Abstract
The implications of thymopoiesis in acquired immunodeficiency syndrome (AIDS) - related opportunistic infections (OI) remain unexplored. We used progressive multifocal leukoencephalopathy (PML), caused by JC virus, as an OI model and we simultaneously investigated thymic output and T-cell responses against JCV in 22 PML patients treated with combined antiretroviral therapy. Thymic output was significantly associated with JCV-specific CD4+ and CD8+ T-cell responses and improved survival. Our data suggest that patients with AIDS-related PML and impaired thymopoiesis are less likely to develop a robust JCV-specific cellular immune response and consequently are at an increased risk for a poor clinical outcome.
Keys words: Human Immunodeficiency Virus, thymus, JC virus, progressive multifocal leukoencephalopathy
INTRODUCTION
Impaired thymic T-cell reconstitution is one of the mechanisms for suboptimal T-cell recovery after effectively inhibiting Human Immunodeficiency Virus (HIV) replication with combined antiretroviral therapy (cART) in patients with acquired immunodeficiency syndrome (AIDS)1–6. The implications of impaired thymopoiesis in AIDS-related opportunistic infections have not yet been elucidated. We hypothesized that thymic contribution to the naïve T-cell pool enhances T-cell responses against opportunistic viruses. We used progressive multifocal leukoencephalopathy (PML), caused by polyomavirus JC (JCV), as an AIDS-defining opportunistic infection model and we investigated the impact of thymic reconstitution in the development of T-cell responses against JCV and in survival from PML.
In the past, radiographic imaging of the thymus and T-cell surface markers have been used for assessing thymic T-cell reconstitution4,7–9. However, given considerable limitations of volumetric imaging studies and the lack of specific cell markers for recent thymic emigrant cells, none of these methods have proven to be a reliable assessment of thymic function10. A more accurate assessment of thymic output may be ascertained by quantification of T-cell receptor rearrangement excision circles (TREC) via polymerase chain reaction (PCR)11–13. TREC are episomal deoxyribonucleic acid (DNA) circles which are stably retained during cell division and generated during the intrathymic rearrangement of T-cell receptor genes. After initiating cART in AIDS patients, quantification of TREC in blood allows monitoring of the thymic contribution to the peripheral pool of naïve T-cells14,15. TREC levels can be influenced by T-cell activation and proliferation in the periphery. Given the profound CD4+ T-cell depletion in patients with AIDS, TREC levels reflect the thymic contribution to the T-cell pool especially when measured early in the course of immune reconstitution5,11,14,15. In the present study, we measured TREC levels in blood in order to assess thymic output in patients with AIDS-related PML. We simultaneously monitored the development of JCV-specific CD4+ and CD8+ T-cell responses using intracellular cytokine staining (ICS) and recorded the clinical outcomes. We paired thymic output with ICS and clinical data in order to investigate the relationship between thymic reconstitution and the development of T-cell responses against JCV, the opportunistic pathogen.
METHODS
This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center. Twenty two patients with AIDS-related PML and five control subjects with AIDS were treated with cART and followed longitudinally at the HIV/Neurology Clinic within our Center. Interferon-γ production from JCV-specific T-cells was detected using ICS, as previously described, after isolating peripheral blood mononuclear cells (PBMC) from whole blood via Ficoll centrifugation16. T-cell activation was assessed using anti-CD25 monoclonal antibodies17. Quantification of TREC in blood was performed as previously described by Douek et al18. We extracted deoxyribonucleic acid (DNA) from the same PBMC obtained for ICS and performed quantitative PCR for the signal-joint (sj)TREC. Results are expressed in sjTREC copies per microgram of PBMC DNA, hereafter abbreviated as TREC cps/μg PBMC DNA13,19. We compared the survival experience in those with high (>2000 copies/μg PBMC DNA) versus low (<2000 copies/μg PBMC DNA) TREC levels using Kaplan-Meier survival analysis based on baseline levels and we used the log-rank test to compare survival experiences. The cut-off TREC level of 2000 copies/μg PBMC DNA has been previously used in thymopoiesis studies19. We used Cox proportional hazards regression models to estimate hazard ratios of death, comparing those with high versus low TREC levels at baseline, and we repeated the analysis accounting for all follow-up TREC measurements. We used Prism 6.0 and STATA 13 software to complete the analyses. Two-sided p-values < 0.05 were considered statistically significant in order to perform logistic regression analysis, Cox proportional hazards regression, and Kaplan-Meier survival analysis.
