Abstract
Adenovirus serotypes have been shown to cause drastic changes in nuclear organization, including the transcription machinery, during infection. This ability of adenovirus to subvert transcription in the host cell facilitates viral replication. Because nuclear actin and nuclear myosin I, myosin V and myosin VI have been implicated as direct regulators of transcription and important factors in the replication of other viruses, we sought to determine how nuclear actin and myosins are involved in adenovirus infection. We first confirmed reorganization of the host’s transcription machinery to viral replication centers. We found that nuclear actin also reorganizes to sites of transcription through the intermediate but not the advanced late phase of viral infection. Furthermore, nuclear myosin I localized with nuclear actin and sites of transcription in viral replication centers. Intriguingly, nuclear myosins V and VI, which also reorganized to viral replication centers, exhibited different localization patterns, suggesting specialized roles for these nuclear myosins. Finally, we assessed the role of actin in adenovirus infection and found both cytoplasmic and nuclear actin likely play roles in adenovirus infection and replication. Together our data suggest the involvement of actin and multiple myosins in the nuclear replication and late viral gene expression of adenovirus.
Keywords: Adenovirus, Nuclear Actin, Nuclear Myosin, Viral Replication
INTRODUCTION
Diverse bacterial and viral pathogens induce actin polymerization of host cells to facilitate infection. In the nuclei of host cells, a pathogenic mechanism for promoting dynamic actin assembly has been described to enable replication in a growing number of viruses. Baculovirus, a large double-stranded DNA virus that replicates inside the host nucleus, has been shown to manipulate nuclear actin for virus gene expression and progeny production [1, 2]. Nuclear actin has also been implicated in herpes viral infection [3, 4]. Replication compartments formed by herpes simplex virus in infected nuclei were shown to move by directed motion and require nuclear actin and myosins [5], and myosin Va shows nuclear enrichment upon herpes simplex virus infection [6]. Furthermore, nuclear actin has been implicated in the nuclear transport of unspliced mRNA from human immunodeficiency virus type 1 (HIV-1) and Mason-Pzifer monkey virus [7, 8]. Although adenovirus infection has been shown to result in loss of nuclear actin from Cajal bodies [9], sites of RNA metabolism that disassemble in the late phase of adenovirus infection [10–12], no studies have directly investigated the role of nuclear actin and myosins in adenovirus infection.
In uninfected cells, nuclear forms of actin and myosins are involved in multiple steps required to produce mature transcripts [13]. Actin and myosins have been shown to interact with several chromatin remodeling complexes [14] and have been implicated in mediating long-range directed movement of chromatin [15, 16]. Nuclear actin has been found to regulate transcription by all three RNA polymerases and is important for pre-initiation complex formation [13]. Nuclear actin is also associated with hnRNP A proteins [17], which along with Cajal bodies are reorganized during adenoviral infection [9, 10]. Moreover, nuclear actin has been linked to the nuclear matrix through its interactions with lamins, emerin, and nuclear scaffolding proteins [18–21]. Recent work on nuclear actin binding proteins has implicated nuclear actin as a potential regulator of nuclear shape and organization [22, 23]. Although the functions of actin and myosins in the nucleus are becoming clearer, the mechanisms by which they operate are largely unknown.
Human adenovirus type 5 (Ad5, family Adenoviridae, genus Mastadenovirus) is a non-enveloped icosahedral virus containing a linear double-stranded DNA molecule that replicates in the cell nucleus [24]. In a productive adenovirus infection there is a dramatic reorganization of the cell nucleus during the intermediate and late phase of lytic infection, whereas early viral gene expression does not alter the nuclear organization of mRNA biogenesis [24–29]. The transition to the late phase of infection is conventionally defined by the onset of viral replication and coincides with the accumulation of viral 72 kD DNA binding protein into spot, ring and crescent-like structures [24, 25, 30, 31]. These virus-induced structures demarcate areas of active viral DNA replication. They also represent nuclear sites where newly replicated adenovirus DNA is heavily transcribed by the host cell’s RNA polymerase II (pol II) and where the resulting pre-mRNAs are processed [24–26, 29, 32]. Adenovirus DNA replication occurs at the surface of these compact ring structures and transcription and splicing are predominantly detected around these structures [24].
Given the dynamic nuclear reorganization and transcriptional reprogramming caused by Ad5 infection and the roles for nuclear actin and nuclear myosins in regulating different aspects of RNA metabolism, we hypothesized that nuclear actin and myosins may be critical for proper viral infection. Indeed, we found that nuclear actin and myosins are components of viral replication centers where they associate with the cell’s transcription machinery. Functionally, the role of actin dynamics in Ad5 infection was found to be especially important because preventing nuclear actin polymerization reduced the progression of viral infection. Together our data provide novel insights into the mechanism of adenovirus infection as well as the regulation of nuclear actin and myosins.
RESULTS
Nuclear reorganization of the cellular transcription machinery after adenovirus infection
Adenoviruses use the host’s cellular RNA polymerase II to transcribe the majority of the viral genome [33]. Immunolocalization studies of adenovirus-infected cells have shown that pol II, nascent transcripts, and splicing factors are redistributed as a consequence of adenovirus infection [24–26, 29]. Areas of active transcription and splicing were shown to localize to the periphery of the viral late phase DNA replication centers (structures marked by 72 kD labeling) and extended further into the surrounding nucleoplasm than replication foci [24].
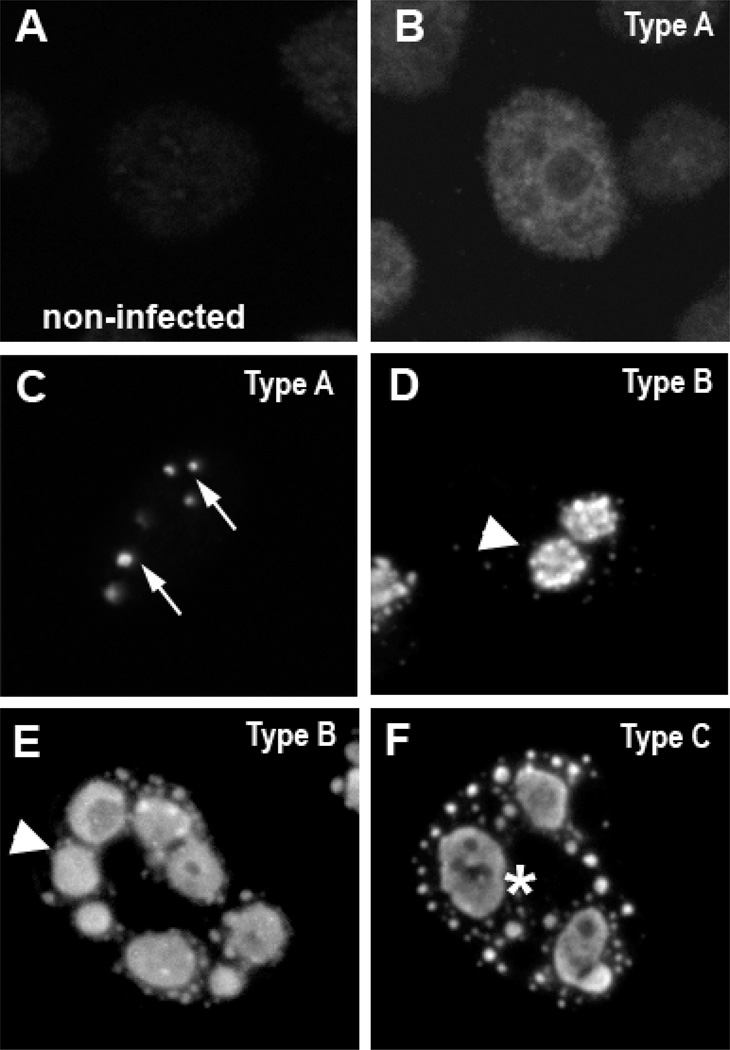
To examine the roles of nuclear actin and nuclear myosins in adenovirus infection, we first examined the spatial distribution of the cellular transcription machinery in adenovirus-infected cells. HeLa cells were infected with the wild-type Ad5 (1000 VP/cell), fixed, permeabilized and stained with an antibody to the viral 72 kD DNA binding protein to determine the subnuclear distribution of viral DNA centers by confocal microscopy. Figure 1 shows the individual patterns of 72 kD protein staining in cells fixed at 21 h post-infection (hpi). They represent the predominant morphological forms of viral DNA centers, which progressively alter as the intermediate and late phases of lytic infection continue [10, 27]. In agreement with these reports [10, 27], we observed that viral infection progressed asynchronously. At 21 hpi, some infected cells showed homogeneous staining of 72 kD protein throughout the nucleus (Fig. 1B). This is indicative of the early phase of infection and is characterized by the production of mRNA and proteins from the early genes (Fig. 1B). Other cells had organized 72 kD protein into distinct spots (Fig. 1C, arrows), and rings (Figs. 1D and E, arrowheads) also known as viral replication centers. The appearance of these structures indicates the onset of viral DNA synthesis that marks the transition to the late phase of the adenovirus life cycle. As the late phase of adenovirus infection continues, enlarged pools of 72 kD protein (Fig. 1F, asterisk), which correspond to increased levels of viral RNA synthesis [10, 24], are detected. To be consistent with previous studies [10, 24, 29] and to facilitate comparison between experiments, we classified 72 kD protein staining patterns as follows: (a) Type A - nuclei with a homogeneous distribution of the 72 kD viral DNA protein (early phase) and nuclei with discrete dots that indicate the transition from the early to intermediate phase (Figs. 1B and C); (b) Type B - nuclei with rings that mark the intermediate phase (Figs. 1D and E), which is also considered the start of the late phase [24–26,29]; (c) Type C - enlarged replication factories typical for the advanced late phase (Fig. 1F).
