Abstract
Background
Critically ill patients appear to be at high risk of developing deep vein thrombosis (DVT) and pulmonary embolism during their stay in the intensive care unit (ICU). However, little is known about the clinical course of venous thromboembolism in the ICU setting. We therefore evaluated, through a systematic review of the literature, the available data on the impact of a diagnosis of DVT on hospital and ICU stay, duration of mechanical ventilation and mortality in critically ill patients. We also tried to determine whether currently adopted prophylactic measures need to be revised and improved in the ICU setting.
Materials and methods
MEDLINE and EMBASE databases were searched up to week 4 of June 2012. Two reviewers selected studies and extracted data. Pooled results are reported as relative risks and weighted mean differences and are presented with 95% confidence intervals (CI).
Results
Seven studies for a total of 1,783 patients were included. A diagnosis of DVT was frequent in these patients with a mean rate of 12.7% (95% CI: 8.7–17.5%). DVT patients had longer ICU and hospital stays compared to those without DVT (7.28 days; 95% CI: 1.4–13.15; and 11.2 days; 95% CI: 3.82–18.63 days, respectively). The duration of mechanical ventilation was significantly increased in DVT patients (weighted mean difference: 4.85 days; 95% CI: 2.07–7.63). DVT patients had a marginally significant increase in the risk of hospital mortality (relative risk 1.31; 95% CI: 0.99–1.74; p=0.06), and a not statistically significant increase in the risk of ICU mortality (RR 1.64; 95% CI: 0.91–2.93; p=0.10).
Conclusions
A diagnosis of DVT upon ICU admission appears to affect clinically important outcomes including duration of ICU and hospital stay and hospital mortality. Larger, prospective studies are warranted.
Keywords: deep vein thrombosis, critically ill patients, outcomes, meta-analysis
Introduction
Critically ill patients are at high risk of developing venous thromboembolism (VTE) during their stay in the intensive care unit (ICU) because of the presence of several risk factors including premorbid medical and surgical conditions, invasive tests and treatments, prolonged immobility (often exacerbated by sedation or paralysis), vascular injury from indwelling central venous catheters, and acute and chronic renal insufficiency1. In addition, critical illness activates the coagulation cascade which may mediate the increased likelihood of VTE2–5. Thus, even when adequate antithrombotic prophylaxis is used, deep vein thrombosis (DVT) may occur in up to 10% of patients6. VTE in critically ill patients may be associated with significant morbidity, including prolonged requirement for ICU and hospital stay and, potentially, increased mortality7.
The diagnosis of DVT and pulmonary embolism (PE) in critically ill patients is, for many aspects, problematic. The performance and interpretation of diagnostic tests for VTE, in particular for PE, in such patients is not well defined: critically ill patients frequently receive mechanical ventilation which reduces the utility of ventilation-perfusion lung scanning, they often have impaired renal function which may preclude intravenous contrast-based testing such as venography or computed tomography pulmonary angiography, the use of pulmonary end-expiratory pressure may cause “false positive” venous ultrasounds, while large central venous catheters may impede blood flow and simulate a thrombus7.
Recent studies suggest that the prevalence of DVT on admission to a medical-surgical ICU may be as high as 10%, and that the incidence of DVT developing during the ICU stay (based on systematic screening) ranges from 8% to 40% 6. These patients have an increased risk of developing PE. Unfortunately, only a few studies have systematically evaluated the incidence of PE in medical-surgical patients with DVT. In a recent, relatively small study on ICU patients, 50% of patients with proximal lower limb DVT and 20% of patients with symptomatic upper limb venous thrombosis have asymptomatic PE at presentation8.
Although multiple criteria for the detection of early DVT have been developed, there is no consensus on the best method of screening and prevention in critically ill patients.
In contrast to the extensive documentation on the short and long-term outcomes of patients with DVT evaluated in other clinical settings, little is known about the clinical course of this disease in the ICU setting. The occurrence of DVT in ICU seems to be a prognostic marker of severity, although its causal relationship with the outcome is not well defined according to available studies. A comprehensive review of data could provide information to support the improvement of prophylactic measures, whether medical and/or mechanical, in order to avoid this frequent complication. Thus, to acquire additional evidence on the real impact of DVT in critically ill patients, we performed a meta-analysis of data available in the literature.
We hypothesized that both clinically undetected and clinically evident VTE would affect the prognosis of critically ill patients.
Materials and methods
Study identification
A systematic search of MEDLINE (1946 to week 4 of June 2012), EMBASE (1980 to week 27 of 2012) was performed using the search terms “DVT”, “venous thrombosis”, “venous thromboembolism”, “ICU”, “intensive care unit”, “medical-surgical ICU”, “quality of life”, “hospitalization length”, “ICU stay”, “hospitality mortality “ “duration of mechanical ventilation”, “ICU mortality”, and “morbidity” both as medical subject headings and keywords.
Study selection
Two Authors (AM and YK) independently reviewed all selected titles and abstracts. Studies were excluded if the title and/or abstract was not appropriate for the aim of the current review. Full texts were subsequently obtained for eligible studies or when the relevance of an article could not be excluded with certainty. Disagreement was resolved by consensus and by opinion of a third reviewer (FD), if necessary.
