Abstract
Background
There are limited published data on the characteristics of blood transfusion recipients in sub-Saharan Africa. This study describes the demographic characteristics of blood transfusion recipients and patterns of blood and blood component use in Zimbabwe.
Materials and methods
Data on the characteristics of the blood transfusion recipients (age, sex, blood group), blood components received (type, quantity), discharge diagnoses and outcomes following transfusion (discharge status, duration of stay in hospital), were retrospectively collected from four major hospitals for the period from January 1, 2012 to December 31, 2012. Diagnoses were grouped into broad categories according to the disease headings of the International Classification of Diseases (ICD-10). Surgical procedures were grouped into broad categories according to organ system using ICD-9.
Results
Most of the 1,793 transfusion recipients studied were female (63.2%) and in the reproductive age group, i.e. 15–49 years (65.3%). The median age of the recipients was 33 years (range, 0–93). The majority of these recipients (n=1,642; 91.6%) received a red blood cell transfusion. The majority of the patients were diagnosed with conditions related to pregnancy and childbirth (22.3%), and diseases of blood and blood-forming organs (17.7%). The median time spent in hospital was 8 days (range, 0–214) and in-hospital mortality was 15.4%.
Discussion
Our sample of blood transfusion recipients were fairly young and most of them received red blood cell transfusions. The majority of patients in the reproductive age group received blood transfusions for pregnancy and childbirth-related diagnoses.
Keywords: blood usage, diagnosis, transfusion, International Classification of Diseases (ICD)
Introduction
Critical review and continuous evaluation of the use of blood and its components are essential activities aimed at improving transfusion practice1. These involve studying the demographic characteristics of blood transfusion recipients, the patterns of blood and blood component use, the clinical conditions and specialties requiring blood transfusion, and the risks associated with blood transfusion in a population. Monitoring blood utilisation is one way of assessing the present and future demands for blood and reducing unnecessary transfusions2. Data on blood utilisation is especially helpful in resource-limited settings in which there are always competing needs for scarce resources. Blood is one such scarce health resource and ensuring its safety and clinical effectiveness requires a great deal of investment, both human and financial. Information on blood utilisation will assist in conducting cost-effectiveness analyses3, establishing clinical practice guidelines, planning efforts for recruitment of new blood donors and streamlining resources for the therapeutic benefit of patients2,4.
A number of studies on blood and blood component utilisation have been carried out in recent years1–3,5–12. Substantial variations in transfusion practices, arising from differences in population age structures, prevalences of conditions requiring transfusion, and levels of health care provision, have been reported4,13,14. However, most of these studies have been conducted in developed countries in which the population and disease burden are different from those in developing countries. Most developed countries have an ageing population, chronic non-infectious diseases and advanced surgical technology3,6,8,12,15, all of which may result in different patterns of blood component use when compared with developing countries. In contrast, the population in developing countries is predominantly young and blood utilisation patterns are likely to be different. There are limited published data on the characteristics of blood transfusion recipients in sub-Saharan Africa. The few studies reported that most of the limited blood supplies in developing countries are used for complications of pregnancy and childbirth, trauma and severe anaemia in childhood16–18. It is, therefore, imperative that blood utilisation studies are carried out in these settings to establish local use patterns in order to be able to meet patients’ needs effectively.
In Zimbabwe, the National Blood Service, Zimbabwe (NBSZ), is responsible for ensuring a safe, adequate, accessible and affordable supply of blood and blood components. Currently, the NBSZ only has complete information on the number and type of blood and its components issued to hospitals; however, the actual consumption data are not readily available at hospital level. Establishing utilisation patterns, especially basic parameters such as the number of patients and components transfused each year, will enable the blood service to dispatch its mandate efficiently and effectively. In this study, the demographic characteristics of patients receiving blood transfusions are described, as are the utilisation patterns of blood and blood components and discharge diagnoses.
Materials and methods
Setting
The study was conducted in four major hospitals, all located in Harare, which had the highest number of units of blood issued nationwide by the NBSZ in 2012; of the four hospital, three were public sector referral hospitals (Parirenyatwa Group of Hospitals, Harare Central Hospital, Chitungwiza Central Hospital) and one was a private hospital (West End Hospital). The four hospitals have a combined capacity of 2,490 beds, annual admissions of 111,794 patients, annual discharges of 100,774 patients, and provide most of the medical and surgical specialties.
Study design
A retrospective analysis of blood transfusion recipient data, covering all blood and blood components transfused and recorded over a 12-month period, was undertaken. The primary data sources were manually completed blood bank registers, from which a sample of blood transfusion recipients was selected.
A computerised standard discharge summary for each patient is routinely completed, at the end of hospital stay, by staff in the medical records department of each hospital. Different systems are in place at the four hospitals, however they were all able to provide all the data fields required for this study. These registries were used to obtain demographic and discharge diagnoses for all the transfused recipients. Data collected from the blood banks were linked with each transfusion recipient’s discharge summary, using the recipient’s full name and hospital number.
Data collection
Data were collected retrospectively from the four hospitals for the 12-month period from 1 January to 31 December, 2012 and covered all blood and blood components recorded in the blood bank registers during this period. A sample size of 600 blood transfusion recipients per hospital, stratified by calendar month was targeted (i.e. 50 blood transfusion recipients were systematically sampled per month for each hospital). The monthly sampling fractions for each hospital were calculated by dividing the target sample size (n=50) by the actual total number of recipients registered each month. Transfusion recipient and blood component information was extracted from the blood bank registers. Data collected from the blood bank registers included full name, hospital number, component type, number of units of component, recipient blood group, donor blood group, date of issue of component and blood bank identification number for each transfusion recipient. Using the recipient’s full name and hospital number, the discharge summaries were extracted from the computerised registries. The variables included in the final dataset were characteristics of the patient (age, sex, blood group), blood components received (type, quantity), primary discharge diagnoses and outcomes following transfusion (discharge status, duration of stay in hospital).