Figure 1.
Nuclear morphology of viral DNA centers. Confocal micrographs of a control (non-infected) HeLa cell (Panel A; no 72 kD staining) and HeLa cells infected with Ad5 wt (1000 VP/cell) and imaged at 21 hpi. Cells were labeled with an antibody against the viral 72 kD DNA binding protein. The homogenous distribution of 72 kD (Type A, Panel B) is consistent with the early stages of viral infection. The onset of viral DNA synthesis marks the transition from the early to the late phase of infection and is characterized by the compaction of viral centers labeled by the anti-72 kD antibody into dots (Type A, Panel C; arrows). The initial part of the late phase is termed the intermediate stage. As the intermediate stage progresses, viral centers labeled by the anti-72 kD antibody grow and are detected as rings (Type B, Panel D and E; arrowheads). Panels D and E also indicate the morphological heterogeneity of the ring structures. As the late phase of adenovirus infection continues, enlarged pools of 72 kD protein become present (Type C, Panel F; asterisk), which correspond to the advanced late phase. Therefore, Type A cells were classified as expressing 72 kD protein in a homogenous nuclear distribution or in discrete dots, indicating the transition from the early into the intermediate phase (i.e. the initial part of the late phase) (Panels B and C). Cells expressing the viral 72 kD DNA binding protein in rings were classified as Type B, indicating the progression of the intermediate phase (Panels D and E) and cells expressing enlarged pools of 72 kD were identified as Type C, corresponding to the advanced stage of the late phase (Panel F).
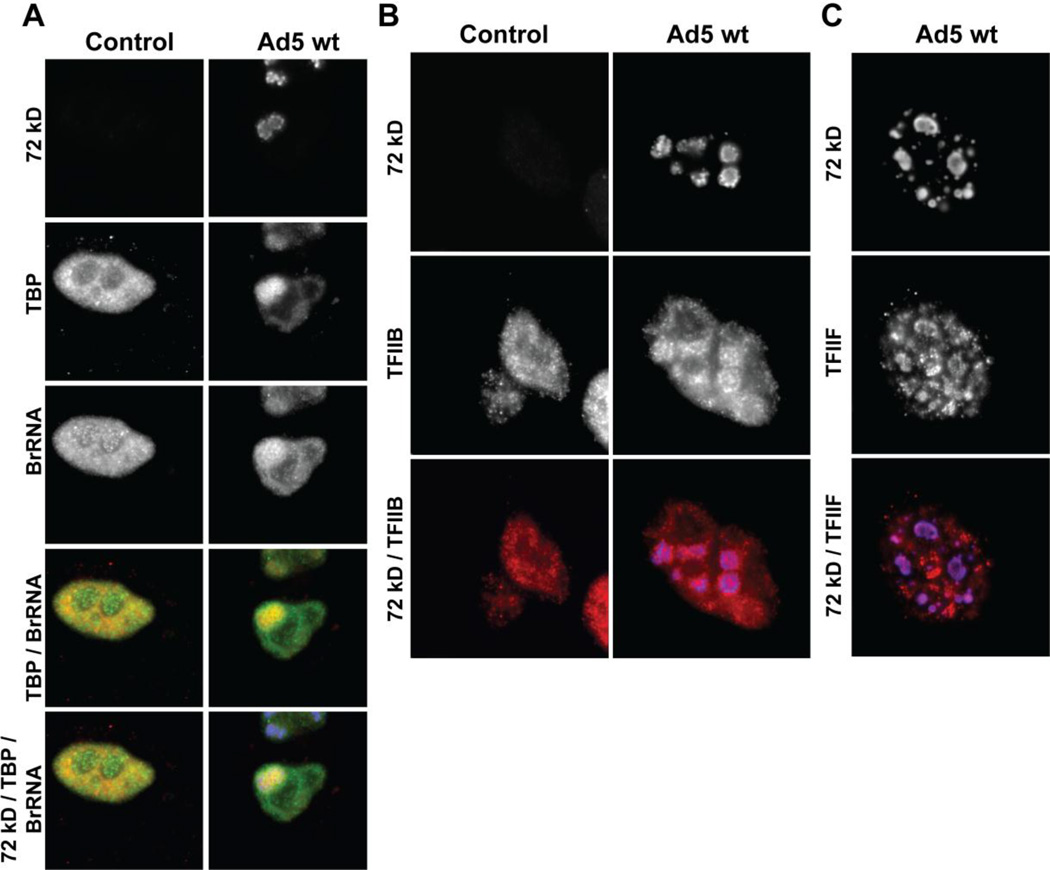
The distribution of adenoviral 72 kD protein in infected cells was then compared to that of the endogenous general transcription factors such as TATA-binding protein (TBP; Fig. 2A), transcription factor IIB (TFIIB; Fig. 2B), transcription factor IIF (TFIIF; Fig. 2C), pol II (Fig. 3), and nascent transcripts (Figs. 2A and 3A). Examination of cells by fluorescence microscopy demonstrated that in control cells, TBP and nascent transcripts are distributed relatively uniformly throughout the nucleus in small foci [also known as transcription factories] (Fig. 2A). In contrast, in infected cells, most of the TBP appears to accumulate at 72 kD labeled viral replication centers (Fig. 2A). The viral replication centers also show the presence of intense BrUTP staining suggesting that active transcription is ongoing at these sites. In agreement, TFIIB (Fig. 2B) and TFIIF (Fig. 2C) also accumulate at ring shaped 72 kD centers, suggesting the presence of transcription initiation complexes.
Figure 2.
Recruitment of the transcription machinery to DNA centers. Confocal micrographs of a control (non-infected) HeLa cell and a cell infected with Ad5 wt (1000 VP/cell) imaged at 21 hpi. (A) Nascent transcripts were labeled using BrUTP in permeabilized cells. Triple staining was performed using anti-BrdU (green), anti-72 kD (blue) and anti-TBP (red) antibodies. (B) Control and infected cells stained with anti-TFIIB and anti-72 kD and (C) anti-TFIIF and anti-72 kD antibodies.
Figure 3.
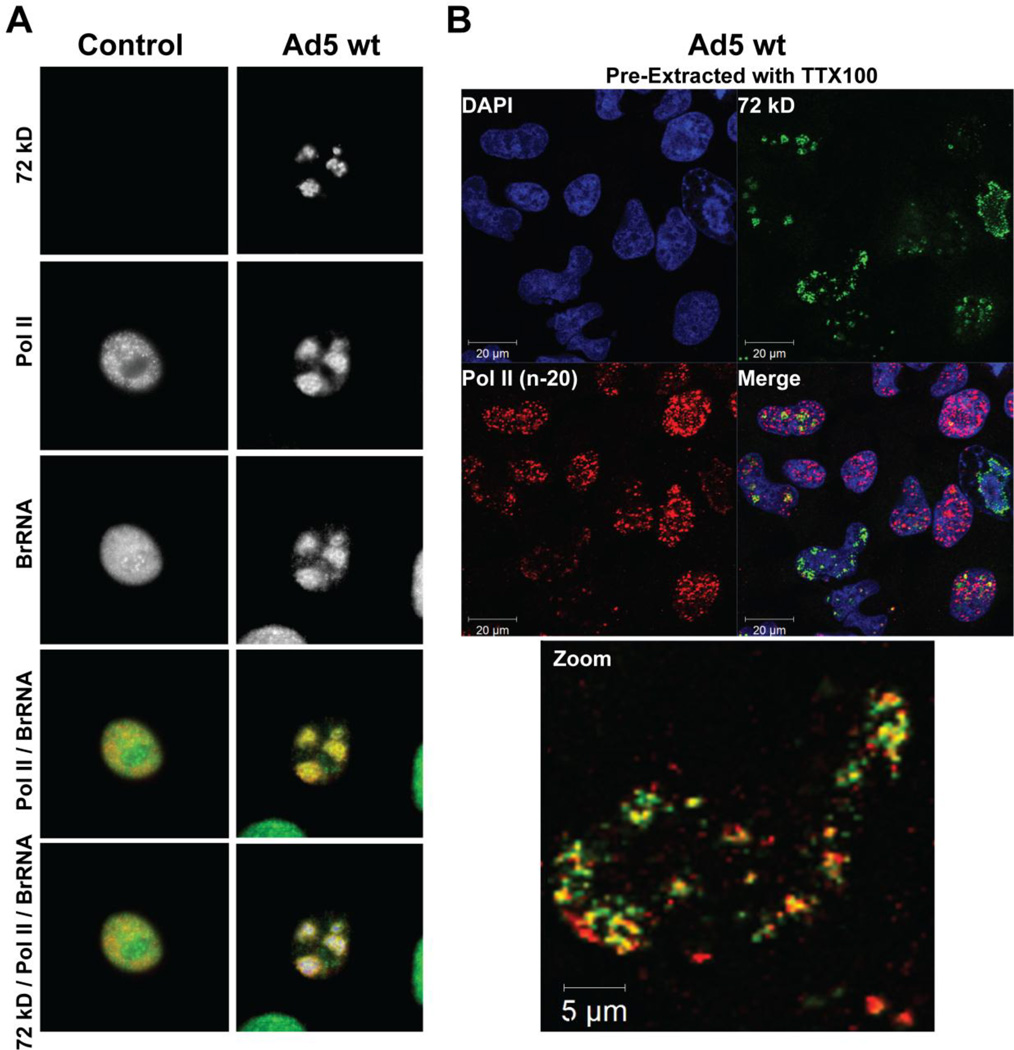
The initiation competent form of pol II accumulates at viral DNA centers. Confocal micrographs of a control (non-infected) HeLa cell and a cell infected with Ad5 wt (1000 VP/cell) and imaged at 21 hpi. (A) Nascent transcripts were labeled using BrUTP in permeabilized cells. Triple staining was performed using anti-BrdU (green), anti-72 kD (blue) and anti-pol II-phosphoserine-5 (H14) (red) antibodies. (B) Pol II remains associated with the viral DNA centers in the nuclear matrix. Ad5 infected cells were pre-extracted with 0.3% Triton X-100 (TTX100) to reveal components of the nuclear matrix, fixed, and subsequently labeled with antibodies against 72 kD protein (green), pol II (red) and counter-stained with DAPI (blue).