English language studies were included if they met the following criteria:
-
the primary outcomes selected were:
duration of ICU or hospital stay,
ICU or hospital mortality,
duration of mechanical ventilation;
the population consisted of adults (over 17 years of age) admitted to a medical-surgical ICU with a DVT diagnosed during the hospital stay or at admission to the ICU - patients receiving thromboprophylaxis at the time of DVT diagnosis were included in the current analysis;
the study design was a prospective/retrospective cohort study or randomised clinical trials that investigated the influence of DVT on one or more of the following outcomes: hospital stay, ICU stay, duration of mechanical ventilation, and mortality.
Studies providing outcomes without specific reference to patients with DVT were excluded.
If the required data could not be located in the published report, we attempted to obtain the necessary information by contacting the corresponding author. Studies not reporting the identified outcomes or without clear information on the analysis of the aforementioned outcomes were excluded. Studies were included when data were expressed as medians and interquartile ranges instead of means ± standard deviations. Patients with PE but without a documented DVT were excluded. The current analysis is focused mainly on DVT because PE is difficult to identify in patients in ICU. Finally, studies on patients with acute spinal cord injury or those who underwent neurosurgery were excluded. To assess the agreement between reviewers for study selection, we used the k statistic, which measures agreement beyond chance9.
Data extraction
Two investigators (AM and FD) independently extracted data from each study. Information on study characteristics, population characteristics and outcomes was extracted. Only cases of DVT were analysed. The outcomes evaluated in ICU patients with and without DVT were duration of mechanical ventilation, ICU stay and hospital stay, and total ICU and hospital mortality. Studies in which outcomes data could not be identified for extraction, and studies that evaluated hospitalised medical patients were excluded. Any disagreements between reviewers were resolved through discussion to reach consensus.
Assessment of study validity
The same two unmasked investigators independently completed the assessment of study validity. Because the use of quality scoring systems or quality scales in observational studies is controversial10, we decided to assess study quality based on the type of study (prospective or retrospective) and selection of patients (consecutive enrolment without potential bias of selection). For each item fulfilled one point was given. A total of two points defined high-quality studies; one or less defined a low-quality study.
Given the characteristics of the included studies, the methodological quality of each study was also evaluated with the Newcastle-Ottawa Scale (NOS), which is specifically developed to assess the quality of non-randomised observational studies11. The scoring system encompasses the following eight items: clear definition of study sample, selection, interventions, outcomes, adequate assessment of the outcome, analyses for comparability, adequate length of follow-up, and appropriate interpretation of results. If an item was adequately addressed, 1 point each was awarded for the first seven specific items and 2 points for analyses for comparability. This results in a quality score between 0 and 9.
Data analysis
The weighted mean proportion of the rate of DVT (prevalence plus incidence) was calculated using a random-effects model. Associations between the presence of DVT and ICU and hospital mortality and the mean difference of duration of mechanical ventilation and of ICU and hospital stay in ICU patients with and without DVT were calculated using a random-effects model (the Der Simionan and Laird method)12. Pooled results are reported as odds ratio (OR) and weighted mean difference (WMD) and are presented with 95% confidence interval (CI) and with two-sided probability values. A probability value of 0.05 or less was considered statistically significant.
The appropriateness of pooling data across studies was assessed using the Cochran Q and I2 test for heterogeneity, which measures the inconsistency across study results and describes the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error13. Finally, funnel plots of effect size against standard error were completed, whenever possible, to assess for the presence of publication bias14.
We used Review Manager (RevMan; version 5.0 for Windows; Oxford, England; The Cochrane Collaboration, 2008) and Stat Direct software (Version 2.7; StatsDirect Ltd, Cheshire, UK) to pool data.
Results
Study identification and selection
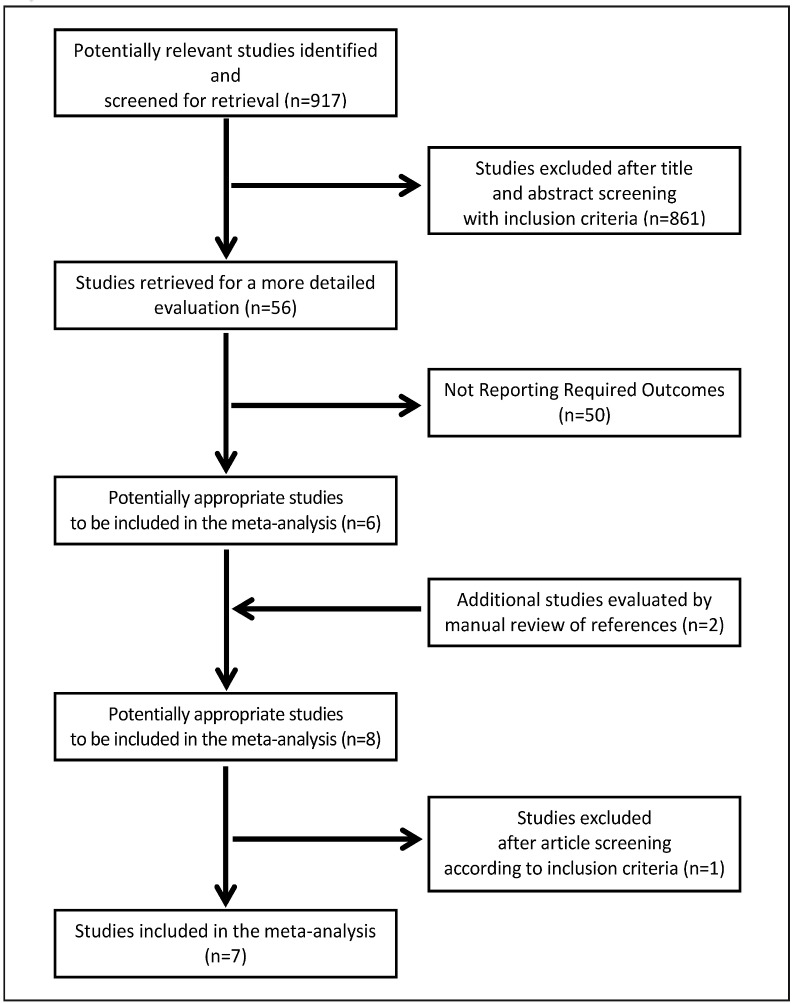
We identified 926 potentially relevant studies from the following databases: 256 from Medline and 670 from EMBASE (Figure 1). After screening the titles and abstracts, 870 were excluded using the predefined inclusion and exclusion criteria; the remaining 56 studies were retrieved for a more detailed evaluation. Manual review of references revealed two additional studies. Fifty of the 58 studies were subsequently excluded after detailed review of the full texts, leaving eight potentially appropriate articles15–23. One study was subsequently excluded since data were not presented in a manner that allowed extraction15–23. Therefore, seven studies were included in the current meta-analysis. Inter-observer agreement for study selection was good, with a k value of 0.74.