Data evaluation
The data collected were computerised using Epi-Info version 719, then transferred to Microsoft Excel 2010 (Redmond, WA, USA) and Stata 1220 for cleaning and statistical analysis. Transfused patients were grouped into broad diagnostic categories according to the 21 (I-XXI) chapters of the International Classification of Diseases (ICD-10). Surgical procedures were grouped into broad categories according to organ system using ICD-9.
Statistical analysis
Categorical demographic and clinical variables (e.g. type of components transfused, in-hospital mortality rates) are summarised using frequency counts and percentages; all continuous measures (e.g. duration of hospital stay) had non-normal distributions so are summarised using medians and ranges. The age groups were categorised into: 0–14, 15–24, 25–54, 55–64 and over 65 years. Data were stratified according to sex, age, and diagnosis of the recipients. Differences between proportions were evaluated using Fisher’s exact test; differences between medians were evaluated using the Mann-Whitney U-test (two groups) or the Kruskal-Wallis test (more than two groups). An a priori significance level of α=0.05 was set for all analyses.
Ethical considerations
Ethical approval was obtained from the Medical Research Council of Zimbabwe (MRCZ/B/402) and the Joint Parirenyatwa Hospital (Harare) and College of Health Sciences Research Ethics Committee (JREC75/12). Additional approval was obtained from each of the participating hospitals.
Results
Blood and blood component distributions
A total of 53,803 units of blood were distributed nationally during the period under study (January–December 2012). More than half of the blood components (n=28,293; 52.6%) were distributed to the four hospitals included in this study. Of these blood components, 23,970 (84.7%) were distributed as red blood cells, 3,014 (10.7%) as fresh-frozen plasma, 954 (3.4%) as platelets, 146 (0.5%) as paediatric packs, 90 (0.3%) as cryoprecipitate and 119 (0.4%) as whole blood.
Transfusion recipients and blood components
Of the 2,400 patients who were systematically selected for the study, discharge summaries were available for 1,793 patients (74.7%). Of these, 1,642 (91.6%) received a red blood cell transfusion. The total number of units transfused to patients in the sample during the study period was 4,249, representing 15.0% of the blood components distributed to the four hospitals. Most of the transfused components were red blood cells (n=3,660; 86.1%) followed by fresh-frozen plasma (n=444; 10.4%), platelets (n=93; 2.2%), cryoprecipitate (n=32; 0.8%) and whole blood (n=20; 0.5%).
Demographic characteristics
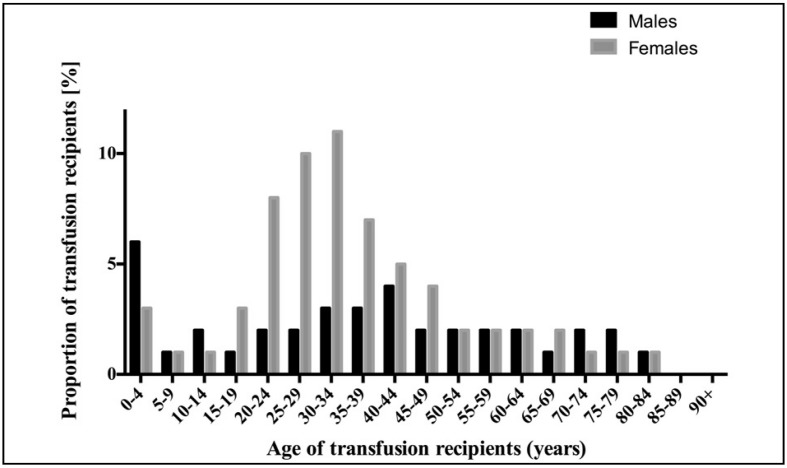
The demographic characteristics and blood groups of the recipients are shown in Table I. Most transfusion recipients were female (1,126; 63.2%), of whom 851 (75.6%) were in the reproductive age group (15–49 years). The median age of the recipients was 33 (range, 0–93) years. The mean age was 35 (standard deviation [SD]: 20) years. Approximately 10% of transfusion recipients were at least 65 years old, while 13.4% recipients were under the age of 15 years. The most common blood group type was O Rhesus positive (531, 45.7%). The distribution of blood transfusion recipients according to sex and age is shown in Figure 1.
Table I.
The distribution of age and ABO blood groups of blood transfusion recipients according to sex.
| Variable | Recipients | Males | Females | |||
|---|---|---|---|---|---|---|
|
|
||||||
| n | % | n | % | n | % | |
| Age range (years) | ||||||
| 0–14 | 238 | 13.4 | 148 | 62.2 | 90 | 37.8 |
| 15–24 | 250 | 14.1 | 58 | 23.2 | 192 | 76.8 |
| 25–54 | 985 | 55.6 | 282 | 28.6 | 703 | 71.4 |
| 55–64 | 115 | 6.5 | 57 | 49.6 | 58 | 50.4 |
| 65+ | 195 | 10.9 | 107 | 54.9 | 88 | 45.1 |
| Not recorded | - | - | - | - | - | - |
|
| ||||||
| Blood group | ||||||
| A+ | 335 | 28.8 | 132 | 39.4 | 203 | 60.6 |
| A− | 12 | 1.0 | 3 | 25.0 | 9 | 75.0 |
| AB+ | 48 | 4.1 | 20 | 41.7 | 28 | 58.3 |
| AB− | 3 | 0.3 | 0 | 0 | 3 | 100 |
| B+ | 209 | 18.0 | 74 | 35.4 | 134 | 64.1 |
| B− | 5 | 0.4 | 3 | 60.0 | 2 | 40.0 |
| O+ | 531 | 45.7 | 208 | 39.2 | 323 | 60.8 |
| O− | 20 | 1.7 | 12 | 60.0 | 8 | 40.0 |
| Not recorded | 630 | - | - | - | - | - |
Table percentages exclude missing values.