Because TBP recruitment to the viral DNA centers was accompanied by the accumulation of nascent transcripts as detected by BrUTP incorporation in permeabilized cells (Fig. 2A), we investigated the presence of active pol II at the viral DNA centers by staining with antibodies to the initiation competent form of pol II (anti-pol II-phosphoserine-5 (H14) antibodies). Immunostaining demonstrated the initiation competent form of pol II was also concentrated at sites of transcription around the viral DNA centers (Fig. 3A). Pol II remained associated with the 72 kD domains in cells pre-extracted to remove soluble nuclear components, suggesting that replication factories are tethered to the nuclear matrix (Fig. 3B). In agreement with previous publications [25, 34], our results indicate that a radical alteration in the distribution of the cellular transcription machinery takes place following viral infection and that active transcription localizes to the periphery of the 72 kD domains in Ad5 infected cells.
Induction of new transcription sites by virus infection causes redistribution of nuclear actin
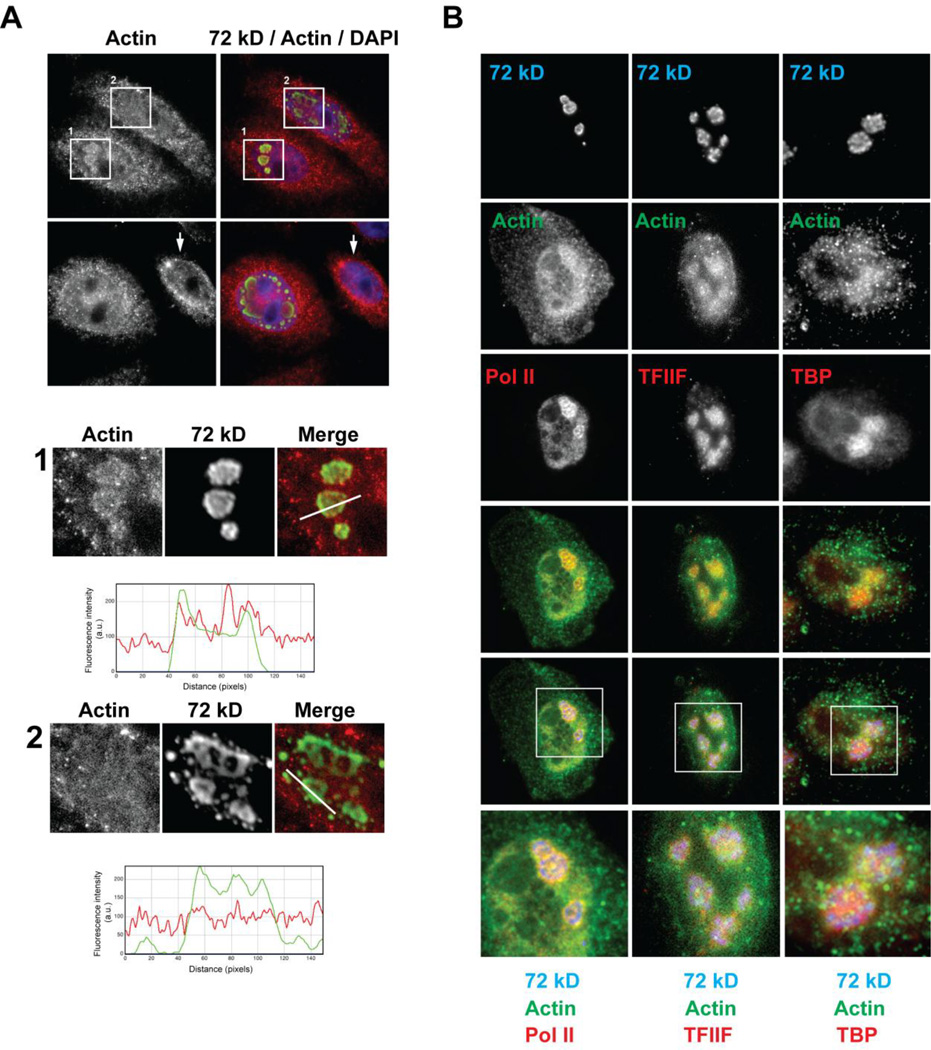
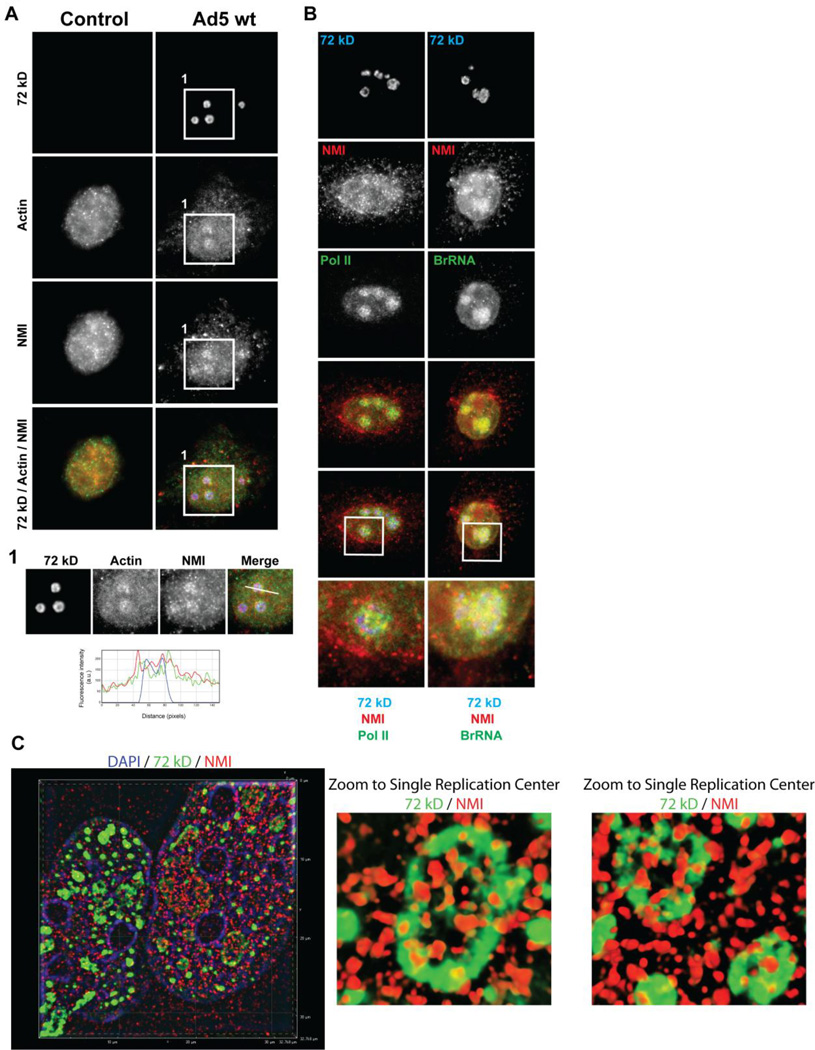
Given the growing literature implicating nuclear actin in viral infections, we assessed the effects of adenovirus infection on the spatial distribution of nuclear actin. We observed that actin concentrates at viral DNA centers present as rings (Type B; Fig. 4A, magnified view 1). However, no accumulation of actin at the viral DNA centers was observed at the larger pools of 72 kD protein (Type C) as the late phase of adenovirus infection continued (Fig. 4A, magnified view 2). We also find that nuclear actin, which accumulates around the viral DNA centers, co-localized with the initiation competent form of pol II (Fig. 4B). Furthermore, aggregates of TBP and TFIIF concentrated at the same viral DNA centers as did actin (Fig. 4B). Together, these data suggested nuclear actin is recruited to viral replication centers present as rings (Type B) where it localizes with the transcription initiation machinery.