Figure 1.
Study progression selection.
Study characteristics
Of the seven included studies, five were prospective17–20,22, one was retrospective21 and one study was both prospective and retrospective16. Results of the study quality assessment according to the NOS are reported in Table I. The characteristics of the included studies are shown in Table II. DVT was diagnosed using Doppler ultrasonography (DUS) in three studies20–22, compression ultrasound (CUS) in three studies16–18 and in one study it was diagnosed using CUS and/or venography19. In all the evaluated studies, screening for DVT was done in all patients irrespectively of clinical symptoms or suspicion. In two studies15,19 routine screening of the lower extremities by CUS was performed within 48 hours of admission to the ICU, in four studies17,18,20,22 it was done every 7 days or sooner, clinically indicated and in one study16 screening was performed twice weekly. The study by Major et al. was excluded from this analysis since the presence of DVT was not systematically determined in the entire study population. PE was diagnosed using pulmonary angiography in three studies19,20,22 ventilation/perfusion lung scan in two studies19–22 and computed tomography pulmonary angiography in two other studies19,20. In contrast to the screening for DVT, these investigations were performed only when there was a clinical suspicion of PE. All seven studies evaluated the efficacy of thromboprophylaxis; unfractionated heparin (UFH) was used in four studies19–22, low molecular weight heparin (LMWH) in three studies17,20,22, UFH and LMWH in one study19 and a sequential compression device (SCD) in six studies16–18,20–22 (Table II). In all these studies, prophylaxis was started on admission to the ICU and continued throughout the ICU stay (thromboprophylaxis was primarily pharmacological, including UFH and LMWH, mechanical prophylaxis included pneumatic compression devices and anti-embolic stockings). The dosage and timing of prophylaxis were not clearly reported in all the analysed studies and it did not allow a specific analysis of this intervention.
Table I.
New Castle Ottawa Scale score of the analysed studies.
Table II.
Study characteristics.
| Study design | Methods used for DVT diagnosis | Methods used for PE diagnosis | Number of patients lost to follow-up or withdrawn | Thromboprophylaxis | Dosage and timing | Number of patients in each study | Timing of DVT/PE screening tests during ICU stay | |
|---|---|---|---|---|---|---|---|---|
| Ibrahim21 (2002 ) | Retrospective | CUS/DUS | n/a | n/a | UFH/SCD | 5,000 IU s.c. twice/daily | 110 | Every 7 days or sooner as clinically indicated |
| Velmahos22 (1998) | Prospective | DUS femoral and popliteal | PA and/or V/Q scan | 4 with IV or IVC thrombi were excluded from final analysis | LDH/SCD bilateral | 5,000 IU every 12 or 8 hours s.c. upon ICU admission | 200 | Every 7 days or sooner as clinically indicated |
| Major20 (2003) | Prospective | DUS. at least every 7 days | PA and/or CT angiogram | 2 excluded due to starting s.c. heparin after admission to ICU: 17 died before discharge from ICU | SCD/UFH/LMWH | n/a | 726 | Every 7 days or sooner as clinically indicated |
| Cook19 (2005) | Prospective | CUS and/or venography | V/Q scan, helical CT, PA | 22 (7.8%): second admission to ICU, trauma, withdrawal of life support. 71 died in ICU, 103 died in hospital, 10 had active bleeding during the study | UFH s.c and i.v/LMWH/warfarin/ antiembolic stocking | UFH 5,000 IU s.c. twice daily | 261 | Within 48 hours of ICU admission, twice weekly, and if DVT/PE was suspected |
| Khouli18 (2006) | Prospective | CUS | n/a | 14 | UFH, SCD, UFH+SCD | 5,000 IU every 8 hours | 141 | Every 7 days or sooner as clinically indicated |
| Joynt17 (2009) | Prospective | CUS and DUS | n/a | n/a | n/a | n/a | 80 | Twice weekly |
| Boddi16 (2010) | Prospective/Retrospective | CUS | n/a | n/a | LMWH and/or elastic compression stocking | 5,000 IU once daily | 375 | Within 48 hours of ICU admission, and twice weekly until discharge |
DVT: deep vein thrombosis; PE: pulmonary embolism; ICU: Intensive Care Unit; CUS: compression ultrasound; DUS: Doppler ultrasound; n/a: not available; UFH: unfractionated heparin; SCD: sequential compression device; IU: International Unit; PA: pulmonary angiography; V/Q: ventilation/perfusion; IV: iliac vein; IVC: inferior vena cava; LDH: low dose heparin; CT: computed tomography; LMWH: low molecular weight heparin; s.c.: sub cutaneous; i.v.: intravenous.
