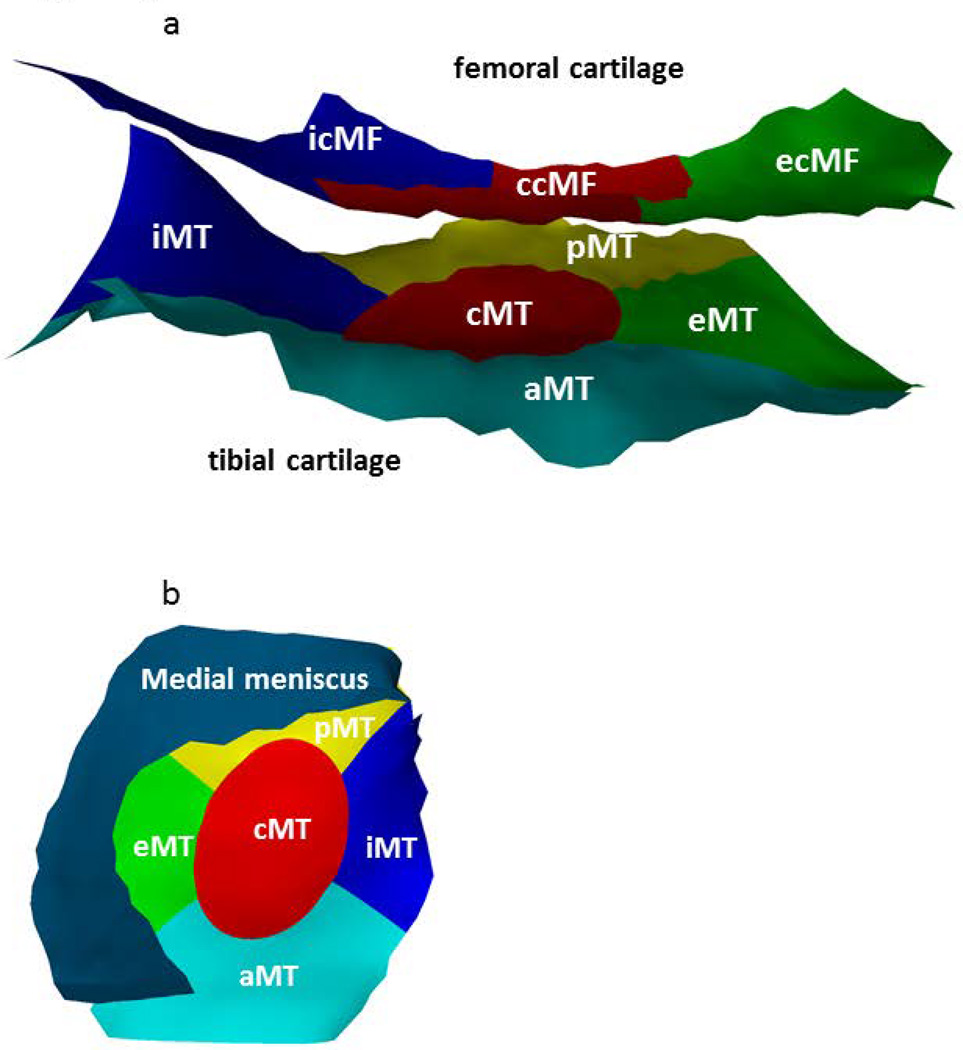
The sagittal DESSwe sequence was used to determine medial femorotibial cartilage thickness as described previously (20). Paired baseline and one-year follow-up images were read by 7 experienced readers with formal training and more than 5 years of experience with cartilage segmentation, with blinding to acquisition order (20). (Fig.1b) After segmentation of the medial tibial and weight-bearing femoral cartilage and quality control of the segmentations by an expert, the cartilage loss was determined across 8 medial femorotibial subregions (software from Chondrometrics GmbH, Ainring, Germany) (18). (Figs. 3) The medial tibial (MT) cartilage was separated in 5 subregions (anterior (aMT), posterior (pMT), central (cMT), external (eMT) and internal (iMT)) and the medial weight-bearing femur (cMF) in 3 subregions (central (ccMF), external (ecMF) and internal (icMF)). These subregional thickness measurements have previously shown to yield good test-retest reproducibility (18) and to be sensitive to one year longitudinal change in knee OA (33).