Figure 1.
The distribution of blood transfusion recipients by age and sex.
Distribution of transfusion recipients by age, component transfused and primary discharge diagnosis
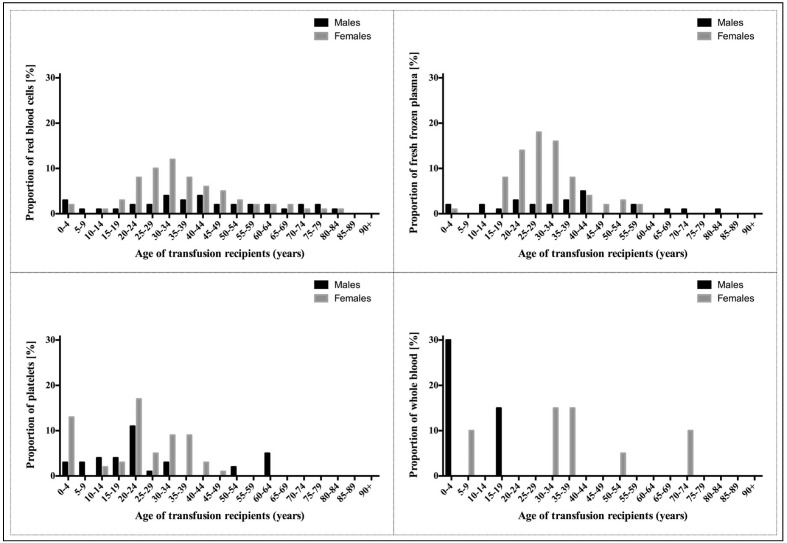
Figure 2 shows recipient age and sex distributions per blood component transfused. Of all the blood components transfused, the majority were transfused to women. Transfusion recipients in the reproductive age group (15–49 years) received 2,560 (69.9%) units of red blood cells, 372 (83.8%) units of fresh-frozen plasma, 62 (66.7%) units of platelets, 9 (45.0%) units of whole blood and 30 (93.8%) units of cryoprecipitate. Paediatric patients below the age of 5 years received 155 (4.2%) units of red blood cells, 13 (2.9%) units of fresh-frozen plasma; 15 (16.1%) units of platelets and 6 (30.0%) units of whole blood. Transfusion recipients aged 65 years or older received the following units: red blood cells (n=394; 10.8%), fresh-frozen plasma (n=16; 3.6%) and whole blood (n=2; 10.0%).
Figure 2.
Age and sex distributions of blood transfusion recipients by blood component transfused.
Discharge diagnoses were available for 91.6% of the blood transfusion recipients, with the rest of the recipients reported as having undergone surgical procedures. The distributions of blood transfusion recipients and blood components transfused over the broad ICD-10 discharge diagnosis categories are shown in Table II. The top five broad ICD-10 diagnostic categories accounting for most blood transfusion recipients were pregnancy and childbirth (n=363; 22.4%), diseases of blood and blood-forming organs (n=287; 17.5%), neoplasms (165; 10.1%), infectious and parasitic diseases (n=146; 9.0%) and diseases of the digestive system (8.2%). Most red blood cells (n=783; 23.9%) and fresh-frozen plasma (205; 46.4%) were used in the pregnancy and childbirth diagnostic category. The category of blood and blood-forming organs accounted for the use of most of the platelets (n=24; 25.8%) and cryoprecipitate (n=28; 87.5%), while most whole blood (n=7; 35.0%) was used in the injury and poisoning category. The median number of units transfused was two for red blood cells, platelets, whole blood and cryoprecipitate and four for fresh-frozen plasma. The principal diagnoses (four character subcategories) associated with high usage of red blood cells, fresh-frozen plasma, platelets, whole blood and cryoprecipitate were unspecified anaemia, false labour (as reported, see discussion), aplastic anaemia, burns, and hereditary factor VIII deficiency, respectively. The 25 most frequently encountered principal diagnoses are shown in Table III.
Table II.
Distributions of blood components transfused over the broad ICD-10 discharge diagnosis categories.