Figure 4.
Ad5 infection results in the redistribution of nuclear actin to viral DNA centers. Confocal micrographs of HeLa cells infected with Ad5 wt (1000 VP/cell) imaged at 21 hpi. (A) Double staining was performed using the 2G2 anti-actin (red) and anti-72 kD antibodies (green). A control non-infected cell is indicated (arrow). Boxed areas 1 and 2 show accumulation of 72 kD protein in rings (Type B) and enlarged pools (Type C) of 72 kD, respectively. (1) Profile plot of fluorescence signal intensities along the white line intersecting viral DNA center in box 1 shows that actin concentrates at the viral DNA centers at the ring stage of infection. (2) Profile plot of fluorescence signal intensities along the white line in box 2 shows no accumulation of actin at the viral DNA centers in Type C nuclei. (B) Triple staining was performed using anti-actin antibody, anti-72 kD antibody and anti-pol II-phosphoserine-5 (H14) or anti-TFIIF or anti-TBP antibody. Magnified views of viral DNA centers as indicated in merged images are shown. Actin co-localizes with the initiation competent form of pol II around the viral DNA centers and accumulates at the same viral DNA centers as TBP and TFIIF.
Effect of adenovirus infection on nuclear myosin distribution
Along with nuclear actin, nuclear forms of myosin I (NMI), V (MV) and VI (MVI) have been implicated in the general regulation of transcription [13, 35–38]. NMI and MV have also been implicated in the active movement of herpes simplex virus replication compartments [5]. Therefore, we next examined the effect of adenovirus infection on the spatial distribution of NMI relative to nuclear actin and viral DNA replication centers (Fig. 5). Control (non-infected) and HeLa cells infected with Ad5 wt (1000 VP/cell) were triple stained using the 2G2 anti-actin antibody, antibodies to NMI and the 72 kD protein. These experiments showed that NMI concentrates at the same viral DNA centers as nuclear actin during Ad5 infection (Fig. 5A). The profile plot (Fig. 5A, bottom) shows the overlap of the anti-NMI (red), anti-nuclear actin (green) and anti-72kD protein (blue) fluorescence intensity peaks along a straight line intersecting the viral DNA centers.
Figure 5.
NMI accumulates with actin, the initiation competent form of pol II and nascent transcripts at the viral DNA centers. Confocal micrographs of a control (non-infected) HeLa cell and a cell infected with Ad5 wt (1000 VP/cell) imaged at 21 hpi. (A) Triple staining using the 2G2 anti-actin antibody (green), anti-NMI antibody (red) and anti-72 kD antibody (blue) showed that NMI concentrates at the same viral DNA centers as nuclear actin during Ad5 infection. (1) A profile plot of the fluorescence signal intensity along the white line intersecting a viral DNA center in the boxed area confirmed the co-localization of nuclear actin, NMI and 72kD protein. (B) Triple staining using antibodies to NMI, 72 kD protein, pol II-phosphoserine-5 (H14) or BrdU to visualize new transcripts was performed. Magnified views of viral DNA centers as indicated in merged images are shown. (C) Superresolution SIM reconstruction images of NMI (red), 72 kD (green), and DAPI (blue) demarcate the close association between NMI and the replication factory periphery.
To determine whether active transcription occurs at the viral DNA centers where NMI accumulates, nascent transcripts were labeled using BrUTP incorporation in permeabilized cells. Triple staining was performed using anti-NMI antibody, anti-72 kD antibody and anti-pol II-phosphoserine-5 (H14) or anti-BrdU antibody. We observed that NMI and the initiation competent form of pol II accumulate at the same viral DNA centers. Moreover, nascent transcripts are present at the viral DNA centers where NMI accumulation is detected (Fig. 5B). Furthermore, to obtain a higher spatial resolution between NMI and the viral replication centers, we performed superresolution structured illumination microscopy (SIM). SIM image reconstructions confirm our confocal analysis and suggest that NMI integrates around the periphery of the viral replication centers (Fig. 5C).
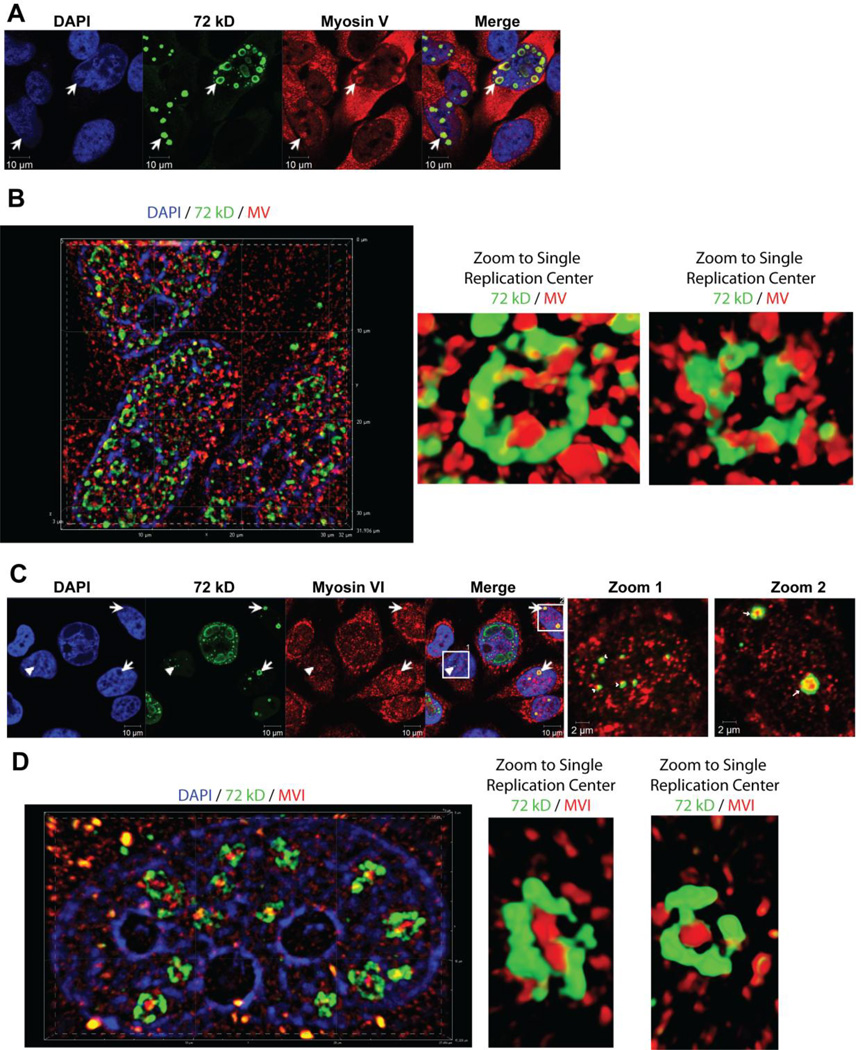
Studies have described the presence of myosin Va and actin in nuclear speckles [35, 39], which also reorganize in the nuclei of adenovirus infected cells [26, 27, 40]. Based on these observations, we evaluated the effect of the adenovirus infection on the spatial distribution of nuclear myosin V. Figure 6A shows that Ad5 infection causes redistribution of nuclear myosin V to the viral DNA centers present as spots and rings (Type A and B; Fig. 6A, arrows), similar to nuclear actin. SIM analysis confirmed this localization and suggests NMI and MV show a comparable staining pattern around viral replication centers (Fig. 6B).
Figure 6.
Redistribution of myosin V and VI following Ad5 infection. Confocal micrographs of HeLa cells infected with Ad5 wt (1000 VP/cell) imaged at 21 hpi. (A) Double staining was performed using anti-MV antibody (red) and anti-72 kD antibody (green) and counter-stained with DAPI (blue). Redistribution of nuclear myosin V to the Type B viral DNA centers is indicated by arrows. (B) Superresolution SIM reconstruction images of MV (red), 72 kD (green), and DAPI (blue) demarcate the close association between MV and the replication factory periphery. (C) Double staining was performed using anti-MVI antibody (red) and anti-72 kD antibody (green) and counter-stained with DAPI (blue). Redistribution of nuclear myosin VI adjacent to type A dots is indicated by arrowhead and localization inside Type B replication centers is marked by arrows. (D) Superresolution SIM reconstruction images of MVI (red), 72 kD (green), and DAPI (blue) show the distribution of MVI within the replication factory.
Myosin VI, a unique minus end directed myosin, has also been described to localize to the nucleus where it is involved in regulating transcription [37]. Therefore, we immunostained for myosin VI in adenovirus infected cells to determine if this myosin is also redistributed upon infection. Surprisingly, we find myosin VI localized adjacent to spots (Type A, Fig. 6C, arrowhead) and concentrated as foci inside small rings (Type B, Fig. 6C, arrows). Indeed, SIM microscopy clearly demonstrated a unique staining pattern for MVI inside the replication factory rings (Fig. 6D).