Fig. 3.
3D reconstruction of a) medial femorotibial cartilage thickness across femorotibial subregions: 5 medial tibial (MT) subregions (anterior (aMT), posterior (pMT), central (cMT), external (eMT), internal (iMT)) and 3 medial weight-bearing femoral cartilage (cMF) subregions (central (ccMF), external (ecMF) and internal (icMF); b) medial meniscus covering the medial femorotibial cartilage including subregions.
Statistical analysis
Mean values and standard deviations (SDs) were determined for all meniscus and cartilage thickness measures in knees with mJSN and in contra-lateral knees without JSN. Longitudinal cartilage loss was measured as the difference between baseline and one year follow-up. Pearson correlation coefficients and their 95% confidence intervals (95% CIs) were computed between baseline meniscus measures and the subregional femorotibial cartilage thickness change, to explore the strength of association between meniscus extrusion and cartilage loss in various subregions. No formal adjustment for multiple comparisons was performed; two-tailed p-values<0.01 were considered statistically significant.
Partial correlation analyses were performed to study the impact of age and BMI on the correlation between subregional cartlage loss and meniscus parameters. Further, stepwise multiple regression models (forward mode) were used to explore whether covariates such as age and BMI provided independent information to meniscus extrusion in the association with cartilage loss.
Results
Demographics
Of the 60 participants included, 22 were men and 38 women. Their mean age was 61.3±9.2years, the body height 1.66±0.1m, the body weight 86.6±13.0kg, and the BMI 31.3±3.9kg/m2. Of the 60 mJSN knees, 43 knees were mJSN grade 1, 14 mJSN grade 2, and 3 mJSN grade 3. No lateral JSN was present in any knee.
Meniscal extrusion
Medial meniscal extrusion in the central slice ranged from 9.9mm to −0.6mm in mJSN knees with a mean of 3.4±2.0mm, and from 5.4mm to −0.75mm in noJSN knees with a mean of 1.8±1.3mm; extrusion distances in the slice 6mm posterior to the center ranged from 8.6mm to 0.15mm in JSN with a mean of 3.2±1.8mm, and from 4.5mm to −1.6mm in noJSN knees with a mean of 1.3±1.4mm. The extrusion area of the medial meniscus was 16.3±7.8% in the noJSN knees and 29.3±13.4% in mJSN knees: the values were 26.5±11.4% (range 5.5–62) in mJSN1, 35.1±11.2% (range 14–50) in mJSN2, and 42.2±32.6% (range 21–80) in mJSN3 knees.
Subregional cartilage thickness loss
The baseline cartilage thickness in the medial subregions is shown in Table 1. Longitudinal cartilage thickness loss (µm) over 1 year was generally greater in mJSN than in noJSN knees (Table 1); it was similar for the total tibia and femur, but was quite variable across the subregions. The greatest rates of cartilage loss in mJSN and noJSN knees were observed in the external and central medial tibia (eMT and cMT) and in the weight-bearing medial femur (ccMF; Table 1). The rate of cartilage loss in eMT did not differ between men and women in JSN knees (p=0.394; unpaired t-test). Also, no significant correlation was observed between cartilage loss in eMT and age (r=0.089; 95% confidence interval [CI] −0.169, 0.335), or eMT and BMI (r=−0.114; 95% confidence interval [CI] −0.358, 0.144). Likewise, no significant relationship was observed between eMT cartilage loss and age, BMI or sex in noJSN knees (data not shown). Partial correlations controlling for age and BMI as covariates were slightly lower than the bivariate ones in the JSN knees and were slightly greater than the bivariate ones in the noJSN knees, but the differences (difference ≤0.06) were minimal. Further, stepwise regression models confirmed that age and BMI did not provide additional information when combined with meniscus extrusion parameters as factors associated with eMT cartilage loss.
Table 1.