| Diagnostic category (ICD-10 codes) | Recipients | Transfused components | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| n | % | RBC | % | FFP | % | PLT | % | WB | % | CRY | % | |
| Pregnancy, childbirth and the puerperium | 363 | 22.4 | 783 | 23.9 | 205 | 46.4 | 14 | 15.1 | 3 | 15.0 | 2 | 6.3 |
| Blood and blood-forming organs | 287 | 17.5 | 610 | 18.3 | 30 | 6.7 | 24 | 25.8 | 4 | 20.0 | 28 | 87.5 |
| Neoplasms | 165 | 10.1 | 378 | 11.4 | 19 | 4.3 | 18 | 19.4 | 0 | 0.0 | 2 | 6.3 |
| Genitourinary system | 130 | 7.9 | 297 | 8.9 | 42 | 9.4 | 1 | 1.1 | 0 | 0.0 | 0 | 0 |
| Digestive system | 133 | 8.2 | 276 | 8.4 | 27 | 6.1 | 5 | 5.4 | 2 | 10.0 | 0 | 0.0 |
| Infectious and parasitic diseases | 146 | 9.0 | 257 | 7.8 | 35 | 7.8 | 8 | 8.6 | 0 | 0.0 | 0 | 0.0 |
| Injury and poisoning | 93 | 5.7 | 163 | 4.9 | 15 | 3.4 | 0 | 0.0 | 7 | 35.0 | 0 | 0.0 |
| Circulatory system | 63 | 3.8 | 128 | 3.8 | 8 | 1.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Respiratory system | 60 | 3.7 | 105 | 3.2 | 11 | 2.5 | 0 | 0.0 | 3 | 15.0 | 0 | 0.0 |
| Endocrine, nutritional and metabolic diseases | 41 | 2.6 | 68 | 2.1 | 12 | 2.7 | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 |
| Nervous system | 30 | 1.8 | 67 | 2.0 | 5 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Symptoms, signs and abnormal clinical and laboratory findings | 32 | 2.1 | 60 | 2.0 | 0 | 0.0 | 4 | 4.3 | 0 | 0.0 | 0 | 0.0 |
| Conditions originating in the perinatal period | 37 | 2.5 | 33 | 1.1 | 27 | 6.1 | 4 | 4.3 | 1 | 5.0 | 0 | 0.0 |
| Skin and subcutaneous tissue | 20 | 1.3 | 31 | 1.1 | 6 | 1.3 | 6 | 6.5 | 0 | 0.0 | 0 | 0.0 |
| Musculoskeletal system and connective tissue | 12 | 0.7 | 15 | 0.5 | 0 | 0.0 | 5 | 5.4 | 0 | 0.0 | 0 | 0.0 |
| Mental and behavioural disorders | 2 | 0.1 | 6 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Factors influencing health status | 2 | 0.1 | 5 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Congenital malformations | 6 | 0.4 | 3 | 0.1 | 2 | 0.4 | 3 | 3.2 | 0 | 0.0 | 0 | 0.0 |
| External causes of morbidity and mortality | 1 | 0.1 | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Ear and mastoid process | 1 | 0.1 | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Total percentages may not add up to 100% due to rounding.
ICD: International Classification of Diseases; RBC: red blood cells; FFP: fresh-frozen plasma; PLT: platelets; WB: whole blood; CRY: cryoprecipitate.
Table III.
The 25 most frequent ICD-10 diagnoses.
| Broad diagnostic category | Four character subcategories | Number of recipients | % | Number of RBC units | RBC% |
|---|---|---|---|---|---|
| Pregnancy, childbirth and the puerperium | Spontaneous abortion | 53 | 3.3 | 127 | 3.9 |
| Post-partum haemorrhage | 54 | 3.3 | 116 | 3.5 | |
| Ectopic pregnancy, unspecified | 29 | 1.8 | 76 | 2.3 | |
| False labour, unspecified | 35 | 2.2 | 52 | 1.6 | |
| Single spontaneous delivery, unspecified | 24 | 1.5 | 47 | 1.4 | |
|
| |||||
| Blood and blood forming organs | Anaemia, unspecified | 211 | 13.0 | 455 | 13.8 |
| Anaemia in other chronic diseases classified elsewhere | 21 | 1.3 | 40 | 1.2 | |
| Aplastic anaemia, unspecified | 11 | 0.7 | 35 | 1.1 | |
| Thrombocytopenia, unspecified | 9 | 0.6 | 21 | 0.6 | |
| Iron deficiency anaemia, unspecified | 3 | 0.2 | 10 | 0.3 | |
|
| |||||
| Neoplasms | Cervix uteri, unspecified | 42 | 2.6 | 106 | 3.2 |
| Leiomyoma of uterus, unspecified | 15 | 0.9 | 36 | 1.1 | |
| Stomach, unspecified | 11 | 0.7 | 24 | 0.7 | |
| Malignant neoplasm of prostate | 10 | 0.6 | 19 | 0.6 | |
| Bladder, unspecified | 7 | 0.4 | 17 | 0.5 | |
|
| |||||
| Genitourinary system | Unspecified kidney failure | 45 | 2.8 | 95 | 2.9 |
| Chronic kidney disease, unspecified | 17 | 1.0 | 46 | 1.4 | |
| Other specified abnormal uterine and vaginal bleeding | 10 | 0.6 | 26 | 0.8 | |
| Excessive and frequent menstruation with regular cycle | 10 | 0.6 | 25 | 0.8 | |
| Acute renal failure, unspecified | 9 | 0.6 | 16 | 0.5 | |
|
| |||||
| Digestive system | Gastrointestinal haemorrhage, unspecified | 62 | 3.8 | 138 | 4.2 |
| Other and unspecified intestinal obstruction | 11 | 0.7 | 20 | 0.6 | |
| Peptic ulcer, site unspecified | 6 | 0.4 | 17 | 0.5 | |
| Duodenal ulcer | 4 | 0.2 | 13 | 0.4 | |
| Peritonitis, unspecified | 6 | 0.4 | 11 | 0.3 | |
ICD: International Classification of Diseases; RBC: red blood cell.
The top five broad ICD-9 procedure categories accounting for most blood transfusion recipients, were surgical procedures related to the digestive system (32.2%), female genital organs (26.2%), musculoskeletal system (17.5%), male genital organs (6.7%) and obstetric procedures (4.7%). Most red blood cells were transfused during surgical procedures on the digestive system (29.8%) followed by operations on female genital organs (26.6%), operations on the musculoskeletal system (18.3%), obstetric procedures (5.6%) and operations on male genital organs (5.4%).