Population characteristics
The number of the subjects enrolled in each study ranged from 110 to 714. The baseline characteristics of the populations are shown in Table IIIa and IIIb. Risk factors that may influence VTE risk, including underlying malignancy and APACHE II score or occurring during ICU stay (mechanical ventilation, use of inotropes/vasopressors and femoral venous catheters) were reported variably and not evaluated by all studies.
Table IIIa.
Population characteristics (1).
| Study | Sample size | Mean age (DVT vs no DVT) | Similarity between study groups | DVT vs no DVT (n.) | Apache II (DVT vs no DVT) Mean±SD | Ventilated (n.) | Isotrope vs Vasopressor (n.) |
|---|---|---|---|---|---|---|---|
| Ibrahim21 (2002) | 110 | 58.7±16.4 | Yes | 26 vs 84 | 21.5±7.5 vs 23.9±8.2 | 110 | n/a |
| Velmahos22 (1998) | 200 | 37 years, 40±15.9 vs 37±15.6 | Yes | 26 vs 174 | ISS used: 23±13 vs 21.2±10.5 | Indirectly measured by PEEP | n/a |
| Major20 (2003) | DVT vs no DVT 12/714 | 37 years, 40±15.9 vs 37±15.6 | People with DVT had elevated APACHE/SAPS | 12 vs 714 | 28.5±2.2 vs 16.4±0.3 | n/a | n/a |
| Cook19 (2005) | 261 | 66.9±15.1 | n/a | 32 vs 229 | 25.5±8.4 | 31 (96.9%) vs 199 (87.7%), p=0.22 | 9 (36%) vs 43 (19.9%) |
| Khouli18 (2006) | 141 | 6,4% (<40 years), 96,6% (>40 years) | Yes | 14 vs 127 | n/a | n/a | n/a |
| Joynt17 (2009) | 80 | 64 (18–78) vs 60 (17–79), p=0,87 | Yes | 15 vs 65 | 21 (9–29) vs 20 (11–32) | 14 (93%) vs 57 (88%) | n/a |
| Boddi16 (2010) | 378 | 61 (46,5–77,5) vs 53 (35–72) | Yes | 12 vs 245 | n/a | 14.5 (6.5–19.75) vs 3 (1.9), p=0.001 | n/a |
Table IIIb.
Population characteristics (2).
| Study | Underlying malignancy (DVT vs no DVT) (n.) | CVC (DVT vs no DVT) (n.) | Renal failure (DVT vs no DVT) (n.) | Exclusion criteria | Type of DVT | Event number |
|---|---|---|---|---|---|---|
| Ibrahim21 (2002) | 8 vs 7 | 17 vs 68 | 3 vs 1 | <18 years, <7 days mechanical ventilation/temporary ICU stay | DVT: upper limbs (5), lower limbs ( 21), calf v (3), proximal to calf (18), occlusive thrombosis (19), catheter (4). PE: 11.5% in pts with DVT. |
Prior DVT in pts with DVT: 2 (7.7%). Prior DVT in pts with no DVT: 1 (1.2%) |
| Velmahos22 (1998) | n/a | 12 (46%) vs 51 (29%) | n/a | ISS <16, pts <12 years, minor or moderate injury | DVT: 3 external Iliac, 20 common femoral, 2 superficial femoral, 23 popliteal. PE: 4 pts clinically diagnosed. |
n/a |
| Major20 (2003) | n/a | n/a | n/a | End stage liver disease, therapeutic anticoagulation prior to admission, known diagnosis of DVT, IVC filter in place prior to admission or one currently placed for PE prophylaxis | DVT and PE | n/a |
| Cook19 (2005) | n/a | 221 (84.7%) vs 83 (38.4%) | Haemodialysis: 4 (16%) vs 13 (6%) | <18 years, ICU <72 hours, pregnancy, life support withdrawal, admitted diagnosis of trauma or orthopaedic surgery | DVT: 9 right-sided, 10 left-sided, 6 bilateral | Only first |
| Khouli18 (2006) | 3/15 (20%), 11/126 (9%) | 96 | n/a | Admission diagnosis of DVT, receiving full anticoagulation, major surgical procedure, contraindications to the use of UFH and SCD | 10 proximal, 4 distal | n/a |
| Joynt17 (2009) | 1 (7%) vs 6 (9%), p=1,0 | n/a | 0 vs 3 | Pregnancy, too unstable for ultrasound, with a diagnosis of DVT | 6 proximal, 9 distal | n/a |
| Boddi16 (2010) | n/a | n/a | n/a | <18 years, recent DVT, active gastroduodenal ulcer, recent haemorrhagic stroke, liver failure, pregnancy, severe renal impairment, congenital or acquired coagulation. | Retrospective: 6 femoral-popliteal veins, 8 subpopliteal, 6 right-sided, 6 left-sided, 2 bilateral. Prospective: 10 proximal, 2 distal. |
n/a |
Data on patients with DVT are reported vs patients without DVT. Third column (mean age): numbers in brackets are the age range, results are reported as mean ± standard deviation (SD); sixth column (Apache II): results are reported as mean ± standard deviation; seventh column (ventilated): numbers in brackets are the percentages of patients.