Subregional cartilage thickness (mm) and loss (µm) in knees ±medial JSN
| Baseline mJSN(mm) |
Baseline noJSN(mm) |
1-year change mJSN (µm) |
1-year change no JSN (µm) |
|
|---|---|---|---|---|
| MFTC | 3.17±0.67 | 3.53±0.52 | −74±182** (−2.0%) | −26±120* (−0.8%) |
| MT | 1.57±0.30 | 1.67±0.27 | −39±85** (−2.5%) | −19±49** (−1.2%) |
| cMF | 1.60±0.42 | 1.86±0.32 | −36±124* (−2.5%) | −7±96 (−0.5%) |
| cMT | 2.03±0.51 | 2.31±0.46 | −76±173**(−3.9%) | −45±98**(−1.7%) |
| eMT | 1.24±0.40 | 1.46±0.22 | −72±151**(−5.6%) | −47±86**(−3.4%) |
| iMT | 1.86±0.43 | 1.85±0.41 | −6±119 (−0.5%) | −16±107 (−1.1%) |
| aMT | 1.39±0.27 | 1.47±0.23 | −14±81 (−0.7%) | −2±57 (−0.1%) |
| pMT | 1.42±0.25 | 1.42±0.24 | −29±88 (−2.1%) | −24±63** (−1.4%) |
| ccMF | 1.61±0.60 | 2.07±0.40 | −63±196*(−3.7%) | −20±170 (−1.0%) |
| ecMF | 1.25±0.35 | 1.41±0.27 | −15±128 (−1.6%) | −7±103 (−0.7%) |
| icMF | 1.90±0.49 | 2.08±0.39 | −28±123(−1.6%) | −4±95 (−0.2%) |
mJSN= medial joint space narrowing; MFTC= medial femorotibial compartment; MT= medial tibia; cMF= central medial femur; c= central, e= external, i= internal a= anterior, p= posterior subregion;
p<0.05;
p< 0.01 for paired t-test between baseline and follow-up
Correlation of meniscal extrusion measures with cartilage loss
In the mJSN knees, negative correlations of the meniscal extrusion in the central slice were observed with cartilage thickness change (a positive correlation between extrusion and cartilage loss), with the relationship reaching statistical significance in the total medial compartment, the external subregion of the medial tibia, and in the central subregion of the weight-bearing femur (Table 2). The strongest correlation was observed for eMT, and that in cMT was lower by comparison. For most subregions, the correlations between extrusion measured in the slice 6mm posterior to the central one and cartilage loss were somewhat weaker than those observed in the central slice (Table 2), whereas those for extrusion measures of the entire meniscus (% area, Table 3) tended to be slightly stronger than those for the central slice. In the noJSN knees, a statistically significant correlation of the meniscal extrusion with cartilage loss was observed only for eMT, in the slice 6mm posterior to the central one (Table 2), whereas that for cMT and other subregions was not significant. The associations between meniscus width and cartilage loss were low and generally did not reach statistical significance, except for the posterior medial tibia in the noJSN knees (Table 3).
Table 2.
Correlation (Pearson r and 95% CI) of medial meniscus extrusion in the central slice and a slice 6mm posterior to the center with subregional femorotibial cartilage loss in knees with and without medial JSN
| Central Slice | Slice 6mm posterior to Center | |||
|---|---|---|---|---|
| mJSN | no JSN | mJSN | no JSN | |
| MFTC | −0.29*[−0.50, −0.04] | −0.01[−0.27, 0.24] | −0.19[−0.42, 0.07] | −0.10[−0.34, 0.16] |
| MT | −0.25[−0.47, 0.01] | −0.03[−0.28, 0.23] | −0.17[−0.41, 0.08] | 0.01[−0.24, 0.27] |
| cMF | −0.24[−0.46, 0.02] | 0.004[−0.25, 0.26] | −0.15[−0.39, 0.11] | −0.13[−0.38, 0.12] |
| cMT | −0.16[−0.40, 0.10] | 0.03[−0.23, 0.28] | −0.09[−0.34, 0.17] | 0.06[−0.19, 0.31] |
| eMT | −0.31*[−0.52, −0.06] | −0.25[−0.47, 0.01] | −0.26*[−0.49, −0.01] | −0.30*[−0.52, −0.05] |
| iMT | −0.03[−0.28, 0.23] | −0.02[−0.28, 0.23] | −0.10[−0.35, 0.15] | 0.11[−0.15, 0.35] |
| aMT | −0.21[−0.44, 0.05] | 0.02[−0.23, 0.28] | −0.14[−0.38, 0.12] | 0.03[−0.22, 0.29] |
| pMT | −0.20[−0.43, 0.06] | 0.09[−0.17, 0.33] | −0.06[−0.31, 0.19] | 0.11[−0.15, 0.35] |
| ccMF | −0.29*[−0.51, −0.04] | 0.02[−0.24, 0.27] | −0.15[−0.39, 0.11] | −0.15[−0.39, 0.11] |
| ecMF | −0.10[−0.35, 0.16] | −0.02[−0.27, 0.24] | 0.05[−0.21, 0.30] | −0.19[−0.42, 0.07] |
| icMF | −0.16[−0.40, 0.10] | −0.03[−0.28, 0.23] | −0.24[−0.47, 0.01] | 0.03[−0.23, 0.28] |
p<0.05; for other abbreviations see Table 1
Table 3.