Role of the nuclear actin in the progression of viral infection
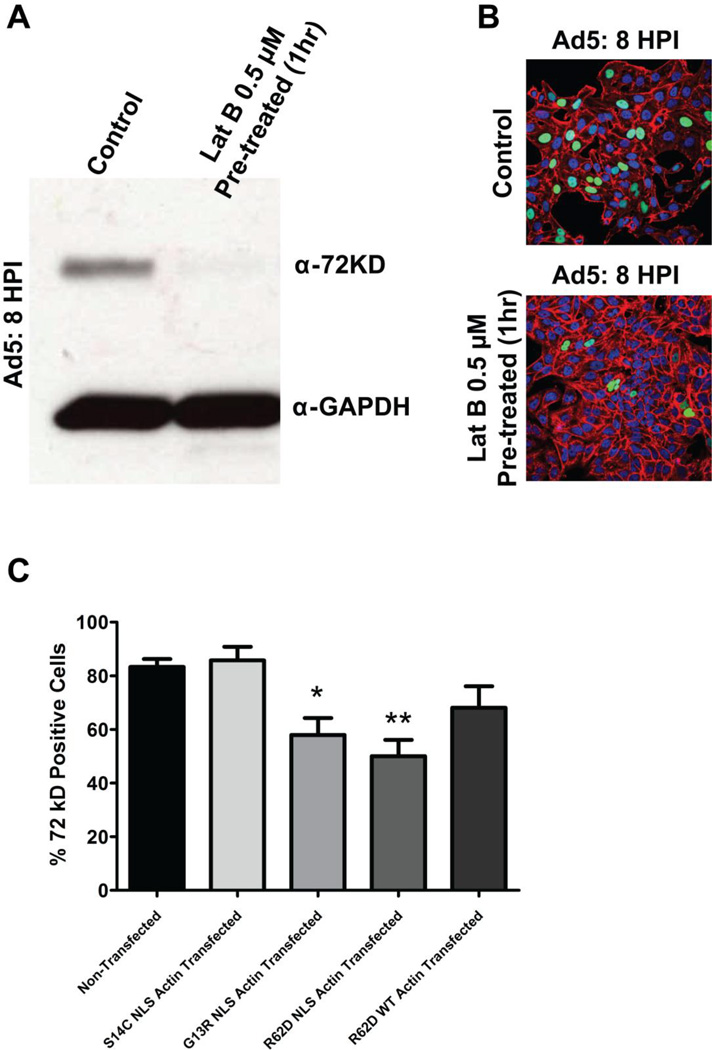
We then investigated if actin is involved in the progression of viral infection by treating cells with Latrunculin B (Lat B). Lat B binds monomeric actin with 1:1 stoichiometry and can be used to block actin polymerization both in vitro and in cells [41]. HeLa cells were treated with Lat B at the final concentration of 0.5 µM for 1 h prior to infection and subsequently washed out after infection. At 8 hpi, western blot analysis or imaging was performed to assess expression of 72 kD protein as a measure of replication potential. We find that the expression of 72 kD protein was dramatically lowered in cells pretreated with Lat B (Fig. 7A). Similarly, immunostaining 8 hpi showed that fewer cells expressed the 72 kD protein when pretreated with Lat B as compared to control cells (Fig. 7B).
Figure 7.
Depolymerizing actin decreases Ad5 replication. (A) HeLa cells were treated with latrunculin B (Lat B; 0.5 mM) for 1 h prior to infection, infected with Ad5 wt (1000 VP/cell), washed out, and extracted in boiling SDS at 8 hpi. A western blot analysis was performed to assess expression of 72 kD protein and GAPDH (loading control). (B) HeLa cells were treated with latrunculin B (Lat B; 0.5 µM) for 1 h prior to infection, infected with Ad5 wt (1000 VP/cell), and washed out. Cells fixed at 8 hpi were stained with anti-72 kD antibody (green), rhodamine phalloidin (red) to mark the F-actin and DAPI (blue). Few cells stain with the anti-72 kD protein antibody when cells are pretreated with Lat B (bottom panel). (C) HeLa cells transfected with indicated constructs for 24 hours were subsequently infected with Ad5 wt (1000 VP/cell) and analyzed at 21 hpi. The percentage of cells expressing 72 kD protein in each condition was scored. Values are means +SEM. Expression of the nuclear actin mutants R26D NLS and G13R NLS that are resistant to polymerization significantly reduced Ad5 replication. *P < 0.05, **P < 0.01, 1way ANOVA, N≥3 independent experiments, > 275 cells/group counted.
To confirm the effects of Lat B and delineate the specific impact of nuclear from cytoplasmic actin polymerization, we transfected cells with constructs encoding actin mutants that resist polymerization (R62D, G13R) or that favor F-actin assembly (S14C) [42, 43] and targeted the mutant actins to the nucleus by attaching a nuclear localization signal (NLS). Cells were infected with Ad5 wt (1000 VP/cell) 24 hours after transfection, fixed at 21 hpi, stained with anti-72 kD and imaged using confocal microscopy. Figure 7C shows the percentage of replication competent cells as gauged by 72 kD expression in each condition. Expression of R26D NLS actin and G13R NLS actin, which retard polymerization, significantly reduced the number of cells undergoing replication. In contrast, the S14C NLS mutant that favors actin polymerization had no effect, suggesting the polymerization of actin is necessary for Ad5 replication. The cytoplasmic R62D actin mutant (without a NLS) also decreased the number of cells with 72 kD staining, but the effect did not reach statistical significance. Thus, Ad5 infection not only depends on cytoplasmic actin dynamics, but actin polymerization also seems to be needed in the nucleus. Surprisingly, cells overexpressing NMI or a mutant NMI with a lower affinity for actin (E407V) [5] or treatment with a potential myosin VI inhibitor [44] did not significantly change 72 kDA expression (not shown).
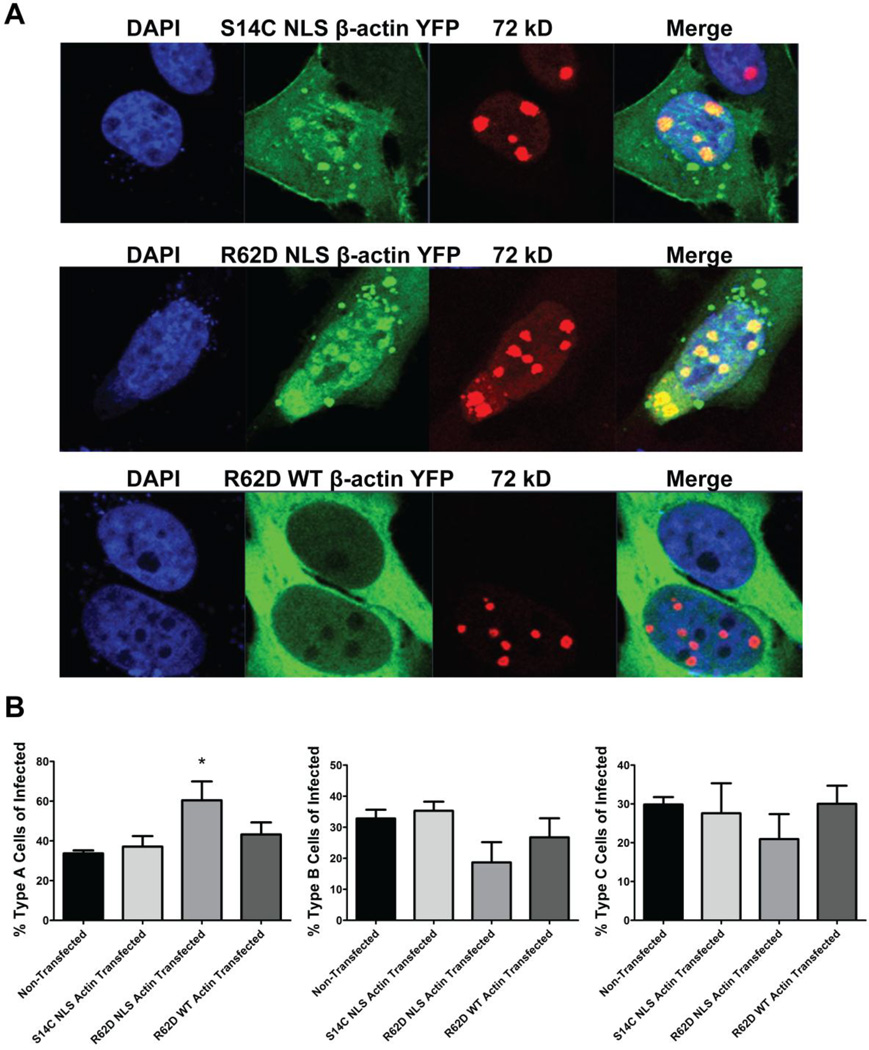
To further analyze the effect of nuclear actin polymerization on the progression of viral infection, we examined the localization of the mutant forms of actin with respect to the viral DNA centers (Fig. 8A). While expression of R62D actin without a NLS showed minimal accumulation in the nucleus and at viral DNA centers, both nuclear actin mutants (S14C NLS and R62D NLS) colocalized with 72 kD at Type B viral replication centers similar to endogenous actin (Fig. 4). Despite this co-localization, we find that the percentage of Ad infected cells with nuclear morphology typical for the late phase of Ad infection (Type B and C; Fig. 8B) was reduced in cells expressing R62D NLS actin (Fig. 8B). Again, the R62D actin mutant without a NLS had less of an effect, and expression of S14C NLS actin had no effect. Correspondingly, expressing R62D NLS actin resulted in a significant increase in the number of cells with nuclear morphology typical for the early phase of Ad infection (Type A; Fig. 8B). These results suggest the expression of the nuclear form of actin that impedes polymerization blocks progression of Ad-infected cells through the intermediate stage of viral infection.