RESULTS
Patient characteristics are shown in table 1. Fifteen patients survived for more than one year after PML onset (PML-survivors) and 7 patients died within one year after disease onset (PML-progressors). There was no significant difference in age among the patient groups and control subjects. Patients in both PML groups had low CD4+ T-cell counts (median below 60 cells/mL) at the time of PML onset and all patients were started on cART within 35 days. PML-survivors had a higher median CD4+ count compared to PML progressors (123 vs 86, p=0.05) at the time of first TREC measurement as well as 6 months after starting cART (301 versus 144, p=0.02). Since clinical factors such as CD4+ count nadir, hepatitis C virus infection or other opportunistic infections have been associated with a poor immunologic response after starting cART, we included these factors in our analysis but did not find significant differences between the PML-survivors and PML-progressors groups20. HIV viral load in blood decreased below 50 copies/mL within 6 months of cART initiation in 12/15 (80%) PML-survivors and 5/7 (71.4%) PML-progressors. Three PML-survivors and two PML-progressors had detectable HIV viremia after six months of treatment; however these patients experienced at least a 2-log drop in HIV viral load within 6 months of starting cART. The cART central nervous system penetration effectiveness (CPE) score was seven for both PML groups indicating that all cART regimens had similar penetration into the brain21. HIV-infected control subjects had similar age, baseline CD4+ counts and time elapsed between cART initiation and TREC PCR compared to PML patients.
Table 1.
HIV-infected patient characteristics.
| PML-survivors (n=15) | PML-progressors (n=7) | P value | Controls (n=5) | |
|---|---|---|---|---|
| Age (median, range) | 43 (21–53) | 44 (41–52) | p=0.1 | 46 (30–53) |
| Gender (male : female) | 14:1 | 6:1 | - | 4:1 |
| Hepatitis C co-infection | 3/15 (20%) | 2/7 (28.6%) | p=1.0 | 1/5 (20%) |
| Other opportunistic infection | 1/15 (6.67%) | 0/7 (0%) | p=1.0 | |
| CD4+ counts nadir (median, range) | 31 (11–131) | 27 (4–88) | p=0.4 | 33 (8–67) |
| CD4+ counts at PML onset | 47 (14–192) | 53 (4–96) | p=0.35 | - |
| CD4+ counts at time of first TREC PCR | 123 (69–301) | 86 (54–251) | p=0.05 | 101 (79–347) |
| CD4+ counts after 6 months of cART | 301 (179–455) | 144 (86–299) | p=0.02 | 325 (350–1024) |
| HIV viral load <50 cps/mL within 6 month of cART | 12/15 (80%) | 5/7 (71.4%) | p=1.0 | 4/5 (80%) |
CD4 counts are expressed in cells/mL, HIV viral loads in copies/mL.
Abbreviations: PML, progressive multifocal leukoencephalopathy; cART combined antiretroviral therapy.
