Abstract
Background
It is very evident that many precautions are taken regarding transfusion of red blood cells in patients with autoimmune haemolytic anaemia. Frequently, considerable efforts are made to examine the indication and serological compatibility prior to transfusion in such patients. However, at times, this may unnecessarily jeopardize patients who urgently require a red blood cell transfusion.
Materials and methods
Thirty-six patients with warm-type autoimmune haemolytic anaemia were included in this study. All patients had reactive serum autoantibodies and required blood transfusion. Standard serological assays were employed for the detection and characterization of antibodies to red blood cells.
Results
A positive direct antiglobulin test was observed in all 36 patients, in addition to detectable antibodies in both the eluate and serum. Significant alloantibodies were detected in the serum samples of three patients (anti-c, anti-JKa, and anti-E). In 32 patients, red blood cell transfusion was administered with no significant haemolytic transfusion reactions due to auto- and/or allo-antibodies. Due to overestimation of positive cross-matches three patients received no transfusion or delayed transfusion and died, and one patient died due to unrecognised blood loss and anaemia which was attributed to an ineffective red blood cell transfusion.
Discussion
Many of the reported recommendations regarding transfusion of red blood cells in autoimmune haemolytic anaemia are highly questionable, and positive serological cross-matches should not result in a delay or refusal of necessary blood transfusions.
Keywords: positive cross-match, serological incompatibility, incompatible RBC transfusion, autoimmune haemolytic anaemia, alloantibodies
Introduction
Patients with autoimmune haemolytic anaemia (AIHA) may frequently develop anaemic hypoxia that cannot be relieved by oxygen administration until therapeutic measures become effective. Apart from red blood cell (RBC) transfusion, no drug is yet available which can immediately stop and/or compensate haemolysis in such patients. During the last two decades, various authors have attempted to encourage clinicians to transfuse patients with decompensated AIHA, even with positive cross-matches due to free autoantibodies1–5. On the other hand, there are many reports which have addressed the risks that should be considered prior to RBC transfusion in these patients2–10.
In practice, these risks are not only challenging for suppliers but also for appliers, particularly in cases in which the patients’ autoantibodies are also present in serum. These antibodies are usually reactive with the vast majority of available RBC, leading to an undesirable impression of an incompatible blood supply and transfusion, respectively. This scenario is rather disconcerting, particularly when the transfusion services cannot exclude the co-existence of alloantibodies. In these situations, unsophisticated published and/or “in-house” recommendations are frequently stressed, i.e. RBC transfusion in these patients is associated with the risk of a haemolytic transfusion reaction due to mismatched blood, and thus the attending physician is responsible for any possible unfavourable transfusion outcome. Similarly, transfusion is only indicated in life-threatening situations, and, if indicated, the total volume transfused should not exceed 1 mL/kg/hour3,4. The aforementioned statements are still routinely used, sometimes resulting in shocking delays and even death of severely affected patients.
In this study, we present data from patients with true (clinically and laboratory verified) AIHA and detectable serum autoantibodies, and highlight that the unique caution associated with RBC transfusion in AIHA is more harmful than useful for these patients.
Materials and methods
All patients included in this study (n=36) had been treated at our institution and/or were referred to our laboratory for serological re-examination since 2000. Only patients with active AIHA, for whom serological and clinical information was available and who had detectable serum autoantibodies and required RBC transfusion, were included. Patients who did not receive transfusions or received compatible RBC transfusion (negative cross-match) have not been considered in this study. Prior to RBC transfusion, all patients were either receiving immunosuppressive therapy, i.e. prednisolone and/or azathioprine or cyclophosphamide, or prednisolone treatment was started with at least 100–250 mg. Serological assays including direct and indirect antiglobulin tests (DAT and IAT, respectively), identification and characterisation of serum and/or eluted antibodies, and antibody adsorption were performed as previously described11–13. This retrospective study was approved by the institutional ethics review board (EA 2/058112).
Results
From clinical and serological view points, all patients had active, warm-type AIHA. The causative autoantibodies were detectable on patients’ RBC and in serum samples in all cases. The vast majority had complement or non-complement activating IgG autoantibodies (n=28). Five patients had mixed autoantibodies (IgG/IgA/IgM), and one patient had strongly reactive IgA autoantibodies. Two patients had only a C3d-positive DAT. Significant alloantibodies were detectable in one case by alloadsorption (anti-E), and in two cases using the tube technique (anti-c in one patient, and anti-Jka in one patient). Four insignificant alloantibodies were also detected (anti-Cw, -P1, -Wra and -Kpa). The remaining 29 patients had no alloantibodies detectable either by the tube technique or by alloadsorption.