Figure 8.
Effects of nuclear targeted, actin polymerization mutants on Ad5 infection. (A) Localization of mutant forms actin with respect to the viral DNA centers. HeLa cells transfected with the indicated constructs for 24 hours were subsequently infected with Ad5 wt (1000 VP/cell), fixed at 21 hpi, labeled with anti-72 kD antibody and DAPI, and analyzed by confocal microscopy. Both nuclear actin mutants S14C NLS and R62D NLS co-localized with the 72 kD protein in nuclei with the morphology of Type B viral DNA centers. (B) Nuclear actin polymerization is necessary for the progression of the viral infection. The percentage of Ad infected cells exhibiting a Type A 72 kD distribution were scored (left). Transfected cells in the late phase of infection, presenting viral replication centers as rings (initial part of the late phase/ intermediate phase, Type B, middle) or as enlarged pools of 72 kD protein (Type C, right) were also quantified. Values are means +SEM. Expression of a polymerization resistant nuclear form of actin mutant (R62D) NLS significantly increases the number of cells in the early phase and initial part of the intermediate phase of infection (Type A) and reduces the percentage of Ad infected cells transitioning to type B and C replication centers. *P < 0.05, 1 way ANOVA, N≥3 independent experiments, > 275 cells/group counted.
DISCUSSION
This study confirms that adenovirus infection causes reorganization of the major sites of transcription in the host cell. In agreement with previous observations, adenoviral subversion of the host nucleus results in the re-localization of general transcription factors and pol II to sites of viral transcription (Figs. 2 and 3). Moreover, we find the novel recruitment of nuclear actin and myosins to these sites of active transcription (Figs. 4–6). Similar to the transcription initiation complex, nuclear actin and myosins associate with specific viral replication centers (classified here as Type B) that are formed in the nuclei of Ad-infected cells during the onset of the late phase of infection. The recruitment of actin and certain myosins to the virus-induced ring structures and previous studies implicating nuclear actin and myosin in transcription [13] suggest roles for these proteins in adenoviral infection. Indeed, we find disruption of both cytoplasmic and nuclear actin has deleterious effects on the ability of Ad5 to infect the host cell, as well as on the progression of late phase of Ad5 infection (Figs. 7 and 8).
Adenovirus infection has been shown to change the distribution of proteins critical for transcription and splicing such as pol II, coilin, and SP35 [10, 26]. These markers for sites of transcription and splicing reorganize from discrete nuclear bodies to the periphery of adenovirus late phase replication compartments. The observation that nuclear actin and myosins are also reorganized to these same sites highlights the importance of nuclear actin and myosins in host cell transcription and splicing. Furthermore, our finding that changes in nuclear actin polymerization are able to alter adenoviral infection confirms the importance of these proteins in the viral life cycle as well. Intriguingly, we found different nuclear myosins are recruited to different parts of viral replication centers, suggesting potentially unique functions for each myosin protein (Figs. 5 and 6). Surprisingly, neither NMI overexpression nor MVI ATPase inhibition caused significant alterations in viral replication (data not shown), however tools to probe the different structural and motor activities of the non-conventional myosins remain lacking [45, 46]. Because nuclear actin, NMI and MVI are known general transcription factors and components of the pre-initiation complex [37, 38, 47], recruitment of these proteins to sites of pol II probably facilitate transcription of the viral genome. On the other hand, we cannot exclude their potential role in viral DNA replication since both of these processes have been localized to the same centers [24]. Nuclear actin and myosins have also been associated as components of the nuclear matrix [13], suggesting that a polymerization competent form of nuclear actin may also be necessary to organize and tether the viral replication compartments to the nuclear matrix (Fig. 3B). The distribution of the nuclear myosin proteins using SIM microscopy hints at the potential for the nuclear myosins to be serving as structural proteins, tethering the components of the viral replication compartments in place (Figs. 5C, 6B, 6D). However, it remains unclear if the interaction between nuclear actin and myosins is a novel method to help organize viral nuclear compartments and/or if these proteins are recruited as a part of the host cell transcription complex.
Our finding that nuclear actin and myosins are involved in adenovirus infection joins a growing list of studies implicating these proteins in the life cycle of viruses that replicate in the nucleus. Indeed, the manipulation of nuclear actin may be a conserved mechanism, since multiple nuclear replicating viruses require nuclear actin and myosins to different extents. Previous studies have shown that baculovirus can manipulate nuclear actin polymerization via Arp2/3 to infiltrate the nucleus [1], and herpes virus may also manipulate nuclear actin polymerization for infection and replication [3–5]. Our results suggest nuclear actin and myosins may be involved in the subversion of transcription from the host to the viral genome, but how adenoviruses regulate nuclear actin and myosins remains unknown. Previous studies have shown that adenovirus is able to actively subvert the host’s actin network. Work in the Ad2 serotype has shown that E4orf4 protein is able to bind Rho GTPases to regulate actin dynamics in the cytoplasm [48]. Because many of the same actin binding proteins, including WASP, Rho, and Rac1, have been shown to regulate actin dynamics in the nucleus as well [22, 49, 50], it is possible that viral proteins regulate nuclear actin dynamics. Also intriguing is the ability of actin to serve as both a substrate and as a co-factor along with viral DNA to activate the adenovirus protease in the late stages of infection [51]. Future studies will be needed to discern how adenoviruses are able to subvert nuclear actin and myosins.
Given the importance of adenoviruses in respiratory disease progression and gene delivery advances, understanding how this virus replicates has significant benefits. Our finding that nuclear actin and myosins are important factors in the infection cycle of adenoviruses provides insights into the necessity of these proteins for both adenoviral infection as well as mammalian cell function.
MATERIALS AND METHODS
Cell culture and adenovirus infection
HeLa cell monolayer cultures were maintained in Dulbecco's modified minimum essential medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), penicillin and streptomycin in a humidified atmosphere containing 5% CO2 at 37°C. Subconfluent cells were infected with wild-type human Adenovirus, Type 5 (Ad5 wildtype, ATCC, catalog No. VR-1516) at a multiplicity of infection of 120 infection units (IFU) per cell (1000 viral particles (VP)/cell). Briefly, cells were washed once with DMEM supplemented with 2% (v/v) FBS and inoculated with virus in DMEM with 2% FBS. During the incubation for 1 h cells were agitated four to five times. Afterwards, the medium was removed and replaced by fresh medium supplemented with 10% serum. Cells were incubated at 37°C until the time for fixation and processing for immunofluorescence.
Antibodies
The following primary antibodies were used: the monoclonal antibody B610 against the viral 72-kDa (72K) DNA binding protein to identify viral replication centers (kindly provided by Dr. Arnold J. Levine, Institute for Advanced Study) [52], the monoclonal antibody to β-actin produced in mouse (clone AC-15, ascites fluid, Sigma Aldrich), the mouse monoclonal antibody 2G2 against actin [53] (kindly provided by Dr. Brigitte Jockusch, Technical University of Braunschweig, Germany), the mouse monoclonal anti-BrdU antibody (clone BMC 9318, Roche) used to detect nascent RNA following Br-UTP incorporation, the rabbit polyclonal antibody specific for TBP (Santa Cruz Biochemical), the mouse monoclonal H14 antibody to phosphorylated serine 5 of the C-terminal domain of pol II (Covance) or the polyclonal pan-Pol II antibody (n-20, Santa Cruz), the rabbit polyclonal anti-TFIIB antibody (Santa Cruz), the rabbit polyclonal anti-TFIIF antibody (Santa Cruz), rhodamine phalloidin (Cytoskeleton Inc.), polyclonal myosin V antibody (kindly provided by Dr. Paul C. Bridgman, Washington University), polyclonal myosin VI antibody (KA-15, Sigma Aldrich) and the rabbit anti-nuclear myosin I (NMI) antibody developed in our laboratory. Secondary antibodies used for immunostaining were goat anti-mouse IgG (H+L), IgG (Fc), or IgM and anti-rabbit IgG conjugated with 488, FITC, Texas Red, Cy2, Cy3, or Cy5 (Jackson ImmunoResearch Laboratories). Antibody validation and immunofluorescence controls can be found in Figure S1 and Table S1.