Outcomes
The mean and median duration of stay in hospital for transfusion recipients was 14 (SD: 18) and 8 (range, 0–214) days, respectively. The in-hospital mortality for transfused patients was 15.9%, while the overall mortality for all the patients was 6.6%. There were statistically significant sex and age differences in both time spent in hospital and survival rates at discharge. The duration of stay in hospital, and in-hospital mortality rate for the transfusion recipients are shown in Table IV.
Table IV.
Comparison of duration of stay in hospital and in-hospital mortality according to sex and age (n=1,793).
| Variables | Outcomes | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Length of stay in hospital | In-hospital mortality | |||||
|
|
||||||
| Recipients, n | Median (range) | p-value | Recipients, n | Rate (%) | p-value | |
| Sex | ||||||
| Male | 630 | 10 (0–145) | 637 | 20.7 | ||
| Female | 1,078 | 7 (0–214) | 0.005a | 1,093 | 13.8 | 0.001c |
| Not recorded | 85 | - | 63 | - | ||
|
| ||||||
| Age range (years) | ||||||
| 0–14 | 231 | 11 (0–145) | 232 | 19.3 | ||
| 15–24 | 238 | 7 (0–176) | 244 | 10.7 | ||
| 25–54 | 949 | 7 (0–198) | 964 | 14.6 | ||
| 55–64 | 118 | 9 (0–214) | 111 | 17.1 | ||
| 65+ | 172 | 12 (0–95) | <0.001b | 189 | 24.3 | 0.011c |
| Not recorded | 85 | - | 53 | - | ||
Mann-Whitney test;
Kruskal-Wallis test;
Fisher’s exact test.
Discussion
This study provides information on the demographic characteristics of blood transfusion recipients and patterns of blood component use in four urban hospitals in Harare. Current information suggests that this is the first attempt at characterising blood component utilisation in Zimbabwe and is one of the few studies of this nature in sub-Saharan Africa. Of the 2,400 patients sampled from the hospital blood banks, discharge records were obtainable for 1,793 patients (74.7%). The discrepancy may have arisen due to erroneous entries of key information (patients’ hospital identification numbers and names) by the hospital staff into the blood bank registers as well as the discharge summaries. It is worth noting that the blood bank registers at the four hospitals are operated manually while computerised registries are in place for recording patients’ discharge summaries. It was not possible to identify patients who received a transfusion using the computerised registries since that information is not captured. While the retrieval rate is acceptably high, it reflects on the need for improved data collection and record keeping within the hospitals. Utilising electronic registries to capture all blood bank records will significantly reduce the discrepancies observed in this study and improve any future look-back activities, which are currently difficult and lack precision. Reviewing and extracting transfusion data from manual registers is labour-intensive, time-consuming and costly, which may explain, in part, the limited number of studies undertaken in sub-Saharan Africa.
The study sample comprised a much younger cohort of transfusion recipients than in studies reported from developed countries in which the majority of transfusion recipients were above the age of 60 years3,6,7,11,21–25. The low median age in this study reflects the age structure of the general population in Zimbabwe, which is characterised by young people, with only 3.6% being over the age of 65 years26. The life expectancy at birth for Zimbabweans is currently estimated at 58 years, while the global population average is 70 years27. Developed countries are predominantly characterised by ageing populations, due to the higher mean life expectancies. An older but comparable transfusion recipient population was reported in Brazil (49 years)28, whereas a much younger recipient population was described from sub-Saharan Africa18,29–31. The Zimbabwe national census statistics states that the male to female ratio in Zimbabwe is 0.95. This may, in part, explain why our sample cohort had more females receiving transfusion. Transfusion recipients in sub-Saharan Africa are mostly children, in malaria endemic areas, and women of childbearing age due to complications of pregnancy17,18,29,30. Receiving a blood transfusion earlier in life may have far-reaching implications for recipients, because of the inherent risks, principally transmission of infectious agents, and adverse reactions associated with blood transfusion. Exposure to these risks, for instance transfusion-transmitted infections, at a younger age may leave the recipient affected for considerably longer than a much older recipient because of differences in life expectancies at the time of exposure. For example, if a young transfusion recipient were to be infected by human immunodeficiency virus, the number of years lived with the disease would be much more than those of an older recipient. On the same note if a younger transfusion recipient were to die from a transfusion-related complication, the number of life years lost would be much greater than the number an older recipient would lose. Unlike the situation in developed countries, most transfusions in developing countries are given early in life, which increases the long-term impact of the transfusions in recipients. The present study included fewer paediatric patients receiving blood transfusion, unlike other studies carried out in sub-Saharan Africa, which included many paediatric patients receiving blood transfusion for anaemia related to malaria18,29,30. This difference can be attributed to the location of the hospitals included in this study, as the four hospitals are at a high altitude and serve a population which live in a relatively malaria-free area.
The distribution of ABO blood groups, among patients included in the study is consistent with that reported in the donor population in Zimbabwe32. Acute shortages of blood of specific groups are often experienced in Zimbabwe making an understanding of the distribution of blood groups among transfusion recipients important. This information is necessary for planning bleeds as well as distributions of blood and blood components; subsequently ensuring that patients receive blood matching their ABO blood group and Rhesus type.