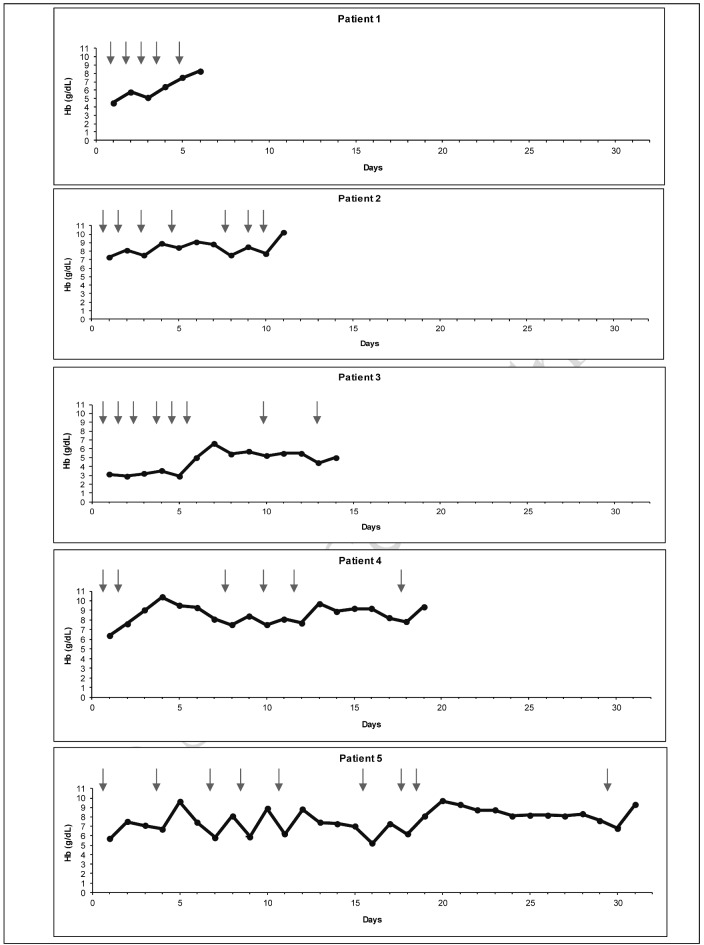
Emergency and/or repeated RBC transfusions were administered in 32 cases without any serious side effects, although transitorily increased haemolysis could not be invariably excluded, as reflected by an increase of lactate dehydrogenase in some instances (Table I). Nevertheless, significant haemolytic transfusion reactions did not occur in any patient. Though RBC transfusion frequently did not result in an adequate increase in haemoglobin concentration, its beneficial effect was evident in all cases. Some patients required repeated transfusions until stabilisation was achieved (Figure 1). Transfusions resulted in minor to significant increases of haemoglobin concentration and transfusions were tolerated in all cases.
Table I.
Effect of serologically incompatible (mismatched) transfusion of red blood cells (RBC) in patients with warm-type autoimmune haemolytic anaemia (AIHA).
| Transfusion series (n)* | Patients (n.) | Effect of RBC transfusions | ||
|---|---|---|---|---|
|
|
||||
| Hb increase (g/dL) | LDH concentration (U/L)** | |||
|
|
||||
| Increased/n. | Decreased/n. | |||
| >10 | 9 | 0.3–4.3 | (45–319)/4 | (31–527)/5 |
| 8 | 3 | 1.8–2.9 | 0 | (27–619)/3 |
| 6 | 4 | 1.7–3.5 | (12)/1 | (61–578)/3 |
| 5 | 2 | 1.2–4.9 | 0 | (18–771)/2 |
| 4 | 1 | 2.1 | (9)/1 | 0 |
| 3 | 3 | 0.7–4.6 | (42–115)/2 | (497)/1 |
| 2 | 7 | 0.2–7.1 | (13–156)/6 | (93)/1 |
| 1 | 3 | 1.4–2.3 | 0 | (67–109)/3 |
Transfusion series ≥2 RBC concentrates.
If measured prior to and after transfusion.
All detectable alloantibodies were considered and serological incompatibility was only related to serum autoantibodies.