DVT: deep vein thrombosis; vs: versus; CVC: central venous catheter; ICU: Intensive Care Unit; PE: pulmonary embolism; n/a: not available; ISS: Injury Severity Score; IVC: inferior vena cava; UFH: unfractionated heparin; SCD: sequential compression device.
A total of 139 episode of DVT were detected in the 1,163 included patients for a mean rate of 12.7% (95% CI: 8.7–17.5%). The rate of DVT was similar when we excluded studies that evaluated the presence of DVT in ICU populations not receiving pharmacological or mechanical antithrombotic prophylaxis (mean rate 12.0%; 95% CI: 7.8–16.9%).
Outcomes
Outcomes assessed during ICU stay in patients with and without DVT were tabulated; information on all relevant outcomes could not be obtained from all studies (Table IV).
Table IV.
Outcomes.
| Study | Duration of mechanical ventilation in days (DVT vs no DVT) | Duration of hospitalisation in days (DVT vs no DVT) | ICU stay in days (DVT vs no DVT) | Hospital mortality rate (DVT vs no DVT) [95% confidence interval] | ICU mortality rate (DVT vs no DVT, n.) [95% confidence intervals] |
|---|---|---|---|---|---|
| Ibrahim21(2002) | 18.9±19.7 vs 14.6±12.9, p=0.310 | 31.4±21.7 vs 27.5±18.2, p=0.375 | 18.6±14.6 vs 15.9±1.04, p=0.388 | 8.9 (34.6%) vs 26.8 (32.1), p=0.815 | n/a |
| Velmahos22 (1998) | Not given^ | 49±32 vs 31±24, p<0.05 | 34±31 vs 19±18, p<0.05 | n/a | 31% (8) vs 18% (31), p= 0.04 |
| Major20 (2003) | n/a | n/a | n/a | n/a | 17% (2) vs 2% (15) p=0.03 |
| Cook19 (2005) | 9** (4–25)* vs 6 (3–13)*, p=0.03 | 51** (24–73)* vs 23** (12–47)*, p<0.001 | 17.5** (8.5–30.5)* vs 9** (5,17)* | 17 (53.1%) vs 85 (37.4%), p=0.04 | -, 8** vs -, 62**, p=0.78 |
| Joynt18 (2006) | 4 [0–14] vs 2 [0–46], p=0.81 | 0 [0–24] vs 0 [0–57], p=0.73 | 4 [5–21] vs 3 [2–61], p=0.89 | 5 (33%) vs 18 (28%), p=0.75 | n/a |
| Khouli17 (2009) | 5.5 [2–20] vs 6 [1–36], p=0.90 | n/a | 8 [3–23] vs 8 [3–36], p=0.52 | 17% [5–51] vs 21% [10–23]; p=0.70 | n/a |
| Boddi16 (2010) | 14.5 [6.5–19.75] vs 3 [1–9], p=0.001 | n/a | 14 [9–26] vs 10 [7–29], p=0.05; 19 [13.5–30] vs 7 [4–15], p=0.001 | n/a | n/a |
The fourth column (ICU stay in days) reports values as mean ± standard deviation, data on interquartile range (*) and median (**) are reported in the last four lines. Square brackets report 95% confidence intervals (CI).
Interquartile range;
median;
necessity for ventilation measured by positive end-expiratory pressure;
-: missing value.
DVT: deep vein thrombosis; vs: versus; ICU: intensive care unit.
Duration of mechanical ventilation
Four studies evaluated the duration of mechanical ventilation9,10,19 in patients with and without DVT (84 and 670 patients, respectively).