Correlation (Pearson r and 95% confidence interval) of percent (%) area of medial meniscus extrusion across the entire tibia and of meniscus width with subregional cartilage loss in knees with and without medial JSN
| % Area of Meniscus Extruded | Meniscus Width | |||
|---|---|---|---|---|
| mJSN | no JSN | mJSN | no JSN | |
| MFTC | −0.28*[−0.50, −0.03] | −0.07[−0.32, 0.19] | 0.15[−0.11, 0.39] | −0.09[−0.34, 0.17] |
| MT | −0.30*[−0.52, −0.05] | −0.02[−0.27, 0.24] | 0.14[−0.12, 0.38] | −0.13[−0.37, 0.13] |
| cMF | −0.19[−0.43, 0.07] | −0.08[−0.33, 0.18] | 0.13[−0.13, 0.37] | −0.04[−0.29, 0.22] |
| cMT | −0.17[−0.41, 0.08] | 0.02[−0.23, 0.27] | 0.06[−0.20, 0.31] | 0.01[−0.24, 0.27] |
| eMT | −0.34*[−0.55, −0.09] | −0.24[−0.47, 0.01] | 0.14[−0.12, 0.38] | −0.02[−0.27, 0.23] |
| iMT | −0.16[−0.40, 0.10] | 0.01[−0.25, 0.26] | 0.04[−0.21, 0.29] | −0.17[−0.41, 0.09] |
| aMT | −0.25[−0.48, 0.001] | 0.01[−0.25, 0.26] | 0.09[−0.16, 0.34] | 0.06[−0.20, 0.31] |
| pMT | −0.21[−0.44, 0.05] | 0.12[−0.14, 0.36] | 0.19[−0.06, 0.43] | −0.30*[−0.51, −0.05] |
| ccMF | −0.18[−0.41, 0.08] | −0.07[−0.32, 0.19] | 0.13[−0.13, 0.37] | −0.07[−0.31, 0.19] |
| ecMF | −0.03[−0.29, 0.22] | −0.11[−0.35, 0.15] | 0.05[−0.21, 0.30] | −0.02[−0.27, 0.24] |
| icMF | −0.24[−0.47, 0.01] | −0.03[−0.28, 0.23] | 0.12[−0.14, 0.36] | −0.002[−0.26, 0.25] |
p<0.05; for abbreviations see Table 1
Discussion
This is the first study to compare quantitative medial meniscal extrusion measures with subsequent medial subregional femorotibial cartilage loss. We hypothesized that the correlation between medial meniscal extrusion and medial femorotibial cartilage loss would be strongest for the external aspect of the medial tibia, a region physiologically covered by the meniscus and hence potentially more vulnerable to mechanical challenge when the meniscus is extruded. Our results confirm the above hypothesis by showing that correlation coefficients between cartilage loss and meniscal extrusion tended to be greater for eMT than for other femorotibial subregions. Similar rates of cartilage loss were observed in the adjacent cMT, which is not physiologically covered by the meniscus, but the correlation was weaker. Further, the correlation was weaker in the external femur (ecMF) than in eMT, potentially because contact of femoral cartilage with the meniscus is more variable with different flexion angles, and femoral cartilage may hence be less dependent on meniscus protection than tibial cartilage. Further, the rate of external femoral cartilage loss was generally less than in eMT.