Immunofluorescence
Ad5 wildtype and mock infected HeLa cells were grown on glass coverslips and harvested at various times post-infection. The cells were washed twice in phosphate buffered saline (PBS) and subsequently fixed in 4% paraformaldehyde in PBS for 15 min at room temperature. After fixation, the cells were permeabilized with 0.5% Triton X-100 in PBS for 15 min at room temperature. Alternatively, the cells were washed twice with PBS, and permeabilized and fixed simultaneously in 4% paraformaldehyde in PBS containing 0.5% Triton X-100 for 15 min at room temperature. Free aldehydes were quenched by incubation with 25 mM glycine in PBS for 15 min at room temperature. After fixation and permeabilization, the cells were rinsed three times in PBS, blocked in PBS containing 1% bovine serum albumin (BSA) and 0.2% fish skin gelatin (FSG) for 20 min, incubated for 1 h with primary antibodies diluted in PBS containing 1% BSA and 0.2% FSG, washed three times for 5 min in PBS containing 1% BSA and 0.2% FSG and incubated with secondary antibodies for 45 min. Alternatively, primary antibodies were fluorescently labeled with Zenon®-labeling technology (Life Technologies) using Zenon® Tricolor Mouse IgG1 Labeling Kit 1 (For Green, Orange and Deep Red Fluorescence Imaging) according to the manufacturer’s instructions. DNA was stained with DAPI (0.07 µg/ml in PBS for 5 min) and the samples were mounted in ProLong® Antifade mounting medium (Life Technologies).
Visualization of transcription sites
The labeling of nascent transcripts with BrUTP was performed as described previously [54]. In brief, cells were lysed by addition of saponin (final concentration 0.25 mg/ml; S-4521; Sigma) in PB-BSA (physiological buffer: 100 mM potassium acetate, 30 mM KCl, 10 mM Na2HPO4, 1 mM MgCl2, 1 mM Na2ATP, 1 mM DTT, and 0.2 mM PMSF, pH 7.4 [34] supplemented with 100 mg/ml BSA and 10 U/ml human placental RNAse inhibitor) for 5 min, rinsed twice by PB-BSA, and preincubated in PB-BSA (5 min, 35°C). Transcription was initiated by adding a transcription mixture to give final concentrations of 100 µM of ATP, CTP, GTP, BrUTP, and 0.4 mM MgCl2 in PB-BSA and incubated for 10 min at 35°C to allow incorporation. To stop the reaction cells were rinsed by ice-cold PB-BSA and immediately fixed in 4% PFA in PBS supplemented with 0.5% Triton X-100 for 15 min. BrRNA was immunolabeled using the mouse monoclonal anti-BrdU antibody. The samples were further processed for immunofluorescence as described above.
Confocal and SIM microscopy
Immunofluorescence samples were examined using a Zeiss LSM 510 or 710 Laser Scanning confocal microscope. The systems were carefully tested for the overlap of optical channels (Figure S1). Image files were processed with Adobe Photoshop or Zen software and the profile plots of fluorescence intensity peaks along profiles spanning the viral DNA center were examined using Image J software. SIM microscopy was performed using a Nikon N-SIM microscope at the UIC confocal microscopy center.
Supplementary Material
HIGHLIGHTS.
Adenovirus infection causes dramatic changes in the host’s transcription machinery
Nuclear actin is recruited to viral centers during the late phase of infection
Nuclear myosin I is recruited with nuclear actin to viral replication centers
Nuclear myosin V and VI are recruited to distinctive regions of viral centers
Cytoplasmic and nuclear actin polymerization are necessary for Ad replication
ACKNOWLEDGMENTS
We thank the UIC Research Resource Center for their help with imaging. This work was funded by the Chicago Biomedical Consortium with support from the Searle Funds at The Chicago Community Trust (to PdeL, Co-PI) and by National Institutes of Health GM80587 to PdeL, as well as an American Heart Association Pre-Doctoral Fellowship (13PRE17050060), a Chicago Biomedical Consortium Scholar award and a UIC Chancellor’s Graduate Research Fellowship to L.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Goley ED, Ohkawa T, Mancuso J, Woodruff JB, D'Alessio JA, Cande WZ, Volkman LE, Welch MD. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-like protein. Science. 2006;314:464–467. doi: 10.1126/science.1133348. [DOI] [PubMed] [Google Scholar]
- 2.Volkman LE. Baculovirus infectivity and the actin cytoskeleton. Current drug targets. 2007;8:1075–1083. doi: 10.2174/138945007782151379. [DOI] [PubMed] [Google Scholar]
- 3.Forest T, Barnard S, Baines JD. Active intranuclear movement of herpesvirus capsids. Nat Cell Biol. 2005;7:429–431. doi: 10.1038/ncb1243. [DOI] [PubMed] [Google Scholar]
- 4.Feierbach B, Piccinotti S, Bisher M, Denk W, Enquist LW. Alpha-herpesvirus infection induces the formation of nuclear actin filaments. PLoS Pathog. 2006;2:e85. doi: 10.1371/journal.ppat.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang L, Godinez WJ, Kim IH, Tektonidis M, de Lanerolle P, Eils R, Rohr K, Knipe DM. Herpesviral replication compartments move and coalesce at nuclear speckles to enhance export of viral late mRNA. Proc Natl Acad Sci U S A. 2011;108:E136–E144. doi: 10.1073/pnas.1103411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts KL, Baines JD. Myosin Va enhances secretion of herpes simplex virus 1 virions and cell surface expression of viral glycoproteins. J Virol. 2010;84:9889–9896. doi: 10.1128/JVI.00732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann W, Reichart B, Ewald A, Muller E, Schmitt I, Stauber RH, Lottspeich F, Jockusch BM, Scheer U, Hauber J, Dabauvalle MC. Cofactor requirements for nuclear export of Rev response element (RRE)- and constitutive transport element (CTE)-containing retroviral RNAs. An unexpected role for actin. J Cell Biol. 2001;152:895–910. doi: 10.1083/jcb.152.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura T, Hashimoto I, Yamamoto A, Nishikawa M, Fujisawa JI. Rev-dependent association of the intron-containing HIV-1 gag mRNA with the nuclear actin bundles and the inhibition of its nucleocytoplasmic transport by latrunculin-B. Genes Cells. 2000;5:289–307. doi: 10.1046/j.1365-2443.2000.00326.x. [DOI] [PubMed] [Google Scholar]
- 9.Gedge LJ, Morrison EE, Blair GE, Walker JH. Nuclear actin is partially associated with Cajal bodies in human cells in culture and relocates to the nuclear periphery after infection of cells by adenovirus 5. Exp Cell Res. 2005;303:229–239. doi: 10.1016/j.yexcr.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 10.James NJ, Howell GJ, Walker JH, Blair GE. The role of Cajal bodies in the expression of late phase adenovirus proteins. Virology. 2010;399:299–311. doi: 10.1016/j.virol.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Rebelo L, Almeida F, Ramos C, Bohmann K, Lamond AI, Carmo-Fonseca M. The dynamics of coiled bodies in the nucleus of adenovirus-infected cells. Mol Biol Cell. 1996;7:1137–1151. doi: 10.1091/mbc.7.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues SH, Silva NP, Delicio LR, Granato C, Andrade LE. The behavior of the coiled body in cells infected with adenovirus in vitro. Mol Biol Rep. 1996;23:183–189. doi: 10.1007/BF00351167. [DOI] [PubMed] [Google Scholar]
- 13.de Lanerolle P, Serebryannyy L. Nuclear actin and myosins: life without filaments. Nat Cell Biol. 2011;13:1282–1288. doi: 10.1038/ncb2364. [DOI] [PubMed] [Google Scholar]
- 14.Kapoor P, Shen X. Mechanisms of nuclear actin in chromatin-remodeling complexes. Trends Cell Biol. 2014;24:238–246. doi: 10.1016/j.tcb.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chuang CH, Carpenter AE, Fuchsova B, Johnson T, de Lanerolle P, Belmont AS. Long-range directional movement of an interphase chromosome site. Curr Biol. 2006;16:825–831. doi: 10.1016/j.cub.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 16.Dundr M, Ospina JK, Sung MH, John S, Upender M, Ried T, Hager GL, Matera AG. Actin-dependent intranuclear repositioning of an active gene locus in vivo. J Cell Biol. 2007;179:1095–1103. doi: 10.1083/jcb.200710058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Percipalle P, Jonsson A, Nashchekin D, Karlsson C, Bergman T, Guialis A, Daneholt B. Nuclear actin is associated with a specific subset of hnRNP A/B-type proteins. Nucleic Acids Res. 2002;30:1725–1734. doi: 10.1093/nar/30.8.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krauss SW, Chen C, Penman S, Heald R. Nuclear actin and protein 4.1: essential interactions during nuclear assembly in vitro. Proc Natl Acad Sci U S A. 2003;100:10752–10757. doi: 10.1073/pnas.1934680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon DN, Zastrow MS, Wilson KL. Direct actin binding to A- and B-type lamin tails and actin filament bundling by the lamin A tail. Nucleus. 2010;1:264–272. doi: 10.4161/nucl.1.3.11799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holaska JM, Wilson KL. An emerin"proteome": purification of distinct emerin-containing complexes from HeLa cells suggests molecular basis for diverse roles including gene regulation, mRNA splicing, signaling, mechanosensing, and nuclear architecture. Biochemistry. 2007;46:8897–8908. doi: 10.1021/bi602636m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro-Lerida I, Pellinen T, Sanchez SA, Guadamillas MC, Wang Y, Mirtti T, Calvo E, Del Pozo MA. Rac1 nucleocytoplasmic shuttling drives nuclear shape changes and tumor invasion. Dev Cell. 2015;32:318–334. doi: 10.1016/j.devcel.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Verboon JM, Rincon-Arano H, Werwie TR, Delrow JJ, Scalzo D, Nandakumar V, Groudine M, Parkhurst SM. Wash interacts with lamin and affects global nuclear organization. Curr Biol. 2015;25:804–810. doi: 10.1016/j.cub.2015.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pombo A, Ferreira J, Bridge E, Carmo-Fonseca M. Adenovirus replication and transcription sites are spatially separated in the nucleus of infected cells. EMBO J. 1994;13:5075–5085. doi: 10.1002/j.1460-2075.1994.tb06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bridge E, Pettersson U. Nuclear organization of adenovirus RNA biogenesis. Exp Cell Res. 1996;229:233–239. doi: 10.1006/excr.1996.0365. [DOI] [PubMed] [Google Scholar]