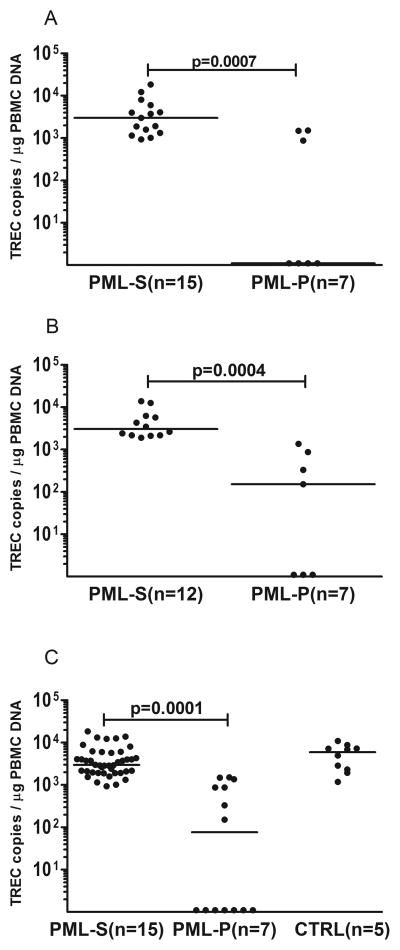
Initial TREC levels in blood were obtained within 45 days (range 38–57) from starting cART for all patients. Repeat TREC levels were measured 180 days (range 167–203) from starting cART for 12 PML-survivors and 7 PML-progressors. All PML-survivors underwent additional testing after one year of starting cART (12 to 58 months). PML-survivors displayed significantly higher TREC levels (median = 3001, range = 931–12201 cps/μg PBMC DNA), compared to PML-progressors (median = undetectable, range = undetectable-1507) upon initial testing (p=0.0007, Figure 1A). T-cell activation at the time of the first TREC PCR was modest and comparable between PML-survivors and PML-progressors (CD4+CD25+ T-cell frequencies ranged from 0.13% to 7.08%, median = 3.68% for PML-survivors and 0.17% to 8.63%, median = 4.11% for PML-progressors, p=0.2). The difference in TREC levels remained significant during repeat testing (p=0.0004 Figure 1B) between PML-survivors (median = 3039, range = 1895–13815) and PML-progressors (median = 152, range = undetectable-1836). There was no significant difference in TREC levels between PML-survivors and control subjects (p=0.3, figure 1C). At the time of the first TREC PCR for each study subject, we performed ICS and we detected a JCV-specific CD4+ T-cell mediated response in 13/15 (86.7%) of PML-survivors and in 3/7 (42.8%) of PML-progressors. In addition, we detected a JCV-specific CD8+ T-cell mediated response in 14/15 (93.3%) PML-survivors and in 2/7 (28.5%) of PML-progressors. Previously, we reported that a cytotoxic T-cell response against JCV is more frequently present in PML-survivors compared to PML-progressors22. We further investigated the relationship between thymic output and the development of JCV-specific T-cell response using logistical regression analysis. Interestingly, we found that initial TREC levels correlated with a detectable CD4+ and CD8+ T-cell response against JCV (p=0.023 and p=0.045 respectively).
Figure 1.
TREC levels in blood of PML-survivors (PML-S), PML-progressors (PML-P) and control subjects (CTRL). TREC levels in blood of PML-survivors, compared to PML-progressors, were significantly higher within 45 days after starting cART (Fig A, p=0.0007) and 180 days after starting cART (Fig B, p=0.0004). TREC levels were overall higher in PML-survivors than PML-progressors (Fig C, 1–58 months of starting cART, p=0.0001) and TREC levels were not significantly different between PML-survivors and control subjects (p=0.3).
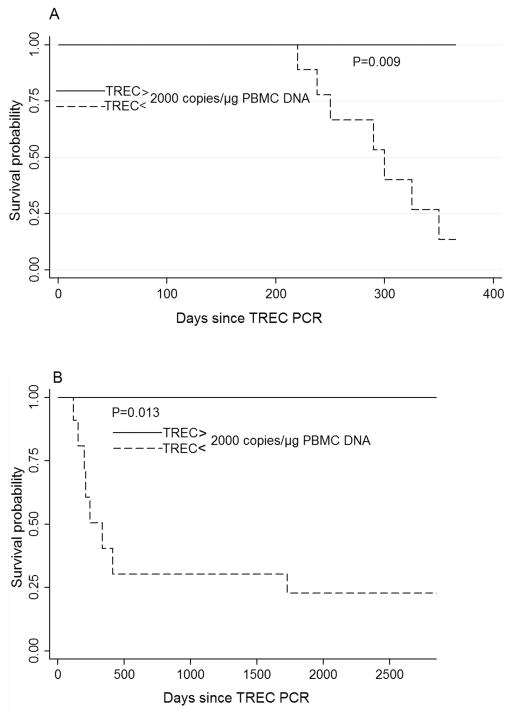
We then performed survival analysis in order to investigate whether initial TREC levels correlate with survival from PML. We found that thymic output above 2000 TREC copies/μg PBMC DNA is strongly associated with survival from PML (p=0.009, hazard ratio = 0.041, 95% confidence interval = 0.01–0.531, Figure 2A). We repeated the survival analysis but allowed TREC levels to vary over time so as to take into account all TREC data measured during repeat testing. Similarly, PML patients who maintained a thymic output above 2000 TREC copies/μg PBMC DNA had improved survival (p=0.013, hazard ratio = 0.071, 95% confidence interval = 0.008–0.579, Figure 2B). The association between TREC levels and survival remained statistically significant and was not materially altered when we adjusted for the number of days elapsed between initiation of cART and TREC PCR.