Our study confirms previous reports that medial meniscus position is an important predictor of structural progression of medial femorotibial OA (15,16). It extends previous findings by using a fully quantitative measurement technology of the meniscus (19,29,30), by exploring it in mJSN and (contra-lateral) noJSN knees, and by studying cartilage loss at a subregional level. Previous studies evaluated the relationship between various grades of meniscus lesions/tears with (subregional) femorotibial cartilage loss (34,35). Crema et al. (34) reported that cartilage loss in the total and external medial tibia was significantly increased with Boston Leads Osteoarthritis Knee Score (BLOKS) (36) grade 3 medial meniscus lesions, and that in the medial femoral condyle with grade 2 lesions. Cartilage loss at the eMT was significantly related to tears of the posterior horn of the meniscus (34), which in other studies was shown to be a cause of meniscus extrusion (7). Chang et al. (35) reported that medial meniscal body tears were associated with cartilage loss in external, central and anterior tibial subregions, and posterior horn tears specifically with posterior tibial subregion loss, independent of tears in the other segments, and also after adjusting for meniscus extrusion. Posterior horn tears were associated with cartilage loss in the underlying subregion (pMT), but not after adjustment for extrusion (35). Cartilage loss in the internal subregions, not covered by the menisci, was not associated with meniscal tears in any segment. The authors concluded that detrimental effects of meniscal tears are not spatially uniform across the tibial and femoral cartilage surfaces, and that some of the effect is experienced locally (35). We previously reported meniscus lesions in the present sample (31) and our results extend these findings by revealing that the relationship between quantitative measures of meniscus extrusion with subregional quantitative cartilage loss is greatest in the external medial tibia. Although no therapeutic options currently exist for maintaining cartilage loss in specific subregions, it may be worth focusing on the external tibial cartilage in knees with meniscus extrusion, once these become available.
In a cross-sectional study, Arno et al. generated computer models and measured the length of meniscal contact and cartilage volume across fifty knees with different OA grades (37). They suggested that cartilage loss initially occurred in regions of direct femorotibial cartilage-cartilage contact, and to spread medially (i.e. to the external subregion) from there when the meniscus showed greater degrees of extrusion (37). Given the cross-sectional nature of their study, however, they were unable to make prospective claims and differentiate cause and effect in the above relationships. Moodie et al. destabilized the medial meniscus in skeletally mature male mice (38), resulting in extrusion of the meniscus; they reported medial cartilage loss especially in the posterior part of the tibial plateau using confocal scanning laser microscopy (CSLM) compared to control mice with a stable meniscus. In our study, the correlation of meniscus extrusion with subsequent cartilage loss in the posterior medial tibia was less than that with the external subregion, and was not greater than for the other femorotibial subregions. However, joint mechanics in the surgical destabilization mouse model likely differ from that in humans.
A limitation of the current study is its limited sample size; in particular, the study had insufficient statistical power to examine the interaction between quantitative meniscus morphology, grade and location of meniscus lesions (39), and quantitative cartilage loss in the various medial femorotibial subregions. Because of the lack of adjustment for meniscal tears, anterior cruciate ligament (ACL) rupture/injury, malalignment and other potential confounders, a causal relation between the meniscal extrusion/position and subsequent cartilage loss cannot be ascertained. Also, the subjects examined here all had a BMI >25, and both knees were frequently painful. Given previous observations of a relationship between meniscus extrusion and frequent pain status (14) and the uncertainty of a relationship with BMI, the results of the current study cannot be generalized to normal-weight subjects without knee pain. However, the sample studied here corresponds to a typical population of OA patients who would see a doctor in primary or secondary care, suffering from knee pain and being overweight, and the knees studied cover a range of radiographic conditions, including knees with advanced structural disease (JSN) and those without. Further, overweight patients with symptomatic knee OA (with or without JSN) correspond to cohorts typically included in clinical trials to study the effect of DMOADs on structural change; in fact the current sample was initially chosen with the attempt to determine optimal recruitment strategies for such a clinical trial (20,21). Previous work has established a relationship between meniscus extrusion and knee pain frequency using a between-knee, within person study design (14). It has also been established that subjects with frequent knee pain experience greater rates of cartilage loss than those without (40). Whether knee pain is associated with increased cartilage loss in certain femorotibial subregions and not in others, however, and to what extent this may be conveyed by meniscus extrusion, remains to be elucidated.