- 26.Bridge E, Xia DX, Carmo-Fonseca M, Cardinali B, Lamond AI, Pettersson U. Dynamic organization of splicing factors in adenovirus-infected cells. J Virol. 1995;69:281–290. doi: 10.1128/jvi.69.1.281-290.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gama-Carvalho M, Condado I, Carmo-Fonseca M. Regulation of adenovirus alternative RNA splicing correlates with a reorganization of splicing factors in the nucleus. Experimental cell research. 2003;289:77–85. doi: 10.1016/s0014-4827(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 28.Puvion-Dutilleul F, Bachellerie JP, Visa N, Puvion E. Rearrangements of intranuclear structures involved in RNA processing in response to adenovirus infection. J Cell Sci. 1994;107(Pt 6):1457–1468. doi: 10.1242/jcs.107.6.1457. [DOI] [PubMed] [Google Scholar]
- 29.Bridge E, Mattsson K, Aspegren A, Sengupta A. Adenovirus early region 4 promotes the localization of splicing factors and viral RNA in late-phase interchromatin granule clusters. Virology. 2003;311:40–50. doi: 10.1016/s0042-6822(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 30.Puvion-Dutilleul F, Puvion E. Replicating single-stranded adenovirus type 5 DNA molecules accumulate within well-delimited intranuclear areas of lytically infected HeLa cells. Eur J Cell Biol. 1990;52:379–388. [PubMed] [Google Scholar]
- 31.Puvion-Dutilleul F, Puvion E. Analysis by in situ hybridization and autoradiography of sites of replication and storage of single- and double-stranded adenovirus type 5 DNA in lytically infected HeLa cells. J Struct Biol. 1990;103:280–289. doi: 10.1016/1047-8477(90)90046-f. [DOI] [PubMed] [Google Scholar]
- 32.Puvion-Dutilleul F, Roussev R, Puvion E. Distribution of viral RNA molecules during the adenovirus type 5 infectious cycle in HeLa cells. J Struct Biol. 1992;108:209–220. doi: 10.1016/1047-8477(92)90021-2. [DOI] [PubMed] [Google Scholar]
- 33.Mathews MB, Shenk T. Adenovirus virus-associated RNA and translation control. J Virol. 1991;65:5657–5662. doi: 10.1128/jvi.65.11.5657-5662.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pombo A, Jackson DA, Hollinshead M, Wang Z, Roeder RG, Cook PR. Regional specialization in human nuclei: visualization of discrete sites of transcription by RNA polymerase III. EMBO J. 1999;18:2241–2253. doi: 10.1093/emboj/18.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pranchevicius MC, Baqui MM, Ishikawa-Ankerhold HC, Lourenco EV, Leao RM, Banzi SR, dos Santos CT, Roque-Barreira MC, Espreafico EM, Larson RE. Myosin Va phosphorylated on Ser1650 is found in nuclear speckles and redistributes to nucleoli upon inhibition of transcription. Cell Motil Cytoskeleton. 2008;65:441–456. doi: 10.1002/cm.20269. [DOI] [PubMed] [Google Scholar]
- 36.Lindsay AJ, McCaffrey MW. Myosin Vb localises to nucleoli and associates with the RNA polymerase I transcription complex. Cell Motil Cytoskeleton. 2009;66:1057–1072. doi: 10.1002/cm.20408. [DOI] [PubMed] [Google Scholar]
- 37.Vreugde S, Ferrai C, Miluzio A, Hauben E, Marchisio PC, Crippa MP, Bussi M, Biffo S. Nuclear myosin VI enhances RNA polymerase II-dependent transcription. Mol Cell. 2006;23:749–755. doi: 10.1016/j.molcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann WA, Vargas GM, Ramchandran R, Stojiljkovic L, Goodrich JA, de Lanerolle P. Nuclear myosin I is necessary for the formation of the first phosphodiester bond during transcription initiation by RNA polymerase II. J Cell Biochem. 2006;99:1001–1009. doi: 10.1002/jcb.21035. [DOI] [PubMed] [Google Scholar]
- 39.Sahlas DJ, Milankov K, Park PC, De Boni U. Distribution of snRNPs, splicing factor SC-35 and actin in interphase nuclei: immunocytochemical evidence for differential distribution during changes in functional states. J Cell Sci. 1993;105(Pt 2):347–357. doi: 10.1242/jcs.105.2.347. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez-Garcia LF, Green SR, Mathews MB, Spector DL. Organization of the double-stranded RNA-activated protein kinase DAI and virus-associated VA RNAI in adenovirus-2-infected HeLa cells. J Cell Sci. 1993;106(Pt 1):11–22. doi: 10.1242/jcs.106.1.11. [DOI] [PubMed] [Google Scholar]
- 41.Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- 42.Posern G, Miralles F, Guettler S, Treisman R. Mutant actins that stabilise F-actin use distinct mechanisms to activate the SRF coactivator MAL. EMBO J. 2004;23:3973–3983. doi: 10.1038/sj.emboj.7600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posern G, Sotiropoulos A, Treisman R. Mutant actins demonstrate a role for unpolymerized actin in control of transcription by serum response factor. Mol Biol Cell. 2002;13:4167–4178. doi: 10.1091/mbc.02-05-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heissler SM, Selvadurai J, Bond LM, Fedorov R, Kendrick-Jones J, Buss F, Manstein DJ. Kinetic properties and small-molecule inhibition of human myosin-6. FEBS Lett. 2012;586:3208–3214. doi: 10.1016/j.febslet.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bond LM, Tumbarello DA, Kendrick-Jones J, Buss F. Small-molecule inhibitors of myosin proteins. Future Med Chem. 2013;5:41–52. doi: 10.4155/fmc.12.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartman MA, Finan D, Sivaramakrishnan S, Spudich JA. Principles of unconventional myosin function and targeting. Annu Rev Cell Dev Biol. 2011;27:133–155. doi: 10.1146/annurev-cellbio-100809-151502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmann WA, Stojiljkovic L, Fuchsova B, Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA, Lessard JL, Hope TJ, Hozak P, de Lanerolle P. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. Nat Cell Biol. 2004;6:1094–1101. doi: 10.1038/ncb1182. [DOI] [PubMed] [Google Scholar]
- 48.Robert A, Smadja-Lamere N, Landry MC, Champagne C, Petrie R, Lamarche-Vane N, Hosoya H, Lavoie JN. Adenovirus E4orf4 hijacks rho GTPase-dependent actin dynamics to kill cells: a role for endosome-associated actin assembly. Mol Biol Cell. 2006;17:3329–3344. doi: 10.1091/mbc.E05-12-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X, Yoo Y, Okuhama NN, Tucker PW, Liu G, Guan JL. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat Cell Biol. 2006;8:756–763. doi: 10.1038/ncb1433. [DOI] [PubMed] [Google Scholar]
- 50.Staus DP, Weise-Cross L, Mangum KD, Medlin MD, Mangiante L, Taylor JM, Mack CP. Nuclear RhoA signaling regulates MRTF-dependent SMC-specific transcription. Am J Physiol Heart Circ Physiol. 2014;307:H379–H390. doi: 10.1152/ajpheart.01002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown MT, McBride KM, Baniecki ML, Reich NC, Marriott G, Mangel WF. Actin can act as a cofactor for a viral proteinase in the cleavage of the cytoskeleton. J Biol Chem. 2002;277:46298–46303. doi: 10.1074/jbc.M202988200. [DOI] [PubMed] [Google Scholar]
- 52.Reich NC, Sarnow P, Duprey E, Levine AJ. Monoclonal antibodies which recognize native and denatured forms of the adenovirus DNA-binding protein. Virology. 1983;128:480–484. doi: 10.1016/0042-6822(83)90274-x. [DOI] [PubMed] [Google Scholar]
- 53.Gonsior SM, Platz S, Buchmeier S, Scheer U, Jockusch BM, Hinssen H. Conformational difference between nuclear and cytoplasmic actin as detected by a monoclonal antibody. J Cell Sci. 1999;112(Pt 6):797–809. doi: 10.1242/jcs.112.6.797. [DOI] [PubMed] [Google Scholar]
- 54.Fuchsova B, Novak P, Kafkova J, Hozak P. Nuclear DNA helicase II is recruited to IFN-alpha-activated transcription sites at PML nuclear bodies. J Cell Biol. 2002;158:463–473. doi: 10.1083/jcb.200202035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.