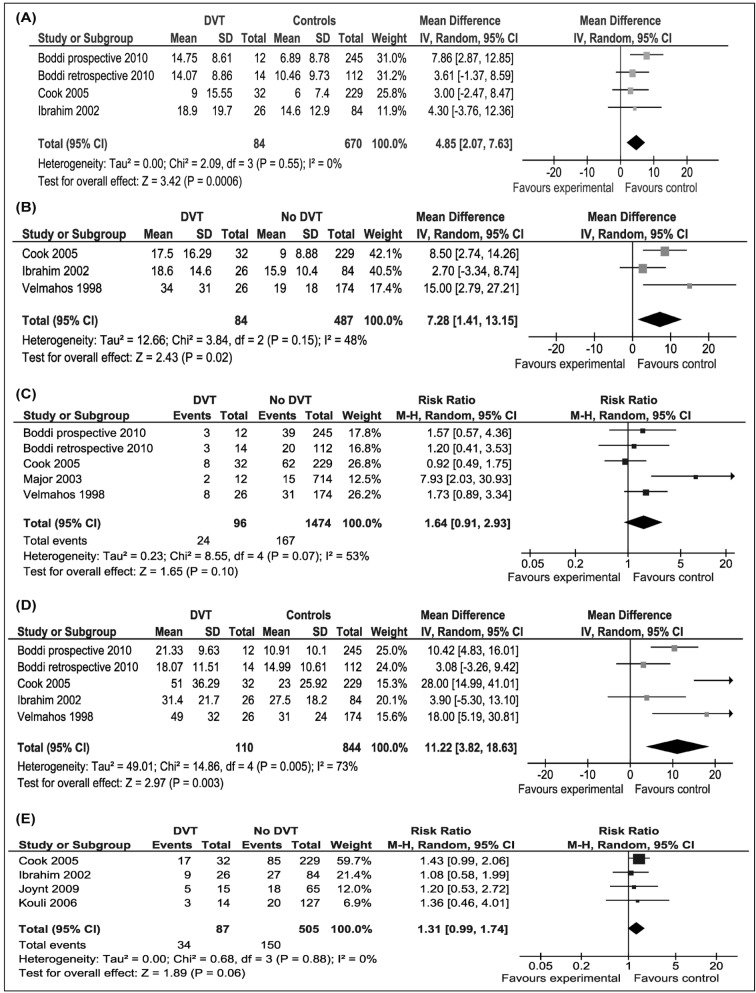
The duration of mechanical ventilation was significantly longer in patients with DVT than in those without DVT (WMD 4.85 days; 95% CI: 2.07, 7.63 days; p=0.006). There was no heterogeneity among the studies (I2=0%; p=0.55) (Figure 2A). Due to the low number of studies, funnel-plot analysis could not be done. Publication bias could not, therefore, be assessed.
Figure 2.
The impact of DVT.
(A) In the ICU on duration of mechanical ventilation; (B) in the ICU on duration of ICU stay; (C) in the ICU on ICU mortality; (D) in the ICU on duration of hospital stay; (E) in the ICU on hospital mortality. DVT: deep vein thrombosis; ICU: Intensive Care Unit.
Duration of stay in hospital and the intensive care unit
Three studies evaluated the duration of ICU stay and five studies the duration of hospital stay (84 patients with and 487 patients without DVT)12–14,18,20.
DVT patients spent a significantly longer time in the ICU than did patients without DVT (WMD 7.28 days; 95% CI: 1.41–13.15 days; p=0.02). Heterogeneity among the studies was moderate, but not significant (I2 =47.9%; p=0.15) (Figure 2B). Due to the low number of studies, funnel-plot analysis could not be done. Publication bias could not, therefore, be assessed.
Hospital stay was significantly longer in DVT patients than in patients without DVT (WMD 11.2 days; 95% CI: 3.82–18.63 days; p=0.003). Heterogeneity among the studies was significant (I2=73%; p=0.005) (Figure 2C,D). Funnel plot of WMD vs standard error appeared symmetric, suggesting absence of a publication bias.
Intensive care unit and hospital mortality
Five studies evaluated ICU mortality (96 patients with and 1474 patients without DVT)9,10,17,18. The data from the study by Boddi et al.16 were not published but were obtained on request from the Authors.
DVT patients had a non-significant increased risk of ICU mortality compared to patients without DVT (RR 1.64; 95% CI, 0.91–2.93; p=0.10). Heterogeneity among the studies was marginally significant (I2= 53%; p=0.07) (Figure 2E). Funnel plot of RR versus standard error appeared symmetric, suggesting absence of a publication bias.
Four studies evaluated hospital mortality (87 patients with and 505 patients without DVT)9,10,17,18 DVT patients had a non-significant increased risk of hospital mortality compared to patients without DVT (RR 1.31; 95% CI, 0.99–1.74; p=0.06). There was no heterogeneity among the studies (I2=0%; p=0.88) (Figure 2E). Due to the low number of studies, funnel-plot analysis could not be done. Publication bias could not, therefore, be assessed.
Discussion
Our systematic review and meta-analysis of the literature confirms that DVT is not uncommon in ICU patients. Although DVT has potentially serious consequences, it is often unrecognised among ICU patients. Unfortunately, physical examination is not useful to rule out a diagnosis of DVT in these patients24. Concern about undiagnosed DVT in the ICU is justified by studies showing that 10% to 100% of DVT identified by screening ultrasound were clinically unsuspected, and it is possible that many mechanically ventilated patients with sudden episodes of hypotension, tachycardia, or hypoxia may have undetected PE25.
In this analysis, designed to explore the impact of a diagnosis of DVT on clinically important outcomes in the ICU, we were able to pool data from seven studies for a total of about 1,800 patients who met the predefined criteria. Statistically significant differences were found in ICU and hospital stay, duration of mechanical ventilation and hospital mortality between patients or without DVT. Furthermore, hospital mortality appeared to be marginally increased in DVT patients in comparison to patients without DVT. On the other hand, although there was a trend, ICU mortality was not significantly different between the two groups. However, these apparently negative results may be explained by the relatively low number of patients included in our meta-analysis.
Our observations are important because the longer duration of stay and duration of mechanical ventilation contributes to increasing health care costs while higher rates of morbid outcomes, including duration of stay and death, are clearly of importance to patients in the ICU. Interestingly, DVT appears common in critically ill patients, despite the routine use of prophylactic measures, even when applied on admission.