Prior to transfusion of RBC, all patients were receiving treatment with immunosuppressive drugs or treatment was started with prednisolone (initially 100–250 mg/day). Increases in haemoglobin concentration are presented in a range of maximum delta values from 0.2 to 7.1 g/dL. Increases (14 patients) and decreases (18 patients) in lactate dehydrogenase are presented as a range of maximum delta values from 9 to 319 U/L and 18 to 771 U/L, respectively. Hb: haemoglobin; LDH: lactate dehydrogenase.
Figure 1.
Effect of red blood cell (RBC) transfusions (represented by arrows) in five representative patients who required repeated transfusions until stabilisation was achieved under treatment with immunosuppressive drugs.
Hb: haemoglobin concentration.
One patient had anaemic shock but survived following uncross-matched transfusion of three Rh(D)-negative units of RBC. This patient (a 16-year old female) was transferred to the university hospital as haemoglobin levels continued to fall from 6.5 to 4.5 and 2.8 g/dL during observation. On arrival, the patient was unconscious and her haemoglobin level was found to be 1.8 g/dL. In addition, the patient had epistaxis and severe autoimmune thrombocytopenia (Evans’ syndrome). Since an emergency RBC transfusion was necessary, the attending physician immediately administered 250 mg prednisolone and transfused unmatched units that were available under intensive care at that hospital. Following administration of the first unit, the patient regained consciousness and rapid improvement was achieved following transfusion of two additional units. On the second day, treatment with prednisolone (100 mg/day) was continued and the patient was discharged from hospital without further complications.
Four patients died due to decompensated haemolysis and anaemia (Table II). One of these patients (patient 1) had diabetes mellitus and coronary heart disease. Unfortunately, this patient was not transfused because her haemoglobin concentration appeared to be stable (>6 g/dL), and the delivered units from the blood service were labelled as incompatible due to serum autoantibodies. Although the attending physicians were informed and a note was attached to the units indicating immediate transfusion of the patient due to a history of coronary artery disease and acute chest pain, the patient did not receive any RBC transfusion. On the following day, she died.
Table II.
Patients who died due to delayed/cautious or no blood transfusion.
| Patient | Age | Sex | Comorbidities | Hb g/dL | Blood transfusion |
|---|---|---|---|---|---|
| 1 | 60 | Female | DM, CAD | 6.5–7.0 | No |
| 2 | 34 | Female | Splenomegaly | <2 | No |
| 3 | 61 | Male | - | 2.4 | Delayed |
| 4 | 53 | Male | Unrecognised bleeding | 2.7 | Inadequate |
DM: diabetes mellitus; CAD: coronary artery disease; Hb: haemoglobin concentration.
The situation in one patient (patient 2, Table II) is well-summarised by a citation from the original report by the attending physician: “The patients’ haemoglobin decreased further; somnolence and hypoxemia were noted. The patient did not receive additional blood transfusions, as the attending haematologist was of the opinion that this would aggravate the underlying condition. On the following day, the patient was intubated and transferred to the intensive care unit at that hospital. On arrival, the patient had metabolic acidosis (pH 7.1), decompensated haemolysis (haemoglobin <2 g/dL), circulatory failure (systolic blood pressure <50 mmHg), and the pupils were found to be enlarged. An emergency splenectomy was performed, but the patient died due to circulatory collapse.”
Patient 3 (Table II) had acute haemolytic anaemia and the causative warm autoantibodies belonged to the IgA class. Unfortunately, the attending physicians did not transfuse the delivered units, although they were informed that the serological incompatibility was solely related to the patient’s autoantibodies, and the in vitro incompatibility did not represent any contraindication to a necessary blood transfusion.
Patient 4 (Table II) has recently been described elsewhere12. In summary, this patient had severe AIHA with massive blood loss which remained unrecognised until death. The attending physicians suggested a haemolytic transfusion reaction because transfusions were ineffective and the patient’s condition continued to deteriorate despite cautiously administered RBC transfusions.
Discussion
Although the information included in this study is partly provocative, the main aim of the study is to minimise or even eliminate the historically implemented caution surrounding RBC transfusion in AIHA. We have previously demonstrated that the incidence of alloimmunisation, as well as the occurrence of adverse haemolytic transfusion reactions, is unnecessarily overestimated in AIHA1,13,14. This is supported by the present data. Indeed, RBC transfusion did not result in a significant increase of haemolysis in a single patient. In contrast, withholding transfusion was dangerous and even fatal in four cases. One patient developed shock as a result of delayed transfusion, another patient died due to anaemia related to haemolysis and uncompensated blood loss, a further patient was not transfused, and one patient received a delayed transfusion. The authors are aware of other patients who have died presumably due to delayed or withheld RBC transfusion as a result of serological incompatibility. Similar denied transfusions in AIHA patients requiring RBC have been reported by Conley and colleagues15. These authors described five patients who had life-threatening anaemia and were not transfused because their attending physicians were concerned that compatible blood could not be obtained. However, the patients were promptly transfused at a tertiary care medical centre.