The majority of the transfusion recipients in the sample (92.6%) received red blood cell transfusions, which is higher percentage than that reported in studies from Brazil (42.0%)28 and Korea (43.3%)1, although it is comparable to the proportions reported from developed countries (56–96%)2,15,24. This is a reflection of the proportions in which blood and blood components are prepared and distributed in Zimbabwe. More than 90% of all collected blood is processed into components and about 87% of the components are distributed as red blood cells33. The distribution of blood components transfused to the recipients in this study was comparable to the distribution of blood components issued to the hospitals included in the study. Currently, the use of whole blood for transfusions is very low in Zimbabwe. Whole blood is only issued for transfusion following special requests by clinicians, mainly for massive haemorrhages and exchange transfusions.
In this survey, women, especially in the reproductive age group (15–49 years), received the majority of the blood and blood components transfused. This is consistent with findings in other countries in sub-Saharan Africa where women receive more blood for pregnancy-related complications, mainly related to intra-partum and post-partum haemorrhage18,29,30. Studies from developed countries found that more men than women receive blood transfusion3,8. This may be attributed to advanced health care services and management practices during pregnancy, which reduce the associated complications requiring transfusion. An assessment of how pregnancy is managed among women in Zimbabwe may assist in developing more effective ways of reducing pregnancy-related complications and subsequently the need for blood transfusion. An understanding of the complications occurring in these young women together with the associated risk factors may go a long way to reducing maternal mortality, which is currently fairly high. It might also help reduce exposure to blood transfusion and hence the associated risks.
The median number of units transfused for each component ranged from two for red blood cells, fresh-frozen plasma, whole blood and cryoprecipitate to four for platelets. These are similar to findings from Brazil (3 units)28, Sweden and Denmark (4 units)15, and Germany (1–4 units)2. In this study the median number of red blood cell units transfused per patient was similar for both males and females, while it increased from one unit for recipients aged less than 15 years to two to four units for recipients over the age of 15 years. The median number of red blood cell units transfused also varied depending on the broad diagnostic category, as reported in the literature2.
The top five diagnoses for which patients received a blood transfusion in Zimbabwe were pregnancy and childbirth, diseases of blood and blood-forming organs, neoplasms, genitourinary system disorders and diseases of the digestive system. In contrast to developing countries, most published studies from African countries9,18,29,30 did not classify the diagnoses according the ICD-10, making direct comparisons with the findings in this study impossible. Only one recent study from Namibia used the ICD-10 classification31 and reported results which are relatively similar to those of our study. This study reported diagnoses in the infectious disease, pregnancy and gastrointestinal categories as accounting for 14.8%, 11.1% and 6.1% of red blood cell units issued, respectively. This underscores the importance of standardising methodologies for undertaking comparative analyses using data from related studies. However, malaria and pregnancy-related conditions were cited among top indications for blood transfusion in the region18,29,30. Studies from non-African countries found that neoplasms, injury, digestive system, and circulatory system as the main diagnoses associated with transfusion1,3,24,28. This strongly demonstrates that blood utilisation patterns vary significantly within regions and according to practices and patients’ characteristics. The disease burdens, levels of organisation and advancement of health care in the different settings also contribute to significant differences in blood utilisation. In developed countries, for example, more resources are available and pregnant women are better cared for, from the onset of pregnancy, which reduces the likelihood of complications and requirements for blood transfusion. Data on the number of pregnant women requiring blood for transfusion is not currently available. However, we recognise the importance of gathering such data especially in the light of the high maternal mortality rate reported in Zimbabwe. Further work addressing the use of blood and blood components in obstetric care is warranted.
In this study, unspecified anaemia was the most common principal diagnosis (73.5%) under diseases of blood and blood-forming organs. This may be due to limited capacity and expertise to make specific diagnoses, thus overestimating diseases of blood and blood-forming organs, while underestimating other diagnoses. This may also be attributed to poor record-keeping and coding by clinicians and medical records staff. False (threatened) labour was provided as a criterion for the discharge diagnoses for transfusion recipients recorded in this study, although it is recognised that blood transfusion has no role in the management of this condition. This is an example of inaccurate, misleading and poor record-keeping, and erroneous coding. A prospective study to evaluate the indications for blood transfusion will assist in classifying transfusion triggers and describing the profiles of individual recipients more precisely. This will also present an opportunity for assessing the use of and adherence to guidelines on prescribing blood in Zimbabwe34. “The National Blood Policy of the Republic of Zimbabwe”35 and “Standards for Blood Donation, Processing and Clinical Transfusion in Zimbabwe”36 were published and accepted for national distribution in 2010. These documents advocate the formation of Hospital Transfusion Committees, which will have the responsibility of assessing the clinical use of blood and blood components in hospitals. In addition, recommendations are made for the establishment of a haemovigilance programme, which will provide surveillance on blood donations and clinical use of blood. Findings from these committees and programmes will ensure a platform for determining adherence to policies, guidelines and practices in the good clinical use of blood, leading to effective auditing and improvements in transfusion practices, nationally. Training hospital personnel, periodic monitoring and evaluation of all activities related to clinical transfusion practices will improve the quality of record keeping, coding and most importantly, patients’ management and care. This would subsequently help enhance the quantity and quality of reports of transfusion-related adverse events, both previously reported as substantially low37.