A limitation is the relatively short follow-up period for determining cartilage loss (i.e. 1 year), and that cartilage loss was only determined in the medial but not in the lateral femorotibial compartment. Given the known relationship between medial JSN and medial cartilage loss, and that between lateral JSN and lateral cartilage loss (41) however, we recommend that future studies exploring the relationship between lateral meniscus extrusion and lateral femorotibial cartilage loss should include knees with lateral JSN. A further limitation of the current study is that segmentation of the meniscus was done using coronal MR imaging, which appears more suitable for examining the body of the meniscus, but not sagittal MR imaging, which is better suited for delineating the anterior and posterior horn. The observed relationships hence apply to medial (external) meniscal extrusion but do not include the effects of anterior or posterior extrusion on (subregional) cartilage loss (17). A strength, of the methodology applied here is that quantitative measurements of medial (external) meniscal extrusion was derived for the entire meniscus and was not confined to single slices, as done in previous studies (15–17). A further strength is that the observed relationships were confirmed for both mJSN and contralateral noJSN knees, as radiographic JSN itself has been shown to be dependent on meniscal extrusion in this (31) and other samples (42). Observations on the relative strength of the association between meniscal extrusion and cartilage loss in mJSN and noJSN knees were not confounded by between-person differences, as they were from the same participants.
In conclusion we find medial meniscus extrusion to be associated with subsequent cartilage loss. The association between quantitative, three-dimensional measures of meniscal extrusion with subregional cartilage loss in the external medial tibial cartilage (eMT) appeared to be greater than for other femorotibial subregions. eMT is a region physiologically covered by the meniscus, and the cartilage in this subregions may be particularly vulnerable to cartilage tissue loss once the meniscus extrudes and the surface is “exposed” to direct, non-physiological, cartilage-cartilage contact.
Significance and Innovations.
First study to associate three-dimensional MRI-based quantitative measures of medial meniscus extrusion with subsequent, longitudinal, subregional cartilage loss in the medial femorotibial joint.
Medial meniscus extrusion is found to be more closely associated with cartilage loss in the external medial tibial cartilage compared with that in other femorotibial subregions
The external tibial cartilage may be particularly vulnerable to thinning once the meniscus extrudes and is “exposed” to direct, non-physiological, cartilage-cartilage contact.
Acknowledgment
The authors thank the following readers at Chondrometrics GmbH: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr for the segmentation of the cartilages from the MRIs. Dr. Susanne Maschek is to be thanked for quality control readings of the segmentations. Further, the authors would like to thank the OAI investigators, clinic staff, and OAI participants at each of the OAI clinical centers for their contributions in acquiring the publicly available clinical and imaging data as well as the team at the OAI coordinating center,
The study and image acquisition was funded by the Osteoarthritis initiative, a public-private partnership comprised of five contracts: N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262. Image analysis of the meniscus was funded by the Paracelsus Medical University Research Fund (PMU FFF R-12702/036/BLO). Image analysis of the cartilage, as previously published and referenced, was funded by Eli Lilly & Co, IN. Ali Guermazi is President and co-owner of the Boston Core Imaging Lab (BICL), a company providing MRI reading services to academic researchers and to industry. He provides consulting services to Novartis, Genzyme, Stryker, MerckSerono and AstraZeneca. Wolfgang Wirth has a part-time appointment with Chondrometrics GmbH, a company providing MR image analysis services, and is co-owner of Chondrometrics GmbH. Felix Eckstein is CEO and co-owner of Chondrometrics GmbH. He provides consulting services to Mariel and MerckSerono.
Footnotes
Conflict of Interest: Katja Bloecker, David Hunter, Herbert Resch and Josef Hochreiter have no competing interests.