Interestingly, there are no reports that definitively demonstrate significant exacerbation of haemolysis in patients with true AIHA. From a clinical viewpoint, the complications described in isolated AIHA patients who received RBC cannot solely be attributed to the transfusion, as has been previously suggested. For example, Petz and Garratty described three patients who died following blood transfusion3. However, one of these patients had human immunodeficiency virus infection and received intravenous immunoglobulin G (IVIgG) on one occasion prior to and also together with RBC transfusion. As can be extracted from the original publication16, this patient appears to have developed disseminated intravascular coagulation following the administration of IVIgG rather than transfusion of RBC: there were no signs of increased haemolysis in the report16. In fact, many reports have demonstrated the association between thromboembolic complications and IVIgG administration17. In the other two patients, death was attributed to new pulmonary emboli. One of these patients had been splenectomised 2 days prior to the development of acute respiratory distress syndrome. It appears rather likely that this patient’s embolus was related to the splenectomy rather than a delayed haemolytic transfusion reaction. Similarly, the complication described in the third patient might be attributed to the severe haemolysis caused by the described IgG and IgM autoantibodies. IgM warm autoantibodies have been repeatedly reported to cause fatal haemolysis3. In addition, transfusion-related acute lung injury cannot be retrospectively excluded in any of the three cases. This syndrome was previously unknown18. Based on our experience1, the results of the present study, and those in isolated reports on AIHA and RBC transfusion19–25 or even incompatible transfusion22, there is no doubt that administration of RBC may at least prevent death due to decompensated haemolysis in severely affected patients. Thus the notion that RBC transfusion may, per se, significantly worsen a patient’s condition must be stroingly circumscribed or overturned. However, in cases in which complement activation is involved, haemolysis may in fact initially slightly or moderately increase, but anaemic hypoxemia, which is more dangerous than a transiently increased haemolysis, can be prevented by continuous transfusion. As soon as the transfused RBC become coated with C3d, they survive haemolysis26, leading to an increase in the haemoglobin concentration and oxygen supply. The capacity of macrophages is not only limited27 but also partly diminished in AIHA patients, due to the fact that haemolysis in these patients is primarily due to cell phagocytosis. The induction of partial macrophage blockage is best demonstrated by the use of anti-D in Rh(D)-positive patients with autoimmune thrombocytopenia28,29.
The issue of alloantibodies being masked by autoantibodies has also been overestimated by somewhat “artificial” findings. The majority of studies reporting on the high incidence of co-existing alloantibodies and autoantibodies largely dealt with serological findings rather than with true AIHA3,30. We, and other groups, have demonstrated the induction of autoantibodies to RBC by transfusion13,31,32. These patients do not have true AIHA and their autoantibodies are usually less harmful than those in true AIHA. In the present study, we identified three alloantibodies which could theoretically have caused haemolytic transfusion reactions if they had been overlooked. Two of these antibodies were easily detected using the tube technique, and the remaining alloantibody became evident following alloadsorption. Even if weak alloantibodies might escape detection prior to transfusion, it is unclear whether such antibodies would actually cause significant haemolysis in patients with active AIHA. Ultimately, the macrophages of these patients are, at least in part, blocked due to ongoing phagocytosis of autologous RBC. In addition, weak alloantibodies are not invariably significant32 and, infrequently, they cause serological rather than haemolytic transfusion reactions1,12,30,33. Finally, we are aware of several reports dealing with haemolytic transfusion reactions due to alloantibodies masked by autoantibodies, but in these cases the patients were found to have “serological AIHA” and not true AIHA33–35.
Conclusions
In summary, we stress the recommendation regarding direct communication between blood suppliers and attending physicians. However, this communication must be made on a scientific basis on both sides. Everybody involved in the treatment of patients with AIHA must be well-informed concerning all aspects of this disorder.
Footnotes
Authorship contributions
YS, MB contributed equally to this work, both design the study, collected and analysed the data and wrote and edited the manuscript; AM collected the data, PA drafted the paper and revised it critically; SA designed the study, wrote and edited the paper and approved the final version.