In-hospital mortality among blood transfusion recipients in this study was lower than that reported in Brazil (24.0%)28, although it was much higher than that in the USA (7.6%)23. Data for patients who did not receive blood transfusion were not available so it was not feasible to assess the association between blood transfusion and in-hospital mortality. The duration of stay in hospital generally provides the level of disease severity of the admission diagnoses and extent of complications that may arise. The duration of stay in hospital for transfusion recipients would be more informative when compared with data for patients who had not received blood transfusions, as this would provide an indication of the additional burden due to transfusion. However, data for patients who did not receive blood transfusion were not available for this study and a comparison was only possible with data for transfusion recipients reported from other countries. In addition, the duration of stay can serve as an indicator of the cost burden of the admitting diagnoses beyond the costs incurred because of transfusion. The median duration of stay in hospital was lower but comparable to that reported for Brazil (13 days; range, 0–278)28. A study by Morton et al. in the USA found that the mean duration of stay in hospital was 9.2 days23. Male transfusion recipients in this study remained in hospital for more days and had a higher mortality rate, at discharge, than women recipients. This may indicate that male recipients had more serious medical conditions, on admission, which required more time to recuperate, with a reduced chance of survival.
The study has a number of potential limitations. First, the data were obtained from four urban hospitals, which may not accurately represent overall transfusion practices in Zimbabwe. A future prospective national study, which incorporates hospitals in all provinces at different locations, will provide a better representation of the patterns of blood utilisation throughout the country. Information on the total number of patients who received transfusions and the actual number of blood components transfused, over a specified period, was not available for this study. This information remains largely unknown because of the unavailability of electronic data systems and reliance on manually kept registers which are often not fully recorded. The number of blood components distributed was, therefore, used as a surrogate measure of consumption. These are key denominators, which are necessary in epidemiological studies and without them it is not possible, from the available data, to accurately extrapolate the information nationally.
Conclusions
In this study sample, blood transfusion recipients were relatively young, which may result in prolonging of the long-term impact of blood transfusion in these recipients. A higher proportion of women received blood transfusion for conditions related to pregnancy and childbirth. Transfusion of red blood cells was predominant. Although this study presents data from a limited number of hospitals and a small proportion of patients who received blood transfusion in Zimbabwe, the findings provide an insight into the characteristics of blood transfusion recipients and form the basis for planning more comprehensive blood utilisation studies in Zimbabwe. The study also highlights the probable differences in utilisation patterns and practices in different settings. This warrants more research in our settings aimed at generating evidence necessary to inform policies and practices.
Acknowledgements
The research leading to these results received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement n. 266194. The Authors wish to thank the T-REC (building research capacity for Blood Transfusion Services in Africa) consortium for its support. The views expressed in the manuscript do not reflect the views nor do they imply endorsement of the suggestion by the funders.
The hospitals’ medical records and blood bank staff are acknowledged for the their assistance in retrieving the data. The Authors wish to thank Nomusa Mukadzambo, an NBSZ Research Data clerk, for the assistance rendered in entering data into Epi-Info.
Footnotes
Authorship contributions
NM had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All the Authors contributed to the design of the study and writing the paper.
The Authors declare no conflict of interest.
References
- 1.Lim YA, Lee WG, Cho SR, et al. A study of blood usage by diagnoses in a Korean university hospital. Vox Sang. 2004;86:54–61. doi: 10.1111/j.0042-9007.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann R, Buscher M, Linhardt C, et al. A survey of blood component use in a German university hospital. Transfusion. 1997;37:1075–83. doi: 10.1046/j.1537-2995.1997.371098016449.x. [DOI] [PubMed] [Google Scholar]
- 3.Borkent-Raven BA, Janssen MP, van der Poel CL, et al. The PROTON study: profiles of blood product transfusion recipients in the Netherlands. Vox Sang. 2010;99:54–64. doi: 10.1111/j.1423-0410.2010.01312.x. [DOI] [PubMed] [Google Scholar]
- 4.Biggin K, Warner P, Prescott R, et al. A review of methods used in comprehensive, descriptive studies that relate red blood cell transfusion to clinical data. Transfusion. 2010;50:711–8. doi: 10.1111/j.1537-2995.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann R, Handtrack D, Zingsem J, et al. A survey of blood utilization in children and adolescents in a German university hospital. Transfus Med. 1998;8:185–94. doi: 10.1046/j.1365-3148.1998.00159.x. [DOI] [PubMed] [Google Scholar]
- 6.Wells AW, Llewelyn CA, Casbard A, et al. The EASTR Study: indications for transfusion and estimates of transfusion recipient numbers in hospitals supplied by the National Blood Service. Transfus Med. 2009;19:315–28. doi: 10.1111/j.1365-3148.2009.00933.x. [DOI] [PubMed] [Google Scholar]
- 7.Vamvakas EC, Taswell HF. Epidemiology of blood transfusion. Transfusion. 1994;34:464–70. doi: 10.1046/j.1537-2995.1994.34694295059.x. [DOI] [PubMed] [Google Scholar]