References
- 1.Krause WR, Pope MH, Johnson RJ, Wilder DG. Mechanical changes in the knee after meniscectomy. J Bone Joint Surg Am. 1976;58:599–604. [PubMed] [Google Scholar]
- 2.Walker PS, Erkman MJ. The role of the menisci in force transmission across the knee. Clin Orthop Relat Res. 1975:184–192. doi: 10.1097/00003086-197506000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Chivers MD, Howitt SD. Anatomy and physical examination of the knee menisci: a narrative review of the orthopedic literature. J Can Chiropr Assoc. 2009;53:319–333. [PMC free article] [PubMed] [Google Scholar]
- 4.Kurosawa H, Fukubayashi T, Nakajima H. Load-bearing mode of the knee joint: physical behavior of the knee joint with or without menisci. Clin Orthop Relat Res. 1980:283–290. [PubMed] [Google Scholar]
- 5.Englund M. The role of biomechanics in the initiation and progression of OA of the knee. Best Pract Res Clin Rheumatol. 2010;24:39–46. doi: 10.1016/j.berh.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Rennie WJ, Finlay DB. Meniscal extrusion in young athletes: associated knee joint abnormalities. AJR Am J Roentgenol. 2006;186:791–794. doi: 10.2214/AJR.04.1181. [DOI] [PubMed] [Google Scholar]
- 7.Choi CJ, Choi YJ, Lee JJ, Choi CH. Magnetic resonance imaging evidence of meniscal extrusion in medial meniscus posterior root tear. Arthroscopy. 2010;26:1602–1606. doi: 10.1016/j.arthro.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Lee DH, Lee BS, Kim JM, Yang KS, Cha EJ, Park JH, et al. Predictors of degenerative medial meniscus extrusion: radial component and knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19:222–229. doi: 10.1007/s00167-010-1274-2. [DOI] [PubMed] [Google Scholar]
- 9.Crema MD, Roemer FW, Felson DT, Englund M, Wang K, Jarraya M, et al. Factors Associated with Meniscal Extrusion in Knees with or at Risk for Osteoarthritis: The Multicenter Osteoarthritis Study. Radiology. 2012;264:494–503. doi: 10.1148/radiol.12110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englund M, Guermazi A, Lohmander LS. The meniscus in knee osteoarthritis. Rheum Dis Clin North Am. 2009;35:579–590. doi: 10.1016/j.rdc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Englund M, Guermazi A, Lohmander SL. The role of the meniscus in knee osteoarthritis: a cause or consequence? Radiol Clin North Am. 2009;47:703–712. doi: 10.1016/j.rcl.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Englund M, Guermazi A, Roemer FW, Aliabadi P, Yang M, Lewis CE, et al. Meniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: The Multicenter Osteoarthritis Study. Arthritis Rheum. 2009;60:831–839. doi: 10.1002/art.24383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englund M, Niu J, Guermazi A, Roemer FW, Hunter DJ, Lynch JA, et al. Effect of meniscal damage on the development of frequent knee pain, aching, or stiffness. Arthritis Rheum. 2007;56:4048–4054. doi: 10.1002/art.23071. [DOI] [PubMed] [Google Scholar]
- 14.Wenger A, Englund M, Wirth W, Hudelmaier M, Kwoh K, Eckstein F. Relationship of 3D meniscal morphology and position with knee pain in subjects with knee osteoarthritis: a pilot study. Eur Radiol. 2012;22:211–220. doi: 10.1007/s00330-011-2234-z. [DOI] [PubMed] [Google Scholar]
- 15.Berthiaume MJ, Raynauld JP, Martel-Pelletier J, Labonte F, Beaudoin G, Bloch DA, et al. Meniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imaging. Ann Rheum Dis. 2005;64:556–563. doi: 10.1136/ard.2004.023796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 18.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 19.Wirth W, Frobell RB, Souza RB, Li X, Wyman BT, Le Graverand MP, et al. A three-dimensional quantitative method to measure meniscus shape, position, and signal intensity using MR images: a pilot study and preliminary results in knee osteoarthritis. Magn Reson Med. 2010;63:1162–1171. doi: 10.1002/mrm.22380. [DOI] [PubMed] [Google Scholar]