The Authors declare no conflict of interest.
References
- 1.Salama A, Berghöfer H, Mueller-Eckhardt C. Red blood cell transfusion in warm-type autoimmune haemolytic anaemia. Lancet. 1992;340:1515–7. doi: 10.1016/0140-6736(92)92766-9. [DOI] [PubMed] [Google Scholar]
- 2.Dacie J. The Haemolytic Anaemias. London: Churchill Livingstone; 1992. [Google Scholar]
- 3.Petz L, Garratty G. Immune Hemolytic Anemias. New York: Chrurchill Livingstone; 2004. [Google Scholar]
- 4.Petz LD. A physician’s guide to transfusion in autoimmune haemolytic anaemia. Br J Haematol. 2004;124:712–6. doi: 10.1111/j.1365-2141.2004.04841.x. [DOI] [PubMed] [Google Scholar]
- 5.Ness PM. How do I encourage clinicians to transfuse mismatched blood to patients with autoimmune hemolytic anemia in urgent situations? Transfusion. 2006;46:1859–62. doi: 10.1111/j.1537-2995.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 6.Rosenfield RE, Jagathambal Transfusion therapy for autoimmune hemolytic anemia. Semin Hematol. 1976;13:311–21. [PubMed] [Google Scholar]
- 7.Jefferies LC. Transfusion therapy in autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 1994;8:1087–104. [PubMed] [Google Scholar]
- 8.Buetens OW, Ness PM. Red blood cell transfusion in autoimmune hemolytic anemia. Curr Opin Hematol. 2003;10:429–33. doi: 10.1097/00062752-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Packman CH. Hemolytic anemia due to warm autoantibodies. Blood Rev. 2008;22:17–31. doi: 10.1016/j.blre.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Barros MM, Blajchman MA, Bordin JO. Warm autoimmune hemolytic anemia: recent progress in understanding the immunobiology and the treatment. Transfus Med Rev. 2010;24:195–210. doi: 10.1016/j.tmrv.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Mayer B, Yürek S, Kiesewetter H, Salama A. Mixed-type autoimmune hemolytic anemia: differential diagnosis and a critical review of reported cases. Transfusion. 2008;48:2229–34. doi: 10.1111/j.1537-2995.2008.01805.x. [DOI] [PubMed] [Google Scholar]
- 12.Bartolmäs T, Mayer B, Yürek S, et al. Paradoxical findings in direct antiglobulin test and classification of agglutinating autoantibodies using eluates and monospecific anti-human globulin sera. Vox Sang. 2015;108:58–63. doi: 10.1111/vox.12187. [DOI] [PubMed] [Google Scholar]
- 13.Ahrens N, Pruss A, Kähne A, et al. Coexistence of autoantibodies and alloantibodies to red blood cells due to blood transfusion. Transfusion. 2007;47:813–6. doi: 10.1111/j.1537-2995.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 14.Mayer B, Salama A. Suspected hemolytic transfusion reaction masking major blood loss in a patient with autoimmune hemolytic disease. Transfusionsmedizin. 2012;2:28–30. In German. [Google Scholar]
- 15.Conley CL, Lippman SM, Ness PM, et al. Autoimmune hemolytic anemia with reticulocytopenia and erythroid marrow. N Engl J Med. 1982;306:281–6. doi: 10.1056/NEJM198202043060507. [DOI] [PubMed] [Google Scholar]
- 16.Bilgrami S, Cable R, Pisciotto P, et al. Fatal disseminated intravascular coagulation and pulmonary thrombosis following blood transfusion in a patient with severe autoimmune hemolytic anemia and human immunodeficiency virus infection. Transfusion. 1994;34:248–52. doi: 10.1046/j.1537-2995.1994.34394196624.x. [DOI] [PubMed] [Google Scholar]
- 17.Katz U, Shoenfeld Y. Review: intravenous immunoglobulin therapy and thromboembolic complications. Autoimmunity. 2005;38:123–37. doi: 10.1191/0961203303lu2168rr. [DOI] [PubMed] [Google Scholar]
- 18.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119:1757–67. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenberghe P, Zachee P, Verstraete S, et al. Successful control of refractory and life-threatening autoimmune hemolytic anemia with intravenous immunoglobulins in a man with the primary antiphospholipid syndrome. Ann Hematol. 1996;73:253–6. doi: 10.1007/s002770050237. [DOI] [PubMed] [Google Scholar]