The results of our systematic review may have important implications for clinical practice. In ICU patients, a DVT surveillance protocol may help in early identification of lower limb thrombi and can help in making decisions related to treatment to prevent progression to PE. However, the choice of the best screening test and the time of screening remain to be established. Venography is still considered the gold standard for the diagnosis of DVT, but this test is invasive and is not a cost-effective method for routine evaluation of DVT. Furthermore, the need for transport, positioning and dye exposure make this test problematic for most ICU patients. Ultrasonography is non-invasive and inexpensive26. However, the sensitivity of screening ultrasound for venous thrombosis in asymptomatic patients was found to be poor for both proximal and distal DVT, because it depends on the number of pathological compression manoeuvres documented in the ultrasound27.
In a prospective cohort study on ICU patients18, bilateral lower extremity compression ultrasound was performed within 48 hours of admission to the ICU, twice weekly, and if VTE was clinically suspected. In this study the authors found that the prevalence of DVT was 2.7% on ICU admission, and the incidence was 9.6% during the ICU stay. A recent prospective study that applied the same ultrasound surveillance protocol, substantially confirmed these data15. Although the clinical implication of some of these events remains to be established, these results suggest the utility of multiple compression ultrasound examinations during ICU stay.
Since DVT also occurred in patients on mechanical and pharmacological thromboprophylaxis, more effective preventive strategies seem to be necessary to reduce VTE in the critically ill population. However, bleeding complications also occurred relatively frequently in this setting, suggesting that thromboprophylaxis must be carefully considered in these patients. Unfortunately, in a recent large randomised controlled trial, LMWH was not superior to UFH in decreasing the incidence of proximal DVT nor in reducing the incidence of major bleeding complications28, although the rate of PE was significantly lower with LMWH than with UFH (1.3 vs 2.3%; hazard ratio, 0.51; 95% CI, 0.30–0.88; p=0.01) The best antithrombotic prophylaxis in this setting does, therefore, remain an unresolved issue.
Our analysis has several limitations: First, we cannot exclude important differences in baseline characteristics and in other concomitant risk factors in patients with and without DVT; thus several other clinical and non-clinical variables could have had an impact on the outcomes, and we could not adjust for them. Second, our systematic review was restricted to case-control studies, and the application of formal meta-analytic methods to observational studies is controversial, since bias implicit in the study design may misrepresent the strength of associations within the data10. To minimise this potential bias, we selected only studies in which the diagnosis of DVT was objectively confirmed. Third, the studies included in our meta-analysis had different inclusion and exclusion criteria, and combining results across studies may be inappropriate. However, the heterogeneity between the studies, calculated using the I2 statistic, was generally low. Furthermore, we decided to perform the analyses using the random-effects model, an approach that accounts for some of the variance between studies. Fourth, since it is recognised that publication bias can affect the results of meta-analyses, we attempted to assess this potential bias using a funnel plot. Funnel plots that considered ICU mortality and mean hospital stay appeared symmetric, suggesting the absence of publication bias for these two outcomes. However, only a few studies have considered mean duration of mechanical ventilation, mean ICU stay and hospital mortality, and funnel-plot analysis could not be done: the presence of publication bias could not, therefore, be excluded for these three outcomes. Fifth, we were unable to perform an adjusted analysis accounting for difference in baseline characteristics and concomitant risk factors (other than DVT) that may contribute to length of stay and mortality among critically ill patients since the contributing studies did not uniformly present data required for such an analysis. This may have led to an overestimate of the WMD and RR estimates for these outcomes. Sixth, our study assumes that ultrasonography is the reference standard for the diagnosis of DVT in critically ill patients; although the reliability of ultrasonography in the ICU has not been tested, it is the dominant method of screening and diagnosis in this setting27. Finally, we cannot conclude that DVT is an independent risk factor for poor outcomes in ICU patients because we did not obtain individual patients’ data from single studies. Despite these limitations, the findings of the present meta-analysis are clinically important, at least insofar as they highlight the (largely undiagnosed) burden of disease attributable to VTE in critically ill patients. They point to the need for further studies in this area, and to the need for more effective strategies to prevent, diagnose and manage DVT in critically ill patients.
Conclusions
In summary, to the best of our knowledge, this is the first systematic review and meta-analysis of the literature investigating the effects of DVT in critically ill patients. Although the strength of our conclusions about the impact of DVT on critically ill patients is limited by the quality of the contributing data and the paucity of eligible studies with control subjects, it is clear that patients who experience DVT have significantly higher rates of morbid outcomes, highlighting the need for further research in this area.
Footnotes
Authors’ contributions
AM, YK and FD extracted, reviewed and statistically evaluated the primary data. AM, MN, FD and SS take primary responsibility for the manuscript. MN, SS, FF, MB and GFG re-evaluated the data and revised the manuscript.