Figure 2.
Kaplan-Meier curves based on TREC levels in blood of patients with AIDS-related PML. Probability of survival was significantly higher in PML patients with TREC > 2000 copies/μg PBMC DNA 45 days after starting cART, compared to PML patients with TREC < 2000 copies/μg PBMC DNA (p=0.009, Fig A). Probability of survival remained significantly higher in patients who maintained TREC >2000 copies/μg PBMC DNA up to 58 months of starting cART (p=0.013, Fig B).
DISCUSSION
In the present study, thymic reconstitution after initiating cART correlates with the development of a cellular immune response against JCV and favors survival from AIDS-related PML. In particular, PML-survivors displayed higher thymic output and robust JCV-specific T-cell responses in comparison to PML-progressors. We estimated thymic output early in the course of immune reconstitution and simultaneously found evidence of modest T-cell activation for all patients. We therefore believe that TREC measurement provides an assessment of thymic output in our cohort and that thymic reconstitution explains, at least in part, the superior CD4+ T-cell recovery in the PML-survivors group. Our findings are in line with previously published data suggesting a central role of the thymus in naïve T-cell recovery in the setting of cART4,5,8,11,23. In animal studies, recent thymic emigrant T-cells have been shown to participate in immune responses against viral pathogens and this is likely due to the reconstitution of a T-cell receptor repertoire with broad antigenic specificity3,11,20,24–26. It is therefore likely that a well-replenished pool of naïve T-cells allows for de novo priming against viruses, such as JCV, and for the development of a virus-specific cellular immune response. Alternatively, JCV-specific T-cells could originate from memory T-cell expansion after starting cART. Nonetheless, memory T-cell depletion is thought to be the cause of AIDS-defining opportunistic infections27. Our findings expand upon the previous observation that subsets of HIV-infected individuals treated with cART exhibit reduced thymic output and immunological failure for reasons that are poorly understood3,4,6,28. In particular, our data suggest that patients with impaired thymopoiesis are likely unable to mount an adequate response against opportunistic viruses such as JCV, which leads to poor clinical outcomes.
Our findings have specific clinical implications. First, TREC levels bear prognostic value since they correlate with survival from AIDS-related PML early during the disease course. This is especially important given the lack of prognostic biomarkers for PML, a devastating brain infection with no specific treatment. TREC are readily quantifiable via PCR, and therefore, constitute a potentially scalable blood test. Second, assessment of thymic output has therapeutic ramifications since it allows for identification of HIV-infected PML patients who could benefit from immunotherapies that stimulate thymic function. One such immunotherapy is recombinant human interleukin-7 (IL-7) which is critical for intrathymic T-cell selection, differentiation, and proliferation10. Investigational use of recombinant IL-7 has been successful in HIV-infected individuals and in PML patients with idiopathic lymphocytopenias29–33. Lastly, since PML is also known to occur in patients with congenital thymic aplasia and in thymectomized individuals, our findings provide insight into the mechanisms of disease in individuals with known thymic dysfunction34,35.
Our study has certain limitations in that our cohort is small and we did not control for multiple covariates. Nonetheless, PML is a rare disease with poorly understood immunopathogenesis and our findings shed light to the mechanisms of T-cell repopulation in AIDS patients who suffer from opportunistic infections such as PML.
In conclusion, in the setting of cART, thymus-dependent T-cell recovery enhances the development of CD4+ and CD8+ T-cell responses against JCV and leads to improved survival from AIDS-related PML. This is the first study to address the implications of thymopoiesis in AIDS-related PML, and the application of our results to other opportunistic infections merits further investigation. Estimating thymic T-cell output early in the course of immune reconstitution has prognostic value in AIDS-related PML and will help screen for patients who might benefit the most from immunotherapies such as recombinant IL-7.