- 20.Perrotta S, Locatelli F, La Manna A, et al. Anti-CD20 monoclonal antibody (Rituximab) for life-threatening autoimmune haemolytic anaemia in a patient with systemic lupus erythematosus. Br J Haematol. 2002;116:465–7. [PubMed] [Google Scholar]
- 21.Eve HE, Rule SA. Autoimmune haemolytic anaemia associated with mantle cell lymphoma. Int J Hematol. 2010;91:322–5. doi: 10.1007/s12185-009-0489-9. [DOI] [PubMed] [Google Scholar]
- 22.Das SS, Zaman RU, Safi M. Incompatible blood transfusion: challenging yet lifesaving in the management of acute severe autoimmune hemolytic anemia. Asian J Transfus Sci. 2014;8:105–8. doi: 10.4103/0973-6247.137445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoba S, Jaye DL, Cohen C, et al. Successful treatment of severe immune hemolytic anemia after allogeneic stem cell transplantation with bortezomib: report of a case and review of literature. Transfusion. 2015;55:259–64. doi: 10.1111/trf.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt ML, Joshi S, DeChristopher PJ, et al. Successful management of concurrent congenital dyserythropoietic anaemia and autoimmune haemolytic anaemia with splenectomy. Br J Haematol. 1998;102:1182–6. doi: 10.1046/j.1365-2141.1998.00892.x. [DOI] [PubMed] [Google Scholar]
- 25.Lucchini G, Masera N, Foti G, et al. A life-threatening paediatric case of acute autoimmune haemolytic anaemia (AIHA) successfully cured by plasma-exchange and combined immunosuppressive treatment. Transfus Apher Sci. 2009;40:115–8. doi: 10.1016/j.transci.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson JP, Frank MM. Studies on the in vivo effects of antibody. Interaction of IgM antibody and complement in the immune clearance and destruction of erythrocytes in man. J Clin Invest. 1974;54:339–48. doi: 10.1172/JCI107769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein HG, Anstee DJ. Mollison’s Blood Transfusion in Clinical Medicine. 11th edition. Oxford: Blackwell Publishing; 2006. [Google Scholar]
- 28.Salama A, Mueller-Eckhardt C, Kiefel V. Effect of intravenous immunoglobulin in immune thrombocytopenia. Lancet. 1983;2:193–5. doi: 10.1016/s0140-6736(83)90175-7. [DOI] [PubMed] [Google Scholar]
- 29.Lazarus AH, Freedman J, Semple JW. Intravenous immunoglobulin and anti-D in idiopathic thrombocytopenic purpura (ITP): mechanisms of action. Transfus Sci. 1998;19:289–94. doi: 10.1016/s0955-3886(98)00043-5. [DOI] [PubMed] [Google Scholar]
- 30.Branch DR, Petz LD. Detecting alloantibodies in patients with autoantibodies. Transfusion. 1999;39:6–10. doi: 10.1046/j.1537-2995.1999.39199116888.x. [DOI] [PubMed] [Google Scholar]
- 31.Salama A, Mueller-Eckhardt C. Delayed hemolytic transfusion reactions. Evidence for complement activation involving allogeneic and autologous red cells. Transfusion. 1984;24:188–93. doi: 10.1046/j.1537-2995.1984.24384225018.x. [DOI] [PubMed] [Google Scholar]
- 32.Zimring JC, Spitalnik SL, Roback JD, Hillyer CD. Transfusion-induced autoantibodies and differential immunogenicity of blood group antigens: a novel hypothesis. Transfusion. 2007;47:2189–96. doi: 10.1111/j.1537-2995.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 33.Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long-term serologic findings, and clinical significance. Transfusion. 1990;30:688–93. doi: 10.1046/j.1537-2995.1990.30891020325.x. [DOI] [PubMed] [Google Scholar]
- 34.Sümnig A, Mayer B, Kiefel V, et al. “Chameleonic” serological findings leading to life-threatening hemolytic transfusion reactions. Transfus Med Hemother. 2014;41(Suppl 1):IMH-P21. doi: 10.1159/000437198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee E, Redman M, Burgess G, Win N. Do patients with autoantibodies or clinically insignificant alloantibodies require an indirect antiglobulin test crossmatch? Transfusion. 2007;47:1290–5. doi: 10.1111/j.1537-2995.2007.01272.x. [DOI] [PubMed] [Google Scholar]