- 20.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Magnetic resonance imaging-based cartilage loss in painful contralateral knees with and without radiographic joint space narrowing: Data from the Osteoarthritis Initiative. Arthritis Rheum. 2009;61:1218–1225. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benichou OD, Hunter DJ, Nelson DR, Guermazi A, Eckstein F, Kwoh K, et al. One-year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010;62:924–931. doi: 10.1002/acr.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloecker K, Guermazi A, Wirth W, Benichou O, Kwoh CK, Hunter DJ, et al. Tibial coverage, meniscus position, size and damage in knees discordant for joint space narrowing - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckstein F, Wirth W, Hunter DJ, Guermazi A, Kwoh CK, Nelson DR, et al. Magnitude and regional distribution of cartilage loss associated with grades of joint space narrowing in radiographic osteoarthritis--data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18:760–768. doi: 10.1016/j.joca.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8:622–630. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloecker K, Englund M, Wirth W, Hudelmaier M, Burgkart R, Frobell RB, et al. Size and position of the healthy meniscus, and its correlation with sex, height, weight, and bone area- a cross-sectional study. BMC Musculoskelet Disord. 2011;12:248. doi: 10.1186/1471-2474-12-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloecker K, Wirth W, Hudelmaier M, Burgkart R, Frobell R, Eckstein F. Morphometric differences between the medial and lateral meniscus in healthy men - a three-dimensional analysis using magnetic resonance imaging. Cells Tissues Organs. 2012;195:353–364. doi: 10.1159/000327012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siorpaes K, Wenger A, Bloecker K, Wirth W, Hudelmaier M, Eckstein F. Interobserver reproducibility of quantitative meniscus analysis using coronal multiplanar DESS and IWTSE MR imaging. Magn Reson Med. 2012;67:1419–1426. doi: 10.1002/mrm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloecker K, Guermazi A, Wirth W, Kwoh CK, Resch H, Hunter DJ, et al. Correlation of semiquantitative vs quantitative MRI meniscus measures in osteoarthritic knees: results from the Osteoarthritis Initiative. Skeletal Radiol. 2014;43:227–232. doi: 10.1007/s00256-013-1769-2. [DOI] [PubMed] [Google Scholar]
- 31.Bloecker K, Wirth W, Hunter DJ, Duryea J, Guermazi A, Kwoh CK, et al. Contribution of regional 3D meniscus and cartilage morphometry by MRI to joint space width in fixed flexion knee radiography--a between-knee comparison in subjects with unilateral joint space narrowing. Eur J Radiol. 2013;82:e832–e839. doi: 10.1016/j.ejrad.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 32.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score) Osteoarthritis Cartilage. 2011;19:990–1002. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crema MD, Guermazi A, Li L, Nogueira-Barbosa MH, Marra MD, Roemer FW, et al. The association of prevalent medial meniscal pathology with cartilage loss in the medial tibiofemoral compartment over a 2-year period. Osteoarthritis Cartilage. 2010;18:336–343. doi: 10.1016/j.joca.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2011;70:74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2008;67:206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 37.Arno S, Walker PS, Bell CP, Krasnokutsky S, Samuels J, Abramson SB, et al. Relation between cartilage volume and meniscal contact in medial osteoarthritis of the knee. Knee. 2012;19:896–901. doi: 10.1016/j.knee.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moodie JP, Stok KS, Muller R, Vincent TL, Shefelbine SJ. Multimodal imaging demonstrates concomitant changes in bone and cartilage after destabilisation of the medial meniscus and increased joint laxity. Osteoarthritis Cartilage. 2011;19:163–170. doi: 10.1016/j.joca.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Bloecker K, Guermazi A, Wirth W, Benichou O, Kwoh CK, Hunter DJ, et al. Tibial coverage, meniscus position, size and damage in knees discordant for joint space narrowing - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21:419–427. doi: 10.1016/j.joca.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63:2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth W, Nevitt M, Hellio Le Graverand MP, Lynch J, Maschek S, Hudelmaier M, et al. Lateral and medial joint space narrowing predict subsequent cartilage loss in the narrowed, but not in the non-narrowed femorotibial compartment--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22:63–70. doi: 10.1016/j.joca.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]