Acknowledgments
S.C. is supported by Harvard University CFAR fund 2P30AI060354-11. I.J.K. is supported in part by NIH National Institute of Neurologic Disorders and Stroke grants R01 NS047029 and R01 NS074995
The authors would like to thank Dr. Daniel Douek for the generous gift of the sjTREC plasmid and Erin Reese and Dr. Murray Allan Mittleman for the biostatistical consultation. This publication was made possible with help from the Harvard University Center for AIDS Research (CFAR), an NIH funded program (P30 AI060354), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIGMS, FIC, and OAR. I.J.K. is supported by NIH National Institute of Neurologic Disorders and Stroke grants R01 NS047029 and R01 NS074995. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources) and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102 and financial contributions from Harvard University and its affiliated academic health care centers.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
References
- 1.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001 Dec 1;167(11):6663–6668. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 2.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004 Dec;21(6):757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Teixeira L, Valdez H, McCune JM, et al. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001 Sep 28;15(14):1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 4.Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis. 2011 Nov;53(9):944–951. doi: 10.1093/cid/cir552. [DOI] [PubMed] [Google Scholar]
- 5.Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. Aids. 2006 Aug 22;20(13):1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 6.Chattopadhyay PK, Douek DC, Gange SJ, Chadwick KR, Hellerstein M, Margolick JB. Longitudinal assessment of de novo T cell production in relation to HIV-associated T cell homeostasis failure. AIDS Res Hum Retroviruses. 2006 Jun;22(6):501–507. doi: 10.1089/aid.2006.22.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolte L, Ryder LP, Albrecht-Beste E, Jensen FK, Nielsen SD. HIV-infected patients with a large thymus maintain higher CD4 counts in a 5-year follow-up study of patients treated with highly active antiretroviral therapy. Scand J Immunol. 2009 Dec;70(6):608–613. doi: 10.1111/j.1365-3083.2009.02328.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Zhao H, Hao Y, et al. Excessive conversion and impaired thymic output contribute to disturbed regulatory T-cell homeostasis in AIDS patients with low CD4 cell counts. Aids. 2013 Apr 24;27(7):1059–1069. doi: 10.1097/QAD.0b013e32835e2b99. [DOI] [PubMed] [Google Scholar]
- 9.Franco JM, Rubio A, Martinez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002 May 15;99(10):3702–3706. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- 10.Politikos I, Boussiotis VA. The role of the thymus in T-cell immune reconstitution after umbilical cord blood transplantation. Blood. 2014 Nov 20;124(22):3201–3211. doi: 10.1182/blood-2014-07-589176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998 Dec 17;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 12.Rallon NI, Lopez M, Lozano S, Sempere-Ortells JM, Soriano V, Benito JM. Longitudinal assessment of interleukin 7 plasma levels in HIV-infected patients in the absence of and under antiretroviral therapy. J Acquir Immune Defic Syndr. 2011 Dec 15;58(5):436–441. doi: 10.1097/QAI.0b013e318231de37. [DOI] [PubMed] [Google Scholar]
- 13.Barreiro P, Pineda JA, Rallon N, et al. Influence of interleukin-28B single-nucleotide polymorphisms on progression to liver cirrhosis in human immunodeficiency virus-hepatitis C virus-coinfected patients receiving antiretroviral therapy. J Infect Dis. 2011 Jun 1;203(11):1629–1636. doi: 10.1093/infdis/jir113. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev. 2007 Apr;216:21–34. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 15.Hazenberg MD, Verschuren MC, Hamann D, Miedema F, van Dongen JJ. T cell receptor excision circles as markers for recent thymic emigrants: basic aspects, technical approach, and guidelines for interpretation. J Mol Med (Berl) 2001 Nov;79(11):631–640. doi: 10.1007/s001090100271. [DOI] [PubMed] [Google Scholar]