- 8.Geißler RG, Franz D, Buddendick H, et al. Retrospective analysis of the blood component utilization in a university hospital of maximum medical care. Transfus Med Hemoth. 2012;39:129–38. doi: 10.1159/000337956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arewa OP. One year clinical audit of the use of blood and blood components at a tertiary hospital in Nigeria. Niger J Clin Pract. 2009;12:429–33. [PubMed] [Google Scholar]
- 10.Balen S, Caser L, Ivankovic E, et al. Evaluation of fresh frozen plasma usage at the University Hospital Center Rijeka. Coll Antropol. 2009;33:1375–81. [PubMed] [Google Scholar]
- 11.Bosch MA, Contreras E, Madoz P, et al. The epidemiology of blood component transfusion in Catalonia, Northeastern Spain. Transfusion. 2011;51:105–16. doi: 10.1111/j.1537-2995.2010.02785.x. [DOI] [PubMed] [Google Scholar]
- 12.Cobain TJ, Vamvakas EC, Wells A, et al. A survey of the demographics of blood use. Transfus Med. 2007;17:1–15. doi: 10.1111/j.1365-3148.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 13.Titlestad K, Georgsen J, Jorgensen J, et al. Monitoring transfusion practices at two university hospitals. Vox Sang. 2001;80:40–7. doi: 10.1046/j.1423-0410.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MF, Yazer MH. Measuring and monitoring blood utilization. Transfusion. 2013;53:3025–8. doi: 10.1111/trf.12342. [DOI] [PubMed] [Google Scholar]
- 15.Kamper-Jørgensen M, Edgren G, Rostgaard K, et al. Blood transfusion exposure in Denmark and Sweden. Transfusion. 2009;49:888–94. doi: 10.1111/j.1537-2995.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- 16.Bugge HF, Karlsen NCT, Oydna E, et al. A study of blood transfusion services at a district hospital in Malawi. Vox Sang. 2013;104:37–45. doi: 10.1111/j.1423-0410.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 17.Schneider WH. History of blood transfusion in sub-saharan Africa. Transfus Med Rev. 2013;27:21–8. doi: 10.1016/j.tmrv.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Lackritz EM, Ruebush TK, 2nd, Zucker JR, et al. Blood transfusion practices and blood-banking services in a Kenyan hospital. AIDS. 1993;7:995–9. doi: 10.1097/00002030-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Dean AG, Arner TG, Sunki GG, et al. Epi Info™, a database and statistics program for public health professionals. CDC; Atlanta: 2011. [Google Scholar]
- 20.StataCorp. Stata Statistical Software: Release 12. StataCorp LP: College Station; 2011. [Google Scholar]
- 21.Cobain TJ, Vamvakas EC, Wells A, et al. A survey of the demographics of blood use. Transfus Med. 2007;17:1–15. doi: 10.1111/j.1365-3148.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamper-Jorgensen M, Ahlgren M, Rostgaard K, et al. Survival after blood transfusion. Transfusion. 2008;48:2577–84. doi: 10.1111/j.1537-2995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 23.Morton J, Anastassopoulos KP, Patel ST, et al. Frequency and outcomes of blood products transfusion across procedures and clinical conditions warranting inpatient care: an analysis of the 2004 healthcare cost and utilization project nationwide inpatient sample database. Am J Med Qual. 2010;25:289–96. doi: 10.1177/1062860610366159. [DOI] [PubMed] [Google Scholar]
- 24.Mathoulin-Pelissier S, Salmi LR, Verret C, et al. Blood transfusion in a random sample of hospitals in France. Transfusion. 2000;40:1140–6. doi: 10.1046/j.1537-2995.2000.40091140.x. [DOI] [PubMed] [Google Scholar]
- 25.Wallis JP, Wells AW, Matthews JN, et al. Long-term survival after blood transfusion: a population based study in the North of England. Transfusion. 2004;44:1025–32. doi: 10.1111/j.1537-2995.2004.03400.x. [DOI] [PubMed] [Google Scholar]
- 26.CIA. The World Factbook. [Accessed on 10/07/2014]. Available from: https://http://www.cia.gov/library/publications/the-world-factbook/geos/zi.html.
- 27.World Health Organization. Life Expectancy. [Accessed on 10/07/2014]. Available from: http://www.who.int/gho/mortality_burden_disease/life_tables/en/
- 28.Goncalez TT, Sabino EC, Capuani L, et al. Blood transfusion utilization and recipient survival at Hospital das Clinicas in Sao Paulo, Brazil. Transfusion. 2012;52:729–38. doi: 10.1111/j.1537-2995.2011.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natukunda B, Schonewille H, Smit Sibinga CT. Assessment of the clinical transfusion practice at a regional referral hospital in Uganda. Transfus Med. 2010;20:134–9. doi: 10.1111/j.1365-3148.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 30.Bugge HF, Karlsen NC, Oydna E, et al. A study of blood transfusion services at a district hospital in Malawi. Vox Sang. 2013;104:37–45. doi: 10.1111/j.1423-0410.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 31.Pitman JP, Wilkinson R, Liu Y, et al. Blood component use in a Sub-Saharan country: results of a 4-year evaluation of diagnoses associated with transfusion orders in Namibia. Transfus Med Rev. 2015;29:45–51. doi: 10.1016/j.tmrv.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Mapako T, Mvere D, Musekiwa Z, et al. Determination of ABO and RhD blood group distribution in the Zimbabwean donor population. Africa Sanguine. 2011;14:4–6. [Google Scholar]
- 33.National Blood Service Zimbabwe. National Blood Service Zimbabwe Annual Report. Harare: National Blood Service Zimbabwe; 2012. [Google Scholar]
- 34.National Blood Service Zimbabwe. Prescribing Blood. Harare: National Blood Service Zimbabwe; 2005. [Google Scholar]
- 35.Ministry of Health and Child Welfare. National Blood Policy of the Republic of Zimbabwe. Harare: Ministry of Health and Child Welfare; 2010. [Google Scholar]
- 36.Ministry of Health and Child Welfare. Standards for Blood Donation, Processing and Clinical Transfusion in Zimbabwe. Harare: Ministry of Health and Child Welfare; 2010. [Google Scholar]
- 37.Mafirakureva N, Khoza S, Mvere DA, et al. Incidence and pattern of 12 years of reported transfusion adverse events in Zimbabwe: a retrospective analysis. Blood Transfus. 2014;12:362–7. doi: 10.2450/2014.0156-13. [DOI] [PMC free article] [PubMed] [Google Scholar]