The Authors declare no conflict of interest
References
- 1.Cook D, Attia J, Weaver B, et al. Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care. 2000;15:127–32. doi: 10.1053/jcrc.2000.19224. [DOI] [PubMed] [Google Scholar]
- 2.Gando S, Kameue T, Nanzaki S, et al. Participation of tissue factor and thrombin in posttraumatic systemic inflammatory syndrome. Crit Care Med. 1997;25:1820–6. doi: 10.1097/00003246-199711000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Crowther MA, Cook DJ, Griffith LE, et al. Neither baseline tests of molecular hypercoagulability nor D-dimer levels predict deep venous thrombosis in critically ill medical-surgical patients. Intensive Care Med. 2005;31:48–55. doi: 10.1007/s00134-004-2467-2. [DOI] [PubMed] [Google Scholar]
- 4.Crowther MA, McDonald E, Johnston M, et al. Vitamin K deficiency and D-dimer levels in the intensive care unit: a prospective cohort study. Blood Coagul Fibrinolysis. 2002;13:49–52. doi: 10.1097/00001721-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg JS, Kearon C, Douketis J, et al. The use of D-dimer testing and impedance plethysmographic examination in patients with clinical indications of deep vein thrombosis. Arch Intern Med. 1997;157:1077–81. [PubMed] [Google Scholar]
- 6.Cook D, Douketis J, Crowther MA, et al. The diagnosis of deep venous thrombosis and pulmonary embolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20:314–9. doi: 10.1016/j.jcrc.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch DR, Ingenito EP, Goldhaber SZ. Prevalence of deep venous thrombosis among patients in medical intensive care. JAMA. 1995;274:335–7. [PubMed] [Google Scholar]
- 8.Cook DJ, Crowther MA, Meade MO, et al. Prevalence, incidence, and risk factors for venous thromboembolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20:309–13. doi: 10.1016/j.jcrc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 9.McGinn T, Wyer PC, Newman TB for the Evidence-Based Medicine Teaching Tips Working Group. Tips for learners of evidence-based medicine: 3. Measures of observer variability (kappa statistic) CMAJ. 2004;171:1369–73. doi: 10.1503/cmaj.1031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. J Am Med Assoc. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Wells G, Shea B. Data extraction for nonrandomised systematic reviews. University of Ottawa; Ottawa: [Accessed on 30/12/2014]. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 12.De Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. Br Med J. 2001;323:101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel R, Cook DJ, Meade MO, et al. Burden of illness in venous thromboembolism in critical care: a multicenter observational study. J Crit Care. 2005;20:341–7. doi: 10.1016/j.jcrc.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Boddi M, Barbani F, Abbate R, et al. Reduction in deep vein thrombosis incidence in intensive care after a clinician education program. J Thromb Haemost. 2010;8:121–8. doi: 10.1111/j.1538-7836.2009.03664.x. [DOI] [PubMed] [Google Scholar]
- 17.Joynt GM, Li TS, Griffith JF, et al. The incidence of deep venous thrombosis in Chinese medical intensive care unit patients. Hong Kong Med J. 2009;15:24–30. [PubMed] [Google Scholar]
- 18.Khouli H, Shapiro J, Pham VP. Efficacy of deep venous thrombosis prophylaxis in the medical intensive care unit. J Intensive Care Med. 2006;21:352–8. doi: 10.1177/0885066606292880. [DOI] [PubMed] [Google Scholar]
- 19.Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med. 2005;33:1565–71. doi: 10.1097/01.ccm.0000171207.95319.b2. [DOI] [PubMed] [Google Scholar]
- 20.Major KM, Wilson M, Nishi GK, et al. The incidence of thromboembolism in the surgical intensive care unit. Am Surg. 2003;69:857–61. [PubMed] [Google Scholar]
- 21.Ibrahim EH, Iregui M, Prentice D, et al. Deep vein thrombosis during prolonged mechanical ventilation despite prophylaxis. Crit Care Med. 2002;30:771–4. doi: 10.1097/00003246-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Velmahos GC, Nigro J, Tatevossian R, et al. Inability of an aggressive policy of thromboprophylaxis to prevent deep venous thrombosis (DVT) in critically injured patients: are current methods of DVT prophylaxis insufficient? J Am Coll Surg. 1998;187:529–33. doi: 10.1016/s1072-7515(98)00223-3. [DOI] [PubMed] [Google Scholar]
- 23.Cafferata HT, Morrison S, Duer C, et al. Venous thromboembolism in trauma patients: standardized risk factors. J Vasc Surg. 1998;28:250–9. doi: 10.1016/s0741-5214(98)70161-2. [DOI] [PubMed] [Google Scholar]
- 24.Crowther MA, Cook DJ, Griffith LE, et al. Deep venous thrombosis: clinically silent in the intensive care unit. J Crit Care. 2005;20:334–40. doi: 10.1016/j.jcrc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Mckelvie PA. Autopsy evidence of pulmonary thromboembolism. Med J Australia. 1994;160:127–8. [PubMed] [Google Scholar]
- 26.Schelling SM, Beyer J, Kakkar AK, et al. Ultrasound screening for asymptomatic deep vein thrombosis after major orthopaedic surgery: the VENUS study. J Thromb Haemost. 2007;5:1431–7. doi: 10.1111/j.1538-7836.2007.02570.x. [DOI] [PubMed] [Google Scholar]
- 27.Schellong S, Hesselschwerdt HJ, Paar WD, et al. Rates of proximal deep vein thrombosis as assessed by compression ultrasonography in patients receiving prolonged thromboprophylaxis with low molecular weight heparin after major orthopedic surgery. Thromb Haemost. 2005;94:532–6. [PubMed] [Google Scholar]
- 28.PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364:1305–14. doi: 10.1056/NEJMoa1014475. [DOI] [PubMed] [Google Scholar]