- 16.Chalkias S, Dang X, Bord E, et al. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014 Jun;75(6):925–934. doi: 10.1002/ana.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biancotto A, Iglehart SJ, Vanpouille C, et al. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008 Jan 15;111(2):699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy Y, Sereti I, Tambussi G, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012 Jul;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy Y, Thiebaut R, Gougeon ML, et al. Effect of intermittent interleukin-2 therapy on CD4+ T-cell counts following antiretroviral cessation in patients with HIV. Aids. 2012 Mar 27;26(6):711–720. doi: 10.1097/QAD.0b013e3283519214. [DOI] [PubMed] [Google Scholar]
- 20.Gazzola L, Tincati C, Bellistri GM, Monforte A, Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009 Feb 1;48(3):328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 21.Fanjul F, Riveiro-Barciela M, Gonzalez J, et al. Evaluation of progressive multifocal leukoencephalopathy treatments in a Spanish cohort of HIV-infected patients: do protease inhibitors improve survival regardless of central nervous system penetration-effectiveness (CPE) score? HIV Med. 2013 May;14(5):321–325. doi: 10.1111/hiv.12008. [DOI] [PubMed] [Google Scholar]
- 22.Gheuens S, Bord E, Kesari S, et al. Role of CD4+ and CD8+ T-cell responses against JC virus in the outcome of patients with progressive multifocal leukoencephalopathy (PML) and PML with immune reconstitution inflammatory syndrome. J Virol. 2011 Jul;85(14):7256–7263. doi: 10.1128/JVI.02506-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro RM, de Boer RJ. The contribution of the thymus to the recovery of peripheral naive T-cell numbers during antiretroviral treatment for HIV infection. J Acquir Immune Defic Syndr. 2008 Sep 1;49(1):1–8. doi: 10.1097/QAI.0b013e318184fb28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kosmrlj A, Read EL, Qi Y, et al. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature. 2010 May 20;465(7296):350–354. doi: 10.1038/nature08997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vrisekoop N, Monteiro JP, Mandl JN, Germain RN. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity. 2014 Aug 21;41(2):181–190. doi: 10.1016/j.immuni.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kou ZC, Puhr JS, Wu SS, Goodenow MM, Sleasman JW. Combination antiretroviral therapy results in a rapid increase in T cell receptor variable region beta repertoire diversity within CD45RA CD8 T cells in human immunodeficiency virus-infected children. J Infect Dis. 2003 Feb 1;187(3):385–397. doi: 10.1086/367674. [DOI] [PubMed] [Google Scholar]
- 27.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2013 Jul;254(1):54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med. 1999 Sep 6;190(5):725–732. doi: 10.1084/jem.190.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carcelain G, Autran B. Immune interventions in HIV infection. Immunol Rev. 2013 Jul;254(1):355–371. doi: 10.1111/imr.12083. [DOI] [PubMed] [Google Scholar]
- 30.Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009 Apr;119(4):997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchetti G, Riva A, Cesari M, et al. HIV-infected long-term nonprogressors display a unique correlative pattern between the interleukin-7/interleukin-7 receptor circuit and T-cell homeostasis. HIV Med. 2009 Aug;10(7):422–431. doi: 10.1111/j.1468-1293.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 32.Sospedra M, Schippling S, Yousef S, et al. Treating Progressive Multifocal Leukoencephalopathy With Interleukin 7 and Vaccination With JC Virus Capsid Protein VP1. Clin Infect Dis. 2014 Dec 1;59(11):1588–1592. doi: 10.1093/cid/ciu682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SY, Choi EC, Woo Jo Y, Henson JW, Kim HS. Transcriptional activation of JC virus early promoter by phorbol ester and interleukin-1beta: critical role of nuclear factor-1. Virology. 2004 Sep 15;327(1):60–69. doi: 10.1016/j.virol.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 34.Squintani G, Ferrari S, Bazzoli E, et al. Progressive multifocal leukoencephalopathy in a patient with Good’s syndrome. Int J Infect Dis. 2010 May;14(5):e444–447. doi: 10.1016/j.ijid.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Prelog M, Egli A, Zlamy M, Hirsch HH. JC and BK polyomavirus-specific immunoglobulin G responses in patients thymectomized in early childhood. J Clin Virol. 2013 Nov;58(3):553–558. doi: 10.1016/j.jcv.2013.08.035. [DOI] [PubMed] [Google Scholar]